Abstract
Objective: Research has proven that the expression of CDC6 is tightly related to tumorigenesis and progression of various tumors. However, the effects of CDC6 in hepatocellular carcinoma remain uncertain. The main purpose of this research is to explore this relationship. Methods: We assessed the expression levels of CDC6 in a serious of cancers from GEPIA database. The expression of CDC6 in hepatocellular carcinoma tissue and normal liver tissue was compared, and further assessed by immunohistochemical staining. Graphpad software was performed for data analysis, and t-test and χ2 analysis were used to investigate the role of CDC6 in hepatocellular carcinoma. Results: The expression level of CDC6 was significantly higher in malignant carcinoid, melanoma, urothelial tumor, and hepatocellular carcinoma in the GEPIA online database. It was related to clinical progression of hepatocellular carcinoma. We found that the expression of CDC6 was correlated with tumor size (P=0.018) and the number of tumor nodes (P=0.003), but not with age, gender and AFP value (P>0.05). Conclusions: The expression level of CDC6 in hepatocellular carcinoma is related tightly to clinical findings. Detecting the expression of CDC6 might provide a new biomarker for patients with hepatocellular carcinoma.
Keywords: CDC6, hepatocellular carcinoma, prognosis
Introduction
Hepatocellular carcinoma is one of the most malignant tumors in the world, because of its high mortality and extreme aggressiveness [1]. The treatment effectiveness of hepatocellular carcinoma has been an urgent issue due to no significant intervention measures. According to the statistics of 2019 in United States [2], cancer was the second most common cause of deathin the USA, behind cardiovascular disease. About 42,030 newly diagnosed hepatocellular carcinoma cases occurred in 2019, while approximately 31780 patients with hepatocellular carcinoma died due to progression. Interestingly, the number of male patients was significantly more than females. There were approximately 42,810 newly diagnosed cases in 2020, and about 30160 died from hepatocellular carcinoma in 2020 [3]. The morbidity and mortality of hepatocellular carcinoma in 2020 were consistent with that of 2019. Among all cancers, death derived from hepatocellular carcinoma was fifth in males and seventh in females respectively. Reducing the high mortality is currently an important aim [4]. Much improvement has been obtained with treatment recently, including surgical resection, neoadjuvant radiotherapy and chemotherapy, targeted therapy, immune-therapy and endocrine therapy [5,6], improving the the prognosis and survival rate. However, the diagnosis of hepatocellular carcinoma still lacks sensitive biomarkers, and a biomarker is needed.
CDC6 (Cell Division Cycle 6) is highly similar to Saccharomyces cerevisiae Cdc6 (a protein critical for the initiation of DNA replication). CDC6 is a regulator at the early steps of DNA replication, and participates in checkpoint controls that ensure DNA replication is completed before mitosis is initiated. Many diseases were found to involve the dysregulation of CDC6, such as Meier-Gorlin Syndrome 5, Meier-Gorlin Syndrome 1, and various tumors [7]. Currently, many scholars have identified that CDC6 participated the prognosis and development of various cancers. For example, Zhao et al identified that CDC6 was up-regulated in glioblastoma multiforme and associated tightly with a poor prognostic signature [8]. Jiang et al have provenm that downregulation of Cdc6 inhibits tumorigenesis of osteosarcoma in vivo and in vitro [9]. Above research all showed that the expression of CDC6 was upregulated in tumors, and knockdown of expression of CDC6 could restrain significantly the carcinogenesis and development of cancer. It is worth noting that CDC6 could serve a biomarker for circulating tumor cells in patients with lung cancer based on the study of An et al [10]. The role of CDC6 in hepatocellular carcinoma remains unclear.
The purpose of this research was to explore expression of CDC6 in hepatocellular carcinoma and its relationship to clinicopathologic data. To further identify the function and effects of CDC6 in hepatocellular carcinoma, this study proposes that CDC6 is highly expressed and associated with prognosis of hepatocellular carcinoma. This may provide a biomarker and treatment target.
Materials and methods
Information of patients
All data in this study were obtained with these patients’ informed consent. All tissues of 90 patients with hepatocellular carcinoma involved in this study were from the Urology department. All tissue collection processes were carried out by scientific methods, and these tissues were confirmed by postoperative pathology.
Antibodies and reagents
IHC Anti-CDC6 antibody (ab109315; rabbit; 1:100), Abcam plc, Cambridge, UK.
Data extraction from GEPIA database
We extracted the differential expression plot of CDC6 between normal liver tissue and hepatocellular carcinoma from GEPIA online database, also the overall survival rate and disease-free survival rate from GEPIA (http://gepia.cancer-pku.cn/detail.php?gene). The expression of CDC6 in various tumors by immunohistochemical staining was obtained from the human protein atlas online database (https://www.proteinatlas.org/).
Immunohistochemical staining
According to the procedures of the immunohistochemical staining kit, paraffin specimens were sliced at 3-5 μm and grilled slices at 70°C for 50 minutes, and soaking in following order, xylene solution (1) for 10 mins, xylene solution (2) for 10 mins, absolute ethanol solution (1) for 5 mins, absolute ethanol solution (2) for 5 mins, 95% alcohol solution for 5 mins, 85% alcohol solution for 5 mins and 75% alcohol solution for 5 mins. We washed in PBS buffer for 2 minutes two times and placed the slices in sodium citrate buffer in the microwave (high heat for 5 mins firstly and then low to medium fire for 7-10 mins). Then, they were placed at room temperature for 2 hours and washed with PBS buffer for 2 mins two times. We removed excess water and dropped H2O2 in a wet box for 20 mins at room temperature, washed again and addeed CDC6 antibody for 4°C overnight. In next day, placed at room temperature for one hour, washed with PBS buffer for 2 mins two times, and added IgG secondary antibodies for 2 hours at room temperature. We washed again with PBS buffer, drop DAB (pre-formulated with liquid A and liquid B), stained at room temperature about 5-15 seconds. The staining reaction was halted with water and we next stained nuclei with hematoxylin solution for 5-8 seconds, followed by a tap water rinse for 2 mins. Soaking was in following order, 75% alcohol solution, 85% alcohol solution, and 95% alcohol solution each for 1 min, absolute ethanol (1) and absolute ethanol (2) each for 3 mins, xylene (1) and xylene (2) each for 5 mins. Finally, neutral gum sealer was applied and slides were observed under the microscope.
Statistical analysis
Statistical analysis was conducted by using the software Graphpad prism 7, and we used t-test for the results comparison between two groups. The relationship between the expression of CDC6 and these clinicopathologic data was investigated using χ2 test.
Results
High expression level of CDC6 is related significantly to prognosis of hepatocellular carcinoma
We analyzed the differential expression of CDC6 in 529 clinical samples (including 369 cases of hepatocellular carcinoma and 160 normal liver tissues) from GEPIA online database. The results demonstrated that the expression level of CDC6 in hepatocellular carcinoma was higher than in normal liver tissues (P<0.05; Figure 1A). Furthermore, we explored the role of CDC6 in the progression of hepatocellular carcinoma, with respect to prognosis, by a Kaplan-Meier curve analysis. The results of these analyses demonstrated that patients with hepatocellular carcinoma with lower expression of CDC6, had a high disease-free survival rate and overall survival rate (Figure 1B). Thus CDC6 is highly expressed in hepatocellular carcinoma and is associated with prognosis.
Figure 1.
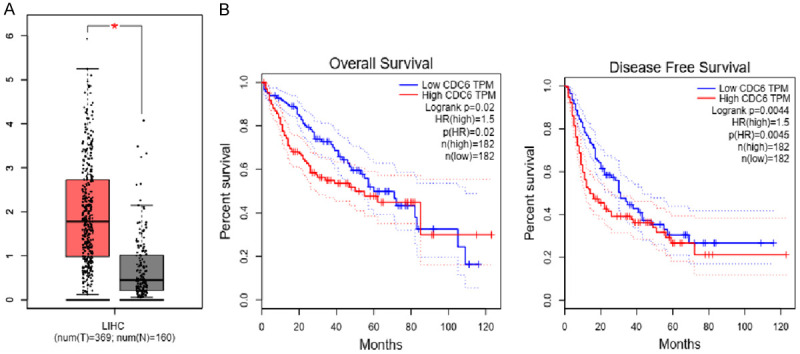
Expression level of CDC6 is positively correlated with poor prognosis of hepatocellular carcinoma. A. The expression of CDC6 in hepatocellular carcinoma and normal liver tissues in GEPIA database. Expression of cancer is higher than in the normal liver tissues. B. Overall and disease-free survival rates of low and high expression of CDC6. The higher the expression of CDC6, lower overall and disease-free survival rates in hepatocellular carcinoma.
Expression of CDC6 in various tumors
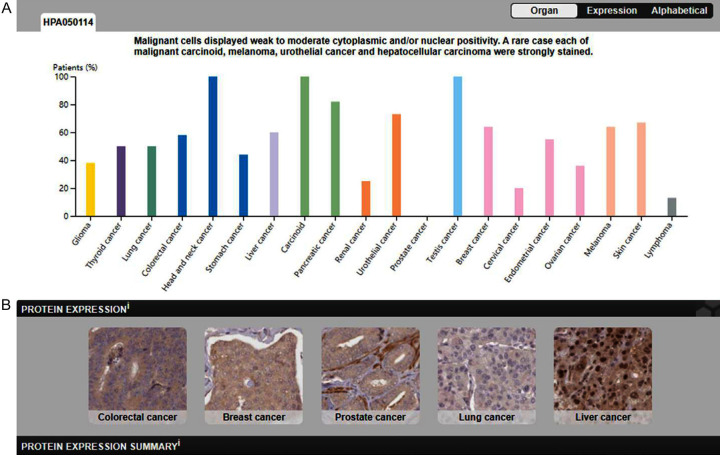
We systematically analyzed the expression level of CDC6 in several common tumors by using the human protein atlas online database. CDC6 had weak to moderate cytoplasmic and/or nuclear positivity. CDC6 was highly expressed in colorectal cancer, carcinoid, and testis cancer, while lowly expressed in renal cancer, cervical cancer and lymphoma, and not detected in prostate cancer (Figure 2A). Then we explored the expression of CDC6 in colorectal cancer, breast cancer, prostate cancer, lung cancer, and liver cancer from the online database. These results were consistent with previous results, and CDC6 was found to stain the cytoplasm (Figure 2B).
Figure 2.
Expression of CDC6 protein in several common tumors and hepatocellular carcinoma (IHC). A. The expression of CDC6 displayed moderate cytoplasmic positivity in most cancers; especially head and neck cancer strongly stained. B. Expression of CDC6 in colorectal cancer, breast cancer, prostate cancer, lung cancer and liver cancer by IHC staining.
High expression of CDC6 in hepatocellular carcinoma
Furthermore, we explore the specific role of CDC6 in hepatocellular carcinoma, whether CDC6 influenced the development and prognosis of hepatocellular carcinoma. An immunohistochemical staining assay was performed to detect the expression level of CDC6 in 90 samples from patients with hepatocellular carcinoma. Results demonstrated that the expression of CDC6 was increased significantly in these patients with hepatocellular carcinoma. We divided these patients into two groups based on high or low intensity of staining (Figure 3A). According to our results, CDC6 mainly stained in the cytoplasm in hepatocellular carcinoma, which was consistent with the results of the database. We further found that the expression of CDC6 was obviously lower in normal liver tissues compared with hepatocellular carcinoma (Figure 3B). The above data implied that the expression levels of CDC6 was associated tightly with the development and prognosis of hepatocellular carcinoma. Consequently, we explored the relationship between the expression of CDC6 and these clinicopathologic data of 90 patients with hepatocellular carcinoma. Five values were queried, including age, gender, number of tumor nodes, tumor size, and AFP value (Table 1). The results demonstrated that the expression level of CDC6 was significantly associated with the number of tumor nodes (P=0.003) and tumor size (P=0.018), but not related to age, gender, or AFP value (P>0.05). Overall, we found that the expression of CDC6 might be involved in tumorigenesis and development of hepatocellular carcinoma, and CDC6 was highly expressed in hepatocellular carcinoma and might serve as a target for treatment.
Figure 3.
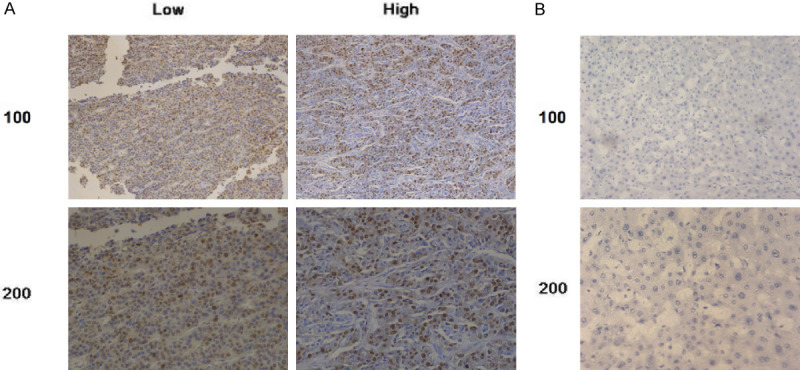
Expression of CDC6 in hepatocellular carcinoma. A. Expression of CDC6 in hepatocellular carcinoma (IHC). Tissues were classified as to low expression and high expression. B. The expression of CDC6 in normal liver tissues was used as a normal control group. The expression of CDC6 in normal liver tissue is low and stain is weak or negative.
Table 1.
Relationships of CDC6 and clinicopathological characteristics in 90 patients with hepatocellular carcinoma
| Feature | All n=90 | CDC6 expression | χ2 | P | |
|---|---|---|---|---|---|
|
| |||||
| Low | High | ||||
| n=42 | n=48 | ||||
| Age (year) | 3.222 | 0.073 | |||
| <56 | 58 | 23 | 35 | ||
| ≥56 | 32 | 19 | 13 | ||
| Gender | 1.286 | 0.257 | |||
| Male | 50 | 26 | 24 | ||
| Female | 40 | 16 | 24 | ||
| Number of tumor nodes | 8.601 | 0.003* | |||
| Single | 43 | 27 | 16 | ||
| Multiple ≥2 | 47 | 15 | 32 | ||
| Tumor size | 5.625 | 0.018* | |||
| <5 cm | 48 | 28 | 20 | ||
| ≥5 cm | 42 | 14 | 28 | ||
| AFP (ng/mL) | 0.294 | 0.588 | |||
| <50 | 38 | 19 | 19 | ||
| ≥50 | 52 | 23 | 29 | ||
stands for P<0.05.
CDC6 may serve as a new biomarker for hepatocellular carcinoma
We propose that CDC6 may serve as a new biomarker for hepatocellular carcinoma according to these results. We analyzed and compared CDC6 as a prognostic-related marker in liver cancer and pancreatic cancer by using an online database analysis. The results showed that CDC6 could be a prognostic biomarker for liver cancer and pancreatic cancer (P<0.001; Figure 4). In summary, we have enough reasons to consider CDC6 to serve a new treatment target and biomarker for hepatocellular carcinoma.
Figure 4.
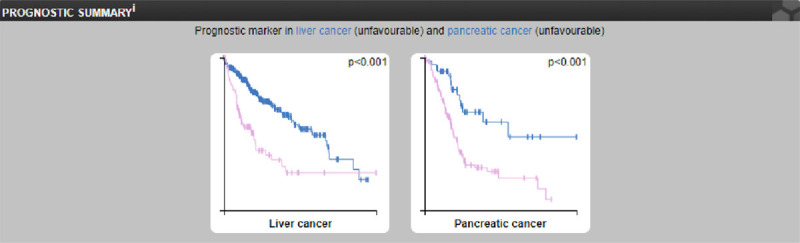
Analysis of the use of CDC6 as a prognostic marker for liver cancer and pancreatic cancer.
Discussion
Hepatocellular carcinoma has high mortality. According to statistical analysis, among all patients dying from cancers, hepatocellular carcinoma accounted for approximately 7% in 2019 [2,11]. While the proportion of hepatocellular carcinoma in 2020 has dropped to 6%, the results demonstrated that the cure rate of hepatocellular carcinoma was increasing daily with progress in medicine [3]. However, it still is a concern fortens of thousands of patients with hepatocellular carcinoma. Incidence and mortality are increasing, though much development. Diagnostic sensitivity and specificity for hepatocellular carcinoma remained low [12]. Diagnosis and screening of hepatocellular carcinoma mainly depend on detecting AFP value [13]; usually, 60-70% patients can be confirmed and found. However, the leftover patients cannot be identified immediately, and the AFP value is interfered by many liver-related diseases and biologic processes, which would influence judgment and strategic decision [14]. Treatment measures of hepatocellular carcinoma have saved a large number of patients, but many were metastatic when diagnosed, or resistant to many drugs and other treatment measures [15]. We lack a sensitive and potential biomarker and target for patients with hepatocellular carcinoma. In this research, we searched the expression of CDC6 protein in hepatocellular carcinoma by online database. Results showed that the expression of CDC6 was higher in hepatocellular carcinoma than normal liver tissue. Immunohistochemical staining assay in this study was confirmatory. These results showed that CDC6 could serve as a biomarker for many patients with hepatocellular carcinoma. CDC6, belonging to one of the most common chromosome replication licensors, is associated with the loading of MCM complex to chromatin [16]. Previous studies identified that CDC6 was a critical component of pre-replication complex (pre-RC) during DNA replication occurring in all eukaryotes [17,18]. The important role of CDC6 in DNA replication implied that it could influence proliferation and transcription by regulating replication-related processes [19,20]. Dysregulation of CDC6 can cause carcinogenesis and development of various tumors, and the proliferation ability would be inhibited significantly when the expression of CDC6 was knocked down [7]. Ke et al have shown that RYBP inhibited the progression of esophageal squamous cell carcinoma by downregulating CDC6 expression to influence the conversion of cell cycles [21]. We analyzed and found that the expression of CDC6 in hepatocellular carcinoma was significantly higher compared with normal liver tissue. We further identified that higher expression level of CDC6 was associated tightly with lower overall survival rate in GEPIA online database. These results implied that the expression of CDC6 might be related to prognosis of hepatocellular carcinoma. CDC6 was associated tightly with overall survival rate of hepatocellular carcinoma, so CDC6 is an essential factor for its progression and development. Kim et al identified that CDC6 mRNA expression was associated tightly with development and aggressiveness of prostate cancer, and this conclusion implied that CDC6 could be an important biomarker for cancers by detecting the mRNA expression level [22]. Its dysregulation also caused progression and development of various tumors, such as colorectal cancer [23], gastric cancer [24,25], pancreatic cancer [26], and glioblastoma [8]. The tumor promoting role of CDC6 has been established. We conclude that CDC6 is highly expressed in hepatocellular carcinoma and associated with prognosis and development. The fact of high expression of CDC6 in hepatocellular carcinoma and relation to progression implies that targeting CDC6 might be a treatment strategy for patients with hepatocellular carcinoma. An inhibitor of CDC6 might restrain the proliferation and progression of hepatocellular carcinoma, and help the overall survival rate. CDC6 biomarker function in hepatocellular carcinoma, was explored using the diagnostic related curve that was simulated in an online database. The results demonstrated that CDC6 was an extremely sensitive diagnosis-related biomarker for hepatocellular carcinoma.
In conclusion, we preliminarily determined that CDC6 could be a new diagnosis-related biomarker and therapy target for hepatocellular carcinoma. However, this conclusion needs to further experiments to strengthen it because of the small sample size and single-center study. We need to store more patient samples and conduct a multicenter and complete study, and we next explore the mechanism between CDC6 on hepatocellular carcinoma. We will explore the change of proliferation and invasion ability after knockdown the expression of CDC6 in hepatocellular carcinoma cell lines, and examine knockdown of CDC6 on tumorigenesis and development in vivo.
Disclosure of conflict of interest
None.
References
- 1.Van Haele M, Moya IM, Karaman R, Rens G, Snoeck J, Govaere O, Nevens F, Verslype C, Topal B, Monbaliu D, Halder G, Roskams T. YAP and TAZ heterogeneity in primary liver cancer: an analysis of its prognostic and diagnostic role. Int J Mol Sci. 2019;20:638. doi: 10.3390/ijms20030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 4.Li Q, Ren B, Gui Q, Zhao J, Wu M, Shen M, Li D, Li D, Chen K, Tao M, Liang R. Blocking MAPK/ERK pathway sensitizes hepatocellular carcinoma cells to temozolomide via downregulating MGMT expression. Ann Transl Med. 2020;8:1305. doi: 10.21037/atm-20-5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng YS, Zhou JM, Sun CH, Zhu J, Yang J, Guo L. The diagnostic value of miR-21 combined with CT in patients with liver cancer. Clin Transl Oncol. 2020;23:1238–1244. doi: 10.1007/s12094-020-02514-4. [DOI] [PubMed] [Google Scholar]
- 6.Guarino M, Caporaso N, Morisco F. Liver resection is always a good choice for hepatocellular carcinoma (HCC) patients regardless of Barcelona Clinic Liver Cancer (BCLC) stage: the therapeutic hierarchy. Ann Transl Med. 2020;8:1282. doi: 10.21037/atm-20-6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim N, Townsend PA. Cdc6 as a novel target in cancer: oncogenic potential, senescence and subcellular localisation. Int J Cancer. 2020;147:1528–1534. doi: 10.1002/ijc.32900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao H, Zhou X, Yuan G, Hou Z, Sun H, Zhai N, Huang B, Li X. CDC6 is up-regulated and a poor prognostic signature in glioblastoma multiforme. Clin Transl Oncol. 2020;23:565–571. doi: 10.1007/s12094-020-02449-w. [DOI] [PubMed] [Google Scholar]
- 9.Jiang W, Yu Y, Liu J, Zhao Q, Wang J, Zhang J, Dang X. Downregulation of Cdc6 inhibits tumorigenesis of osteosarcoma in vivo and in vitro. Biomed Pharmacother. 2019;115:108949. doi: 10.1016/j.biopha.2019.108949. [DOI] [PubMed] [Google Scholar]
- 10.An C, Liu G, Cheng S, Pang B, Sun S, Zhang Y, Pan Z, Kang X. A pilot study of cdc6 as a biomarker for circulating tumor cells in patients with lung cancer. J Clin Lab Anal. 2020;34:e23245. doi: 10.1002/jcla.23245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69:438–451. doi: 10.3322/caac.21583. [DOI] [PubMed] [Google Scholar]
- 12.Giannini EG, Bucci L, Garuti F, Brunacci M, Lenzi B, Valente M, Caturelli E, Cabibbo G, Piscaglia F, Virdone R, Felder M, Ciccarese F, Foschi FG, Sacco R, Svegliati Baroni G, Farinati F, Rapaccini GL, Olivani A, Gasbarrini A, Di Marco M, Morisco F, Zoli M, Masotto A, Borzio F, Benvegnù L, Marra F, Colecchia A, Nardone G, Bernardi M, Trevisani F. Patients with advanced hepatocellular carcinoma need a personalized management: a lesson from clinical practice. Hepatology. 2018;67:1784–1796. doi: 10.1002/hep.29668. [DOI] [PubMed] [Google Scholar]
- 13.Sun B, Huang Z, Wang B, Yu Y, Lin S, Luo L, Wang Y, Huang Z. Significance of glypican-3 (GPC3) expression in hepatocellular cancer diagnosis. Med Sci Monit. 2017;23:850–855. doi: 10.12659/MSM.899198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu R, Wang J, Huang X, Zhang Q, Xie Y, Pang L, Bai L, Zhou J. Clinical value of spectral CT imaging combined with AFP in identifying liver cancer and hepatic focal nodular hyperplasia. J BUON. 2019;24:1429–1434. [PubMed] [Google Scholar]
- 15.Vitale A, Trevisani F, Farinati F, Cillo U. Treatment of hepatocellular carcinoma in the precision medicine era: from treatment stage migration to therapeutic hierarchy. Hepatology. 2020;72:2206–2218. doi: 10.1002/hep.31187. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt JM, Bleichert F. Structural mechanism for replication origin binding and remodeling by a metazoan origin recognition complex and its co-loader Cdc6. Nat Commun. 2020;11:4263. doi: 10.1038/s41467-020-18067-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bömer M, Pérez-Salamó I, Florance HV, Salmon D, Dudenhoffer JH, Finch P, Cinar A, Smirnoff N, Harvey A, Devoto A. Jasmonates induce Arabidopsis bioactivities selectively inhibiting the growth of breast cancer cells through CDC6 and mTOR. New Phytol. 2021;229:2120–2134. doi: 10.1111/nph.17031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu Y, Tareen A, Sheu YJ, Ireland WT, Speck C, Li H, Joshua-Tor L, Kinney JB, Stillman B. Evolution of DNA replication origin specification and gene silencing mechanisms. Nat Commun. 2020;11:5175. doi: 10.1038/s41467-020-18964-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parker MW, Bell M, Mir M, Kao JA, Darzacq X, Botchan MR, Berger JM. A new class of disordered elements controls DNA replication through initiator self-assembly. Elife. 2019;8:e48562. doi: 10.7554/eLife.48562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun S, Zhang X, Xu M, Zhang F, Tian F, Cui J, Xia Y, Liang C, Zhou S, Wei H, Zhao H, Wu G, Xu B, Liu X, Yang G, Wang Q, Zhang L, Gong Y, Shao C, Zou Y. Berberine downregulates CDC6 and inhibits proliferation via targeting JAK-STAT3 signaling in keratinocytes. Cell Death Dis. 2019;10:274. doi: 10.1038/s41419-019-1510-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ke Y, Guo W, Huang S, Li Y, Guo Y, Liu X, Jin Y, Ma H. RYBP inhibits esophageal squamous cell carcinoma proliferation through downregulating CDC6 and CDC45 in G1-S phase transition process. Life Sci. 2020;250:117578. doi: 10.1016/j.lfs.2020.117578. [DOI] [PubMed] [Google Scholar]
- 22.Kim YH, Byun YJ, Kim WT, Jeong P, Yan C, Kang HW, Kim YJ, Lee SC, Moon SK, Choi YH, Yun SJ, Kim WJ. CDC6 mRNA expression is associated with the aggressiveness of prostate cancer. J Korean Med Sci. 2018;33:e303. doi: 10.3346/jkms.2018.33.e303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu Y, Wang L, Li Z, Wan Z, Shao M, Wu S, Wang G. Potential prognostic and diagnostic values of CDC6, CDC45, ORC6 and SNHG7 in colorectal cancer. Onco Targets Ther. 2019;12:11609–11621. doi: 10.2147/OTT.S231941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao B, Zhang J, Chen X, Xu H, Huang B. Mir-26b inhibits growth and resistance to paclitaxel chemotherapy by silencing the CDC6 gene in gastric cancer. Arch Med Sci. 2019;15:498–503. doi: 10.5114/aoms.2018.73315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X, Zhang M, Guo Q, Hu X, Zhao Z, Ni L, Liu L, Wang X, Wang Z, Tong D, Chang S, Cao Y, Huang C. MicroRNA-1297 inhibits proliferation and promotes apoptosis in gastric cancer cells by downregulating CDC6 expression. Anticancer Drugs. 2019;30:803–811. doi: 10.1097/CAD.0000000000000776. [DOI] [PubMed] [Google Scholar]
- 26.Youn Y, Lee JC, Kim J, Kim JH, Hwang JH. Cdc6 disruption leads to centrosome abnormalities and chromosome instability in pancreatic cancer cells. Sci Rep. 2020;10:16518. doi: 10.1038/s41598-020-73474-6. [DOI] [PMC free article] [PubMed] [Google Scholar]