Abstract
This study investigates the incidence of myocarditis and pericarditis emergency department or inpatient hospital encounters before COVID-19 vaccine availability (January 2019–January 2021) and during a COVID-19 vaccination period (February-May 2021) in a large US health care system.
Rare cases of cardiac inflammation following SARS-CoV-2 vaccination have been reported.1,2,3,4 We reviewed the clinical records of vaccine recipients to identify cases of postvaccination myocarditis or pericarditis.
Methods
Forty hospitals in Washington, Oregon, Montana, and Los Angeles County, California, that were part of the Providence health care system and used the same electronic medical record (EMR) were included. All patients with documented COVID-19 vaccinations administered inside the system or recorded in state registries at any time through May 25, 2021, were identified. Vaccinated patients who subsequently had emergency department or inpatient encounters with diagnoses of myocarditis, myopericarditis, or pericarditis were ascertained from EMRs (see eTables 1 and 2 in the Supplement for exclusions and definitions).
The monthly rates of first-time hospital diagnoses (excluding patients with previous diagnoses in January 2018–January 2019) in January 2019 through January 2021 (prevaccine period) and February through May 2021 (vaccine period) were compared.
The Wilson method was used to calculate 95% confidence intervals for single proportions. Change in incidence between periods and 95% confidence intervals for incidence were assessed using an exact rate ratio test assuming Poisson distribution, with a 2-sided P < .05 defining statistical significance. R version 2021 statistical software (R Foundation) was used. The Providence institutional review board approved the study with a waiver of informed consent.
Results
Among 2 000 287 individuals receiving at least 1 COVID-19 vaccination, 58.9% were women, the median age was 57 years (interquartile range [IQR], 40-70 years), 76.5% received more than 1 dose, 52.6% received the BNT162b2 vaccine (Pfizer/BioNTech), 44.1% received the mRNA-1273 vaccine (Moderna), and 3.1% received the Ad26.COV2.S vaccine (Janssen/Johnson & Johnson). Twenty individuals had vaccine-related myocarditis (1.0 [95% CI, 0.61-1.54] per 100 000) and 37 had pericarditis (1.8 [95% CI, 1.30-2.55] per 100 000).
Myocarditis occurred a median of 3.5 days (IQR, 3.0-10.8 days) after vaccination (mRNA-1273 vaccine, 11 cases [55%]; BNT162b2 vaccine, 9 cases [45%]) (Table). Fifteen individuals (75%; 95% CI, 53%-89%) were male, and the median age was 36 years (IQR, 26-48 years). Four persons (20%; 95% CI, 8%-42%) developed symptoms after the first vaccination and 16 (80%; 95% CI, 58%-92%) developed symptoms after the second. Nineteen patients (95%; 95% CI, 76%-99%) were admitted to the hospital. All were discharged after a median of 2 days (IQR, 2-3 days). There were no readmissions or deaths. Two patients received a second vaccination after onset of myocarditis; neither had worsening of symptoms. At last available follow-up (median, 23.5 days [IQR, 4.8-41.3 days] after symptom onset), 13 patients (65%; 95% CI, 43%-82%) had symptom resolution and 7 (35%; 95% CI, 18%-57%) were improving.
Table. Characteristics of Post–COVID-19 Vaccination Myocarditis and Pericarditis Casesa.
| Characteristics | Myocarditis (n = 20) | Pericarditis without myocarditis (n = 37) |
|---|---|---|
| Immunizations at symptom onset | ||
| 1 | 4 (20) | 15 (40.5) |
| 2 | 16 (80) | 22 (59.5) |
| Vaccine received most recently before symptom onset | ||
| Ad26.COV2.S | 0 | 2 (5.4) |
| mRNA-1273 | 11 (55) | 12 (32.4) |
| BNT162b2 | 9 (45) | 23 (62.2) |
| Time from most recent immunization to symptom onset, median (IQR), d | 3.5 (3-10.8) | 20 (6-41) |
| Age, median (IQR), y | 36 (26.3-48.3) | 59 (46-69) |
| Sex | ||
| Female | 5 (25) | 10 (27) |
| Male | 15 (75) | 27 (73) |
| Race and ethnicityb | ||
| White | 19 (95) | 31 (83.8) |
| Asian | 0 | 2 (5.4) |
| Latinx | 0 | 2 (5.4) |
| Black | 0 | 0 |
| Other | 0 | 2 (5.4) |
| Unknown | 1 (5) | 0 |
| Encounter state | ||
| California | 1 (5) | 7 (18.9) |
| Montana | 0 | 1 (2.7) |
| Oregon | 8 (40) | 8 (21.6) |
| Washington | 11 (55) | 21 (56.8) |
| Comorbidities | ||
| Alcohol or drug dependence | 4 (20) | 5 (13.5) |
| Coronary artery disease | 1 (5) | 4 (10.8) |
| Cancer | 2 (10) | 5 (13.5) |
| Heart failure | 0 | 2 (5.4) |
| Cirrhosis | 0 | 1 (2.7) |
| Chronic kidney disease | 1 (5) | 4 (10.8) |
| COPD | 0 | 4 (10.8) |
| Diabetes | 2 (10) | 4 (10.8) |
| Hypertension | 5 (25) | 18 (48.6) |
| Autoimmune disease | 0 | 3 (8.1) |
| Case management | ||
| Admitted to hospital | 19 (95) | 13 (35.1) |
| Intensive care unit stay | 2 (10) | 1 (2.7) |
| Treated for heart failurec | 8 (40) | 5 (13.5) |
| Colchicine | 9 (45) | 20 (54.1) |
| NSAIDs | 15 (75) | 18 (48.6) |
| Systemic steroids | 0 | 4 (10.8) |
| Length of stay, median (IQR), d | 2 (2-3) | 1 (1-2) |
| Laboratory findings (highest value during hospital visit) | ||
| ALT ≥50 U/L | 1 (5) | 2 (5.4) |
| AST ≥50 U/L | 6 (30) | 1 (2.7) |
| Creatinine ≥1.2 mg/dL | 1 (5) | 4 (10.8) |
| Hemoglobin <9 g/dL | 0 | 0 |
| White blood cell count ≥12 000/μL | 3 (15) | 8 (21.6) |
| Absolute neutrophils, median (IQR), ×109/L | 5 (3.5-7.5) | 7 (5-8) |
| Absolute lymphocytes, median (IQR), ×109/L | 2 (1.5-2) | 2 (1-2) |
| Platelets <100×103/μL | 0 | 0 |
| Platelets ≥400×103/μL | 0 | 2 (5.4) |
| ESR ≥30 mm/h | 0 | 5 (13.5) |
| Elevated troponin level | 19 (95) | 0 |
| Temperature ≥38 °C | 0 | 0 |
| Bundle branch block | 1 (5) | 2 (5.4) |
| ST elevation | 9 (45) | 14 (37.8) |
| PR depression | 0 | 7 (18.9) |
| Corrected QT interval, median (IQR), ms | 444 (425-467) | 425 (413-457) |
| Ejection fraction <50% | 5 (25) | 3 (8.1) |
| Clinical status at last follow-up | ||
| Resolved | 13 (65) | 7 (18.9) |
| Improved | 7 (35) | 23 (62.2) |
| Persistent | 0 | 2 (5.4) |
| Insufficient documentation | 0 | 5 (13.5) |
| Time from symptom onset to last follow-up, median (IQR), d | 23.5 (4.8-41.3) | 28 (7-53) |
| Returned to hospital for same symptoms | 1 (5) | 1 (2.7) |
| Died | 0 | 0 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; COPD, chronic obstructive pulmonary disease; ESR, erythrocyte sedimentation rate; IQR, interquartile range; NSAID, nonsteroidal anti-inflammatory drug.
Data are No. (%) of patients unless otherwise specified.
Race and ethnicity information was obtained from electronic medical records, where this information is assigned by either patients or health care registration personnel; these data are reported owing to the possibility of racial and ethnic differences in rates of postvaccination myocarditis and/or pericarditis.
Treated with diuretics, β-blockers, angiotensin-converting enzyme inhibitors, or angiotensin receptor blockers.
Pericarditis developed after the first immunization in 15 cases (40.5%; 95% CI, 26%-57%) and after the second immunization in 22 cases (59.5%; 95% CI, 44%-74%) (mRNA-1273 vaccine, 12 cases [32%]; BNT162b2 vaccine, 23 cases [62%]; Ad26.COV2.S vaccine, 2 cases [5%]). Median onset was 20 days (IQR, 6.0-41.0 days) after the most recent vaccination. Twenty-seven individuals (73%; 95% CI, 57%-85%) were male, and the median age was 59 years (IQR, 46-69 years). Thirteen (35%; 95% CI, 22%-51%) were admitted to the hospital, none to intensive care. Median stay was 1 day (IQR, 1-2 days). Seven patients with pericarditis received a second vaccination. No patient died. At last available follow-up (median, 28 days; IQR, 7-53 days), 7 patients (19%; 95% CI, 9%-34%) had resolved symptoms and 23 (62%; 95% CI, 46%-76%) were improving.
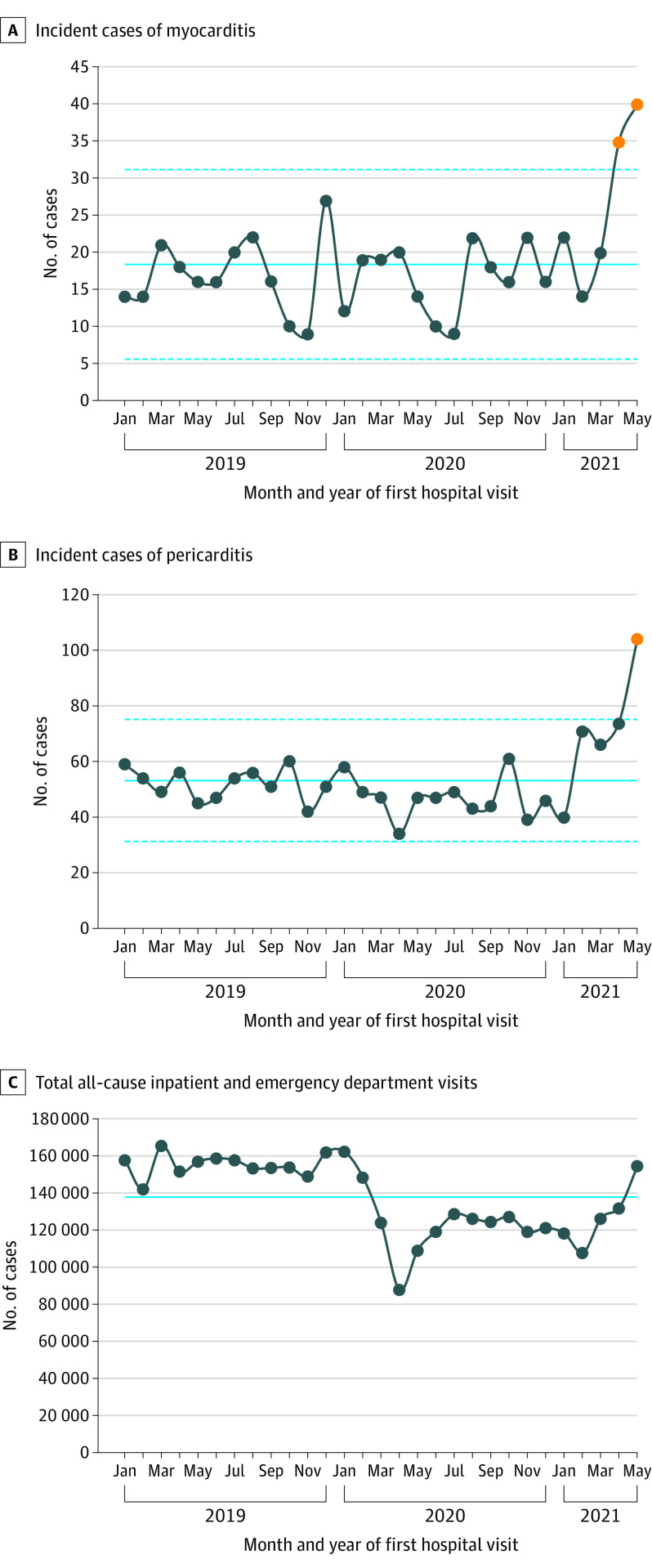
The mean monthly number of cases of myocarditis or myopericarditis during the prevaccine period was 16.9 (95% CI, 15.3-18.6) vs 27.3 (95% CI, 22.4-32.9) during the vaccine period (P < .001) (Figure). The mean numbers of pericarditis cases during the same periods were 49.1 (95% CI, 46.4-51.9) and 78.8 (95% CI, 70.3-87.9), respectively (P < .001).
Figure. Monthly Number of Inpatient and Emergency Department Cases of Myocarditis and Pericarditis at 40 Hospitals in the Western US.
A statistical process control c-chart was used for panels A and B, with control limits at ±3 times the standard deviation of the overall count. Orange circles are counts outside the control limits. Solid lines are incidence over the entire time frame; dashed lines are the ±3 sigma (control limits) about the incidence over the entire time frame.
Discussion
Two distinct self-limited syndromes, myocarditis and pericarditis, were observed after COVID-19 vaccination. Myocarditis developed rapidly in younger patients, mostly after the second vaccination. Pericarditis affected older patients later, after either the first or second dose.
Some vaccines are associated with myocarditis,5 including mRNA vaccines,1,2,3,4 and the Centers for Disease Control and Prevention recently reported a possible association between COVID-19 mRNA vaccines and myocarditis, primarily in younger male individuals within a few days after the second vaccination, at an incidence of about 4.8 cases per 1 million.6 This study shows a similar pattern, although at higher incidence, suggesting vaccine adverse event underreporting. Additionally, pericarditis may be more common than myocarditis among older patients.
Study limitations include cases missed in outside care settings and missed diagnoses of myocarditis or pericarditis (which would underestimate the incidence), as well as inaccurate EMR vaccination information. Temporal association does not prove causation, although the short span between vaccination and myocarditis onset and the elevated incidence of myocarditis and pericarditis in the study hospitals lend support to a possible relationship.
Section Editors: Jody W. Zylke, MD, Deputy Editor; Kristin Walter, MD, Associate Editor.
eTable 1. Exclusion Criteria
eTable 2. Definitions
References
- 1.Montgomery J, Ryan M, Engler R, et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol. Published online June 29, 2021. doi: 10.1001/jamacardio.2021.2833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim HW, Jenista ER, Wendell DC, et al. Patients with acute myocarditis following mRNA COVID-19 vaccination. JAMA Cardiol. Published online June 29, 2021. doi: 10.1001/jamacardio.2021.2828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bautista García J, Peña Ortega P, Bonilla Fernández JA, et al. Acute myocarditis after administration of the BNT162b2 vaccine against COVID-19. Rev Esp Cardiol (Engl Ed). Published online April 27, 2021. doi: 10.1016/j.recesp.2021.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosner CM, Genovese L, Tehrani BN, et al. Myocarditis temporally associated with COVID-19 vaccination. Circulation. Published online June 16, 2021. doi: 10.1161/CIRCULATIONAHA.121.055891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Su JR, McNeil MM, Welsh KJ, et al. Myopericarditis after vaccination, Vaccine Adverse Event Reporting System (VAERS), 1990-2018. Vaccine. 2021;39(5):839-845. doi: 10.1016/j.vaccine.2020.12.046 [DOI] [PubMed] [Google Scholar]
- 6.Wallace M, Oliver S. COVID-19 mRNA vaccines in adolescents and young adults: benefit-risk discussion. Slide 28. Published June 23, 2021. Accessed July 7, 2021. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-06/05-COVID-Wallace-508.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Exclusion Criteria
eTable 2. Definitions