Abstract
Background: Adropin, a peptide translated from the Energy Homeostasis Associated gene (ENHO), was mainly expressed in the liver and was a regulator in metabolic and energy homeostasis. This study aims to investigate the correlation between adropin and histological characteristics of the liver, and the clinical relevance of adropin in patients with metabolic dysfunction-associated fatty liver disease (MAFLD).
Methods: A total of 62 subjects, including 32 healthy controls and 30 MAFLD patients, were enrolled in this case-control study. The MAFLD patients were further divided into two subgroups, including NGT-M group and T2DM-M group. Serum adropin levels, metabolic parameters and intrahepatic lipids, the liver ENHO mRNA expressions and histological characteristics were investigated.
Results: MAFLD patients showed significantly lower circulating adropin compared with healthy controls (2.02 ± 2.92 vs. 5.52 ± 0.65 ng/mL, P < 0.0001). Subgroup analysis exhibited dramatically declined serum adropin levels in T2DM-M patients compared with NGT-M group (0.51 ± 0.73 vs. 4.00 ± 3.52 ng/mL, P < 0.001). H&E and Oil Red O staining show exacerbated steatohepatitis in T2DM-M patients in contrast with NGT-M group. Furthermore, serum adropin concentrations were negatively correlated with intrahepatic triglyceride (TG), total cholesterol (TC), and NAFLD activity score (NAS) (TG: r = −0.495; TC: r = −0.392; NAS: r = −0.451; all P < 0.05).
Conclusions: MAFLD patients showed significantly lower adropin levels than the healthy controls, especially in T2DM patients. Adropin maybe a potential biomarker for predicting the development of MAFLD, especially in T2DM individuals.
Keywords: metabolic dysfunction-associated fatty liver disease, adropin, triglyceride, steatohepatitis, type 2 diabetes mellitus
Introduction
Metabolic dysfunction-associated fatty liver disease (MAFLD), formerly named non-alcoholic fatty liver disease (NAFLD), affecting a quarter of the general population, which has become the most common liver disease (Sarin et al., 2020; Eslam et al., 2020b). The newly proposed diagnostic criteria for MAFLD irrespective of alcohol intake or other concomitant liver diseases. The criteria are principally based on an evidence of hepatic steatosis and include one of the following three criteria: overweight/obesity, presence of type 2 diabetes mellitus (T2DM), or evidence of metabolic dysfunction (Eslam et al., 2020a). The fundamental pathophysiological change of hepatic steatosis is accumulated free fatty acids (FFAs) and triglycerides (TGs) in hepatocytes. Steatohepatitis is the severely advanced stage of hepatic steatosis and is typically characterized by steatosis, lobular inflammation, and ballooning with or without peri-sinusoidal fibrosis (Castera et al., 2019). Many biomarkers which can be used to predict steatohepatitis in patients with MAFLD have been reported. However, considering the invasiveness and expensive cost of liver biopsy, a noninvasive, economical, easily accessible, highly sensitive, and specific biomarker is urgently needed to predict the development of MAFLD.
Adropin, a secreted peptide encoded by the Energy Homeostasis Associated (ENHO) gene (Kumar et al., 2008), was mainly expressed in the brain and liver. Recent studies showed beneficial effects of adropin on improving glucose homeostasis, dyslipidemia, obesity-associated hyperinsulinemia, and energy homeostasis. Clinical studies suggested that serum adropin was reduced in many diseases, such as NAFLD, T2DM, diabetic nephropathies, coronary atherosclerosis, hypertension, and polycystic ovary disease (Jasaszwili et al., 2020; Ye et al., 2021). Nevertheless, the correlation between adropin and different glucose tolerance in MAFLD patients remains unclear. On the basis of previous findings, we hypothesized that adropin might be involved in the pathogenesis of MAFLD and the serum adropin could be served as a predictive factor of MAFLD. Thus, this study was designed to investigate the correlation between adropin and histological characteristics of the liver, and the clinical relevance of adropin in MAFLD patients.
Materials and Methods
Study Design
From July 2019 to January 2020, 30 MAFLD patients were histologically confirmed with hepatic steatosis, which were enrolled in China-Japan Friendship Hospital. MAFLD patients were divided into two subgroups, including NGT-M group (13 normal glucose tolerance, NGT) and T2DM-M group (17 type 2 diabetes mellitus patients, T2DM). No drug intervention was given in patients with T2DM. We enrolled 32 sex and age matched healthy controls who were free from diagnosed hepatic steatosis based on the results of upper abdomen ultrasonography and routine physical examination at Beijing Chao-yang Hospital Affiliated to Capital Medical University from June 2018 to October 2019. All subjects had to meet the following exclusion criteria: alcohol consumption (≥140 g/wk in men and ≥70 g/wk in women), chronic viral hepatitis, autoimmune hepatitis, drug-induced liver disease, primary biliary cirrhosis, biliary obstruction, Wilson's disease, and α-1 antitrypsin deficiency-associated liver disease. All participants completed a uniform questionnaire containing questions about the histories of present and past illnesses and medical therapy. Thirty MAFLD patients were asked to undergo intra-operative liver biopsy at the time of admission. The study was approved by the human research ethics committee of the China-Japan Friendship Hospital (2019-103-K71-1), according to the principles of the Declaration of Helsinki. Written informed consent was obtained from all subjects.
Human Liver Tissues and Serum
MAFLD patients (n = 30) from the department of general surgery collaboration with China-Japan Friendship Hospital underwent intra-operative liver biopsies during sleeve gastrectomy intervention, following the recommendations by the American Association for the Study of Liver Diseases (Rockey et al., 2009). The histological characteristics of the liver were graded according to a histological scoring system for NAFLD (Kleiner et al., 2005). NAFLD activity score (NAS) was calculated by adding these three items. Serum samples were centrifuged at 3,000 rpm for 15 min, and the obtained serum and liver tissues were immediately snap-frozen and stored at −80°C until use.
Anthropometric and Biochemical Measurements
Height, weight and waist/hip circumference were measured light clothes on and without shoes, and the body mass index (BMI, kg/m2) was calculated. Fasting blood samples were obtained after overnight fast for the measurement of plasma glucose, C-peptide, insulin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), total cholesterol (TC), triglyceride (TG), high-density lipoprotein-cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c) levels, apoprotein A1 (Apo-A1), apoprotein B (Apo-B), non-esterified fatty acid (NEFA), small dense-low-density lipoprotein cholesterol (sd-LDL). Homeostasis model assessment insulin resistance index (HOMA-IR) was calculated according to the following formula: FINS (μIU/mL) × FBG (mmol/L)/22.5. A 75-g oral glucose tolerance test (OGTT) was performed, plasma glucose levels were measured at 30, 60, 120, and 180 min after oral glucose, the levels of C-peptide and insulin were also detected at 60, 120, and 180 min after oral glucose. Human serum adropin was measured with an enzyme-linked immunosorbent assay (ELISA) kit (Phoenix Pharmaceuticals, Belmont, CA, USA, EK-032-35), according to the manufacturer's protocol.
Histological Staining
Fresh liver tissue was immediately fixed in 10% phosphate-buffered formalin and 4% neutral-buffered formalin solution for 24 h, embedded in paraffin, sectioned at 6 μm, and then deparaffinized in xylene and rehydrated through a series of decreasing concentrations of ethanol. The sections were prepared for hematoxylin and eosin (H&E) staining, which demarcates fat degeneration. Oil Red O staining was conducted on frozen-sections embedded in OCT to determine hepatic steatosis. Images were captured with a light microscope (Olympus, Tokyo, Japan).
Liver Lipid Analysis
Hepatic triglycerides and cholesterol, free cholesterol was extracted in 50 mM Tris buffer, homogenized, and incubated at 37°C with shaking overnight. The triglyceride and cholesterol, free cholesterol measurement kit (Solarbio, Beijing, China) were used following the manufacturer's instruction to measure lipid contents.
RNA Extraction and qRT-PCR
Total RNA was extracted from human liver tissues using Trizol Reagent (Life Technologies, Carlsbad, CA), then reverse transcripted into cDNA with GoScript reverse transcriptase (Promega, Madison, WI), and prepared for RT-qPCR analysis with SYBR green premix (TaKaRa, Nojihigashi, Kusatsu, Shiga, Japan). RT-qPCR assays were performed on CFX Connect Real-Time System (BIO-RAD, CA). Values are expressed as fold change over control, β-Actin (Actb) was used for normalization to quantify relative mRNA expression levels, relative changes in mRNA expression were calculated using the comparative cycle method (2−ΔΔCt). Real-time PCR primer sequences are shown in Supplementary Table 1.
Statistical Analysis
Normally distributed variables were expressed as mean values ± standard deviation of mean (SD). Non-normally distributed variables were described as medians (25th, 75th percentiles), including TG, FINS and HOMA-IR, NEFA. Categorical variables are expressed as n (number) with percentage (%). Comparisons between two groups were performed by Student's t-test for continuous variables and χ2 analyses for categorical variables. The correlations between adropin and other parameters were analyzed by Pearson's or Spearman's coefficient. Data were analyzed using the IBM SPSS statistical software (version 24.0; SPSS Inc., Chicago, IL, USA) and GraphPad Prism software (version 7.0; CA, USA). Statistically significant was considered at p < 0.05 (two-tailed).
Results
Clinical Characteristics of the Study Participants
Clinical characteristics including anthropometric parameters and biochemical indexes in both control subjects and MAFLD patients are shown in Table 1. The MAFLD patients had significantly higher levels of BMI, WHR, HbA1c, FBG, FINS, HOMA-IR, TG, ALT, AST, and GGT but a lower level of HDL-c compared with healthy controls (All p < 0.01). The serum adropin level of MAFLD patients was significantly lower than that of control subjects (2.02 ± 2.92 ng/ml vs. 5.52 ± 0.65 ng/ml, p < 0.001).
Table 1.
Anthropometric parameters and biochemical indexes in healthy controls and MAFLD patients.
| Control (n = 32) | MAFLD (n = 30) | P-value | |
|---|---|---|---|
| Sex (male), n (%) | 11 (34) | 10 (33) | 0.931 |
| Age (years) | 35.1 ± 4.8 | 35.1 ± 9.5 | 0.989 |
| BMI (Kg/m2) | 21.46 ± 1.61 | 36.88 ± 5.79 | <0.001 |
| Waist circumference (cm) | 75.47 ± 8.41 | 115.30 ± 12.67 | <0.001 |
| WHR | 0.91 ± 0.02 | 0.95 ± 0.07 | <0.01 |
| HbA1c (%) | 5.10 ± 0.44 | 6.54 ± 1.93 | <0.001 |
| FBG (mM) | 4.74 ± 0.49 | 7.11 ± 2.84 | <0.001 |
| FINS (μU/mL) | 5.50 (4.90–8.00) | 15.89 (12.94–20.32) | <0.001 |
| HOMA-IR | 1.27 (0.87–1.85) | 4.28 (3.76–5.57) | <0.001 |
| TC (mM) | 4.68 ± 0.59 | 4.67 ± 0.73 | 0.950 |
| TG (mM) | 0.90 (0.68–1.18) | 1.88 (0.94–2.41) | <0.001 |
| LDL-C (mM) | 2.61 ± 0.56 | 2.78 ± 0.55 | 0.307 |
| HDL-C (mM) | 1.56 ± 0.33 | 0.91 ± 0.17 | <0.001 |
| Apo-A1 (g/L) | 1.41 ± 0.23 | 1.37 ± 0.23 | 0.592 |
| Apo-B (g/L) | 0.78 ± 0.13 | 0.79 ± 0.15 | 0.916 |
| sd-LDL (mM) | 0.83 ± 0.25 | 0.79 ± 0.45 | 0.638 |
| NEFA (mM) | 0.49 (0.30–0.57) | 0.50 (0.40–0.55) | 0.219 |
| ALT (IU/L) | 16.75 ± 6.91 | 51.00 ± 30.52 | <0.001 |
| AST (IU/L) | 19.56 ± 5.00 | 49.79 ± 25.79 | <0.001 |
| GGT (IU/L) | 15.56 ± 6.20 | 44.88 ± 37.15 | <0.001 |
| Adropin (ng/ml) | 5.52 ± 0.65 | 2.02 ± 2.92 | <0.001 |
Values are presented as mean ± SD (standard deviation of mean) for normally distributed data or medians (25th, 75th percentiles) for non-normally distributed data.
Apo-A1, apoprotein A1; Apo-B, apoprotein B; BMI, body mass index; FBG, fasting blood glucose; FINS, fasting insulin; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance; LDL-C, low-density lipoprotein cholesterol; NEFA, non-esterified fatty acid; sd-LDL, small dense-low-density lipoprotein cholesterol; TG, triglycerides; TC, total cholesterol; WHR, waist to hip ratio.
P-values in bold indicate significance. The P-value was assessed using Student's t-test or Mann–Whitney U-test.
Subgroup Analysis in MAFLD Patients With Different Glucose Tolerance
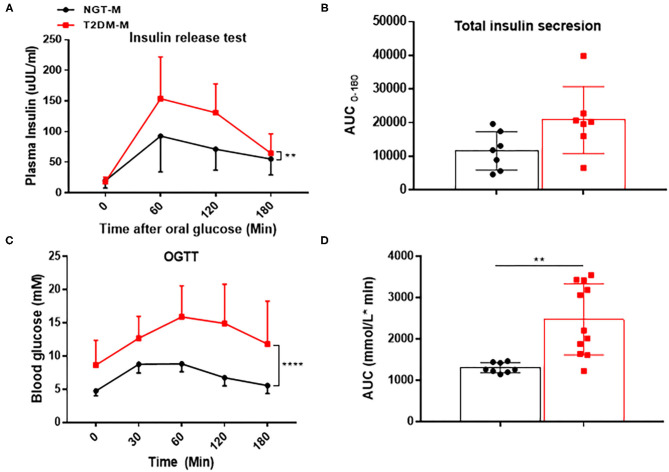
To investigate the effect of abnormal glucose metabolism on adropin in MAFLD patients, we performed a subgroup analysis. As Table 2 showed, the two subgroups were similar in terms of age, BMI and WHR (All p > 0.05). However, the T2DM-M group had significantly higher levels of HbA1c, FBG, HOMA-IR, TG, and ALT than those of NGT-M group (All p < 0.05). Insulin release test and AUC suggested that insulin release peak at 1 h but not return to fasting level at 3 h in T2DM-M group (Figures 1A,B). OGTT and AUC showed that glucose tolerance was severely impaired in T2DM-M group compared with NGT-M subjects (Figures 1C,D). Notably, serum adropin level was significantly reduced in T2DM-M group compared with NGT-M group (0.51 ± 0.73 vs. 4.00 ± 3.52 ng/ml, p < 0.001).
Table 2.
Anthropometric parameters and biochemical indexes among NGT and T2DM groups in MAFLD patients.
| MAFLD | P-value | ||
|---|---|---|---|
| NGT (n = 13) | T2DM (n = 17) | NGT vs. T2DM | |
| Sex (male), n (%) | 5 (38) | 5 (29) | 0.602 |
| Age (years) | 32.2 ± 9.5 | 37.3 ± 9.1 | 0.150 |
| Weight (Kg) | 110.90 ± 21.97 | 99.89 ± 17.09 | 0.132 |
| BMI (Kg/m2) | 38.66 ± 6.76 | 35.51 ± 4.67 | 0.133 |
| Waist circumference (cm) | 121.20 ± 12.9 | 110.9 ± 10.85 | 0.025 |
| WHR | 0.97 ± 0.06 | 0.94 ± 0.08 | 0.266 |
| HbA1c (%) | 5.36 ± 0.26 | 7.48 ± 2.18 | 0.015 |
| FBG (mM) | 5.54 ± 1.87 | 8.49 ± 2.86 | 0.003 |
| FINS (μU/mL) | 15.89 (12.09–19.47) | 15.69 (13.23–23.90) | 0.439 |
| HOMA-IR | 3.69 (2.20–4.38) | 5.21 (4.18–9.64) | 0.020 |
| TC (mM) | 4.62 ± 0.69 | 4.70 ± 0.79 | 0.820 |
| TG (mM) | 0.96 (0.78–1.86) | 2.11 (1.83–3.69) | 0.037 |
| LDL-C (mM) | 2.73 ± 0.57 | 2.81 ± 0.56 | 0.769 |
| HDL-C (mM) | 0.98 ± 0.21 | 0.85 ± 0.10 | 0.116 |
| Apo-A1 (g/L) | 1.46 ± 0.32 | 1.30 ± 0.09 | 0.138 |
| Apo-B (g/L) | 0.77 ± 0.19 | 0.85 ± 0.14 | 0.335 |
| sd-LDL (mM) | 0.68 ± 0.44 | 0.86 ± 0.46 | 0.415 |
| NEFA (mM) | 0.50 (0.40–0.50) | 0.50 (0.48–0.63) | 0.632 |
| ALT (IU/L) | 37.50 ± 14.85 | 65.40 ± 33.98 | 0.047 |
| AST (IU/L) | 47.25 ± 25.58 | 52.33 ± 26.88 | 0.640 |
| GGT (IU/L) | 41.43 ± 19.82 | 50.88 ± 49.86 | 0.647 |
| Adropin (ng/ml) | 4.00 ± 3.52 | 0.51 ± 0.73 | <0.001 |
| NAS | 6.68 ± 1.61 | 6.94 ± 1.77 | 0.681 |
Values are presented as mean ± SD (standard deviation of mean) for normally distributed data or medians (25th, 75th percentiles) for non-normally distributed data.
Apo-A1, apoprotein A1; Apo-B, apoprotein B; BMI, body mass index; FBG, fasting blood glucose; FINS, fasting insulin; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance; LDL-C, low-density lipoprotein cholesterol; NAS, NAFLD activity score; NEFA, non-esterified fatty acid; sd-LDL, small dense-low-density lipoprotein cholesterol; TG, triglycerides; TC, total cholesterol; WHR, waist to hip ratio.
P-values in bold indicate significance. The P-value was assessed using Student's t-test or Mann–Whitney U-test.
Figure 1.
The indicators of insulin resistance in MAFLD patients. (A,B) Insulin release test and the respective analysis of the area under the curve (AUC) (n = 7). (C,D) Oral glucose tolerance test (OGTT) and the respective analysis of the area under the curve (AUC) (n = 8–11). The data are expressed as the mean ± SD, **P < 0.01, ****P < 0.0001.
Correlations of Serum Adropin Levels and Liver ENHO Expressions With Metabolic Parameters in MAFLD Subjects
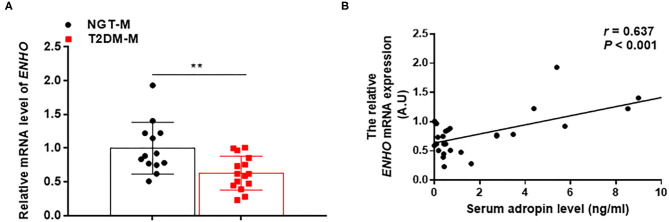
To investigated the relationship between adropin and metabolic parameters in MAFLD patients, a linear correlation analysis was performed. As summarized in Table 3, serum adropin levels were negatively correlated with BMI, FBG, FINS, HbA1c and HOMA-IR, TG, Apo-B, NEFA, ALT, AST, and GTT, in spite of these differences did not reach the statistical significance. Interestingly, serum adropin levels were positively correlated with HDL-C and Apo-A1 (All p < 0.001). Furthermore, liver ENHO mRNA expression was obviously downregulated in T2DM-M group compared with NGT-M group (0.63 ± 0.06 vs. 1.00 ± 0.11, p < 0.01) (Figure 2A). Liver ENHO mRNA expression was positively correlated with Apo-A1 (r = 0.497, p < 0.05). Circulating adropin level was positively associated with liver ENHO mRNA expression (r = 0.637, p < 0.001; Figure 2B).
Table 3.
Correlation of serum adropin level and liver ENHO mRNA expression with metabolic indicators in MAFLD patients.
| Variables | Serum adropin level (ng/ml) | Liver ENHO mRNA expression (A.U) | ||
|---|---|---|---|---|
| r | P | r | P | |
| BMI (Kg/m2) | −0.014 | 0.940 | −0.016 | 0.937 |
| Waist circumference (cm) | 0.044 | 0.817 | 0.106 | 0.590 |
| WHR | 0.057 | 0.765 | 0.149 | 0.746 |
| HbA1c (%) | −0.318 | 0.198 | −0.218 | 0.385 |
| FBG (mM) | −0.358 | 0.052 | −0.152 | 0.439 |
| FINS (μU/mL) | −0.166 | 0.510 | −0.247 | 0.323 |
| HOMA-IR | −0.308 | 0.215 | −0.310 | 0.211 |
| TC (mM) | 0.190 | 0.450 | −0.237 | 0.344 |
| TG (mM) | −0.244 | 0.362 | −0.352 | 0.182 |
| LDL-C (mM) | 0.018 | 0.944 | −0.348 | 0.157 |
| HDL-C (mM) | 0.785 | 0.000 | 0.380 | 0.119 |
| Apo-A1 (g/L) | 0.780 | 0.000 | 0.497 | 0.036 |
| Apo-B (g/L) | −0.007 | 0.976 | −0.283 | 0.256 |
| sd-LDL (mM) | 0.128 | 0.625 | −0.189 | 0.484 |
| NEFA (mM) | −0.256 | 0.322 | 0.058 | 0.838 |
| ALT (IU/L) | −0.369 | 0.131 | −0.336 | 0.187 |
| AST (IU/L) | −0.067 | 0.754 | −0.008 | 0.971 |
| GGT (IU/L) | −0.028 | 0.918 | 0.280 | 0.311 |
Correlations between serum adropin or liver ENHO mRNA expression and anthropomorphic, blood chemistry data were calculated by Pearson's or Spearman's coefficient (r).
Apo-A1, apoprotein A1; Apo-B, apoprotein B; BMI, body mass index; FBG, fasting blood glucose; FINS, fasting insulin; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance; LDL-C, low-density lipoprotein cholesterol; NEFA, non-esterified fatty acid; sd-LDL, small dense-low-density lipoprotein cholesterol; TG, triglycerides; TC, total cholesterol; WHR, waist to hip ratio.
P-values in bold indicate significance.
Figure 2.
Adropin expression in the liver was lower in MAFLD subjects. (A) Quantitative real-time RT-PCR analysis of ENHO mRNA expression in the liver from MAFLD patients (n = 13–15). (B) Correlations of liver ENHO mRNA expression with serum adropin levels. The data are expressed as the mean ± SD, **P < 0.01.
Histological Features of the Liver in Patients With MAFLD
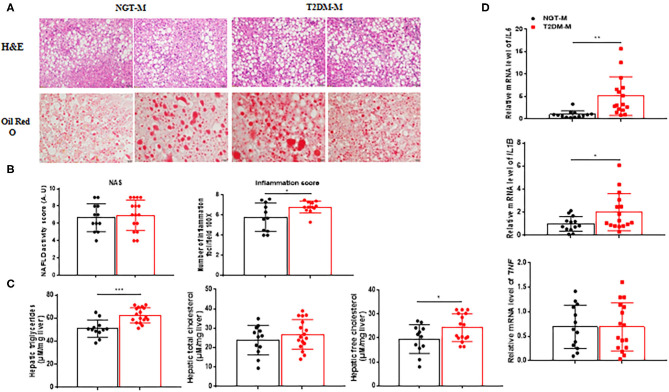
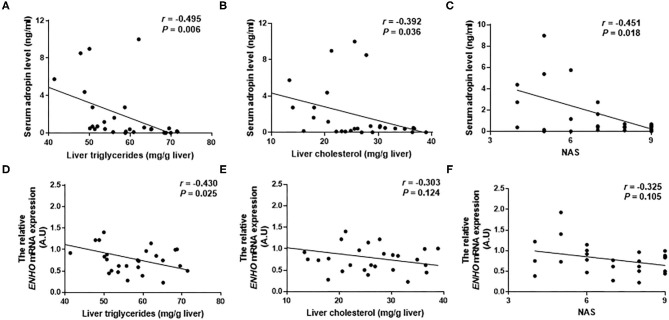
T2DM-M patients exhibited more severe hepatic macrosteatosis, inflammation, and ballooning (Figure 3A), concurrent with a higher NAS and inflammation score (Figure 3B). Oil Red O staining demonstrated a more lipid accumulation in the T2DM-M patients (Figure 3A). Hepatic TG and free cholesterol were significantly higher in the T2DM-M group than those in the NGT-M group (Figure 3C). Moreover, the pro-inflammation genes (TNF, IL1B, and IL6) mRNA expression in the liver were higher in the T2DM-M group compared with NGT-M group (Figure 3D). Besides, serum adropin levels were significantly inverse correlated with intrahepatic TG (r = −0.495, p = 0.006; Figure 4A), TC (r = −0.392, p = 0.036; Figure 4B), and NAS (r = −0.451, p = 0.018; Figure 4C). Liver adropin levels were negatively correlated with intrahepatic TG (r = −0.430, p = 0.025; Figure 4D), TC (r = −0.303, p = 0.124; Figure 4E), and NAS (r = −0.325, p = 0.105; Figure 4F). We have analyzed the association between adropin and liver pro-inflammation genes. As shown in Supplementary Figures 1A–F, the mRNA expressions of IL6 and IL1B were negatively correlated with adropin, but there were no statistically difference.
Figure 3.
Steatohepatitis was exacerbated in T2DM-M group compared with NGT-M group. (A) Representative liver HandE staining (magnification, ×200; scale bar: 50 μm) and Oil Red O staining (magnification, ×400; scale bar: 20 μm) (n = 13–17). (B) Hepatic histological analysis of HandE staining (NAS and lobular inflammation score) (n = 11–16). (C) Hepatic triglycerides and (free) cholesterol levels (n = 12–17). (D) Quantitative real-time RT-PCR analysis of TNF IL1B IL6 mRNA expressions in the liver from MAFLD patients (n = 13–16). The data are expressed as the mean ± SD, *P < 0.05, **P < 0.01, and ***P < 0.001.
Figure 4.
The correlation between serum adropin/liver adropin levels and the level of liver triglycerides (A,D), and liver cholesterol (B,E), and NAFLD activity score (C,F) in MAFLD patients.
Discussion
Our results indicate that serum adropin levels and liver ENHO mRNA expressions were significantly decreased in T2DM-M patients. These data verified the inverse association between circulating adropin and intrahepatic TG, TC, and NAS. As far as we known, few researches have been done to investigate the association of adropin with intrahepatic lipid contents in adult MAFLD patients. Overall, lower serum level of adropin may be a practical biomarker that can be used to detect the progression of MAFLD.
Several previous studies consistently showed that adropin played a critical role in maintaining glucose homeostasis, increasing the glucose utilization, improving glucose tolerance, and reducing insulin resistance (Butler et al., 2019; Jasaszwili et al., 2020). Adropin deficiency exacerbated the dysregulated glucose homeostasis and insulin resistance in diet-induced obesity (DIO) mice. Adropin injection significantly reduced fasting blood glucose, inhibited hepatic glucose production, lowered fasting insulin level, and improved insulin resistance in high-fat diet mice. In vitro study showed that adropin effects on glucose production were restricted in insulin resistance hepatocytes induced by palmitic acid or high glucose (Chen et al., 2017, 2020). Chronic consumption of high fat/high sucrose diets resulting in hepatic insulin resistance suppressed liver adropin expression, and could contribute to the dysregulation of glucose metabolism (Banerjee et al., 2020). Most clinical studies confirmed a dramatically decline of adropin in patients with T2DM when compared with healthy subjects (Wu et al., 2014; Chen et al., 2017). In accordance with prior studies, we found that serum adropin level and liver adropin expression was significantly decreased in T2DM-M patients. Moreover, serum adropin levels were positively correlated with liver adropin expression. Therefore, the lower serum adropin level of MAFLD patients may indicate more serious insulin resistance and glucose intolerance. All these data imply a closely connection between adropin and glucose homeostasis in MAFLD patients.
Adropin is required for metabolic homeostasis and is involved in preventing dyslipidaemia (Kumar et al., 2008). Animal studies suggested that adropin could suppress fatty acid oxidation (Gao et al., 2014). In human subjects, circulating adropin was negatively correlated with the levels of plasma TG, apolipoprotein B, and LDL-c and was positively correlated with the HDL-c level (Butler et al., 2012). Our findings are consistent with previous studies that serum adropin concentrations were inversely associated with these indices of blood lipid. It is important to note that liver TG and TC was inversely correlated with serum adropin level, but only liver TG was significantly negative correlated with liver ENHO mRNA expression. We believed that two reasons might explain these differences. On the one hand, circulating adropin levels may associate with the discrepancy of the equilibrium between cholesterol synthesis and processing of circulating lipoprotein particles (Ghoshal et al., 2018). On the other hand, liver ENHO mRNA expression is regulated by the biological clock and nutrition status. Experiments based on mice overexpressing adropin unable to prove the role for adropin in regulating cholesterol uptake from the diet, clearance from the circulation or cholesterol biosynthesis (Ghoshal et al., 2018). The precise effect of adropin in lipid metabolism is still unclear. Further studies examining the relationship between adropin expression in liver tissues and intrahepatic TG and TC are required. Our study showed that circulating adropin was negatively correlated with intrahepatic TG and TC.
Genetically engineered animals provided strong evidence indicating that adropin contributes to the modulation of inflammation. Chen et al. (2019) demonstrated that serum adropin concentrations were obviously decreased in steatohepatitis, adropin deficient mice showed exacerbated hepatic steatosis, inflammation and fibrosis when fed methionine-choline deficient (MCD) or western diets. Serum adropin level was lower in B-ultrasound or liver biopsy diagnosed NAFLD patients, and was associated with the severity of NAFLD (Sayin et al., 2014; Kutlu et al., 2019). Adropin treatment decreased the expression of the proinflammatory cytokines Il1b, Il6, and Tnfa in mice with MCD diet-induced steatohepatitis (Chen et al., 2019). Thus, adropin as a potential anti-inflammatory factor plays an important role in the process of steatohepatitis. Herein, we included patients with biopsy-proven hepatic steatosis to explore the association between adropin and liver injury. We found that both serum adropin level and liver ENHO mRNA expression were negatively associated with ALT and NAS. The proinflammatory genes (TNF, IL6, and IL1B) were increased in T2DM-M patients compared with those in NGT-M group. In addition, the serum adropin levels and liver ENHO mRNA expressions were dramatically decreased in T2DM-M patients compared with NGT-M group. Thus, our findings pointed out the potential of adropin in identifying MAFLD, especially in T2DM individuals.
A limitation of this study is the cross-sectional design which harbored a small sample size, and the liver samples from patients who volunteered to have a liver biopsy, which may lead to selection bias. Hence, more large and random liver-biopsy hepatic steatosis populations are needed to further confirm our results and conclusions.
Conclusions
To sum up, the current findings provide further indication of the significance of adropin in maintaining metabolic homeostasis. Adropin may participate in the pathogenesis of MAFLD and can be served as a predictive biomarker of MAFLD. Nevertheless, more in vivo experiments and a large-scale clinical trial is required to confirm these data in MAFLD.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the China-Japan Friendship Hospital (2019-103-K71). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
GW, JL, HM, and AQ: conceived and designed the experiments. NL, GX, and AQ: analyzed the data. NL, GX, AQ, and BZ: contributed reagents materials analysis tools. NL: wrote the paper. GW, JL, and AQ: revised and adapted the manuscript. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Glossary
Abbreviations
- Apo-A1
apoprotein A1
- Apo-B
apoprotein B
- BMI
body mass index
- ELISA
enzyme-linked immunosorbent assay
- ENHO
energy homeostasis associated gene
- FBG
fasting blood glucose
- FINS
fasting insulin
- FFAs
free fatty acids
- HbA1c
glycated hemoglobin
- HDL-C
high-density lipoprotein cholesterol
- HOMA-IR
homeostasis model assessment of insulin resistance
- LDL-C
low-density lipoprotein cholesterol
- MAFLD
metabolic dysfunction-associated fatty liver disease
- MCD
methionine-choline deficient
- NAFLD
nonalcoholic fatty liver disease
- NAS
NAFLD activity score
- NEFA
non-esterified fatty acid
- NGT
normal glucose tolerance
- OGTT
oral glucose tolerance test
- sd-LDL
small dense-low-density lipoprotein cholesterol
- T2DM
type 2 diabetes mellitus
- TG
triglycerides
- TC
total cholesterol
- WHR
waist to hip ratio.
Footnotes
Funding. The Chinese National Natural Science Foundation (No. 81770792, 81972137) and Beijing Municipal Natural Science Foundation (No. Z201810025038) to GW.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2021.696163/full#supplementary-material
References
- Banerjee S., Ghoshal S., Stevens J. R., McCommis K. S., Gao S., Castro-Sepulveda M., et al. (2020). Hepatocyte expression of the micropeptide adropin regulates the liver fasting response and is enhanced by caloric restriction. J. Biol. Chem. 295, 13753–13768. 10.1074/jbc.RA120.014381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler A. A., Tam C. S., Stanhope K. L., Wolfe B. M., Ali M. R., O'Keeffe M., et al. (2012). Low circulating adropin concentrations with obesity and aging correlate with risk factors for metabolic disease and increase after gastric bypass surgery in humans. J. Clin. Endocrinol. Metab. 97, 3783–3791. 10.1210/jc.2012-2194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler A. A., Zhang J., Price C. A., Stevens J. R., Graham J. L., Stanhope K. L., et al. (2019). Low plasma adropin concentrations increase risks of weight gain and metabolic dysregulation in response to a high-sugar diet in male nonhuman primates. J. Biol. Chem. 294, 9706–9719. 10.1074/jbc.RA119.007528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castera L., Friedrich-Rust M., Loomba R. (2019). Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology 156, 1264–1281.e1264. 10.1053/j.gastro.2018.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Zeng K., Liu Q. C., Guo Z., Zhang S., Chen X. R., et al. (2017). Adropin deficiency worsens HFD-induced metabolic defects. Cell Death Dis. 8:e3008. 10.1038/cddis.2017.362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Chen S., Shen T., Yang W., Chen Q., Zhang P., et al. (2020). Adropin regulates hepatic glucose production via PP2A/AMPK pathway in insulin-resistant hepatocytes. FASEB J. 34, 10056–10072. 10.1096/fj.202000115RR [DOI] [PubMed] [Google Scholar]
- Chen X., Xue H., Fang W., Chen K., Chen S., Yang W., et al. (2019). Adropin protects against liver injury in nonalcoholic steatohepatitis via the Nrf2 mediated antioxidant capacity. Redox Biol. 21:101068. 10.1016/j.redox.2018.101068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslam M., Newsome P. N., Sarin S. K., Anstee Q. M., Targher G., Romero-Gomez M., et al. (2020a). A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J. Hepatol. 73, 202–209. 10.1016/j.jhep.2020.07.045 [DOI] [PubMed] [Google Scholar]
- Eslam M., Sanyal A. J., George J., International Consensus P. (2020b). MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 158, 1999–2014.e1991. 10.1053/j.gastro.2019.11.312 [DOI] [PubMed] [Google Scholar]
- Gao S., McMillan R. P., Jacas J., Zhu Q., Li X., Kumar G. K., et al. (2014). Regulation of substrate oxidation preferences in muscle by the peptide hormone adropin. Diabetes 63, 3242–3252. 10.2337/db14-0388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoshal S., Stevens J. R., Billon C., Girardet C., Sitaula S., Leon A. S., et al. (2018). Adropin: an endocrine link between the biological clock and cholesterol homeostasis. Mol. Metab. 8, 51–64. 10.1016/j.molmet.2017.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasaszwili M., Billert M., Strowski M. Z., Nowak K. W., Skrzypski M. (2020). Adropin as a fat-burning hormone with multiple functions-review of a decade of research. Molecules 25:549. 10.3390/molecules25030549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner D. E., Brunt E. M., Van Natta M., Behling C., Contos M. J., Cummings O. W., et al. (2005). Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41, 1313–1321. 10.1002/hep.20701 [DOI] [PubMed] [Google Scholar]
- Kumar K. G., Trevaskis J. L., Lam D. D., Sutton G. M., Koza R. A., Chouljenko V. N., et al. (2008). Identification of adropin as a secreted factor linking dietary macronutrient intake with energy homeostasis and lipid metabolism. Cell Metab. 8, 468–481. 10.1016/j.cmet.2008.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutlu O., Altun O., Dikker O., Aktas S., Ozsoy N., Arman Y., et al. (2019). Serum adropin levels are reduced in adult patients with nonalcoholic fatty liver disease. Med. Princ. Pract. 28, 463–469. 10.1159/000500106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockey D. C., Caldwell S. H., Goodman Z. D., Nelson R. C., Smith A. D., American Association for the Study of Liver D . (2009). Liver biopsy. Hepatology 49, 1017–1044. 10.1002/hep.22742 [DOI] [PubMed] [Google Scholar]
- Sarin S. K., Kumar M., Eslam M., George J., Al Mahtab M., Akbar S. M. F., et al. (2020). Correction to lancet gastroenterol hepatol 2020; 5: 167–228. Lancet Gastroenterol. Hepatol. 5:e2. 10.1016/S2468-1253(19)30342-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayin O., Tokgoz Y., Arslan N. (2014). Investigation of adropin and leptin levels in pediatric obesity-related nonalcoholic fatty liver disease. J. Pediatr. Endocrinol. Metab. 27, 479–484. 10.1515/jpem-2013-0296 [DOI] [PubMed] [Google Scholar]
- Wu L., Fang J., Chen L., Zhao Z., Luo Y., Lin C., et al. (2014). Low serum adropin is associated with coronary atherosclerosis in type 2 diabetic and non-diabetic patients. Clin. Chem. Lab. Med. 52, 751–758. 10.1515/cclm-2013-0844 [DOI] [PubMed] [Google Scholar]
- Ye Z., Zhang C., Zhao Y. (2021). Potential effects of adropin on systemic metabolic and hormonal abnormalities in polycystic ovary syndrome. Reprod. Biomed. Online 42,1007–1014. 10.1016/j.rbmo.2021.01.020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.