Introduction
Lenvatinib is an oral multiple tyrosine receptor kinase inhibitor currently approved by the US Food and Drug Administration for the treatment of thyroid cancer, renal cell carcinoma, hepatocellular carcinoma (HCC), and endometrial carcinoma.1 Its mechanism of action is selective inhibition of vascular endothelial growth factor receptors (VEGFRs) 1 to 3, fibroblast growth factor receptors 1 to 4, RET, KIT, and platelet-derived growth factor receptor-α. Cutaneous adverse events are common among antiangiogenic treatments and include psoriasiform eruptions, which have been observed with other VEGFR inhibitors.2,3 Here, we report, to our knowledge, a novel case of psoriasiform drug eruption following lenvatinib therapy.
Case report
A 66-year-old Chinese man with a history of chronic hepatitis type B, liver cirrhosis, HCC, and end-stage renal disease on hemodialysis presented to our dermatology clinic with a 6-week history of a progressive, pruritic eruption on his upper and lower extremities, trunk, abdomen, hands, and feet, in addition to a desquamative, tender palmoplantar eruption. Both eruptions began 2 weeks after initiating lenvatinib therapy at the dosage of 12 mg orally once daily for progressive and metastatic HCC. He had previously received other liver-directed therapy, including radiofrequency ablation, transcatheter arterial chemoembolization, radioembolization with yttrium-90, stereotactic body radiotherapy, nivolumab (which was discontinued due to disease progression), and microwave ablation.
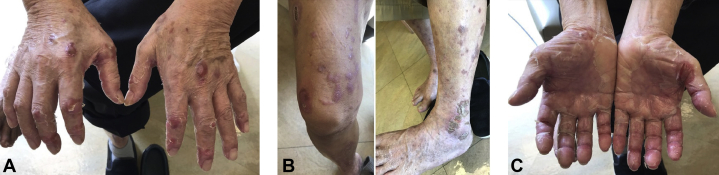
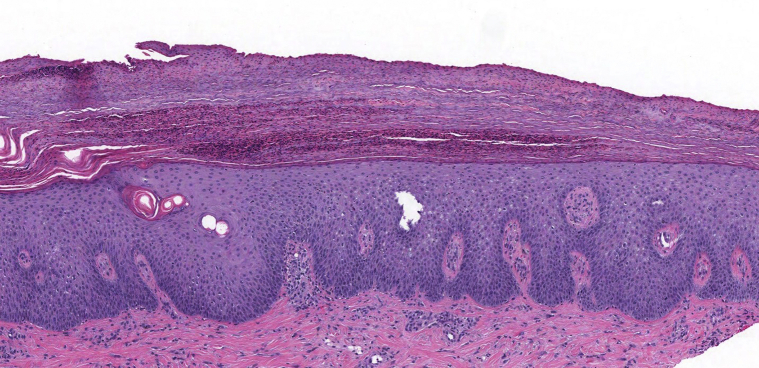
On physical examination, the patient had numerous well-demarcated, pruritic, scaly plaques on the upper and lower extremities, trunk, abdomen, and dorsal sides of hands and feet (Fig 1). The patient also had a tender, red, and desquamating acral erythema. No nail changes were noted. A skin biopsy of the right thigh demonstrated regular epidermal hyperplasia with hypogranulosis beneath the confluent parakeratosis, where there were collections of neutrophils (Fig 2). The patient was given a topical high-potency corticosteroid in addition to a drug holiday of 3 weeks. During this time, his eruption and associated pruritus improved, and he was reinitiated on a lower dose of lenvatinib at 8 mg orally once daily. His skin lesions continued to improve while on a lower dose of lenvatinib, and the patient was tapered down to a medium-potency topical corticosteroid and referred to phototherapy. Due to the progression of his oncologic disease, lenvatinib was later discontinued in favor of cabozantinib.
Fig 1.
The patient had well-demarcated, erythematous, psoriasiform plaques present on the (A) dorsal side of the hands, (B) lower extremities, and dorsal side of the feet, along with (C) cooccurring palmar-plantar dysesthesia.
Fig 2.
A punch biopsy from the right thigh. There was regular epidermal hyperplasia with hypogranulosis beneath confluent parakeratosis, where there were collections of neutrophils. Neutrophils were seen within the epidermis. (Original magnification: ×10.)
Discussion
The vascular endothelial growth factor (VEGF) signaling pathway is a key regulator of tumor vasculature, mediating endothelial cell proliferation, migration, vascular permeability, and vasodilation.4 Lenvatinib and sorafenib are closely related multikinase inhibitors (MKIs) that inhibit VEGFR. However, lenvatinib differs from other MKIs, particularly sorafenib, in its ability to inhibit fibroblast growth factor receptor signaling, which is associated with the activation of cellular cascades and responses such as cell growth, proliferation, differentiation, apoptosis, and survival.5
Currently, lenvatinib is approved by the US Food and Drug Administration for the treatment of advanced endometrial carcinoma, unresectable HCC, advanced renal cell carcinoma, and differentiated thyroid cancer. Although dermatologic adverse events, including palmar-plantar dysesthesia, hair and skin discoloration, and xerosis, are commonly seen with lenvatinib and other MKIs, to our knowledge, no reports on psoriasiform drug eruption associated with lenvatinib exist.2 Adverse events related to lenvatinib typically occur early in the course of therapy within the first 2 months of initiation.6 In this case, the psoriasiform eruption and cooccurring palmar-plantar dysesthesia emerged after 6 weeks of lenvatinib therapy, suggesting a temporal link. Furthermore, the patient had no prior history of psoriasis or psoriasiform-like rashes prior to the lenvatinib therapy.
The pathogenesis of more common cutaneous toxicities, such as acral erythema, of VEGFR inhibitors is suggested to be due to epidermal cell apoptosis or interactions between endothelial cells and stroma; however, the literature remains inconclusive to date.2 In psoriasis pathogenesis, VEGF has been implicated as it is overexpressed on psoriatic keratinocytes, contributes to epidermal hyperplasia, and induces angiogenesis.3 Therefore, it seems paradoxical that psoriasiform eruptions have been observed following sorafenib and, now, lenvatinib therapy, given that both block VEGFR signaling. Furthermore, reports that describe the treatment with sorafenib and sunitinib, both related MKIs, exist, leading to the remission of existing psoriasis.7,8 Current hypotheses regarding the pathogenesis of sorafenib-induced psoriasiform reactions involve the hypoxia-inducible factor pathway, which is upstream of VEGF. Sorafenib upregulates HIF-2α, which has been found to be upregulated in psoriatic plaques.9 Currently, there are sparse data in the literature regarding the specific effect of lenvatinib on the hypoxia-inducible factor pathways and circulating VEGF. Lenvatinib has more immunomodulatory effects than sorafenib and has been shown to increase the proportion of T lymphocytes compared with controls.10 Psoriasis is largely T lymphocyte–mediated, so an upregulation of T cells is a possible mechanism by which lenvatinib causes psoriasiform dermatitides. The underlying biologic mechanisms by which VEGFR inhibitors such as lenvatinib induce or exacerbate psoriasis remain unclear and may differ between MKIs due to the variability in their targets and affinities and discrete immunomodulatory effects.
In conclusion, cutaneous toxicities are frequently reported side effects of MKIs. Although reactions such as the psoriasiform drug eruption described in this case are not life-threatening, they are impactful on patients' quality of life as well as the continuation of the oncologic medication. With the increasing use of targeted therapies such as lenvatinib, dermatologists will play an important role in the early recognition and management of adverse cutaneous reactions. Early assessment of skin-related toxicities is critical to mitigate the reaction without compromising treatment of the malignancy.
Conflicts of interest
None disclosed.
Footnotes
Author Sally and Dr Ugonabo are cofirst authors.
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Hao Z., Wang P. Lenvatinib in management of solid tumors. Oncologist. 2020;25(2):e302–e310. doi: 10.1634/theoncologist.2019-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cubero D.I.G., Abdalla B.M.Z., Schoueri J. Cutaneous side effects of molecularly targeted therapies for the treatment of solid tumors. Drugs Context. 2018;7:212516. doi: 10.7573/dic.212516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ensslin C.J., Kao P.H., Wu M.Y. Psoriasiform drug eruption secondary to sorafenib: case series and review of the literature. Cutis. 2019;104(3):E11–E15. [PubMed] [Google Scholar]
- 4.Ellis L.M., Hicklin D.J. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8(8):579–591. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- 5.Casadei Gardini A., Puzzoni M., Montagnani F. Profile of lenvatinib in the treatment of hepatocellular carcinoma: design, development, potential place in therapy and network meta-analysis of hepatitis B and hepatitis C in all phase III trials. Onco Targets Ther. 2019;12:2981–2988. doi: 10.2147/OTT.S192572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haddad R.I., Schlumberger M., Wirth L.J. Incidence and timing of common adverse events in lenvatinib-treated patients from the SELECT trial and their association with survival outcomes. Endocrine. 2017;56(1):121–128. doi: 10.1007/s12020-017-1233-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Kester M.S., Luelmo S.A.C., Vermeer M.H., Blank C., van Doorn R. Remission of psoriasis during treatment with sorafenib. JAAD Case Rep. 2018;4(10):1065–1067. doi: 10.1016/j.jdcr.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Narayanan S., Agarwal N., Callis-Duffin K., Batten J. Improvement of psoriasis during sunitinib therapy for renal cell carcinoma. Am J Med Sci. 2010;339(6):580–581. doi: 10.1097/MAJ.0b013e3181dd1aa5. [DOI] [PubMed] [Google Scholar]
- 9.Yiu Z.Z., Ali F.R., Griffiths C.E. Paradoxical exacerbation of chronic plaque psoriasis by sorafenib. Clin Exp Dermatol. 2016;41(4):407–409. doi: 10.1111/ced.12788. [DOI] [PubMed] [Google Scholar]
- 10.Kimura T., Kato Y., Ozawa Y. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1-6 hepatocellular carcinoma model. Cancer Sci. 2018;109(12):3993–4002. doi: 10.1111/cas.13806. [DOI] [PMC free article] [PubMed] [Google Scholar]