Abstract
Background
Salvage endoscopic nasopharyngectomy has better survival prognosis and fewer complications in the management of early stage rNPC, compared to re-irradiation. However, the treatment modality of advanced recurrent nasopharyngeal carcinoma (rNPC) remains controversial. Thus, the purpose of this study was to investigate the demographics, clinical outcomes, and prognostic factors associated with salvage endoscopic nasopharyngectomy in advanced rNPC.
Methods
This study conducted a retrospective analysis of advanced rNPC patients who underwent salvage surgery betweenm January 2014 and December 2019. The overall survival (OS) and progression-free survival (PFS) were analyzed. Univariable and multivariable analyses of OS and PFS were performed using the Cox regression model. The predicted values of the parameters were determined by means of the receiver operating characteristic (ROC) curve analysis.
Results
Among the 120 patients included, there were 75 patients with rT3 stage and 45 patients with rT4 stage. With the median follow-up time of 18 months,the 3 -year OS and PFS were 55.2% and 29.4%, respectively. Multivariate analyses showed that the rNPC patients with older age, low BMI (Body Mass Index), rT4 stage, tumor necrosis, and tumor invasion into the ICA was predictive of worse OS, whereas low BMI and rT4 stage were associated with worse PFS. In addition, the rT stage was identified as a better predictor of OS (area under the ROC curve: 0.669; P=0.003) than the other clinical features.
Conclusions
Salvage treatment using endoscopic nasopharyngectomy appears to be an effective treatment in the management of patients with advanced rNPC. In addition, case matching studies and prospective studies with larger clinical samples are required to further evaluate the efficacy of endoscopic surgery compared with re-irradiation in advanced rNPC.
Keywords: recurrent nasopharyngeal carcinoma, endoscopic, nasopharyngectomy, survival, prognostic factors
Introduction
Nasopharyngeal carcinoma (NPC), an epithelial malignant tumor originating from the mucosal lining of the nasopharynx, is endemic in Southern China and Southeast Asia (1). As a result of the advancements in the field of intensity-modulated radiotherapy (IMRT) in the management of patients with NPC, local recurrence remains one of the most important modes of treatment failure. Overall, 10% to 15% of the patients who undergo definitive radiotherapy will display local recurrence after undergoing treatment (2). Re-irradiation with IMRT for the management of recurrent nasopharyngeal carcinoma (rNPC) is often accompanied by serious complications, such as radiation necrosis of the bone, multiple cranial nerve dysfunction, and brain necrosis, which damages the quality of life of the patients and even leads to death (3). Moreover, Yu et al. reported that treatment with re-irradiation for the management of rNPC could only improve the survival rates in the patients with rT1-2 disease, whereas no significant changes were observed in the patients with rT3-4 disease (4).
Several recent studies have investigated the employment of salvage endoscopic nasopharyngectomy in the management of rNPC, in view of the fact that it might have better survival prognosis and fewer complications, compared to re-irradiation (5, 6). Liu et al. reported that endoscopic surgery significantly improved the overall survival (OS), compared to IMRT, in the patients with early stage rNPC (tumors confined to the nasopharyngeal cavity, the postnaris or nasal septum, the superficial parapharyngeal space, or the base of the sphenoid sinus) (7). Furthermore, a previous study by the authors on patients with rNPC showed that the site-specific and sinonasal-related quality of life were maintained after endoscopic nasopharyngectomy, compared to the preoperative quality of life. Endoscopic surgery appears to be a valuable therapeutic option in the management of patients with rNPC (8).
In scenarios involving advanced rNPC (rT3 and rT4 stages), endoscopic surgery is a challenging endeavor, owing to the frequent invasion into the internal carotid artery (ICA), skull base, orbit, infratemporal fossa, dura mater, cranial nerves, etc. Previous literature reports on the subject are few and involved small sample sizes. Consequently, the survival prognosis and the independent prognostic factors remain controversial (9, 10). The current study retrospectively collected the data pertaining to 120 patients with advanced rNPC who underwent endoscopic nasopharyngectomy during the time period from January 2014 to December 2019. To the best of our knowledge, the present study involves the highest number of reported cases of endoscopic surgery for the management of advanced rNPC. The present study focused on the demographics, clinical outcomes, and prognostic factors pertaining to these patients with rNPC.
Methods
Study Population
The present study performed a retrospective chart review of 120 patients who were diagnosed with advanced rNPC and underwent endoscopic nasopharyngectomy for the management of the same at the Department of Otorhinolaryngology of the Affiliated Eye, Ear, Nose, and Throat Hospital at Fudan University during the time period from January 2014 to December 2019. The patients with T1 and T2 rNPC, and missing data pertaining to important variables were excluded from the study. The reasons for the missing data pertaining to important variables included incomplete preoperative clinical data and inability to contact the patient (e.g., phone number was no longer valid). In addition, there are also some cases without surgically resectable for patients with advanced rNPC: (1) The tumors extensively invaded the intracranial structure, especially the important blood vessels and nerves; (2) The tumors invaded the intracranial structure with obvious radiation brain edema after previous radiotherapy; (3) distant metastasis; (4) unresectable neck lymph node metastasis. The tumor margin to the ICA of less than 0.5 cm was considered as tumor invasion into the ICA (7). The current study was approved by the Institutional Review Board of the Affiliated Eye, Ear, Nose, and Throat Hospital at Fudan University.
Salvage Endoscopic Nasopharyngectomy
The decision to perform salvage endoscopic nasopharyngectomy was based on the location and extent of the tumor, taking into account the patient preferences and consultations with the radiation oncologists and surgeons. In case of the lesions involving the sphenoid sinus, bilateral sphenoidal sinuses were opened, and the septum was removed. The posterior end of the nasal septum was removed, and the base of the sphenoid sinus was ground off to outline the sphenoid sinus and nasopharynx. In order to remove the tumors invading the base of the middle cranial fossa and infratemporal fossa, a modified Caldwell-Luc approach through the anterior wall of the ipsilateral maxillary sinus into the infratemporal fossa and the base of the middle cranial fossa was often required to create a better surgical space and to complete the surgical procedure. The external pterygoid plate was abraded, and the foramen ovale and the main trunk of the mandibular nerve were exposed backwards. Bleeding from the pterygoid venous plexus was arrested by packing with quick yarn. The lingual and inferior alveolar nerves on the posteromedial side of the external pterygoid muscle were located, and the middle meningeal artery and sphenoid spine were exposed posteriorly.
The skull base bone can be divided into cancellous substance and cortex. When the tumor invades the cancellous bone of skull base, sometimes it is not easy to distinguish from the inflammatory granulation tissue after radiation. Pathological biopsy can be taken during the operation to determine whether there is tumor invasion, so as to ensure the negative margin. When the tumor invades the cortex of the skull base, surgeons can judge the extent of tumor invasion by endoscopic visual field and grind the invaded bone to the normal boundary by drill.
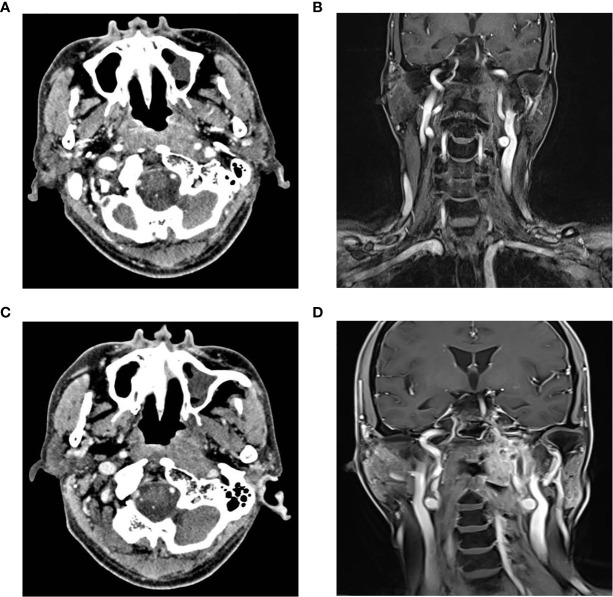
In cases with tumor invasion into the internal carotid artery (ICA), some patients underwent balloon occlusion test (BOT) of the ICA prior to the surgical procedure. If BOT was negative, ICA occlusion could be performed immediately, and the lesion could be removed during surgery ( Figure 1 ). The parapharyngeal, petrosal, foramen, and clival segments of the ICA were exposed, and the related ICA was resected, in accordance with the extent of invasion. If BOT was positive, bypass surgery between the external carotid artery and middle cerebral artery was performed prior to the tumor resection. Subsequently, the rNPC resection was performed after two weeks. In order to prevent postoperative complications, such as wound infection caused by the extensive exposure of the skull base, nasal free mucosal flap, septal pedicled mucosal flap, or temporal muscle flap were used to repair the skull base defect ( Figure 2 ).
Figure 1.
Imaging examination of ICA before and after embolization (A) Horizontal enhanced CT showed that the tumor partially wrapped the left ICA; (B) Coronal enhanced MRI exhibited that the tumor invaded the left pharyngeal recess, parapharyngeal space, the longus capitis, sphenoid body and skull base, and partially surrounded the left ICA; (C, D) Horizontal enhanced CT and coronal enhanced MRI showed the undeveloped cavenous, lacerum, petrous and cervical segments of the left ICA. CT, Computer Tomography; MRI, Magnetic resonance imaging; ICA, Internal carotid artery.
Figure 2.
Reconstruction of skull base defect after salvage surgery for recurrent nasopharyngeal carcinoma: (A) nasal free flap; (B) septal pedicled flap; and (C) temporal muscle flap. ICA, Internal carotid artery.
Clinical Data
The current study retrieved the following clinical data pertaining to the patients: the demographic and clinical features such as the age, sex, history of smoking, alcohol consumption, diabetes, hypertension, and body mass index (BMI); tumor-related features including the number of radiotherapy sessions before surgery, preoperative combined chemotherapy, the time interval between recurrence and the last session of radiotherapy, rT stage, status of lymph node metastasis (LNM) at the time of recurrence, pathological type, status of tumor necrosis, surgical margins, tumor invasion into the ICA, the use of pedicled flaps to repair the skull base defect, postoperative adjuvant therapy, progression-free survival (PFS) and overall survival (OS); and serological parameters, i.e., hemoglobin (Hb) levels, neutrophil-to-lymphocyte ratio (NLR), and serum alkaline phosphatase levels.
Statistical Analysis
The curves of OS and PFS were plotted using the Kaplan–Meier method, and the significance of the differences among rNPC patients with regard to the prognostic factors was analyzed using the Log-rank tests. A Cox regression model was used to perform multivariate survival analyses. The predicted values of the parameters were determined by means of the receiver operating characteristic (ROC) curve analysis. The follow-up period was defined as the time period from the initial diagnosis at our institution to the date of death or last contact.
Results
Study Population
A summary of the details regarding the patients involved in the present study is presented in Table 1 . A total of 120 patients were identified; 88 (73.3%) male subjects and 32 (26.7%) female subjects. Among the aforementioned patients, the number of patients with age ≥50 years, a history of smoking, alcohol consumption, diabetes, and hypertension were sixty-five, thirty, twenty-one, four, and thirty, respectively. In the present study, the normal range of BMI was set at 18.5–24.9 kg/m2; 26, 80, and 14 patients had high, normal, and low BMI, respectively. Regarding the serologic parameters, forty-two, forty, and six patients had low hemoglobin (<120 g/L) levels, high NLR (≥6), and low serum alkaline phosphatase (<50 mmol/L) levels, respectively. The time interval between recurrence and the last radiotherapy session was more than three years in sixty-eight patients.
Table 1.
Characteristics of patients with advanced rNPC.
| Characteristics | Total = 120 | % |
|---|---|---|
| Gender | ||
| Male | 88 | 73.3 |
| Female | 32 | 26.7 |
| Age (years) | ||
| ≥50 | 65 | 54.2 |
| <50 | 55 | 18.3 |
| Smoking history | ||
| Yes | 30 | 25.0 |
| NO | 90 | 75.0 |
| Drinking history | ||
| Yes | 21 | 17.5 |
| No | 99 | 82.5 |
| Diabetes mellitus | ||
| Yes | 116 | 96.7 |
| No | 4 | 3.3 |
| Hypertension | ||
| Yes | 90 | 75.0 |
| No | 30 | 25.0 |
| BMI | ||
| 18.5–24.9 | 80 | 66.7 |
| <18.5 | 14 | 11.7 |
| >24.9 | 26 | 21.7 |
| Hemoglobin | ||
| ≥120 | 78 | 65.0 |
| <120 | 42 | 35.0 |
| NLR | ||
| ≥6 | 40 | 33.3 |
| <6 | 80 | 66.7 |
| Alkaline phosphatase | ||
| <50 | 6 | 5.0 |
| ≥50 | 114 | 95.0 |
| Period between recurrence and the last session of radiotherapy (year) | ||
| ≥3 | 68 | 56.7 |
| <3 | 52 | 43.3 |
| Preoperative combined chemotherapy | ||
| Yes | 99 | 82.5 |
| No | 21 | 17.5 |
| Histological subtype | ||
| WHO type II | 43 | 35.8 |
| WHO type III | 77 | 64.2 |
| rT stage | ||
| rT3 | 75 | 62.5 |
| rT4 | 45 | 37.5 |
| LNM | ||
| Yes | 28 | 23.3 |
| NO | 92 | 76.7 |
| Tumor necrosis | ||
| Yes | 64 | 53.3 |
| No | 56 | 46.7 |
| Tumor invasion of the ICA | ||
| No invasion | 79 | 65.8 |
| Invasion | 28 | 23.3 |
| Invasion, but ICA was embolized | 13 | 10.8 |
| The use of pedicled flap | ||
| Yes | 55 | 45.8 |
| No | 65 | 54.2 |
| Surgical margin | ||
| Negative margin | 85 | 70.8 |
| Positive margin | 35 | 29.2 |
| Postoperative radiotherapy | ||
| No | 112 | 93.3 |
| Yes | 8 | 6.7 |
| Postoperative chemotherapy | ||
| No | 94 | 78.3 |
| Yes | 26 | 21.7 |
| Postoperative PD-1 treatment | ||
| No | 111 | 92.5 |
| Yes | 9 | 7.5 |
| All patients with follow-up (mean months of survival) range) |
18 (2-81) | |
| Outcome | ||
| Remission | 39 | 32.5 |
| Deceased | 40 | 33.3 |
| Alive with disease | 41 | 34.2 |
rNPC, recurrent nasopharyngeal carcinoma; BMI, Body mass index; NLR, Neutrophil to lymphocyte ratio; LNM, lymph node metastasis; ICA, Internal carotid artery.
Among the study subjects, 99 patients received adjuvant chemotherapy along with preoperative radiotherapy. The most frequent histological subtype of the tumor encountered in the current study was World Health Organization (WHO) type III (n=77, 64.2.0%), followed by WHO type II (n=43, 45.8%). In the current study, the tumors were staged on the basis of the rTNM staging system by the American Joint Committee on Cancer (AJCC/UICC) (7th edition, published in 2010) as follows: rT3 (n=75) and rT4 (n=45). The current study detected lymph node metastases in 28 patients (23.3%). The presence of tumor necrosis was observed in 64 patients (53.5%).
Preoperative tumor invasion into the ICA was observed in 41 patients (34.2%), including 13 cases with ICA embolization and 28 cases without ICA embolization. Moreover, in 79 patients, the tumor did not invade the ICA (63.8%). Among the 120 patients, 84 (70.0%) underwent repair of the postoperative nasopharyngeal defect. The pedicled flap was used in 55 cases (45.8%), including 41 cases of pedicled septal mucosal flap and 14 cases of temporal muscle flap. The pedicled flap was not employed to repair skull base defect in 65 patients, including 29 cases that underwent repair using nasal free mucosal flap and 36 cases without any intervention. Furthermore, eight, twenty-six, and nine patients received postoperative radiotherapy, chemotherapy, and programmed cell death protein 1 (PD-1) treatment, respectively.
Among the study subjects, 85 (70.8%) patients had negative surgical margins. The relationship between surgical margin and ICA embolization in patients with tumor invasion of the ICA is shown in Table 2 . A higher rate of negative margins (11/13, 84.6%) was observed in the patients with ICA embolization, compared to those without embolization (15/28, 53.6%). However, there was no significant difference between the two groups (P=0.084).
Table 2.
The relationship between surgical margin and ICA embolization in rNPC patients with tumor invasion of ICA.
| Embolization of ICA | Surgical margin | P value | |
|---|---|---|---|
| Negative margin | Positive margin | ||
| No (n) | 15 | 13 | 0.084 |
| Yes (n) | 11 | 2 | |
rNPC, recurrent nasopharyngeal carcinoma; ICA, Internal carotid artery.
Postoperative Complications
In this study, no patient died during the perioperative period. Although the post-operative period was uneventful, there still remain some complications. Most patients have wound scab, dry nose and headache in the first few days after surgery. The use of antibiotics and nasal irrigation gradually relieved these symptoms in about 4 weeks. In addition, 16 cases (13.3%) developed nasopharyngeal necrosis, which is a serious postoperative complication caused by surgical wound infection and difficult to heal due to previous radiotherapy. 15 patients (12.5%) had dyskinesia of masticatory muscle or limitation of mouth opening due to the mandibular branch injury of trigeminal nerve. One patient occurred cerebral infarction after ICA embolization.
Overall Survival and Progression-Free Survival
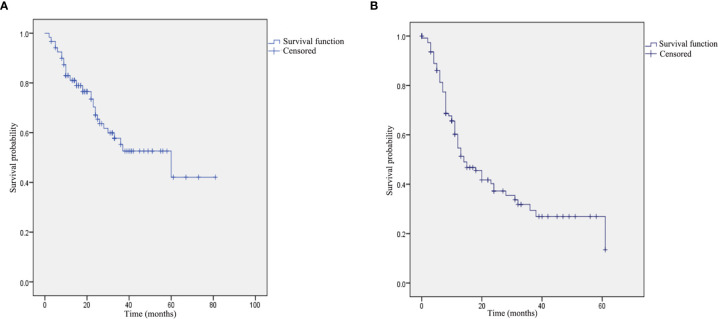
The median duration of follow-up in the current study was 18 months (range: 2–81 months). Among the 40 patients who expired during the course of the study, 19 patients expired due to tumor progression at the local site (n=12) or the brain (n=5), the cause of mortality in 15 patients was postoperative ICA hemorrhage, three patients expired due to lung metastasis, one patient died as a result of skull base necrosis, one patient died of brain necrosis, and one patient expired by reason of cerebral infarction. Remission was confirmed by means of physical examination and enhanced magnetic resonance in 39 patients (32.5%), and 41 patients (34.2%) survived with the disease. The 1-year,2-year and 3-year OS pertaining to all the patients were 81.1%, 67.1% and 55.2%, respectively. In addition, the 1-year, 2-year and 3-year PFS were 54.7%, 37.3% and 29.4%, respectively ( Figure 3 ).
Figure 3.
Kaplan–Meier curve pertaining to the survival in patients with advanced recurrent nasopharyngeal carcinoma: (A) overall survival: and (B) progression-free survival.
Prognostic Analysis
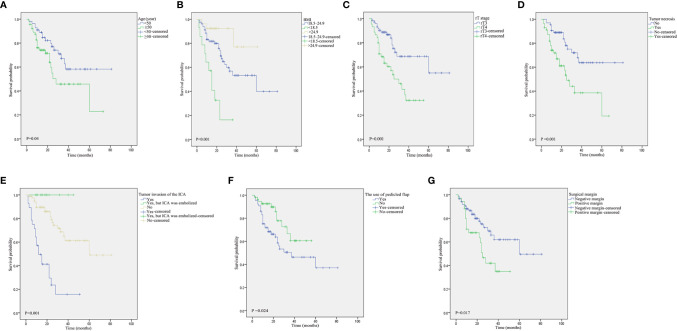
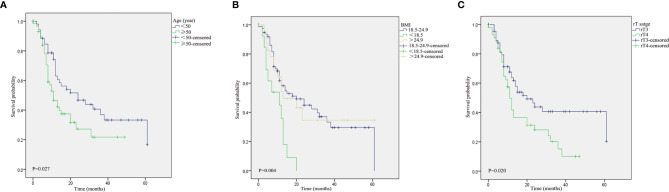
The log-rank tests for prognostic factors pertaining to advanced rNPC are shown in Table 3 . Age ≥50 years ( Figure 4A ), low BMI ( Figure 4B ), rT4 disease ( Figure 4C ), tumor necrosis ( Figure 4D ), tumor invasion into the ICA ( Figure 4E ), the use of pedicled flap ( Figure 4F ) and positive surgical margins ( Figure 4G ) were associated with a poor prognosis of OS. In addition, Age ≥50 years ( Figure 5A ), low BMI ( Figure 5B ) and rT4 disease ( Figure 5C ) were found to be significantly associated with worse PFS.
Table 3.
Log-rank test of prognostic factors for rNPC.
| Prognostic factors | OS (%) | P value | PFS (%) | P value | ||||
|---|---|---|---|---|---|---|---|---|
| 1-year | 2-year | 3-year | 1-year | 2-year | 3-year | |||
| Sex | 0.110 | 0.640 | ||||||
| Male | 83.5 | 73.8 | 60.4 | 56.9 | 36.0 | 31.4 | ||
| Female | 74.1 | 61.4 | 45.5 | 48.1 | 39.2 | 23.5 | ||
| Age (year) | 0.040 | 0.027 | ||||||
| ≥50 | 74.1 | 54.9 | 45.7 | 46.6 | 27.2 | 21.7 | ||
| <50 | 88.9 | 77.1 | 62.7 | 63.0 | 46.7 | 37.0 | ||
| Smoking history | 0.120 | 0.127 | ||||||
| Yes | 89.3 | 80.3 | 70.3 | 55.6 | 41.1 | 33.2 | ||
| NO | 78.2 | 63.4 | 51.8 | 51.4 | 24.0 | 18.0 | ||
| Drinking history | 0.220 | 0.846 | ||||||
| Yes | 90.5 | 90.5 | 77.6 | 54.1 | 37.1 | 29.8 | ||
| NO | 79.1 | 63.2 | 51.9 | 57.1 | 38.1 | 28.6 | ||
| Diabetes mellitus | 0.926 | 0.815 | ||||||
| Yes | 100 | 50 | 50 | 66.7 | 33.3 | 33.3 | ||
| NO | 80.5 | 67.3 | 55.4 | 54.0 | 37.2 | 29.3 | ||
| Hypertension | 0.847 | 0.670 | ||||||
| Yes | 82.9 | 78.0 | 59.4 | 54.6 | 31.9 | 31.9 | ||
| No | 80.4 | 63.9 | 54.4 | 54.6 | 38.8 | 28.9 | ||
| BMI | <0.001 | 0.004 | ||||||
| 18.5–24.9 | 81.7 | 69.5 | 53.2 | 58.2 | 44.9 | 33.3 | ||
| <18.5 | 57.1 | 32.7 | 16.3 | 35.9 | 0 | 0 | ||
| >24.9 | 92.3 | 92.3 | 92.3 | 54.4 | 34.6 | 34.6 | ||
| Hemoglobin | 0.461 | 0.115 | ||||||
| ≥120 | 81.5 | 67.9 | 57.5 | 59.4 | 41.2 | 33.7 | ||
| <120 | 80.2 | 64.9 | 47.3 | 45.9 | 32.2 | 16.1 | ||
| NLR | 0.137 | 0.644 | ||||||
| ≥6 | 72.5 | 56.1 | 42.1 | 38.7 | 38.7 | 29.0 | ||
| <6 | 83.4 | 70.2 | 58.7 | 59.0 | 36.7 | 29.6 | ||
| Alkaline phosphatase | 0.899 | 0.428 | ||||||
| <50 | 83.3 | 83.3 | 41.7 | 60.0 | 60.0 | 60.0 | ||
| ≥50 | 81.0 | 66.2 | 55.6 | 54.3 | 36.1 | 28.0 | ||
| Preoperative combined chemotherapy | 0.067 | |||||||
| chemotherapy | 0.541 | |||||||
| Yes | 84.1 | 69.2 | 57.4 | 52.4 | 40.7 | 31.6 | ||
| No | 66.7 | 55.6 | 41.7 | 65.9 | 13.8 | 13.8 | ||
| Period between recurrence and the last | ||||||||
| session of radiotherapy (year) | 0.433 | 0.291 | ||||||
| ≥3 | 80.4 | 72.4 | 68.8 | 60.6 | 43.7 | 30.9 | ||
| <3 | 81.8 | 62.9 | 43.2 | 47.9 | 30.6 | 27.2 | ||
| Histological subtype | 0.406 | 0.340 | ||||||
| WHO type II | 73.9 | 63.5 | 57.7 | 59.0 | 44.1 | 35.3 | ||
| WHO type III | 86.6 | 69.2 | 54.5 | 52.2 | 34.1 | 26.0 | ||
| rT stage | 0.001 | 0.020 | ||||||
| rT3 | 88.8 | 75.3 | 68.8 | 63.7 | 43.8 | 40.6 | ||
| rT4 | 68.4 | 53.7 | 36.9 | 41.8 | 28.2 | 15.1 | ||
| LNM | 0.216 | 0.159 | ||||||
| Yes | 78.2 | 60.0 | 40.0 | 55.7 | 28.6 | 14.3 | ||
| No | 81.9 | 69.2 | 60.0 | 54.1 | 40.4 | 33.1 | ||
| Tumor necrosis | 0.001 | 0.698 | ||||||
| Yes | 71.8 | 53.4 | 38.6 | 58.7 | 36.2 | 25.6 | ||
| No | 88.8 | 78.0 | 67.9 | 50.4 | 39.6 | 39.6 | ||
| Tumor invasion of the ICA | <0.001 | 0.192 | ||||||
| No invasion | 89.6 | 79.2 | 65.1 | 57.6 | 39.6 | 34.8 | ||
| Invasion | 49.5 | 23.6 | 15.7 | 39.9 | 23.3 | 11.7 | ||
| Invasion, but ICA was embolized | 100 | 100 | 100 | 68.6 | 57.1 | 28.6 | ||
| The use of pedicled flap | 0.024 | 0.513 | ||||||
| Yes | 92.6 | 77.8 | 60.5 | 50.9 | 33.7 | 23.6 | ||
| No | 71.8 | 58.7 | 50.5 | 57.6 | 40.2 | 34.0 | ||
| Surgical margin | 0.017 | 0.139 | ||||||
| Negative margin | 86.6 | 75.2 | 61.9 | 55.2 | 43.0 | 36.3 | ||
| Positive margin | 67.8 | 50.8 | 41.9 | 53.4 | 25.1 | 15.1 | ||
| Postoperative radiotherapy | 0.655 | 0.200 | ||||||
| No | 81.5 | 68.3 | 55.6 | 56.6 | 38.1 | 29.6 | ||
| Yes | 75.0 | 50.0 | 50.0 | 31.3 | 31.3 | 31.3 | ||
| Postoperative chemotherapy | 0.134 | 0.248 | ||||||
| No | 79.1 | 65.8 | 51.1 | 58.3 | 39.1 | 34.0 | ||
| Yes | 87.7 | 71.7 | 71.7 | 42.4 | 31.4 | 16.7 | ||
| Postoperative PD-1 treatment | 0.238 | 0.675 | ||||||
| No | 79.5 | 66.2 | 53.7 | 54.9 | 38.3 | 32.5 | ||
| Yes | 100 | 83.3 | 83.3 | 51.9 | 25.9 | 25.9 | ||
rNPC, recurrent nasopharyngeal carcinoma; OS, Overall survival; PFS, Progress free survival; BMI, Body mass index; NLR, Neutrophil to lymphocyte ratio; LNM, lymph node metastasis; ICA, Internal carotid artery.
Figure 4.
Kaplan–Meier curves pertaining to the overall survival in patients with recurrent nasopharyngeal carcinoma: (A) age (≥50 vs. <50 years); (B) body mass index (BMI); (C) rT stage (rT3 vs. rT4); (D) tumor necrosis; (E) tumor invasion into the ICA; (F) the use of pedicled flap; and (G) surgical margins.
Figure 5.
Kaplan–Meier curves pertaining to the progression-free survival in patients with recurrent nasopharyngeal carcinoma: (A) age (≥50 vs. <50 years); (B) body mass index (BMI); and (C) rT stage (rT3 vs. rT4).
The variables that were considered to be significant in the Cox univariate analyses were age, BMI, rT stage, tumor necrosis, tumor invasion into the ICA, the use of pedicled flaps to repair the defect, and positive surgical margins. Subsequently, Cox multivariate analyses were performed using the aforementioned seven variables. A total of five variables (age, BMI, T stage, tumor necrosis, and tumor invasion into the ICA) were proven to be independent prognostic factors in the multivariate Cox regression model ( Table 4 ). Moreover, based on the factors affecting OS, the predictive values were analyzed by means of the ROC analysis, which revealed that the rT stage was the best predictor for OS. The area under the ROC curve for rT stage with regard to the OS was 0.669 (95% confidence interval [CI], 0.563–0.774; P=0.003). The prognostic values pertaining to the other factors are shown in Figure 6 . In addition, multivariable analysis revealed that patients with BMI and rT stage were significantly related to PFS ( Table 5 ).
Table 4.
Univariate and multivariate Cox regression analyses of OS in patients with rNPC.
| Variable | Univeriate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| HR | 95%CI | P | HR | 95%CI | P | ||
| Age (year) | |||||||
| ≥50 | 1 | Reference | 1 | Reference | |||
| <50 | 0.517 | 0.272-0.985 | 0.045 | 0.462 | 0.235-0.908 | 0.025 | |
| BMI | |||||||
| 18.5–24.9 | 1 | Reference | 1 | Reference | |||
| <18.5 | 3.693 | 1.745-7.817 | 0.001 | 2.981 | 1.282-6.931 | 0.011 | |
| >24.9 | 0.348 | 0.105-1.147 | 0.083 | 0.570 | 0.159-2.047 | 0.388 | |
| T stage | |||||||
| rT3 | 1 | Reference | 1 | Reference | |||
| rT4 | 2.827 | 1.483-5.391 | 0.002 | 2.396 | 1.140-5.037 | 0.021 | |
| Tumor necrosis | |||||||
| Yes | 1 | Reference | 1 | Reference | |||
| No | 0.361 | 0.189-0.688 | 0.002 | 0.488 | 0.249-0.960 | 0.038 | |
| Tumor invaded the ICA | |||||||
| No invasion | 1 | Reference | 1 | Reference | |||
| Invasion | 5.164 | 2.713-9.827 | 0.000 | 2.445 | 1.093-5.469 | 0.030 | |
| Invasion, but ICA was embolized. | <0.001 | / | 0.968 | <0.001 | / | 0.971 | |
| Surgical margin | |||||||
| Negative margin | 1 | Reference | 1 | Reference | |||
| Positive margin | 2.108 | 1.123-3.959 | 0.020 | 1.017 | 0.478-2.162 | 0.965 | |
| The use of pedicled flap | |||||||
| Yes | 1 | Reference | 1 | Reference | |||
| No | 2.179 | 1.085-4.379 | 0.029 | 1.405 | 0.666 -2.968 | 0.372 | |
| Sex | |||||||
| Male | 1 | Reference | |||||
| Female | 1.667 | 0.876-3.171 | 0.119 | ||||
| Smoking history | |||||||
| Yes | 1 | Reference | |||||
| No | 2.064 | 0.805-5.295 | 0.132 | ||||
| Drinking history | |||||||
| Yes | 1 | Reference | |||||
| No | 2.439 | 0.751-7.926 | 0.138 | ||||
| Diabetes mellitus | |||||||
| Yes | 1 | Reference | |||||
| No | 0.911 | 0.124-6.685 | 0.911 | ||||
| Hypertension | |||||||
| Yes | 1 | Reference | |||||
| NO | 0.933 | 0.455-1.910 | 0.849 | ||||
| Hemoglobin | |||||||
| <120 | 1 | Reference | |||||
| ≥120 | 0.783 | 0.405-1.511 | 0.466 | ||||
| NLR | |||||||
| ≥6 | 1 | Reference | |||||
| <6 | 0.593 | 0.294-1.198 | 0.145 | ||||
| Alkaline phosphatase | |||||||
| <50 | 1 | Reference | |||||
| ≥50 | 1.096 | 0.264-4.552 | 0.900 | ||||
| The time between recurrence and the last radiotherapy (year) | |||||||
| ≥3 | 1 | Reference | |||||
| <3 | 1.282 | 0.684-2.405 | 0.438 | ||||
| Preoperative combined chemotherapy before surgery | |||||||
| Yes | 1 | Reference | |||||
| No | 1.922 | 0.937-3.941 | 0.075 | ||||
| Histological subtype | |||||||
| WHO type II | 1 | Reference | |||||
| WHO type III | 0766 | 0.405-1.447 | 0.411 | ||||
| LNM | |||||||
| Yes | 1 | Reference | |||||
| No | 0.657 | 0.334-1.292 | 0.068 | ||||
| Postoperative radiotherapy | |||||||
| No | 1 | Reference | |||||
| Yes | 1.305 | 0.401-4.240 | 0.658 | ||||
| Postoperative chemotherapy | |||||||
| No | 1 | Reference | |||||
| Yes | 0.498 | 0.195-1.273 | 0.145 | ||||
| Postoperative PD-1 treatment | |||||||
| No | 1 | Reference | |||||
| Yes | 0.324 | 0.044-2.366 | 0.267 | ||||
rNPC, recurrent nasopharyngeal carcinoma; OS, Overall survival; BMI, Body mass index; NLR, Neutrophil to lymphocyte ratio; LNM, lymph node metastasis; ICA, Internal carotid artery; HR, hazard ratio; CI, confidence interval.
Figure 6.

Receiver operating characteristic analysis revealed that the rT stage was the best predictor for overall survival (OS). The area under the ROC curve for rT stage was 0.669 (P = 0.003).
Table 5.
Univariate and multivariate Cox regression analyses of PFS in patients with rNPC.
| Variable | Univeriate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P | HR | 95%CI | P | |
| Age (year) | ||||||
| ≥50 | 1 | Reference | 1 | Reference | ||
| <50 | 0.580 | 0.352-0.957 | 0.033 | 0.610 | 0.368-1.010 | 0.055 |
| BMI | ||||||
| 18.5–24.9 | 1 | Reference | 1 | Reference | ||
| <18.5 | 2.795 | 1.445-5.409 | 0.002 | 2.462 | 1.267-4.784 | 0.008 |
| >24.9 | 1.019 | 0.544-1.908 | 0.953 | 0.904 | 0.480-1.703 | 0.755 |
| T stage | ||||||
| rT3 | 1 | Reference | 1 | Reference | ||
| rT4 | 1.750 | 1.075-2.850 | 0.024 | 1.648 | 1.010-2.689 | 0.046 |
| Sex | ||||||
| Male | 1 | Reference | ||||
| Female | 0.881 | 0.511-1.518 | 0.648 | |||
| Smoking history | ||||||
| Yes | 1 | Reference | ||||
| No | 0.662 | 0.384-1.142 | 0.138 | |||
| Drinking history | ||||||
| Yes | 1 | Reference | ||||
| No | 0.849 | 0.477-1.839 | 0.849 | |||
| Diabetes mellitus | ||||||
| Yes | 1 | Reference | ||||
| No | 0.849 | 0.207-3.480 | 0.849 | |||
| Hypertension | ||||||
| Yes | 1 | Reference | ||||
| No | 0.677 | 0.496-1.578 | 0.677 | |||
| Hemoglobin | ||||||
| <120 | 1 | Reference | ||||
| ≥120 | 0.670 | 0.401-1.119 | 0.126 | |||
| NLR | ||||||
| ≥6 | 1 | Reference | ||||
| <6 | 0.872 | 0.482-1.579 | 0.652 | |||
| Alkaline phosphatase | ||||||
| <50 | 1 | Reference | ||||
| ≥50 | 1.732 | 0.424-7.084 | 0.444 | |||
| The time between recurrence and the | ||||||
| last radiotherapy (year) | ||||||
| ≥3 | 1 | Reference | ||||
| <3 | 1.292 | 0.794-2.101 | 0.303 | |||
| Preoperative combined chemotherapy before surgery | ||||||
| Yes | 1 | Reference | ||||
| No | 1.211 | 0.645-2.275 | 0.551 | |||
| Histological subtype | ||||||
| WHO type II | 1 | Reference | ||||
| WHO type III | 1.289 | 0.755-2.200 | 0.353 | |||
| LNM | ||||||
| Yes | 1 | Reference | ||||
| No | 0.688 | 0.402-1.175 | 0.171 | |||
| Postoperative radiotherapy | ||||||
| No | 1 | Reference | ||||
| Yes | 0.559 | 0.223-1.402 | 0.215 | |||
| Postoperative chemotherapy | ||||||
| No | 1 | Reference | ||||
| Yes | 1.374 | 0.790-2.390 | 0.261 | |||
| Postoperative PD-1 treatment | ||||||
| No | 1 | Reference | ||||
| Yes | 0.839 | 0.361-1.947 | 0.683 | |||
| Tumor necrosis | ||||||
| Yes | 1 | Reference | ||||
| No | 0.910 | 0.558-1.483 | 0.705 | |||
| Tumor invaded the ICA | ||||||
| No invasion | 1 | Reference | ||||
| Invasion | 1.554 | 0.888-2.718 | 0.122 | |||
| Invasion, but ICA was embolized. | 0.774 | 0.306-1.959 | 0.589 | |||
| Surgical margin | ||||||
| Negative margin | 1 | Reference | ||||
| Positive margin | 1.444 | 0.875-2.383 | 0.150 | |||
| The use of pedicled flap | ||||||
| Yes | 1 | Reference | ||||
| No | 0.853 | 0.523-1.391 | 0.853 | |||
rNPC, recurrent nasopharyngeal carcinoma; PFS, progress free survival; BMI, Body mass index; NLR, Neutrophil to lymphocyte ratio; LNM, lymph node metastasis; ICA, Internal carotid artery; HR, hazard ratio; CI, confidence interval.
Discussion
Aggressive salvage treatment is recommended for the management of locally rNPC, owing to the fact that many patients can still achieve long-term survival (4). The most common therapeutic modalities used for the management of rNPC are salvage surgery and re-irradiation with IMRT. Moreover, several studies have reported that IMRT is the most effective treatment for the management of advanced rNPC. Salvage surgery is only employed in the scenarios involving resectable, early-stage rNPC, such as rT1 disease, rT2–3 disease with limited parapharyngeal space involvement, or disease confined to the base of the sphenoid sinus (11, 12). However, advanced rNPC is characteristically more radioresistant, compared to the primary tumor, which can be attributed to the poor blood supply and hypoxia, and a suboptimal dose distribution resulting from the protection of critical structures. Re-irradiation for the management of these bulky tumors warrants a higher radiation dose and wider treatment range to include a 1–1.5 cm margin, thereby compromising adjacent critical structures, which leads to severe radiotherapy-related toxicity and poor survival outcomes (13–15).
Over the recent years, several studies have suggested that salvage surgery could be used in patients with advanced rNPC (9, 10, 12, 16). Wong et al. reported favorable patient outcomes in fifteen patients with advanced rNPC (two rT3 and thirteen rT4 tumors) who underwent surgery by means of the endoscopic approach; the two-year OS was 66.7% (10). In our study, the three-year OS in patients with advanced rNPC was 55.2%, which was higher, compared to the patients who underwent salvage IMRT (17–19). Furthermore, the three-year OS in patients with rT3 and rT4 tumors were 68.8% and 36.9%, respectively, which were higher, compared to the patients who underwent salvage IMRT pertaining to rT3 and rT4 tumors, i.e., of 49.5% and 33.9%, reported by Hua et al. (18) However, many patients who received salvage surgery were given postoperative multimodal treatment including chemotherapy, radiotherapy or anti-PD-1 drugs. In addition, this study lacked a comparison cohort between salvage surgery and IMRT. Thus, case matching studies and prospective studies with larger clinical samples are required to further evaluate the efficacy of endoscopic surgery compared with re-irradiation in advanced rNPC.Postoperative ICA hemorrhage is one of the major causes of mortality after salvage surgery in patients with advanced rNPC. Consequently, tumor invasion into the ICA presents a difficult undertaking with regard to surgery. During the early years of the current research, the present study adopted the vidian nerve and eustachian tube as consistent and reliable ICA markers in the patients undergoing such high-risk surgical procedures (20). According to the relationship between the aforementioned markers, the parapharyngeal segment and petrous segment can be safely identified during the surgical procedure. However, the tumor resection had limitations; the tumor close to the ICA could only be removed as much as possible. Inevitably, some patients had tumor recurrence or postoperative ICA hemorrhage, which affects the OS in patients with advanced rNPC.
Recently, the authors proposed a new surgical procedure for the management of rNPC, namely, the embolization of ICA and the resection of tumor invading the ICA. This innovative technique can expand the scope of surgical resection and completely remove the tumor on the ICA, so as to ensure the negative margin, and reduce the probability of residual tumor and recurrence. In addition, it can avoid the occurrence of ICA hemorrhage in the process of tumor dissection, thus reduce the risk of surgery. More importantly, preoperative radiotherapy and previous surgical trauma have a great influence on the blood supply to the skull base, and postoperative cavity infection can occur easily. When the focus of infection invades the ICA, it leads to fatal ICA hemorrhage. Consequently, ICA embolization can also prevent postoperative ICA rupture. In this series, the three-year OS rate pertaining to the patients without ICA invasion, invasion into ICA, and embolization of ICA were 65.1%, 15.7%, and 100%, respectively. Multivariate Cox regression analysis showed that ICA invasion was an independent risk factor for OS. Hence, it was concluded that the patients with rNPC with ICA invasion who undergo preoperative ICA embolization might have a better survival prognosis after salvage nasopharyngectomy.
BMI is a simple weight-for-height calculation that is often used to evaluate the nutritional status in adults. Several studies have reported that lower BMI is an independent risk factor for tumor recurrence or distant metastasis after radiotherapy in NPC patients (21, 22). A previous study regarding metastatic NPC by Li et al. demonstrated that the overweight/obese status was associated with longer OS, compared to the underweight or normal weight status. Overweight patients experience adverse treatment outcomes to a lesser extent and are less likely to experience malnutrition, cachexia, and increased tolerance to cancer treatment (23). The current study observed that rNPC patients with low BMI displayed worse OS after endoscopic surgery, compared to the patients with normal BMI. The patients with low BMI should receive further assessment, intensive counseling, and nutrition support. However, the current study did not observe any significant difference between the patients with high BMI and normal BMI with regard to OS, which was consistent with our preliminary report (24, 25).
Conclusion
Generally, Salvage treatment using endoscopic nasopharyngectomy appears to be an effective treatment in the management of patients with advanced rNPC. The independent risk factors pertaining to the OS were age (above 50 years), low BMI, rT4 stage, tumor necrosis, and tumor invasion into the ICA. In addition, case matching studies and prospective studies with larger clinical samples are required to further evaluate the efficacy of endoscopic surgery compared with re-irradiation in advanced rNPC.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving humans were reviewed and approved by the Institutional Review Board of AEENTH at Fudan University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
DW, HY, and XS conceived, designed, and supervised the study. WL conceived and designed the study, performed the statistical analysis, and drafted the manuscript. HZ conceived and designed the study and drafted the manuscript. HLu acquired the data. YG and HLi drafted the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 81870703), Shanghai Shen Kang Hospital Development Center (SHDC12018118), Clinical Research Plan of SHDC (SHDC2020CR2005A), Research Units of New Technologies of Endoscopic Surgery in Skull Base Tumor (2018RU003) supported by the Chinese Academy of Medical Sciences, Science and Technology Commission of Shanghai Municipality (20Y11902000, 21ZR1411700), and Shanghai Municipal Health Commission (201940143).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank all the participants for their contribution and participation.
References
- 1. Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal Carcinoma. Lancet (2019) 394:64–80. 10.1016/S0140-6736(19)30956-0 [DOI] [PubMed] [Google Scholar]
- 2. Kong L, Lu JJ. Reirradiation of Locally Recurrent Nasopharyngeal Cancer: History, Advances, and Promises for the Future. Chin Clin Oncol (2016) 5:26. 10.21037/cco.2016.03.19 [DOI] [PubMed] [Google Scholar]
- 3. Lu TX, Mai WY, Teh BS, Zhao C, Han F, Huang Y, et al. Initial Experience Using Intensity-Modulated Radiotherapy for Recurrent Nasopharyngeal Carcinoma. Int J Radiat Oncol Biol Phys (2004) 58:682–7. 10.1016/S0360-3016(03)01508-6 [DOI] [PubMed] [Google Scholar]
- 4. Yu KH, Leung SF, Tung SY, Zee B, Chua DT, Sze WM, et al. Survival Outcome of Patients With Nasopharyngeal Carcinoma With First Local Failure: A Study by the Hong Kong Nasopharyngeal Carcinoma Study Group. Head Neck (2005) 27:397–405. 10.1002/hed.20161 [DOI] [PubMed] [Google Scholar]
- 5. You R, Zou X, Hua YJ, Han F, Li L, Zhao C, et al. Salvage Endoscopic Nasopharyngectomy Is Superior to Intensity-Modulated Radiation Therapy for Local Recurrence of Selected T1-T3 Nasopharyngeal Carcinoma - A Case-Matched Comparison. Radiother Oncol (2015) 115:399–406. 10.1016/j.radonc.2015.04.024 [DOI] [PubMed] [Google Scholar]
- 6. Hao CY, Hao SP. The Management of rNPC: Salvage Surgery vs. Re-Irradiation. Curr Oncol Rep (2020) 22:86. 10.1007/s11912-020-00949-0 [DOI] [PubMed] [Google Scholar]
- 7. Liu YP, Wen YH, Tang J, Wei Y, You R, Zhu XL, et al. Endoscopic Surgery Compared With Intensity-Modulated Radiotherapy in Resectable Locally Recurrent Nasopharyngeal Carcinoma: A Multicentre, Open-Label, Randomised, Controlled, Phase 3 Trial. Lancet Oncol (2021) 22:381–90. 10.1016/S1470-2045(20)30673-2 [DOI] [PubMed] [Google Scholar]
- 8. Li W, Lu H, Liu J, Liu Q, Wang H, Zhang H, et al. Quality of Life Following Salvage Endoscopic Nasopharyngectomy in Patients With Recurrent Nasopharyngeal Carcinoma: A Prospective Study. Front Oncol (2020) 10:437. 10.3389/fonc.2020.00437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wong EHC, Liew YT, Loong SP, Prepageran N. Five-Year Survival Data on the Role of Endoscopic Endonasal Nasopharyngectomy in Advanced Recurrent Rt3 and Rt4 Nasopharyngeal Carcinoma. Ann Otol Rhinol Laryngol (2020) 129:287–93. 10.1177/0003489419887410 [DOI] [PubMed] [Google Scholar]
- 10. Wong EHC, Liew YT, Abu Bakar MZ, Lim EYL, Prepageran N. A Preliminary Report on the Role of Endoscopic Endonasal Nasopharyngectomy in Recurrent Rt3 and Rt4 Nasopharyngeal Carcinoma. Eur Arch Otorhinolaryngol (2017) 274:275–81. 10.1007/s00405-016-4248-2 [DOI] [PubMed] [Google Scholar]
- 11. Lee AWM, Ng WT, Chan JYW, Corry J, Makitie A, Mendenhall WM, et al. Management of Locally Recurrent Nasopharyngeal Carcinoma. Cancer Treat Rev (2019) 79:101890. 10.1016/j.ctrv.2019.101890 [DOI] [PubMed] [Google Scholar]
- 12. Chan JYW, Wong STS, Wei WI. Surgical Salvage of Recurrent T3 Nasopharyngeal Carcinoma: Prognostic Significance of Clivus, Maxillary, Temporal and Sphenoid Bone Invasion. Oral Oncol (2019) 91:85–91. 10.1016/j.oraloncology.2019.02.023 [DOI] [PubMed] [Google Scholar]
- 13. Wang Y, Wang ZQ, Jiang YX, Wang FH, Luo HY, Liang Y, et al. A Triplet Chemotherapy Regimen of Cisplatin, Fluorouracil and Paclitaxel for Locoregionally Recurrent Nasopharyngeal Carcinoma Cases Contraindicated for Re-Irradiation/Surgery. Expert Opin Pharmacother (2016) 17:1585–90. 10.1080/14656566.2016.1204293 [DOI] [PubMed] [Google Scholar]
- 14. Yue Q, Zhang M, Chen Y, Zheng D, Chen Y, Feng M. Establishment of Prognostic Factors in Recurrent Nasopharyngeal Carcinoma Patients Who Received Salvage Intensity-Modulated Radiotherapy: A Meta-Analysis. Oral Oncol (2018) 81:81–8. 10.1016/j.oraloncology.2018.04.017 [DOI] [PubMed] [Google Scholar]
- 15. Tian YM, Huang WZ, Yuan X, Bai L, Zhao C, Han F. The Challenge in Treating Locally Recurrent T3-4 Nasopharyngeal Carcinoma: The Survival Benefit and Severe Late Toxicities of Re-Irradiation With Intensity-Modulated Radiotherapy. Oncotarget (2017) 8:43450–7. 10.18632/oncotarget.15896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen Z, Qiu Q. Analysis of Clinical Efficacy and the Quality of Life After Endoscopic Nasopharyngectomy for Residual or Recurrent Nasopharyngeal Carcinoma. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi (2015) 50:896–903. 10.3760/cma.j.issn.1673-0860.2015.11.004 [DOI] [PubMed] [Google Scholar]
- 17. Kong F, Zhou J, Du C, He X, Kong L, Hu C, et al. Long-Term Survival and Late Complications of Intensity-Modulated Radiotherapy for Recurrent Nasopharyngeal Carcinoma. BMC Cancer (2018) 18:1139. 10.1186/s12885-018-5055-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hua YJ, Han F, Lu LX, Mai HQ, Guo X, Hong MH, et al. Long-Term Treatment Outcome of Recurrent Nasopharyngeal Carcinoma Treated With Salvage Intensity Modulated Radiotherapy. Eur J Cancer (2012) 48:3422–8. 10.1016/j.ejca.2012.06.016 [DOI] [PubMed] [Google Scholar]
- 19. Chan OS, Sze HC, Lee MC, Chan LL, Chang AT, Lee SW, et al. Reirradiation With Intensity-Modulated Radiotherapy for Locally Recurrent T3 to T4 Nasopharyngeal Carcinoma. Head Neck (2017) 39:533–40. 10.1002/hed.24645 [DOI] [PubMed] [Google Scholar]
- 20. Liu J, Sun X, Liu Q, Wang D, Wang H, Ma N. Eustachian Tube as a Landmark to the Internal Carotid Artery in Endoscopic Skull Base Surgery. Otolaryngol Head Neck Surg (2016) 154:377–82. 10.1177/0194599815616799 [DOI] [PubMed] [Google Scholar]
- 21. Tang LQ, Li CF, Li J, Chen WH, Chen QY, Yuan LX, et al. Establishment and Validation of Prognostic Nomograms for Endemic Nasopharyngeal Carcinoma. J Natl Cancer Inst (2016) 108:djv291. 10.1093/jnci/djv291 [DOI] [PubMed] [Google Scholar]
- 22. Huang PY, Zeng Q, Cao KJ, Guo X, Guo L, Mo HY, et al. Ten-Year Outcomes of a Randomised Trial for Locoregionally Advanced Nasopharyngeal Carcinoma: A Single-Institution Experience From an Endemic Area. Eur J Cancer (2015) 51:1760–70. 10.1016/j.ejca.2015.05.025 [DOI] [PubMed] [Google Scholar]
- 23. Li W, Shen LJ, Chen T, Sun XQ, Zhang Y, Wu M, et al. Overweight/obese Status Associates With Favorable Outcome in Patients With Metastatic Nasopharyngeal Carcinoma: A 10-Year Retrospective Study. Chin J Cancer (2016) 35:75. 10.1186/s40880-016-0139-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li W, Lu H, Liu J, Liu Q, Wang H, Zhang H, et al. A Novel Nomogram to Predict Survival in Patients With Recurrent Nasopharyngeal Carcinoma After Salvage Endoscopic Surgery. Oral Oncol (2020) 111:104922. 10.1016/j.oraloncology.2020.104922 [DOI] [PubMed] [Google Scholar]
- 25. Li W, Lu H, Wang H, Zhang H, Sun X, Hu L, et al. Salvage Endoscopic Nasopharyngectomy in Recurrent Nasopharyngeal Carcinoma: Prognostic Factors and Treatment Outcomes. Am J Rhinol Allergy (2021) 35:1458–66. 10.1177/1945892420964054 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.