Abstract
Background
Liver cirrhosis is an end-stage multiple liver disease. Mesenchymal stem cells (MSCs) are an attractive cell source for reducing liver damage and regressing fibrosis; additional therapies accompanying MSCs can potentially enhance their therapeutic effects. Kampo medicines exhibit anti-inflammatory and anti-oxidative effects. Here, we investigated the therapeutic effect of MSCs combined with the Kampo medicine Juzentaihoto (JTT) as a combination therapy in a carbon tetrachloride (CCl4)-induced cirrhosis mouse model.
Methods
C57BL/6 mice were administered JTT (orally) and/or MSCs (one time, intravenously). The levels of liver proteins were measured in the sera. Sirius Red staining and hydroxyproline quantitation of hepatic tissues and immune cells were conducted, and their associated properties were evaluated. Liver metabolomics of liver tissues was performed.
Results
JTT monotherapy attenuated liver damage and increased serum albumin level, but it did not effectively induce fibrolysis. JTT rapidly reduced liver damage, in a dose-dependent manner, after a single-dose CCl4 administration. Furthermore, JTT-MSC combination therapy attenuated liver damage, improved liver function, and regressed liver fibrosis. The combination increased the CD4+/CD8+ ratio. JTT had stronger effects on NK and regulatory T cell induction, whereas MSCs more strongly induced anti-inflammatory macrophages. The combination therapy further induced anti-inflammatory macrophages. JTT normalized lipid mediators, and tricarboxylic acid cycle- and urea cycle-related mediators effectively.
Conclusions
The addition of JTT enhanced the therapeutic effects of MSCs; this combination could be a potential treatment option for cirrhosis.
Keywords: Juzentaihoto, Cirrhosis, Mesenchymal stem cells, Macrophage, Fibrosis
Highlights
-
•
Juzentaihoto (JTT) enhanced the therapeutic effects of mesenchymal stem cells (MSCs).
-
•
JTT induced NK and regulatory T cells, whereas MSCs induced anti-inflammatory macrophages.
-
•
JTT normalized lipid mediators, the tricarboxylic acid cycle, and urea cycle-related mediators.
-
•
This combination could be a potential treatment option against cirrhosis therapy.
1. Introduction
The liver can be damaged by several factors, including hepatitis virus infection, alcohol, and non-alcoholic steatohepatitis (NASH). Although the liver has high regenerative potential and can exhibit spontaneous fibrosis regression (also known as fibrolysis), chronic liver damage can lead to fibrosis and cirrhosis [1]. The standard treatment for advanced cirrhosis is liver transplantation; however, this procedure is invasive, and the availability of liver donors is scarce. Cirrhosis treatments that can suppress the activation of hepatic stellate cells (HSCs) and fibrogenesis, as well as induce fibrolysis and anti-inflammatory macrophages, are desired [2].
Mesenchymal stem cells (MSCs), multipotent stem cells that can be differentiated into osteocytes, chondrocytes, and adipocytes, can be harvested from not only bone marrows but also medical wastes, such as adipose tissues, umbilical cord tissues, and dental pulp, and are therefore an attractive therapy for cirrhosis. MSCs exhibit anti-oxidative and anti-inflammatory effects, which can potentially suppress fibrogenesis and induce fibrolysis [[3], [4], [5]]. Briefly, their anti-oxidative effect can reduce hepatocyte damage and inactivate immune cells, thereby suppressing HSC activation and fibrogenesis. In addition, MSCs can activate anti-inflammatory macrophages that lead to fibrolysis [[6], [7], [8]]. However, to enhance the therapeutic effect of MSCs, they are administered in combination with conventionally used drugs.
The Kampo medicine Juzentaihoto (JTT) is a dried and powdered hot-water-extract of 10 crude drugs mixed at the following ratio: Astragali radix (3.0), Cinnamomi cortex (3.0), Rehmanniae radix (3.0), Paeoniae radix (3.0), Cnidii rhizoma (3.0), Atractylodis lanceae rhizoma (3.0), Angelicae radix (3.0), Ginseng radix (3.0), Poria (3.0), and Glycyrrhizae radix (1.5) [9,10]. JTT is traditionally administered to patients with anemia, anorexia, or fatigue [11], and used to restore physical strength post surgery and alleviate potential adverse effects of anti-cancer drugs and radiation therapy [12,13]. Reportedly, components of these herbs exhibit anti-inflammatory and anti-oxidative effects and can inhibit the activation of pro-inflammatory cytokines and immunomodulate macrophages [9]. However, the therapeutic effect of JTT for liver damage and fibrosis remains poorly understood.
In this study, we aimed to evaluate JTT monotherapy in a CCl4-induced liver cirrhosis mouse model. We believe that JTT can also enhance the therapeutic effects of MSCs; hence, we determined the effect of MSC and JTT as a combination therapy in this model. Finally, we evaluated immune cells following therapy and conducted metabolome analysis using mouse liver samples.
2. Methods
2.1. Animals and environmental conditions
C57BL/6 male mice were purchased from Charles River Laboratories (Yokohama, Japan) and housed in a specific pathogen-free environment and maintained under standard conditions with a 12/12-h day/night cycle. The mice had access to food and water ad libitum. All animal experiments were conducted in compliance with the regulations and approved by the Institutional Animal Care and Committee of Niigata University.
2.2. Dietary JTT supplementation
JTT was provided by Tsumura & Co. (Tokyo, Japan) and mixed with the standard diet CE-2 (CLEA Japan, Inc., Tokyo, Japan) at 3 % (normal dose) or 1.5 % (low dose).
2.3. MSCs
Human bone marrow-derived (BM-MSCs; passage 2) and adipose tissue-derived MSCs (AD-MSCs; passage 2) were obtained from PromoCell (Heidelberg, Germany; C-12977). The cells were expanded through passage 4 in StemPro MSC SFM XenoFree medium (Thermo Fisher Scientific, Waltham, MA, USA) under hypoxic (5 %) conditions in the presence of 5 % CO2 at 37 °C.
The morphology, proliferation potential, adherence rate, and viability of the cells were tested using PromoCell. The cells were analyzed by flow cytometry using a comprehensive panel of markers, CD73/CD90/CD105 and CD14/CD19/CD34/CD45/HLA-DR. Adipogenic, osteogenic, and chondrogenic differentiation assays were performed for each lot in the absence of antibiotics and antimycotics.
2.4. Mouse cirrhosis model
Ten-week-old male mice were intraperitoneally injected with 1 mL/kg CCl4 dissolved in corn oil (both from Fujifilm Wako Pure Chemical Industries Ltd., Osaka, Japan) at a 1:10 volumetric ratio, twice weekly until euthanasia. One week was necessary to achieve the effective blood concentration; therefore, 7 weeks after CCl4 administration, CE-2 was continued with JTT (3 % or 1.5 %) or without JTT for 4 weeks. In addition, 1 × 106 MSCs were administered 8 weeks after CCl4 administration. During JTT and/or MSC treatment, CCl4 was also administered until euthanasia. Three days after the final CCl4 administration, serum and fibrosis analyses were performed. In the single-dose CCl4 injection experiment, CE-2 with JTT (3 %) was administered 7 days before CCl4 administration (same dose as described above). Thirty-six hours after CCl4 administration, serum and tissues were obtained following euthanasia.
2.5. Serum analyses
Serum levels of alanine aminotransferase (ALT), aspartate transaminase (AST), and albumin (ALB) were determined by Oriental Yeast Co., LTD. Nagahama LSL (Nagahama, Japan) using cardiac blood samples.
2.6. Sirius Red staining
To quantify fibrosis, tissues fixed with 10 % formalin were cut into 4-μm thick sections and stained with Sirius Red. Micrographs were captured from each section randomly (×40, 10 fields/mouse) using an all-in-one microscope (BZ-9000, Keyences, Osaka, Japan). Quantitative analysis of the fibrotic areas was performed using ImageJ (version 1.6.0 20, National Institutes of Health, Bethesda, MD, USA).
2.7. Hydroxyproline assay
Liver samples (20 mg) were homogenized and assayed using the QuickZyme Hydroxyproline Assay kit (QuickZyme Bioscience, Zernikedreef, Netherlands) according to the manufacturer's instructions. The liver samples were extracted and their absorbances were measured at 570 nm using Thermo Scientific Multiskan FC (Thermo Fisher Scientific, Waltham, MA, USA). Data are expressed as the mass of hydroxyproline per 1 mg liver tissue.
2.8. Flow cytometry
The mice were anesthetized with isoflurane (Fujifilm Wako Pure Chemical Industries Ltd., Osaka, Japan), and their livers were perfused with PBS. The livers were finely chopped and further dissociated in gentleMACS C tube (Miltenyi Biotec, Bergisch Gladbach, Germany) using the Liver Dissociation Kit (Miltenyi Biotec) and gentleMACS (Miltenyi Biotec) according to the manufacturers’ instructions. The dissociated cells were washed and stained with appropriate antibodies (Supplementary Table 1), and then analyzed using a BD FACSAria™ III (Becton Dickinson, Franklin Lakes, NJ, USA). Dead cells were excluded by 7-amino-actinomycin D (Becton Dickinson) counterstaining. Data were analyzed using FlowJo 10.7.1 software (Becton Dickinson).
2.9. Liver metabolomics
Hepatic hydrophilic metabolites and lipid mediators were measured by liquid chromatography–tandem mass spectrometry (LC–MS/MS) and gas chromatography–tandem mass spectrometry (GC–MS/MS). Analyses were conducted as previously described [14] using the Method Packages (Shimadzu, Kyoto, Japan), which contains a mass spectral library, method files specifying the analytical conditions, and data analysis parameters. A more detailed protocol is described in Supplementary Methods 1.
2.10. Statistical analyses
Statistical analyses were performed using GraphPad Prism8 (GraphPad Software Inc., La Jolla, CA, USA), R (R Foundation, Vienna, Austria), and Microsoft Excel (Microsoft Corporation, Redmond, WA, USA). Data are presented as mean ± SD. The results were assessed using Welch's t-test. Differences between groups were analyzed using Welch's one-way ANOVA. Metabolomics data were processed using R. Plots, charts, and heatmaps were prepared using R and Microsoft Excel. R was used to calculate the statistical significance of differences between two points using Mann–Whitney U test. Differences were considered significant at p < 0.05.
3. Results
3.1. JTT attenuates liver damage but does not regress fibrosis
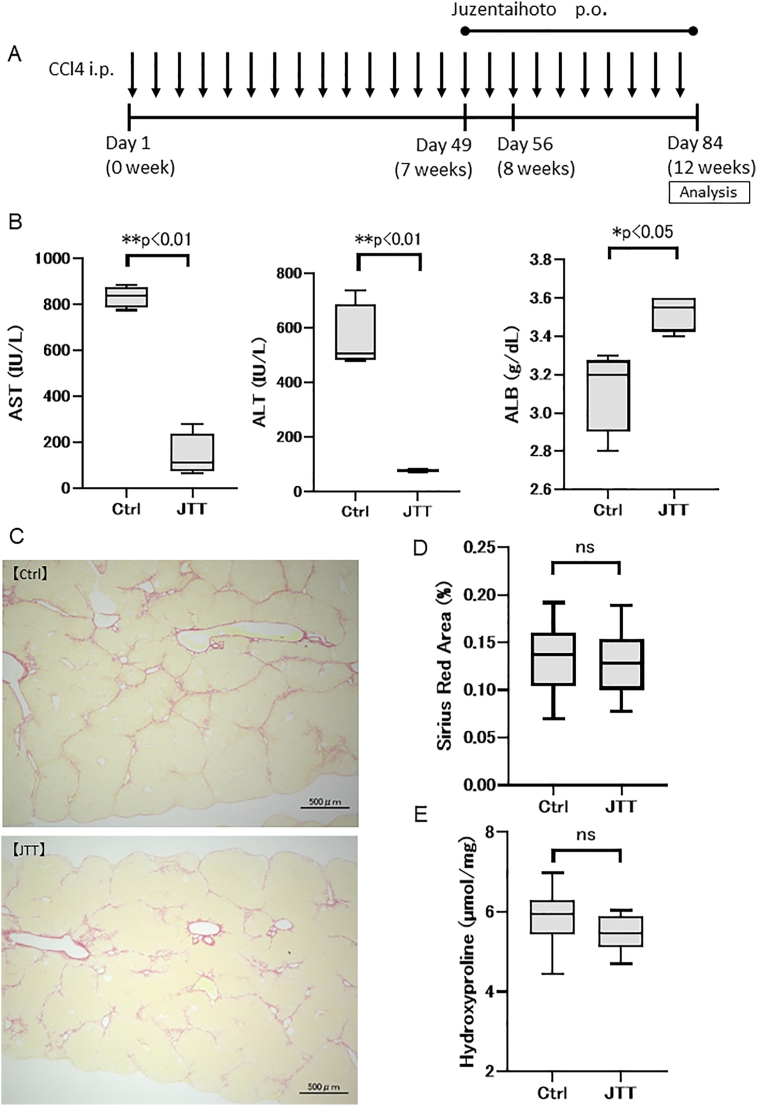
To evaluate the therapeutic effect of JTT, 3 % JTT in a standard diet was administered for the last 5 weeks of the 12-week CCl4 (twice weekly) cirrhosis model (Fig. 1A). The serum levels of AST (Ctrl group; 834.5 ± 40.6 IU/L, JTT group; 141.3 ± 81.9 IU/L, p < 0.01) and ALT (Ctrl group; 557.5 ± 106.4 IU/L, JTT group; 77.8 ± 4.7 IU/L, p < 0.01) significantly decreased in the JTT group compared with those in the Ctrl group (Fig. 1B). In addition, the serum ALB level significantly increased (Ctrl group; 3.13 ± 0.19 g/dL, JTT group; 3.53 ± 0.08 g/dL, p < 0.05) in the JTT group relative to that in the Ctrl group (Fig. 1B). Sirius Red staining and hydroxyproline quantitation showed that the stained areas (Ctrl group; 0.14 ± 0.03%, JTT group; 0.13 ± 0.03%, p = 0.56; Fig. 1C and D) and hydroxyproline level (Ctrl group; 5.86 ± 0.66 nmol/mg, JTT group; 5.47 ± 0.43 nmol/mg, p = 0.16; Fig. 1E) did not significantly decrease following JTT treatment. These results revealed that the oral intake of JTT effectively attenuated liver damage and increased ALB production; however, it did not effectively regress fibrosis.
Fig. 1.
Therapeutic effect of Juzentaihoto (JTT) (5-week oral administration) in a CCl4-induced cirrhosis mouse model. (A) Experimental scheme. (B) Serum levels of aspartate transaminase (AST), alanine transaminase (ALT), and albumin (ALB) on day 84. (C) Sirius Red staining of the liver tissue. Scale bar = 500 μm. (D) Sirius Red staining. (E) Quantification of hydroxyproline. n = 4 in each group. Data are presented as mean ± SD. ∗∗p < 0.01, ∗p < 0.05. Ctrl, control; ns, not significant.
3.2. JTT reduces fibrogenesis by reducing liver damage
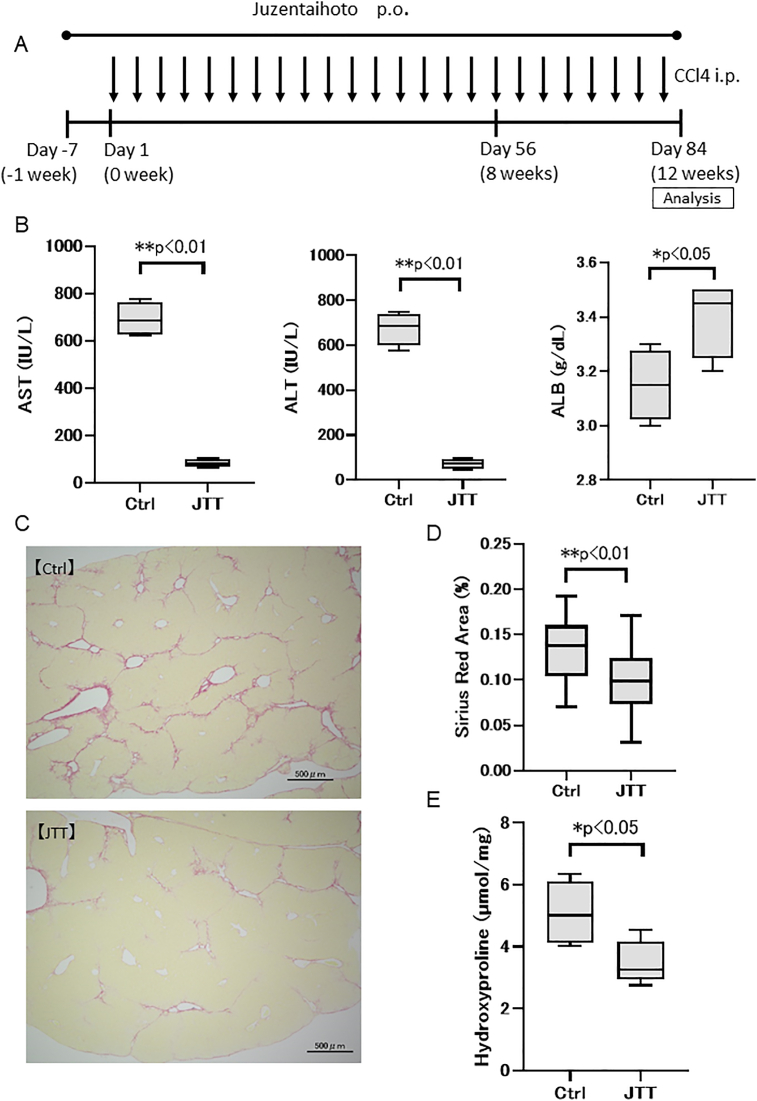
In the aforementioned experiment, we could not confirm whether fibrosis had been regressed by JTT intake. Therefore, 3 % JTT was administered a week before the 12-week CCl4 administration (Fig. 2A). The serum level of AST (Ctrl; 691.3 ± 64.3 IU/L, JTT group; 84.0 ± 16.6 IU/L, p < 0.01) and ALT (Ctrl group; 673.8 ± 64.3 IU/L, JTT group; 72.5 ± 18.6 IU/L, p < 0.01) was significantly decreased, whereas that of ALB (Ctrl group; 3.15 ± 0.11 g/dL, JTT group; 3.40 ± 0.12 g/dL, p < 0.05) was significantly increased by JTT treatment (Fig. 2B). Sirius Red-stained areas (Ctrl group; 0.14 ± 0.03%, JTT group; 0.09 ± 0.04%, p < 0.01) and hydroxyproline levels (Ctrl group; 5.09 ± 0.91 nmol/mg, JTT group; 3.50 ± 0.62 nmol/mg, p < 0.05) decreased significantly with JTT treatment (Fig. 2C–E). These data, together with those described above, indicate that JTT can reduce fibrogenesis by attenuating liver damage. However, we suspected that JTT possesses a weak ability to induce fibrolysis in this model.
Fig. 2.
Therapeutic effects of Juzentaihoto (JTT) (13-week oral administration) in a CCl4-induced cirrhosis mouse model. (A) Experimental scheme. (B) Serum levels of aspartate transaminase (AST), alanine transaminase (ALT), and albumin (ALB) on day 84. (C) Sirius Red staining of the liver tissue. Scale bar = 500 μm. (D) Sirius Red staining. (E) Quantification of hydroxyproline. n = 4 in each group. Data are presented as mean ± SD. ∗∗p < 0.01, ∗p < 0.05. Ctrl, control; ns, not significant.
3.3. JTT attenuates short-term liver damage after single-dose CCl4 treatment
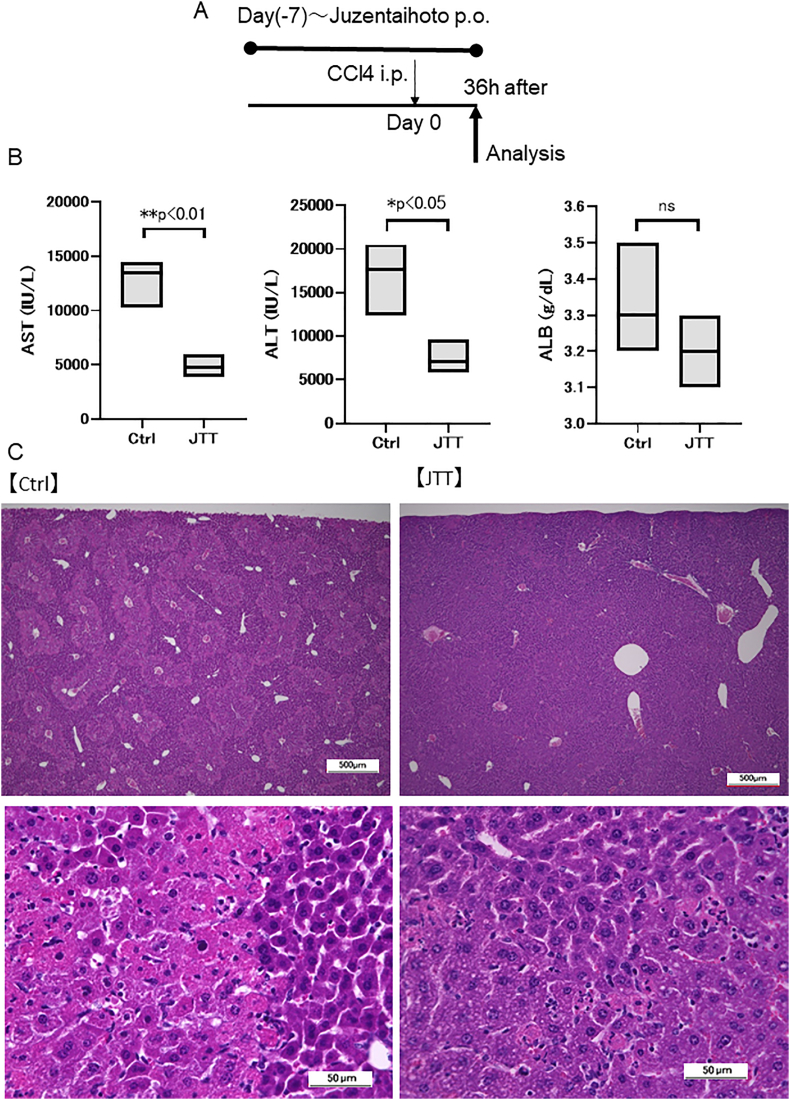
To determine whether JTT can reduce short-term liver damage, 3 % JTT was administered one week before single-dose CCl4 treatment (Supplementary Fig. 1A). While the serum ALB level did not significantly change, the serum AST (Ctrl group; 12,758 ± 1797 IU/L, JTT group; 4870 ± 856 IU/L, p < 0.01) and ALT levels (Ctrl group; 16,885 ± 3357 IU/L, JTT group; 7538 ± 1606 IU/L, p < 0.05) significantly decreased (Supplementary Fig. 1B) with a corresponding decrease in the damage areas in the liver tissues (Supplementary Fig. 1C). These results indicate that on achieving effective blood concentration, JTT could promptly/effectively reduce liver damage.
3.4. JTT induces therapeutic effects in a dose-dependent manner
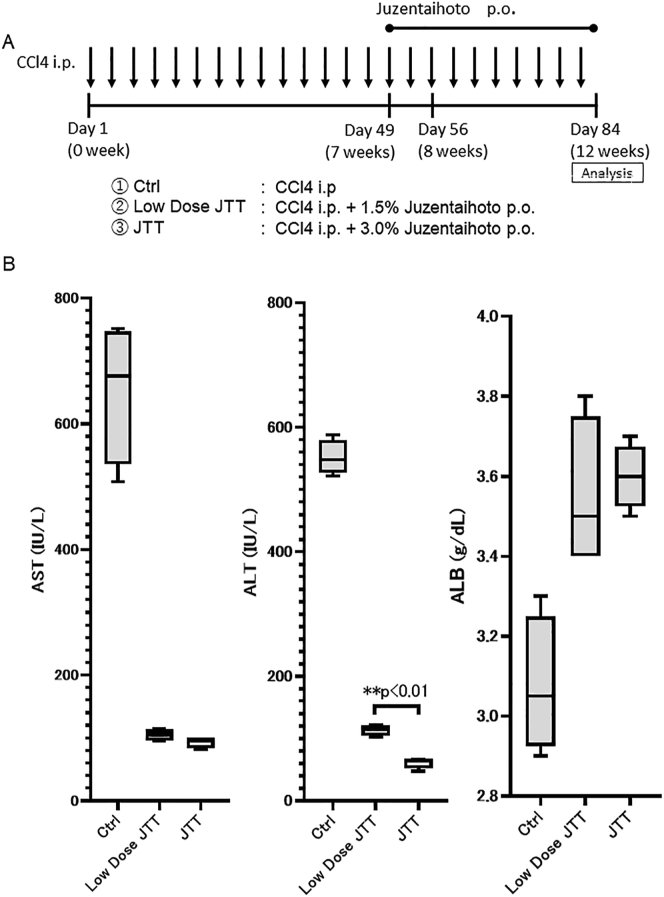
We evaluated the dose dependency of JTT treatment in the 12-week model. Accordingly, 3 % (JTT) or 1.5% (low-dose JTT) JTT, or Ctrl diet, was initiated 7 weeks post CCl4 administration and continued until euthanasia (Fig. 3A). The serum level of ALB significantly increased, whereas that of AST and ALT significantly decreased in both low-dose JTT and JTT groups compared with those in the Ctrl group. Moreover, the serum ALT level in the JTT group was significantly lower than that in the low-dose JTT group (JTT group; 61 ± 7.6 IU/L, low-dose JTT group; 113 ± 7.4 IU/L, p < 0.01; Fig. 3B). These results indicate that JTT could effectively attenuate liver damage even at a low dose and that its therapeutic effect was dose dependent.
Fig. 3.
Therapeutic effect of low-dose Juzentaihoto (JTT) (five-week oral administration) in a CCl4-induced cirrhosis mouse model. (A) Experimental scheme. (B) Serum levels of aspartate transaminase (AST), alanine transaminase (ALT), and albumin (ALB) on day 84. Data are presented as mean ± SD. ∗∗p < 0.01. Ctrl, control; ns, not significant.
3.5. Combination therapy of JTT and MSC attenuates liver damage and regresses fibrosis
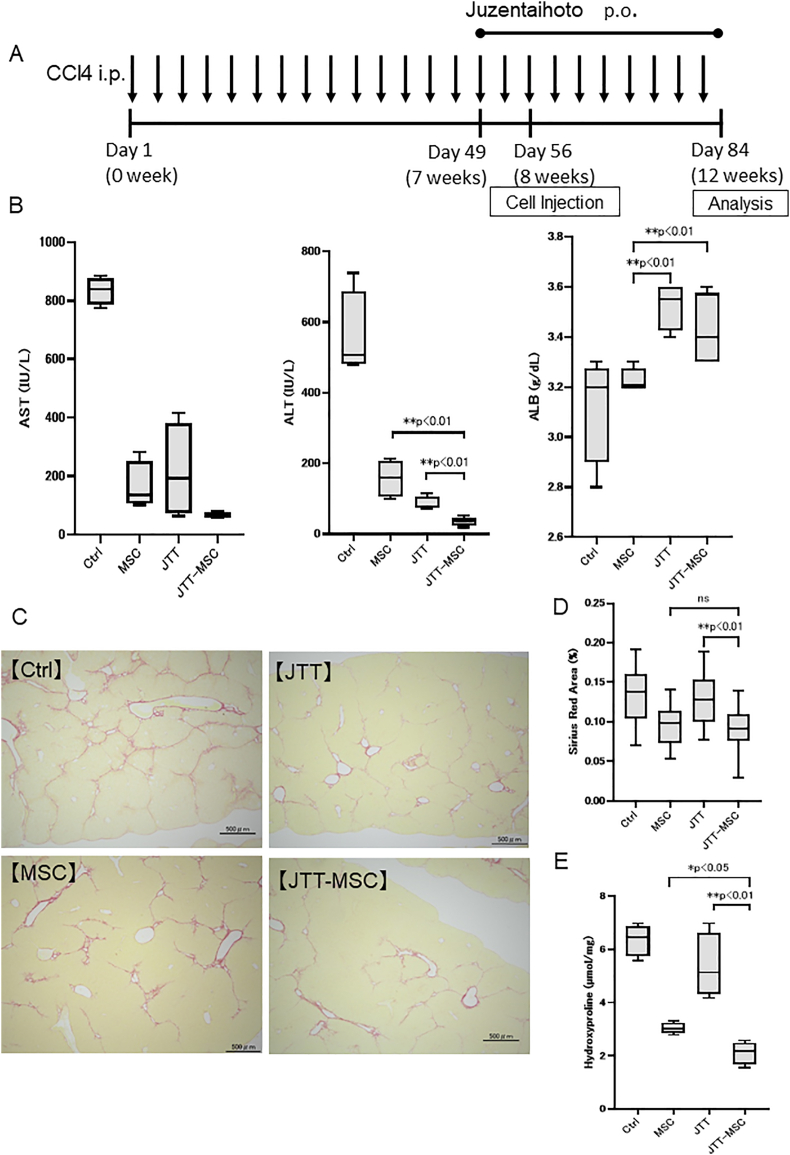
We evaluated the effect of combining JTT with MSCs using the 12-week CCl4 (twice weekly) cirrhosis model by comparing it with the no treatment (Ctrl) group, MSC group (one-time injection of MSCs at 8 weeks after CCl4 administration), and JTT group (3 % of JTT with standard diet started from 7 weeks until sacrifice; Fig. 4A). While both MSC and JTT groups presented significantly decreased serum AST and ALT levels relative to the Ctrl group, the JTT-MSC group exhibited an additional decrease in these levels (ALT, MSC group; 157 ± 48.1 IU/L, JTT-MSC group; 35 ± 11.3 IU/L, p < 0.01, JTT group; 85 ± 17.3 IU/L, JTT-MSC group; 35 ± 11.3 IU/L, p < 0.01). Moreover, the JTT and JTT-MSC groups presented significantly increased serum ALB level compared with the MSC and Ctrl groups (MSC group; 3.23 ± 0.04 g/dL, JTT group; 3.56 ± 0.08 g/dL, p < 0.01, MSC group; 3.23 ± 0.04 g/dL, JTT-MSC group; 3.43 ± 0.13 g/dL, p < 0.01; Fig. 4B). We further evaluated fibrosis by calculating Sirius Red-stained areas and quantifying liver hydroxyproline amount. We found that the MSC and JTT-MSC combination reduced fibrosis relative to the JTT and Ctrl groups (Sirius Red, JTT group; 0.13 ± 0.03%, JTT-MSC group; 0.09 ± 0.03%, p < 0.01, hydroxyproline, JTT group; 5.36 ± 1.06 nmol/mg, JTT-MSC group; 2.11 ± 0.37 nmol/mg, p < 0.01), suggesting that MSC therapy could regress fibrosis more efficiently (Fig. 4C–E). Overall, JTT and MSCs attenuated liver damage individually, at different points of action, and therefore, could attenuate liver damage, improve liver function, and regress liver fibrosis, resulting in better therapeutic effect.
Fig. 4.
Therapeutic effect of Juzentaihoto (JTT) (five-week oral administration) and/or mesenchymal stem cells (MSCs) in aCCl4-induced mouse cirrhosis model. (A) Experimental scheme. (B) Serum levels of aspartate transaminase (AST), alanine transaminase (ALT), and albumin (ALB) on day 84. (C) Sirius Red staining of the liver tissue. Scale bar = 500 μm. (D) Frequency of Sirius Red-stained areas. (E) Quantification of hydroxyproline. n = 4 in each group. Data are presented as mean ± SD. ∗∗p < 0.01, ∗p < 0.05. Ctrl, control; ns, not significant; JTT-MSC, JTT and MSC combination therapy.
3.6. The effect of combination therapy against immune cells in the liver
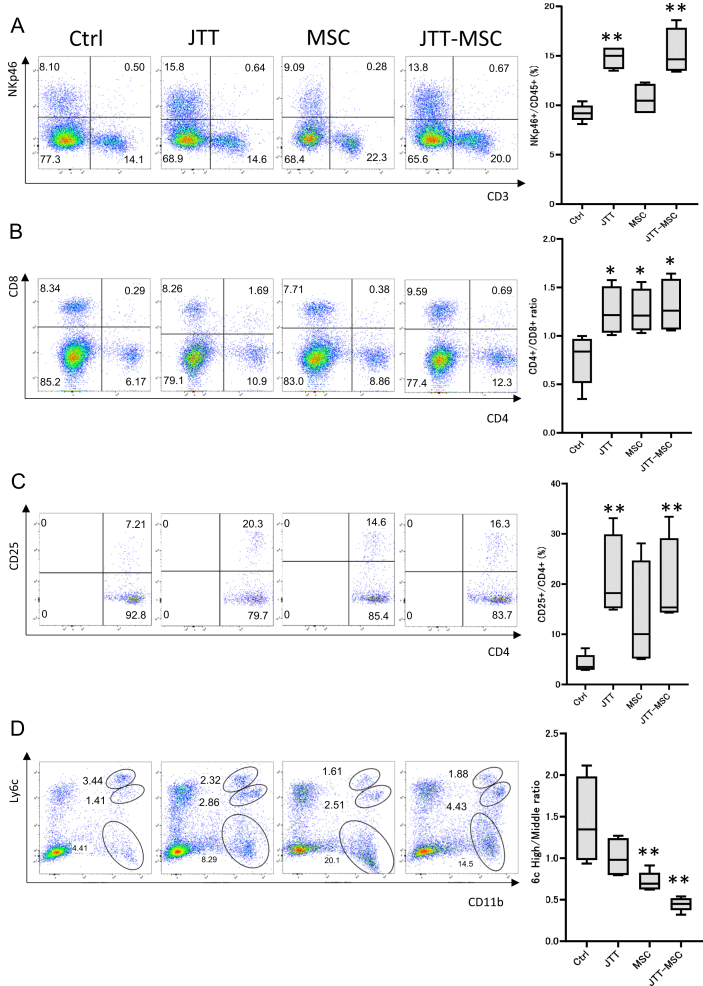
To elucidate the modes of action by which JTT and MSC induced therapeutic effects, we performed flow cytometry to evaluate the following ratios: NKp46+ (NK cells)/CD45+, CD4+/CD8+, CD4+CD25+ (regulatory T, Treg cells) [15,16]/CD4+, and CD11b+Ly6chigh (pro-inflammatory macrophages)/CD11b+Ly6cmiddle (anti-inflammatory macrophages) [17]. While both JTT and MSC individual treatments increased the CD4+/CD8+ ratio, JTT efficiently increased the NKp46+/CD45+ and CD4+CD25+/CD4+ ratios, suggesting that JTT could efficiently increase NK cells and Treg cells in the liver. In contrast, MSC treatment decreased the CD11b+Ly6chigh/CD11b+Ly6cmiddle ratio efficiently compared with the JTT group. Combination therapy of JTT-MSC decreased this ratio further, suggesting that MSCs possess a stronger ability to induce anti-inflammatory macrophages (Fig. 5A–D). While the effects against immune cells in the liver by JTT and MSC were different, JTT-MSC combination therapy affected the immune response, inducing the production of Treg cells and anti-inflammatory macrophages.
Fig. 5.
Flow cytometry of immune cells in the liver after control, JTT, MSC, and JTT-MSC treatments. The panels represent scatter plots of normal saline (NS) control, JTT-treated, MSC-treated, and JTT-MSC combination-treated groups (left to right), and the analyzed data of these panels are shown on the right. (A) CD3 (x-axis) versus NKp46 (y-axis). Analyzed data of NKp46+ cells/CD45+ cells (right). (B) CD4 (x-axis) versus CD8 (y-axis). Analyzed data of CD4+/CD8+ (right). (C) CD4 (x-axis) versus CD25 (y-axis). Analyzed data of CD25+/CD4+ (right). (D) CD11b (x-axis) versus Ly6c (y-axis). Analyzed data of Ly6high/Ly6cmiddle in CD11b+ cells (right). n = 4 in each group. Data are presented as mean ± SD. ∗∗p < 0.01, ∗p < 0.05. Ctrl, control; ns, not significant; JTT-MSC, JTT and MSC combination therapy.
3.7. JTT partially restores the lipid level, tricarboxylic acid, and urea cycle more effectively than MSCs
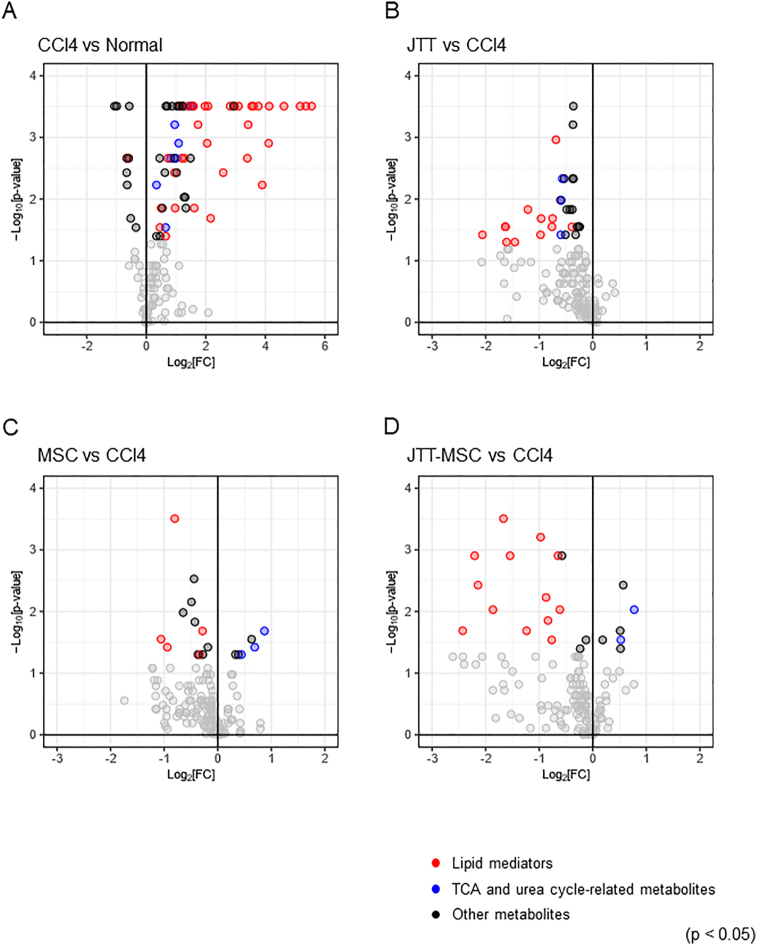
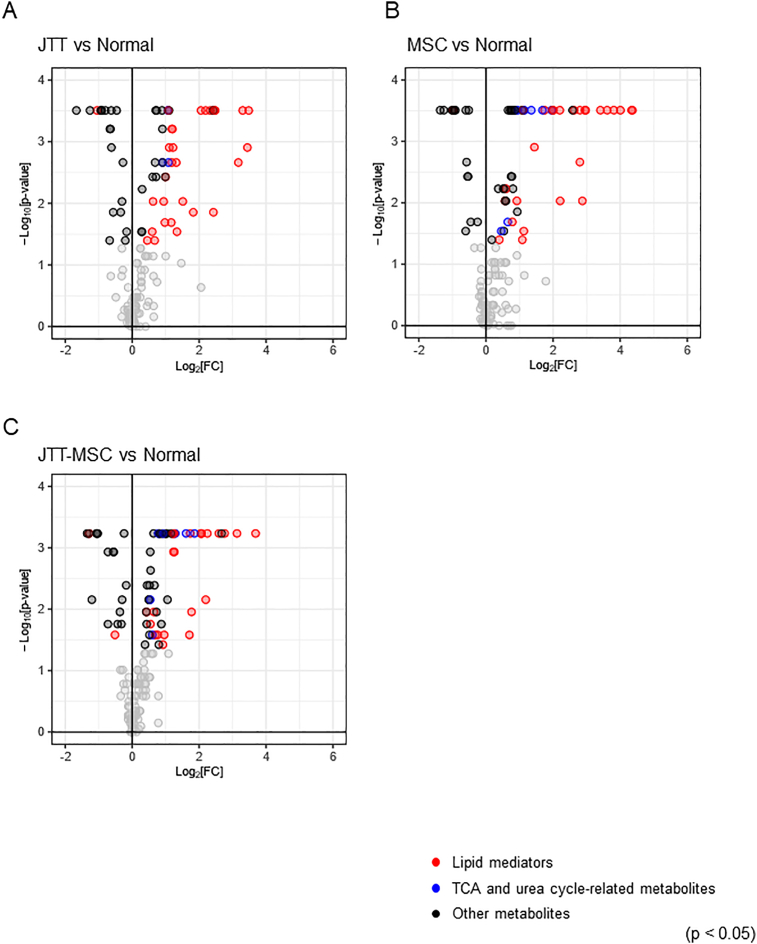
We conducted metabolome analyses of normal liver and CCl4-administered cirrhotic liver with and without treatment (JTT, MSC, and JTT-MSC combination groups). First, we conducted the following pairwise comparisons of liver metabolites: CCl4-no treatment vs. normal, CCl4 with JTT vs. normal, CCl4 with MSC vs. normal, and CCl4 with JTT-MSC vs. normal. We found that the levels of 61 metabolites were significantly increased, and those of 10 metabolites were decreased by CCl4 treatment (Fig. 6A, Supplementary data 1, p < 0.05). The number of metabolites exhibiting a log2 fold-change of ±3 or more was 13 in the CCl4 treatment group, whereas it was four in the CCl4 with JTT, six in the CCl4 with MSC, and two in the CCl4 with JTT-MSC groups (Supplementary Figs. 2A–C). Next, to compare the effect of each treatment, we conducted pairwise comparisons of these metabolites between the CCl4-no treatment group and each drug treatment group.
Fig. 6.
Volcano plots of the liver metabolome analysis. (A) No treatment versus CCl4-induced, (B) JTT versus CCl4, (C) MSC versus CCl4, and (D) JTT-MSC versus CCl4. Plots represent log2 fold-change value versus −log10 p-value. Black, red, and blue dots indicate p < 0.05, and others are indicated in gray. n = 8 in each group. Red and blue, and black dots represent the metabolite classes (lipid mediators, TCA and urea cycle-related metabolites, and others, respectively). JTT-MSC, JTT and MSC combination therapy.
Regarding the other treatments, JTT decreased the levels of liver metabolites, especially lipid mediators, and tricarboxylic acid (TCA) cycle- and urea cycle-related metabolites (Fig. 6B, Supplementary data 1, levels of 29 metabolites decreased, p < 0.05), whereas there was a slight change in the MSC group (Fig. 6C, Supplementary data 1, levels of six metabolites increased, and those of 12 metabolites decreased, p < 0.05). The JTT-MSC combination also decreased liver lipid mediator levels (Fig. 6D, Supplementary data 1, levels of six metabolites increased, and those of 16 metabolites decreased, p < 0.05).
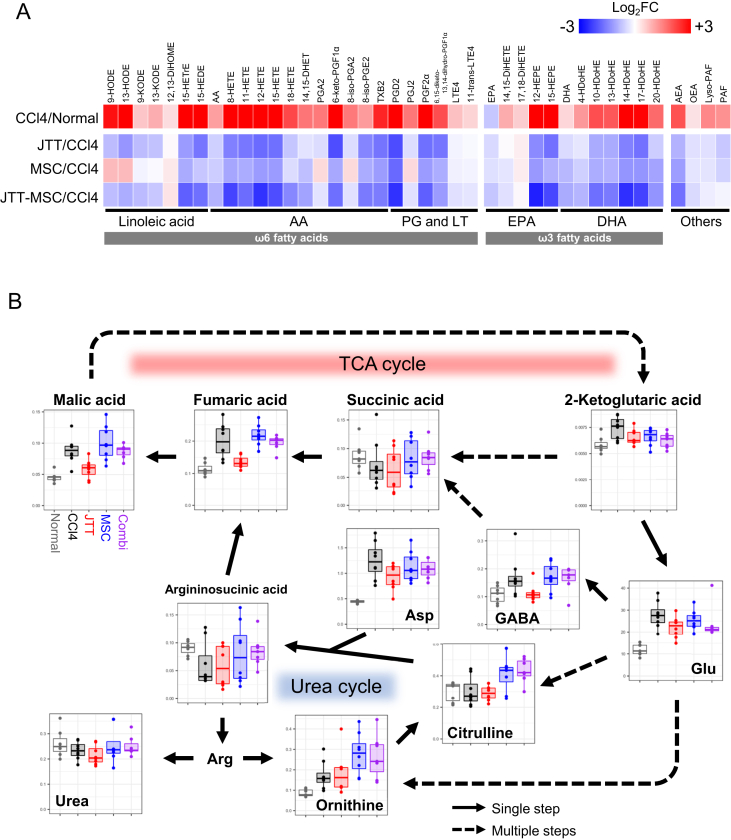
Heatmaps of individual lipid mediators show that the CCl4 treatment affected a wide range of lipid mediators, and JTT and MSC suppressed many of them. The effect of JTT treatment on ω6 fatty acids tended to be stronger than that of the CCl4 with MSC treatment according to the changes in 9-HODE, 13-HODE, PGA2, 8-iso-PGA2, and PGJ2. The effect of the combination was comparable to that of the JTT treatment (Fig. 7A). The pathway analysis of the TCA and urea cycles showed that while the changes after CCl4 with MSC treatment were insignificant, CCl4 with JTT significantly reduced metabolite levels, particularly the levels of malic acid and fumaric acid. In addition, 2-ketoglutaric acid, glutamic acid, gamma-aminobutyric acid, and aspartic acid were also apparently decreased in the CCl4 with JTT group (Fig. 7B). Hence, we conclude that JTT is more effective metabolically than MSCs and that JTT supports and strengthens the therapeutic effects of MSCs.
Fig. 7.
Analysis of lipid mediators and hydrophilic metabolites in the liver. (A) Heatmap showing the fold-change of each of the common metabolites in the liver after treatment with CCl4 (CCl4/Normal), CCl4 with JTT (JTT/CCl4), CCl4 with MSC (MSC/CCl4), and CCl4 in JTT-MSC (JTT-MSC/CCl4). Red indicates an increase in metabolite fold change or upregulation; blue indicates downregulation of a specific metabolite. n = 8. AA, arachidonic acid; AEA, arachidonoyl ethanolamide; DHA, docosahexaenoic acid; DHET, dihydroxy-eicosatrienoic acid; DiHETE, dihydroxy-eicosatetraenoic acid; DiHOME, dihydroxy-octadecenoic acid; EPA, eicosapentaenoic acid; EpOM, epoxy-octadecenoic acid; HDoHE, hydroxy-docosahexaenoic acid; HEDE, hydroxy-eicosadienoic acid; HEPE, hydroxy-eicosapentaenoic acid; HETE, hydroxy-eicosatetraenoic acid; HETrE, hydroxy-eicosatrienoic acid; HODE, hydroxy-octadecadienoic acid; KODE, keto-octadecadienoic acid; LT, leukotriene; OEA, oleoylethanolamide; PAF, platelet-activating factor; PG, prostaglandin; TX, thromboxane. (B) Pathway analysis of the TCA cycle- and urea cycle-related metabolites. Metabolite levels in each group are represented using box plots as normalized areas in the LC/MS or GC/MS analysis. Gray, black, red, blue, and purple indicate the results of the Wild, CCl4, JTT, MSC, and JTT-MSC groups, respectively. Arg, arginine; Asp, aspartic acid; GABA, γ-aminobutyric acid; Glu, glutamic acid; TCA, tricarboxylic acid.
4. Discussion
In the present study, we elucidated that JTT and MSC combination therapy attenuates liver damage, improves liver function, and regresses liver fibrosis. Although both JTT and MSCs attenuated liver damage by increasing the ratio of CD4+/CD8+, the two treatments had different modes of action. JTT increased serum ALB with an increase in NK cells and Treg cells. In addition, JTT effectively normalized lipid levels, TCA cycle, and urea cycle. However, MSC treatment reduced fibrosis while increasing anti-inflammatory macrophages. Overall, we consider that these different modes of action effectively regressed the damage in our cirrhosis model.
Our results after injecting MSCs were consistent with those of a previous study [6]. Xenogenic human MSCs are useful for their effect on murine liver injury models [7,8]. Watanabe et al. reported that MSCs migrate primarily into the lung after injection, act as “conducting cells” by producing humoral factors, affect “effector cells,” and exert therapeutic effects [6]. During cirrhosis, macrophages are the main effector cells [18]. MSCs have been reported to induce anti-inflammatory macrophage activation, confirmed in the current study; anti-inflammatory macrophages are essential for improving fibrosis and tissue repair. They possess phagocytic ability and produce matrix metalloproteinases (MMPs) [4,6]. In contrast, MSCs have been reported to exert therapeutic potential when host inflammation activity is high, that is, MSCs can be influenced by their circumstances and change their therapeutic potential [3]. Although several clinical studies have confirmed the therapeutic effects of MSCs, only a few MSC therapies have been approved. Hence, to clarify these discrepancies, we attempted the JTT and MSC combination therapy.
JTT has been used or expected to be used in several clinical settings. Kawai et al. reported that JTT combined with chemotherapy improved the progression-free survival of patients with postoperative recurrence of non-small cell lung cancer by preventing nutritional disorders [19]. Hisha et al. reported that an n-hexane extract from JTT had hematopoietic stem cell–stimulatory activity. The authors also fractionated the extract and found that oleic acid and linolenic acid are potent hematopoietic stem cell stimulators [20]. In an animal study, Choi et al. reported that JTT reduced cancer-induced anorexia and cachexia in mice [21]. Furthermore, Ohnishi et al. reported that JTT inhibits liver metastasis of colon-cancer cells in mice [13].
With respect to hepatology, Shosaikoto, a Kampo medicine, is commonly used. Shosaikoto is composed of seven crude drugs: Bupleuri radix (7.0), Pinelliae tuber (5.0), Scutellariae radix (3.0), Zizyphi fructus (3.0), Ginseng radix (3.0), Glycyrrhizae radix (2.0), and Zingiberis rhizoma (1.0). It has been reported to exert anti-inflammatory and anti-fibrotic effects experimentally and clinically [[22], [23], [24]]. Recently, Takahashi et al. reported that JTT and Shosaikoto inhibited necroinflammation and fibrosis in a nonalcoholic steatohepatitis (NASH) model [25]. As JTT and Shosaikoto share two herbs (Ginseng radix and Glycyrrhizae radix), we considered that the therapeutic effects observed in the present study are attributable to these herbs. Reportedly, the extracts from Ginseng radix exhibit anti-oxidative activities, whereas glycyrrhizin extracted from Glycyrrhizae radix demonstrates anti-inflammatory activities [9]. As each crude-drug component contains numerous ingredients and exerts therapeutic effects by themselves, it remains difficult to elucidate whether only Ginseng radix or Glycyrrhizae radix is effective. However, we consider that their anti-inflammatory and anti-oxidative effects would be their key modes of action.
In this study, we conducted flow cytometry of immune cells in the liver and found that both JTT and MSC individually increased the CD4+/CD8+ ratio. In addition, JTT increased NK cells and Treg cells. MSC therapy increased anti-inflammatory macrophages more effectively than JTT therapy. Ishikawa et al. reported that JTT significantly increased the serum IL-12 and IFN-γ levels and induced NK cell activity, which increased the inhibitory action of anti-PD-1 antibody on B16 cell metastasis [12]. This is consistent with our finding that NK cells increased in the liver. As IFN-γ has been reported to kill activated hepatic stellate cells [26] and strengthen the anti-inflammatory effects of MSCs [8], it is possible that IFN-γ and other factors induced by JTT also enhance the therapeutic effects of MSCs. Induction of Treg cells by JTT has not been reported yet. We could not determine whether JTT induced Treg cells directly or indirectly. Further research in this regard is needed. However, the effects of JTT against macrophages have been reported. Liu et al. reported that JTT promoted the differentiation of transplanted bone marrow cells into microglia in the injured mouse brain [9]. Takaoka et al. reported that glycolipids of JTT stimulated macrophages [27]. Furthermore, Onishi et al. reported that JTT activated macrophages and T cells and prevented the metastasis of colon cancer cells to the liver in mice [13]. However, none of these reports mentioned macrophage polarity. JTT may have additional effects on macrophages that facilitate tissue repair by MSCs.
Interestingly, both MSCs and JTT decreased the serum AST and ALT to similar levels; however, MSCs did not strongly increase the serum ALB level compared with JTT. To the best of our knowledge, this phenomenon has not been reported yet. ALB is produced by hepatocytes, and therefore, we considered that JTT affects the function of hepatocytes. Although we could not elucidate the underlying mechanism, our findings demonstrate that JTT affected metabolites such as lipid mediators, and those related to the TCA cycle and urea cycle in the liver. Altered arachidonic acid metabolism, a shift from the TCA cycle to aerobic glycolysis, and hyperammonemia due to reduced urea synthesis are characteristics of hepatic fibrosis [28]. Glutamine synthesis, as an alternative pathway for ammonia detoxification, is observed in patients with cirrhosis [29]. Moreover, amino acid imbalance in patients with cirrhosis disturbs the TCA cycle of dendritic cells [30]. In the current study, lipid mediators that are major arachidonic acid metabolites; TCA cycle-related metabolites such as malic acid, fumaric acid, and aspartic acid; and the urea cycle-related metabolite, glutamine, were increased in CCl4-administered cirrhotic livers. These findings are consistent with the previous reports mentioned herein. Therefore, the partially restored metabolic function of the liver mediated by JTT could be associated with improved liver health. Normalization of liver function by JTT together with the anti-fibrotic effects induced by MSCs effectively attenuated cirrhosis in the present study.
This study had some discussable concerns. MSCs produce several biologically active factors, whereas JTT contains numerous compounds, thereby making it difficult to specifically identify the moieties associated with the observed therapeutic effects. However, such multicomponent mixtures may be beneficial for appropriate immunomodulation. Recently, multiple studies have purified and characterized Kampo medicinal compounds by high-pressure liquid chromatography [27]. It has been reported that MSC-derived exosomes are attributable to the function of MSCs [5]. The levels of proteins, miRNAs, and lipids of the secreted exosomes have been elucidated. Specific combinations of substances will be used as novel therapeutic strategies in the future to treat various diseases.
Author contributions
SN and AT: conception and design; ST, TW, MO, SM, TI, TS, and MK: generation, collection, analysis, and interpretation of data; TN, KO, MN and NF: analysis of the metabolic profile. SN and AT: writing of the manuscript; KT and ST: supervision of the study.
Declaration of competing interest
This research was funded by Tsumura & Co. TN, KO, MN, and NF are employees of Tsumura & Co.; ST received grant support from Tsumura & Co.
Acknowledgments
We thank Mr. Takao Tsuchida for his cooperation in the preparation of pathological tissues. This research was supported by Tsumura & Co.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.reth.2021.07.002.
Contributor Information
Atsunori Tsuchiya, Email: atsunori@med.niigata-u.ac.jp.
Shuji Terai, Email: terais@med.niigata-u.ac.jp.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Fig. 1.
Therapeutic effect of Juzentaihoto (JTT) in a single-dose CCl4-induced cirrhosis mouse model. (A) Experimental scheme. (B) Serum levels of aspartate transaminase (AST), alanine transaminase (ALT), and albumin (ALB) of Ctrl and JTT groups at 36 h after CCl4 administration. (C) Hematoxylin and eosin staining (upper panels; scale bar: 500 μm, lower panels; scale bar: 50 μm) of Ctrl and JTT groups. n = 3 in each group. Data are presented as mean ± SD. ∗∗p < 0.01, ∗p < 0.05. Ctrl, control; ns, not significant.
Supplementary Fig. 2.
Volcano plots of the liver metabolome analysis. (A) JTT versus no treatment (Normal), (B) MSC versus Normal, and (C) JTT-MSC versus Normal. Plots represent log2 fold-change value versus the −log10 p-value. Black, red, and blue dots indicate p < 0.05, and others are indicated in gray; n = 8 in each group. Red, blue, and black dots represent the metabolite classes (lipid mediators, TCA and urea cycle-related metabolites, and others, respectively). JTT-MSC, JTT and MSC combination therapy.
Supplementary Table 1.
Antibodies used in flow cytometry. Supplementary Methods 1. Detailed procedure for liver metabolomics.
References
- 1.Schuppan D., Ashfaq-Khan M., Yang A.T., Kim Y.O. Liver fibrosis: direct antifibrotic agents and targeted therapies. Matrix Biol. 2018;68–69:435–451. doi: 10.1016/j.matbio.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Terai S., Tsuchiya A. Status of and candidates for cell therapy in liver cirrhosis: overcoming the "point of no return" in advanced liver cirrhosis. J Gastroenterol. 2017;52:129–140. doi: 10.1007/s00535-016-1258-1. [DOI] [PubMed] [Google Scholar]
- 3.Tsuchiya A., Kojima Y., Ikarashi S., Seino S., Watanabe Y., Kawata Y. Clinical trials using mesenchymal stem cells in liver diseases and inflammatory bowel diseases. Inflamm Regen. 2017;37:16. doi: 10.1186/s41232-017-0045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsuchiya A., Takeuchi S., Watanabe T., Yoshida T., Nojiri S., Ogawa M. Mesenchymal stem cell therapies for liver cirrhosis: MSCs as "conducting cells" for improvement of liver fibrosis and regeneration. Inflamm Regen. 2019;39:18. doi: 10.1186/s41232-019-0107-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsuchiya A., Takeuchi S., Iwasawa T., Kumagai M., Sato T., Motegi S. Therapeutic potential of mesenchymal stem cells and their exosomes in severe novel coronavirus disease 2019 (COVID-19) cases. Inflamm Regen. 2020;40:14. doi: 10.1186/s41232-020-00121-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watanabe Y., Tsuchiya A., Seino S., Kawata Y., Kojima Y., Ikarashi S. Mesenchymal stem cells and induced bone marrow-derived macrophages synergistically improve liver fibrosis in mice. Stem Cells Transl Med. 2019;8:271–284. doi: 10.1002/sctm.18-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kojima Y., Tsuchiya A., Ogawa M., Nojiri S., Takeuchi S., Watanabe T. Mesenchymal stem cells cultured under hypoxic conditions had a greater therapeutic effect on mice with liver cirrhosis compared to those cultured under normal oxygen conditions. Regen Ther. 2019;11:269–281. doi: 10.1016/j.reth.2019.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takeuchi S., Tsuchiya A., Iwasawa T., Nojiri S., Watanabe T., Ogawa M. Small extracellular vesicles derived from interferon-gamma pre-conditioned mesenchymal stromal cells effectively treat liver fibrosis. NPJ Regen Med. 2021;6:19. doi: 10.1038/s41536-021-00132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu H., Wang J., Sekiyama A., Tabira T. Juzen-taiho-to, an herbal medicine, activates and enhances phagocytosis in microglia/macrophages. Tohoku J Exp Med. 2008;215:43–54. doi: 10.1620/tjem.215.43. [DOI] [PubMed] [Google Scholar]
- 10.Yoshioka H., Fukaya S., Miura N., Onosaka S., Nonogaki T., Nagatsu A. Suppressive effect of Kampo formula "Juzen-taiho-to" on carbon tetrachloride-induced hepatotoxicity in mice. Biol Pharm Bull. 2016;39:1564–1567. doi: 10.1248/bpb.b16-00421. [DOI] [PubMed] [Google Scholar]
- 11.Liu H., Wang J., Tabira T. Juzen-Taiho-to, an herbal medicine, promotes the differentiation of transplanted bone marrow cells into microglia in the mouse brain injected with fibrillar amyloid beta. Tohoku J Exp Med. 2014;233:113–122. doi: 10.1620/tjem.233.113. [DOI] [PubMed] [Google Scholar]
- 12.Ishikawa S., Ishikawa T., Tezuka C., Asano K., Sunagawa M., Hisamitsu T. Efficacy of Juzentaihoto for tumor immunotherapy in B16 melanoma metastasis model. Evid Based Complement Alternat Med. 2017;2017:6054706. doi: 10.1155/2017/6054706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohnishi Y., Fujii H., Hayakawa Y., Sakukawa R., Yamaura T., Sakamoto T. Oral administration of a Kampo (Japanese herbal) medicine Juzen-taiho-to inhibits liver metastasis of colon 26-L5 carcinoma cells. Jpn J Canc Res. 1998;89:206–213. doi: 10.1111/j.1349-7006.1998.tb00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitagawa H., Ohbuchi K., Munekage M., Fujisawa K., Kawanishi Y., Namikawa T. Data on metabolic profiling of healthy human subjects' plasma before and after administration of the Japanese Kampo medicine maoto. Data Brief. 2019;22:359–364. doi: 10.1016/j.dib.2018.11.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen L., Huang H., Zhang W., Ding F., Fan Z., Zeng Z. Exosomes derived from T regulatory cells suppress CD8+ cytotoxic T lymphocyte proliferation and prolong liver allograft survival. Med Sci Mon Int Med J Exp Clin Res. 2019;25:4877–4884. doi: 10.12659/MSM.917058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu D., Fu J., Jin L., Zhang H., Zhou C., Zou Z. Circulating and liver resident CD4+CD25+ regulatory T cells actively influence the antiviral immune response and disease progression in patients with hepatitis B. J Immunol. 2006;177:739–747. doi: 10.4049/jimmunol.177.1.739. [DOI] [PubMed] [Google Scholar]
- 17.Clements M., Gershenovich M., Chaber C., Campos-Rivera J., Du P., Zhang M. Differential Ly6C expression after renal ischemia-reperfusion identifies unique macrophage populations. J Am Soc Nephrol. 2016;27:159–170. doi: 10.1681/ASN.2014111138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moroni F., Dwyer B.J., Graham C., Pass C., Bailey L., Ritchie L. Safety profile of autologous macrophage therapy for liver cirrhosis. Nat Med. 2019;25:1560–1565. doi: 10.1038/s41591-019-0599-8. [DOI] [PubMed] [Google Scholar]
- 19.Kawai H., Saito Y. Combination of Juzentaihoto and chemotherapy improves the prognosis of patients with postoperative recurrence of non-small cell lung cancer. Mol Clin Oncol. 2020;13:13. doi: 10.3892/mco.2020.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hisha H., Yamada H., Sakurai M.H., Kiyohara H., Li Y., Yu C. Isolation and identification of hematopoietic stem cell-stimulating substances from Kampo (Japanese herbal) medicine, Juzen-taiho-to. Blood. 1997;90:1022–1030. [PubMed] [Google Scholar]
- 21.Choi Y.K., Jung K.Y., Woo S.M., Yun Y.J., Jun C.Y., Park J.H. Effect of Sipjeondaebo-tang on cancer-induced anorexia and cachexia in CT-26 tumor-bearing mice. Mediat Inflamm. 2014;2014:736563. doi: 10.1155/2014/736563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shimizu I. Sho-saiko-to: Japanese herbal medicine for protection against hepatic fibrosis and carcinoma. J Gastroenterol Hepatol. 2000;15(Suppl):D84–D90. doi: 10.1046/j.1440-1746.2000.02138.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee J.K., Kim J.H., Shin H.K. Therapeutic effects of the oriental herbal medicine Sho-saiko-to on liver cirrhosis and carcinoma. Hepatol Res. 2011;41:825–837. doi: 10.1111/j.1872-034X.2011.00829.x. [DOI] [PubMed] [Google Scholar]
- 24.Ono M., Miyamura M., Kyotani S., Saibara T., Ohnishi S., Nishioka Y. Effect of Sho-saiko-to extract on HGF and TGF-beta levels of intraorgans in liver-injured rats after partial hepatectomy. J Pharm Pharmacol. 2000;52:111–118. doi: 10.1211/0022357001773599. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi Y., Soejima Y., Kumagai A., Watanabe M., Uozaki H., Fukusato T. Inhibitory effects of Japanese herbal medicines sho-saiko-to and juzen-taiho-to on nonalcoholic steatohepatitis in mice. PloS One. 2014;9 doi: 10.1371/journal.pone.0087279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radaeva S., Sun R., Jaruga B., Nguyen V.T., Tian Z., Gao B. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology. 2006;130:435–452. doi: 10.1053/j.gastro.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 27.Takaoka A., Iacovidou M., Hasson T.H., Montenegro D., Li X., Tsuji M. Biomarker-guided screening of Juzen-taiho-to, an oriental herbal formulation for immunostimulation. Planta Med. 2014;80:283–289. doi: 10.1055/s-0033-1360391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang M.L., Yang S.S. Metabolic signature of hepatic fibrosis: from individual pathways to systems biology. Cells. 2019;8 doi: 10.3390/cells8111423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blanco Vela C.I., Bosques Padilla F.J. Determination of ammonia concentrations in cirrhosis patients-still confusing after all these years? Ann Hepatol. 2011;10:S60–S65. [PubMed] [Google Scholar]
- 30.Kakazu E., Kondo Y., Kogure T., Ninomiya M., Kimura O., Ueno Y. Plasma amino acids imbalance in cirrhotic patients disturbs the tricarboxylic acid cycle of dendritic cell. Sci Rep. 2013;3:3459. doi: 10.1038/srep03459. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.