Abstract
Phosphoinositides play a pivotal role in the maturation of nascent phagosomes into microbicidal phagolysosomes. Following degradation of their contents, mature phagolysosomes undergo resolution, a process that remains largely unexplored.
Here we studied the role of phosphoinositides in phagolysosome resolution. PtdIns4P, which is abundant in maturing phagolysosomes, was found to be depleted as they tubulate and resorb. Depletion was caused, in part, by transfer of phagolysosomal PtdIns4P to the endoplasmic reticulum, a process mediated by ORP1L, a RAB7 effector. ORP1L formed discrete tethers between the phagolysosome and the endoplasmic reticulum, resulting in distinct regions where PtdIns4P depletion and enrichment alternated. Tubules emerged from PtdIns4P-rich regions, where ARL8b and SKIP accumulated. SKIP binds preferentially monophosphorylated phosphoinositides, of which PtdIns4P is most apparent in phagolysosomes, contributing to their tubulation. Accordingly, premature hydrolysis of PtdIns4P impaired SKIP recruitment and phagosome resolution. Thus, resolution involves phosphoinositides and tethering of phagolysosomes to the endoplasmic reticulum.
INTRODUCTION
Phagocytosis is an essential component of the innate immune response. Professional phagocytes, such as macrophages, neutrophils and dendritic cells effectively internalize, degrade and dispose of pathogenic microorganisms1–3. Additionally, phagocytosis is central for tissue homeostasis: in excess of 200 billion effete and necrotic cells are cleared from the human body every day4–6.
Phagosome formation is initiated by receptor engagement and clustering, followed by actin rearrangement and scission of the nascent vacuole from the plasma membrane (PM)2,3,7. Once formed, the phagosome undergoes rapid remodeling through interaction with components of the endocytic pathway. Through this transition —known as phagosome maturation— the phagosome acquires microbicidal and degradative properties8–10. Phagosome formation and maturation have been studied extensively. By contrast, much less is known about the concluding stage, namely phagosome resolution, whereby phagolysosomes and their contents are resorbed3,11 and antigens are presented to lymphoid cells3,12,13. Despite its obvious importance, the molecular basis of phagosome resolution has not been explored; our current view of phagolysosome resolution is limited to speculation and extrapolation from processes such as lysosome turnover and autophagy.
Phagosome formation and maturation are associated with acute changes in phosphoinositide metabolism14–16. Formation of phagosomes is accompanied by focal generation of PtdIns(3,4,5)P3 and disappearance of PtdIns(4,5)P217–19, which are required for actin restructuring, pseudopod extension and vacuolar sealing17,20,21. The newly formed PtdIns(3,4,5)P3 disappears promptly from the sealed phagosome, followed immediately by a burst of PtdIns3P formation22,23. The latter persists for several minutes, coinciding with the early stages of the maturation sequence. PtdIns4P undergoes multiphasic changes during phagosome formation and maturation: its concentration, which is sizeable in the PM, increases momentarily in the phagosomal membrane upon scission, followed by an abrupt decline. PtdIns4P is then undetectable during early maturation, but reappears when PtdIns3P disappears, reaching a concentration higher than that in the PM24.
Little is known about the function of PtdIns4P in phagocytosis24,25 and there are no known PtdIns4P effectors associated with phagosomes. Because it was preliminarily detected in tubular structures emanating from phagolysosomes24, we speculated that PtdIns4P might play a role in resolution. Here we describe yet another phase in the metabolism of PtdIns4P during phagocytosis, the underlying mechanism, and its relationship to phagosome resolution.
RESULTS
Phagosome resolution.
Phagosome fission events have been reported26, but the steps leading to phagolysosome resolution have not been studied. We therefore initially documented the progressive fragmentation of phagosomes formed by RAW264.7-macrophages that had ingested IgG-opsonized sheep erythrocytes (IgG-SRBC). To visualize the phagosomes, the macrophages were transfected with soluble (cytosolic) GFP, while the SRBC were covalently labeled with tetramethylrhodamine (TAMRA, Fig. 1a). Based on the size of the phagosomes and the integrity of their cargo, we identified three stages of late maturation, all occurring after fusion with lysosomes had taken place (i.e. >20 min after target engulfment): stage I consisted of homogeneously sized (4–5 µm) vacuoles containing seemingly intact, undigested SRBC; stage II consisted of largely intact phagosomes containing partially digested SRBC, with small fragmented structures in close proximity; in stage III, the phagocytic vacuoles were mostly fragmented into smaller vesicles containing SRBC remnants, dispersed throughout the cytoplasm (Fig. 1a). Resolution occurred progressively, with ≈70% of the phagosomes having fragmented (stage III) after ≈3 h (Fig. 1c). Electron microscopy (Fig. 1b) confirmed that stage I phagolysosomes contained mostly intact SRBC, and that some of the SRBC had undergone lysis by stage II, evident by the appearance of areas of reduced electron density indicative of hemoglobin leakage. Hemoglobin-rich vesicles and tubules were also seen in the vicinity of phagosomes at this stage. Multiple smaller vesicles of intermediate electron density were seen at stage III. That these derived from fragmentation of phagosomes was verified labeling the SRBC with cationized ferritin prior to phagocytosis. As shown in Figs. 1b and S1a, ferritin was readily discernible in the vesicles. Phagosome fragmentation was further confirmed using LAMP1 tagged with photo-activatable GFP (LAMP1-PAGFP). We assessed vesiculation by exclusively photo-activating the membrane of phagolysosomes (Fig. S1b) at early stages and following the distribution of PAGFP-positive membranes over time. As shown in Fig. S1c, LAMP1-PAGFP-containing vesicles derived from the phagosome appeared during resolution.
Figure 1. Phagosomal resolution is characterized by tubular fission events.
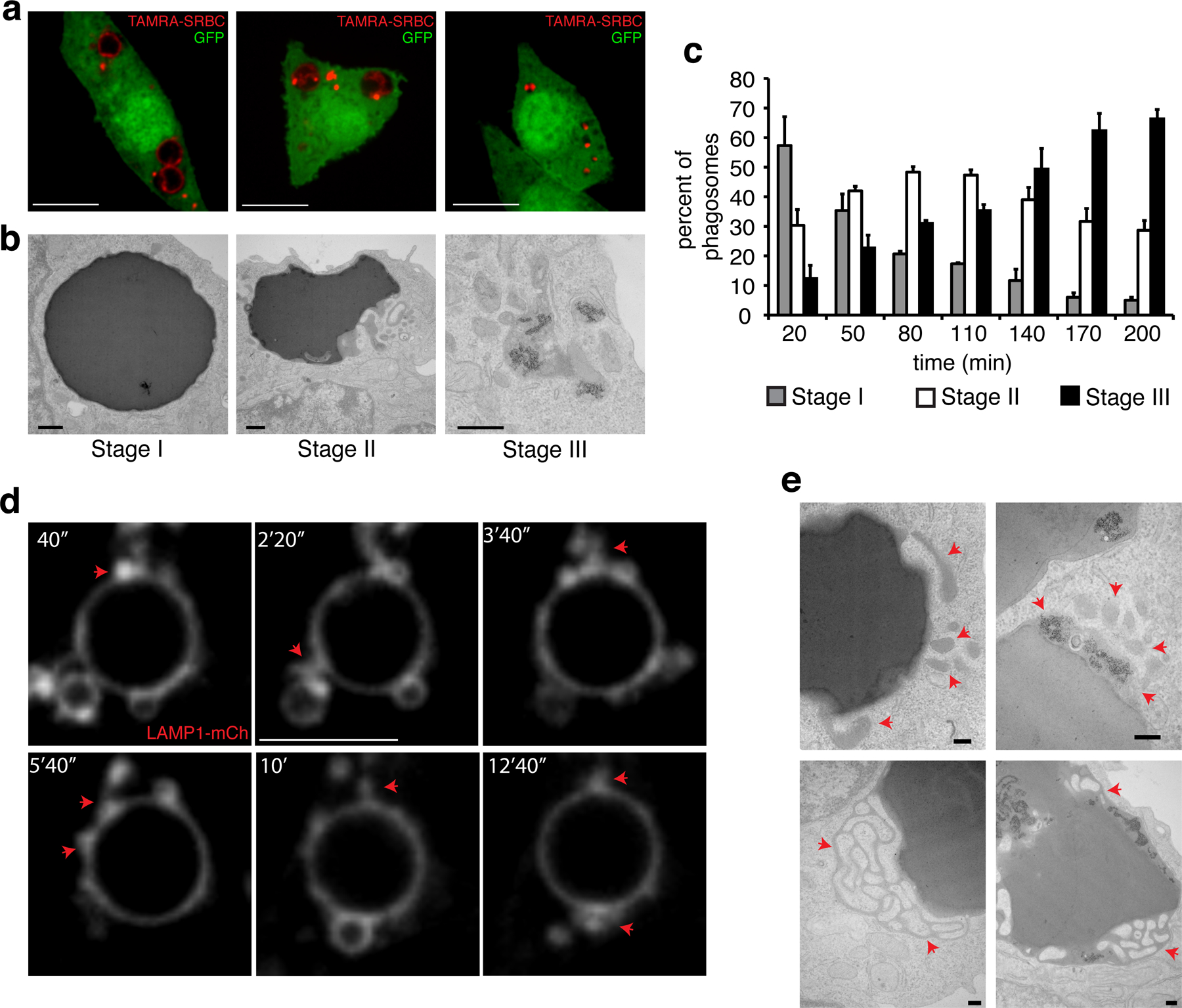
a) Confocal slices of GFP-expressing RAW macrophages after phagocytosis of TAMRA-labeled SRBC; micrographs are representative of three stages of phagosomal resolution defined based on the integrity of the target and of the phagosome; scale bars = 10 µm. b) Representative transmission electron micrographs of RAW macrophages during the three stages of resolution of SRBC-containing phagosomes; for stage III, SRBC were pre-labeled with ferritin; scale bars = 200 nm. c) Assessment of phagosome resolution in RAW macrophages over time. Resolution was assessed by evaluating 100 cells containing individual phagosomes per time point in each of 3 independent experimental replicates. Shown are means and error bars represent SD. d) Confocal slices representative of membrane remodeling in phagolysosomes of RAW macrophages expressing LAMP1-mCh; scale bars = 5 µm. e) Representative scanning electron micrographs of structures budding and extruding from resolving phagosomes with either unlabeled (left) or ferritin-labeled (right) SRBC; scale bars = 200 nm. In (d) and (e) arrows indicate tubulo-vesicular structures emanating from the phagosomes. The two rightmost images in (e) show phagosomes containing SRBC that had been coated with cationized ferritin, which appears as electron-dense puncta.
The dynamic nature of remodeling was visualized using fluorescently-tagged LAMP1. We initially detected fusion of LAMP1-bearing vesicles with maturing phagosomes, followed by the extension of tubular protrusions from mature phagolysosomes, some of which underwent fission or formed blebs (Fig. 1d; Movie 1). Often, areas of the phagosomal membrane where LAMP1 appeared thicker were noted. Because these were not well resolved by optical microscopy, we analyzed their structure by electron microscopy. They consisted of intricate tubular networks identified as connected to the phagosome by the electron density of hemoglobin (Fig. 1e, bottom left) and/or ferritin (bottom right).
PtdIns4P dynamics during phagosome resolution.
Because phagolysosome formation is associated with reacquisition of PtdIns4P24,25, we speculated that this phospholipid may play a role in remodeling and resolution. As shown in Figs. 2a–b, the highest concentration of PtdIns4P —visualized using the 2xP4M biosensor27— was attained 20–30 min after particle internalization, when phagolysosome resolution begins. After reaching peak levels, PtdIns4P gradually disappeared over the next 45 min (Fig. 2a–b; Movie 2). This decrease was characterized by preferential depletion of PtdIns4P in discrete regions of the phagosomal membrane, with persistence in the adjacent regions (Fig. 2a). Notably, PtdIns4P-positive vesicular and tubular structures emanated from the areas of the phagosome where the phosphoinositide was enriched (Fig. 2c). Similar observations were made using another PtdIns4P-selective probe based on SidC. To obtain better spatio-temporal resolution, we turned to lattice light-sheet microscopy. The three-dimensional images acquired by this method showed striking tubulation events (Fig. 2d, S2a). A fraction of the tubules approached the Golgi complex, others seemed directed to the PM.
Figure 2. Dynamics and distribution of PtdIns4P during initiation of phagosome resolution.
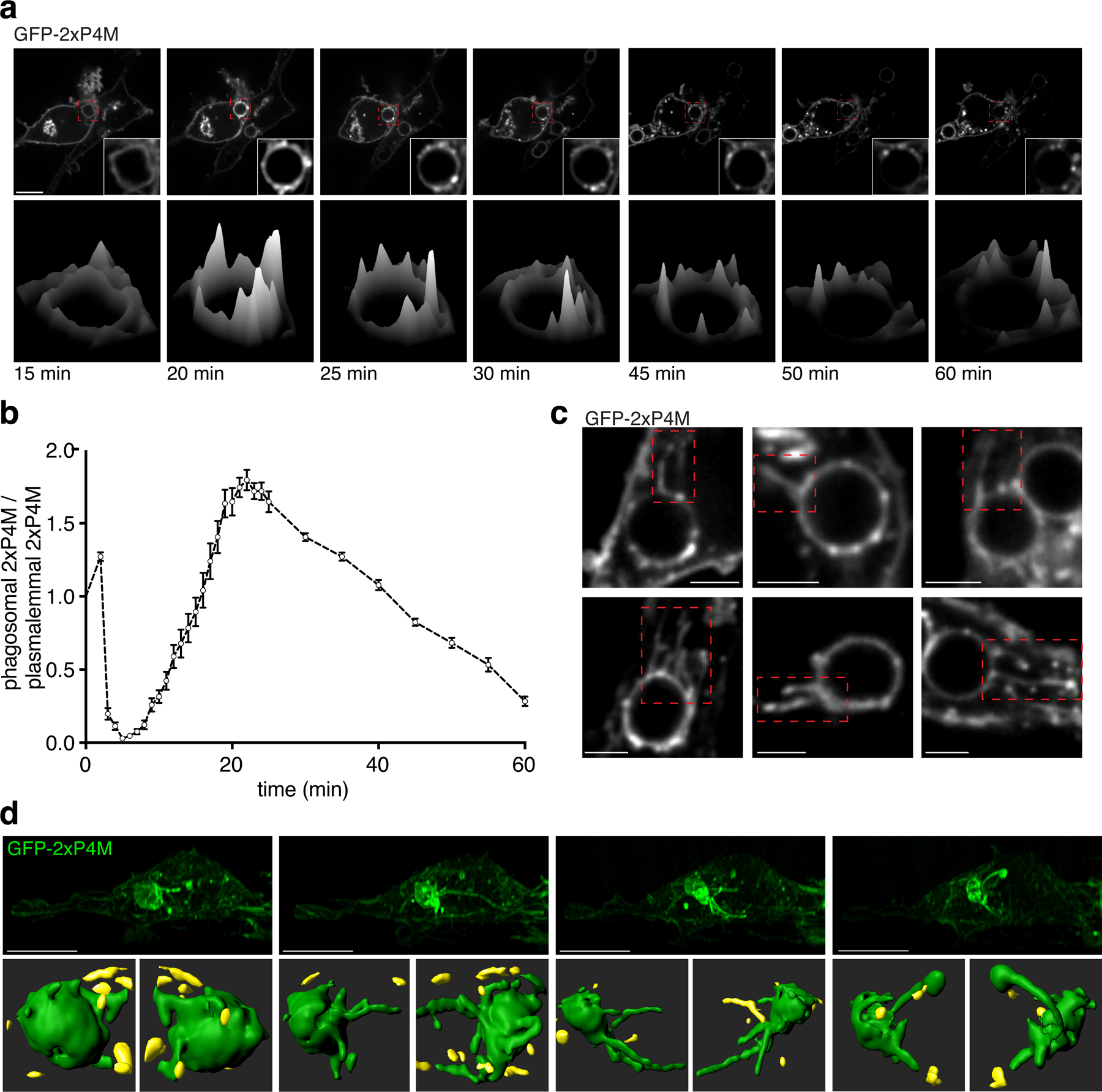
a) Top: time-lapse gallery of confocal micrographs acquired during phagocytosis of SRBC by RAW macrophages transiently expressing GFP-2xP4M showing multiphasic PtdIns4P dynamics; bottom: time-lapse surface intensity plots corresponding to the phagosome in the insets above; scale bar = 10 µm. b) Summary of tetra-phasic changes of PtdIns4P during phagocytosis; for quantitation, phagosomal fluorescence intensity was normalized to plasmalemmal fluorescence intensity; shown are means of 10 experimental replicates, error bars represent SEM. c) Confocal micrographs showing phagosomal tubules decorated with GFP-2xP4M. d) Top: side view of lattice light-sheet (LLS) time-lapse micrographs of RAW macrophages expressing GFP-2xP4M containing phagolysosomes; bottom: 3D surface reconstructions of GFP-2xP4M-positive phagosomes during tubulation for resolution. 3D reconstructions correspond to LLS micrographs on top; green surfaces are part of the phagosome and yellow surfaces are other compartments. Top scale bars = 10 µm; bottom scale bars = 3 µm.
PtdIns4P degradation mechanism.
We next investigated the mechanism underlying the inhomogeneous distribution of PtdIns4P. First, we considered that PI4K2A —the kinase shown earlier to be responsible for the reappearance of PtdIns4P in phagolysosomes24,25— might redistribute and ultimately detach from the membrane. However, we found the kinase to remain associated with the phagolysosomal membrane throughout the period when PtdIns4P was depleted (Fig. S2b). This suggested that variations in the rate and site of PtdIns4P removal were responsible for its inhomogeneous depletion. PtdIns4P consumption could be catalyzed by lipid phosphatases. SAC2 (INPP5F), which functions as an inositol 4-phosphatase in early endosomes, could in principle play this role. We therefore assessed its presence in late phagosomes. GFP-SAC2 was mostly found in phagosomes during early maturation stages (5–10 min; Fig. S2c–d), almost invariably co-localizing with RAB5 and rarely with LAMP-1 (Fig. S2c,e). During late stages (40–60 min), when phagosomes were predominantly LAMP1-positive, the phosphatase was seldom detected, suggesting that it is not involved in the disappearance of PtdIns4P (Fig. S2c–d).
Hence, we turned our attention to a potential lipid-transport mechanism, namely oxysterol-binding protein-related protein 1L (ORP1L). ORP1L contains a series of ankyrin repeats that bind active RAB728 and is therefore anticipated to associate with phagolysosomes, which are RAB7-positive. In addition, ORP1L has a pleckstrin-homology (PH) domain that binds phosphoinositides28, an FFAT motif that can associate with the endoplasmic reticulum (ER) proteins VAPA/B29–31, and an OSBP-related domain (ORD) shown to bind and translocate sterols31–37 (Fig. 3a). Of note, while the ORD domain of ORP1L can indeed bind cholesterol, it was found to bind PtdIns4P with even higher affinity36. Because SAC1 —the main 4-phosphatase of mammalian cells37— localizes to the ER39, we hypothesized that ORP1L might mediate PtdIns4P transfer from phagolysosomes to the ER, where it would be degraded by SAC140. Analogous non-vesicular lipid transport mechanisms have been reported in other compartments41–45.
Figure 3. ORP1L accumulates in phagosomes during late maturation.
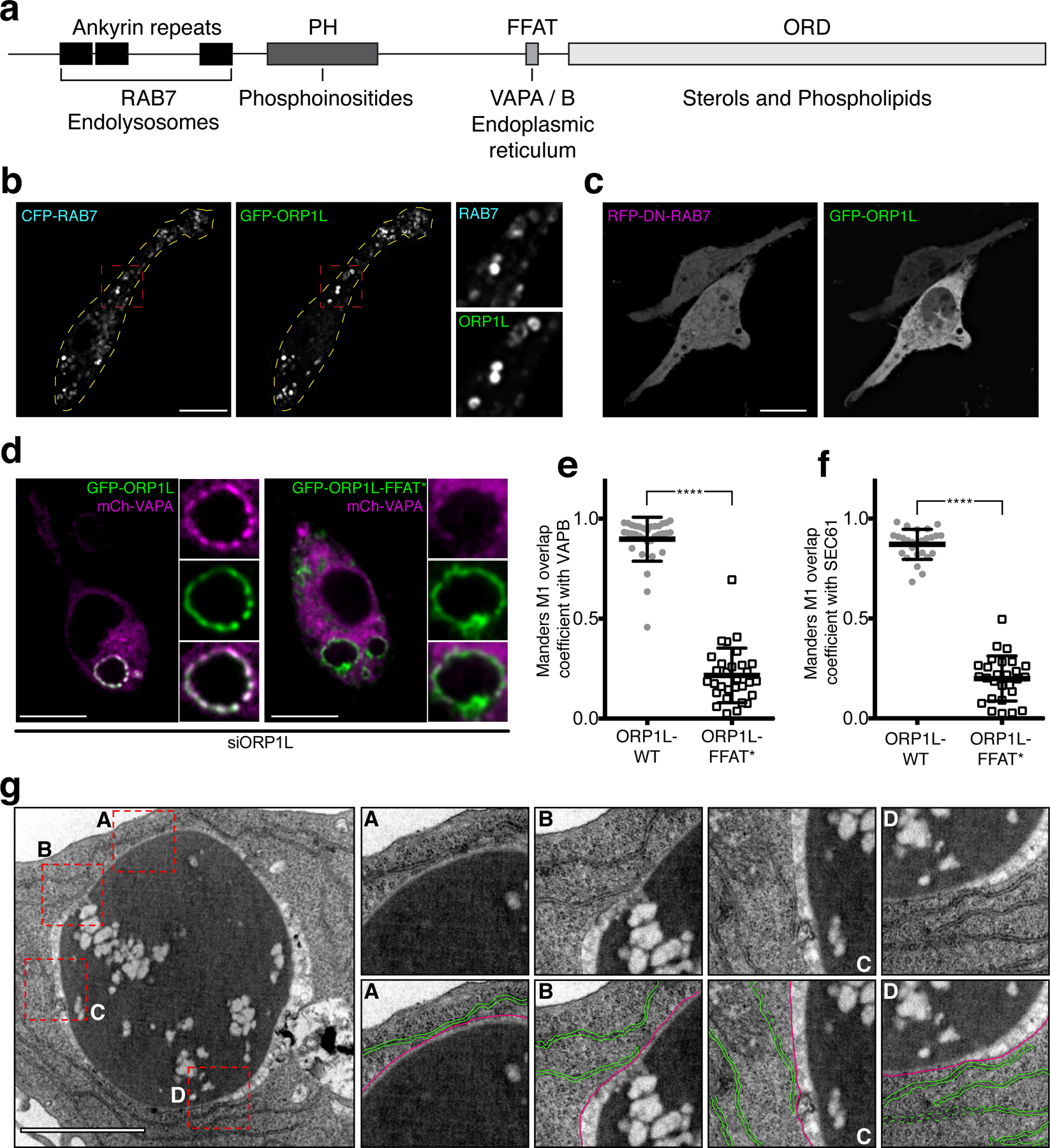
a) Schematic representation of functional domains of ORP1L. b) Confocal micrographs of RAW macrophages co-expressing GFP-ORP1L and RFP-RAB7 in RAW macrophages; insets are magnifications of red boxes; dotted yellow lines outline the cell periphery. c) Confocal micrographs of RAW macrophages co-expressing GFP-ORP1L and dominant-negative RFP-RAB7(T22N). d) Representative confocal micrograph of RAW macrophages co-expressing mCh-VAPA GFP-ORP1L (left) or mCh-VAPA and GFP-ORP1L-FFAT* (right) after 30 min of phagocytosis; insets show magnifications of the phagosome from the main micrographs. e) Graph showing the means and individual values of a Manders co-localization analysis of mCh-VAPB with either GFP-ORP1L (gray circles) or GFP-ORP1L-FFAT* (white squares); error bars in e-f represent standard deviations. Here and in subsequent figures ****p ≤ 0.0001. f) Graph showing the means and individual values of a Manders co-localization analysis of mCh-SEC61 with either GFP-ORP1L (gray circles) or GFP-ORP1L-FFAT* (white squares). g) Transmission electron micrograph of a RAW macrophage after 40 min of phagocytosis showing contacts between the ER and phagosomal membranes. Insets are magnifications of dotted red boxes. Bottom inset panels highlight ER membranes (colored in green) in contact with the phagosomal membrane (colored in magenta). Scale bar = 1 µm.
To assess this hypothesis we determined whether ORP1L was expressed in myeloid cells. Consistent with transcriptomic data46, we found ORP1L protein to be expressed in monocytes and markedly upregulated upon differentiation to macrophages (Fig. S3a); expression of the short variant of ORP1 (ORP1S) was negligible. In primary macrophages, endogenous ORP1L localized to endomembranes, as did GFP-tagged ORP1L expressed in RAW264.7-macrophages (Fig. S3b). The endomembrane compartment corresponded to late endosomes/lysosomes, as it was RAB7-positive (Fig. 3b). That interaction of its ankyrin repeats with RAB7 was responsible for ORP1L association with endo/lysosomes was verified by expressing RAB7(T22N), a dominant-negative form, which displaced of ORP1L to the cytosol (Fig. 3c).
In primary macrophages ORP1L decorated the phagolysosomal membrane (Fig. S3c top), as did GFP-tagged ORP1L in RAW264.7-macrophages (Fig. S3c, bottom). ORP1L was first detected on phagosomes ≈15 min after formation, attaining maximal levels after ≈30 min (Fig. S3d), paralleling the course of acquisition of RAB7. It is noteworthy that while GFP-ORP1L initially associated with phagosomes in a discontinuous, “patchy” pattern resembling that of the endogenous protein, the continued recruitment of the ectopically (over)expressed protein gradually made the pattern more continuous (Movie 3).
We investigated the cause of the patchy distribution of ORP1L. Its FFAT motif is anticipated to associate with VAPA/B, forming membrane contacts with the ER. We validated this notion using a gibberellin-based heterodimerization system47 to recruit ORP1L-derived soluble constructs (lacking the ankyrin repeats) to the PM. Recruitment of a construct containing the wildtype FFAT sequence forced the apposition of VAPB to the PM (Fig S4b), but this failed to occur when ORP1L contained a mutated FFAT sequence (D478A, designated FFAT*) unable to bind VAPA/B (Fig S4c).
We predicted that ORP1L would mediate the formation of contacts between late phagosomes and the ER. Accordingly, VAPA and VAPB were found to line the phagolysosomal membrane (Figs. 3d–e, S3e), where they co-localized with phagosomal GFP-ORP1L. This close association was mediated by the FFAT motif, since it was eliminated when full-length ORP1L containing the D478A mutation (ORP1L-FFAT*) was expressed in cells where the endogenous (wildtype) ORP1L had been knocked down (Figs. 3d–e). Moreover, when co-expressed in RAW264.7-macrophages, mCherry-SEC61 (an ER-resident protein) and GFP-ORP1L –but not ORP1L-FFAT*– similarly co-localized along the phagosome periphery (Figs. 3f, S3f). Further evidence was obtained by electron microscopy: multiple contact sites between the ER and phagolysosomes form during the late maturation stages (Figs. 3g, S3h).
ORP1L-dependent phagolysosome-to-ER PtdIns4P transport.
We next tested whether ORP1L plays a role in the disappearance of PtdIns4P. To this end, we co-expressed 2xP4M and ORP1L. Strikingly, we observed that the ORP1L-rich regions of phagosomes were preferentially depleted of PtdIns4P (Figs. 4a, S5a; Movie 4). The mutual exclusion was exacerbated over time, as ORP1L association increased. Eventually, ORP1L accumulated throughout most of the phagosome, at which time PtdIns4P was virtually undetectable (Figs. 4a, S5a).
Figure 4. ORP1L expression accelerates late PtdIns4P disappearance in maturing phagosomes.
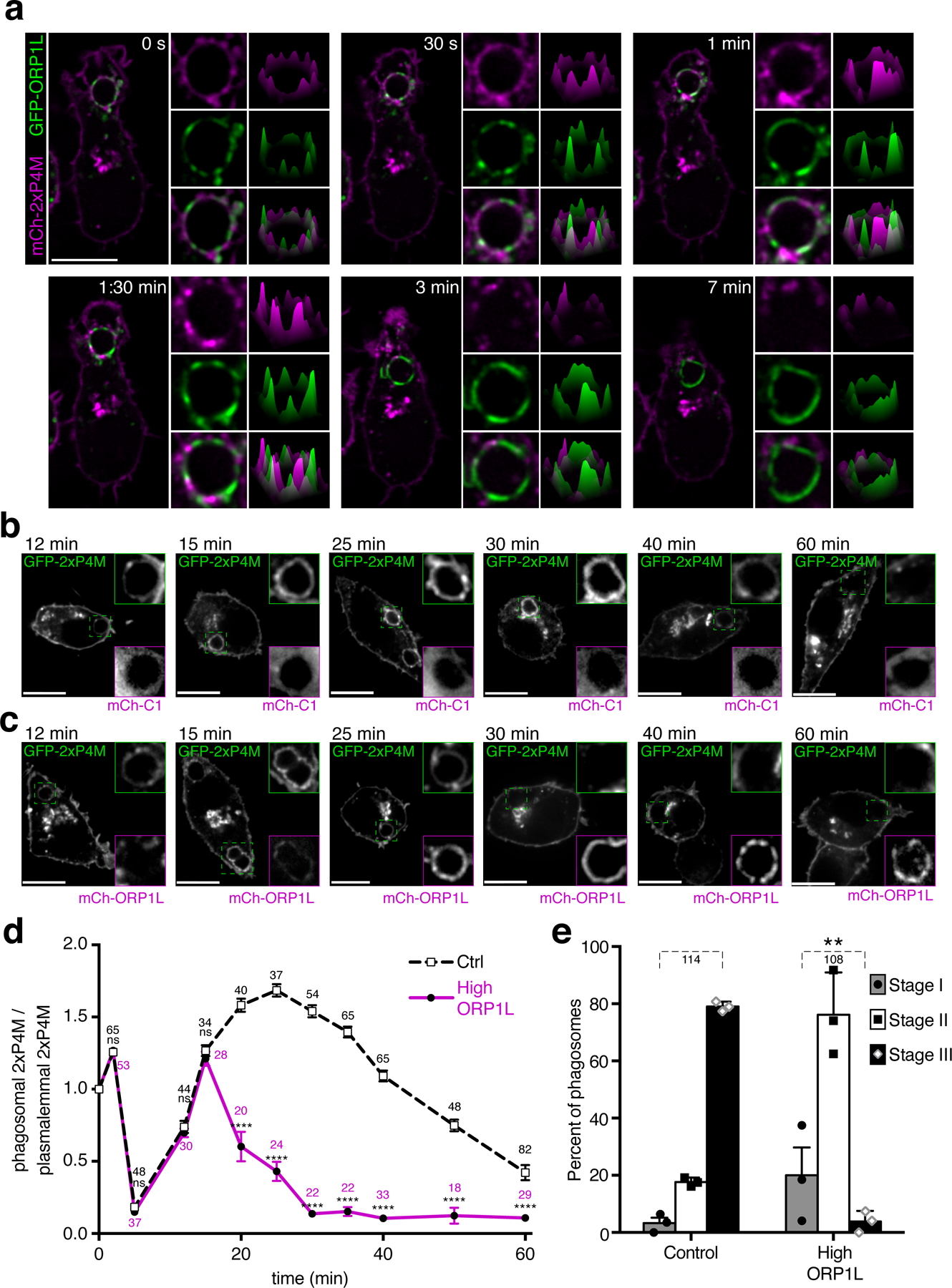
a) Representative time-lapse confocal micrographs of RAW macrophages co-expressing mCh-2xP4M and GFP-ORP1L, starting 20 min after phagocytosis; first column of insets show magnifications of phagosomes in the main micrograph; second column of insets shows surface intensity plots corresponding to these phagosomes. b) Representative confocal micrographs of RAW macrophages co-expressing GFP-2xP4M and a control mCh vector at different times during phagosome maturation; insets show magnifications of dash-line boxes; green frame for the GFP channel and magenta frame for the mCh channel c) Representative confocal micrographs of RAW macrophages co-expressing GFP-2xP4M and mCh-ORP1L at different times during phagosome maturation. Scale bars = 10 µm in a-c. d) Black dotted line: PtdIns4P dynamics during phagocytosis in cells expressing a control mCh vector; magenta line: PtdIns4P dynamics during phagocytosis in cells expressing mCh-ORP1L; means of 3 experimental replicates; error bars represent SEM; a two-way ANOVA with multiple comparisons, using Sidak correction was used to assess significance, assuming statistical independence between groups. Here and in subsequent figures **p ≤ 0.01; the number of phagosomes quantified for each condition is indicated. e) Assessment of phagosomal resolution in cells expressing mCh-ORP1L or mCh alone (Control). Maturation stages were defined as in Fig. 1. Data are means ± SD; significance was calculated by two-tailed unpaired t tests. The number of phagosomes assessed in 3 independent experiments for each condition is indicated.
These observations suggest a role for ORP1L in the clearance of PtdIns4P, presumably by transfer to the ER. If correct, this model predicts that excessive recruitment of ORP1L would cause faster and more homogeneous removal of phagosomal PtdIns4P. Accordingly, PtdIns4P disappearance occurred considerably faster in cells expressing mCherry-tagged ORP1L than in cells transfected with unconjugated mCherry (Fig. 4b–d). Notably, resolution of phagosomes in macrophages expressing high levels of ORP1L was significantly impaired even 4.5 h after phagocytosis (Figs. 4e, S5b), suggesting that PtdIns4P plays an active role in resolution.
To more directly ascertain its role in PtdIns4P catabolism, we inactivated the Osbpl1a gene in RAW264.7-macrophages using CRISPR/Cas9. The effectiveness of the deletion was validated at both the genomic level and by immunoblotting (Fig. S6a,b). We then compared the kinetics of PtdIns4P clearance from phagosomes formed by wildtype and ORP1L-deficient cells (Fig. 5a–b). ORP1L knockout cells cleared PtdIns4P more slowly than wildtype cells; the overall difference in the rate of disappearance was highly significant (Fig. 5b). Similar results were obtained with three separate Osbpl1a knockout cell lines (Fig. S6c–d).
Figure 5. ORP1L transports PtdIns4P from maturing phagosomes to the ER.
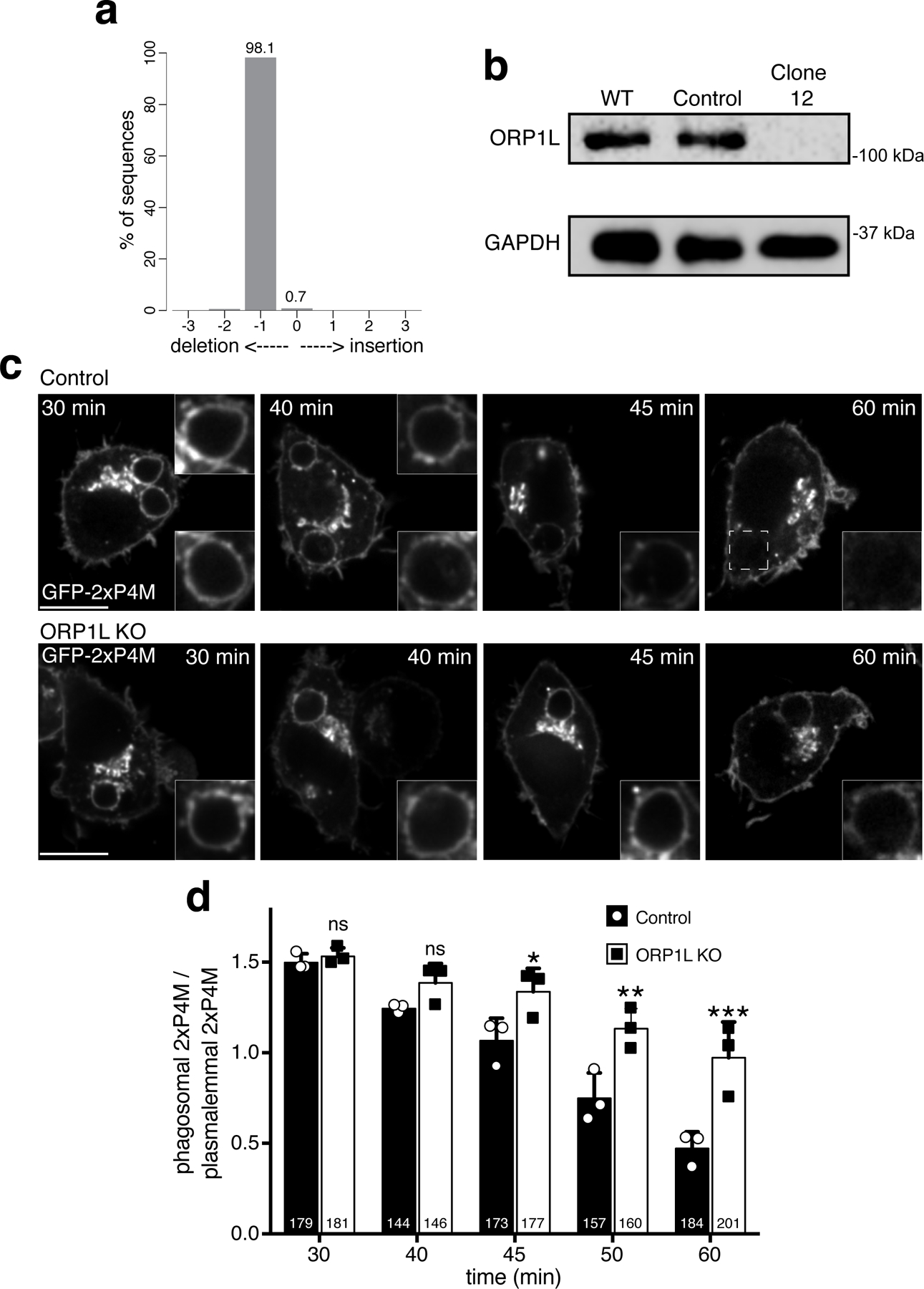
a) Top: Representative confocal micrographs of control RAW macrophages expressing GFP-2xP4M at different times during phagosome maturation. Bottom: representative confocal micrographs of ORP1L KO RAW macrophages expressing GFP-2xP4M at different times during maturation. Scale bar = 10 µm. b) PtdIns4P dynamics in maturing phagosomes of control cells (black bars) and ORP1L KO cells (white bars); shown are means of normalized fluorescence intensity values of phagosomes per time-point, from 3 separate experiments (the number of individual phagosomes quantified is indicated within the bars); error bars represent SD; ***p ≤ 0.001 and ** p≤0.01, determined by two-way ANOVA comparing cell lines with multiple comparisons, using Sidak correction assuming statistical independence between groups. c) Representative confocal micrographs after 30 min of phagocytosis of: control RAW macrophages co-transfected with GFP-2xP4M and an mCh-vector and treated with siRNA targeting ORP1L (first column); in the following columns RAW macrophages were treated with siRNA targeting ORP1L and co-transfected with FP-2xP4M and different ORP1L constructs tagged with fluorescent proteins. d) Normalized FP-2xP4M fluorescence intensity of individual phagosomes of RAW macrophages expressing the indicated constructs. Data points are the number of experiments indicated in parentheses; medians with interquartile ranges are indicated. Significance calculated by two-tailed unpaired t tests. Scale bars = 10 µm.
Based on its ability to form membrane contacts, the preferential depletion of PtdIns4P at these sites, and by analogy with related proteins36,48–50, we assumed that ORP1L transfers PtdIns4P from phagolysosomes to the ER. Previous studies have shown that sterols bind to a hydrophobic pocket in the ORD domain of ORP1L31,33–37. Interestingly, this pocket contains two conserved histidines that could coordinate the binding of PtdIns4P48–50. We mutated these two histidines (Fig. S6e) and assessed the ability of the resulting mutant ORP1L (HH651/652AA; called ORD* in Fig. 5c) to accelerate the clearance of the phosphoinositide from phagolysosomes, as shown for wildtype ORP1L (Fig. 4b–d). In this and subsequent experiments where mutant forms of ORP1L were expressed, endogenous ORP1L was silenced using siRNA to minimize its countervailing effects. As shown in Figs. 5c–d, S6k, elimination of these histidine residues did not interfere with recruitment of ORP1L-ORD* to phagolysosomes but eliminated its ability to stimulate the clearance of PtdIns4P.
We next assessed whether the FFAT motif is also required for PtdIns4P depletion. Fig. 5c–d show that the FFAT* mutant was incapable of accelerating PtdIns4P disappearance, presumably because linkage with the ER was impaired (Figs. 3d–e, S6i). We interpret these results to mean that, after establishment of membrane contacts, the ORD domain of ORP1L transfers PtdIns4P to the ER, where it is hydrolyzed by SAC1. In principle, degradation of PtdIns4P by SAC1 could occur in trans. We tested this possibility using ORP1L-ORD*. While this mutant retains the ability to interact with VAPA/B, it cannot bind and transfer PtdIns4P. Expression of ORP1L-ORD* in ORP1L KO cells did not cause a significant reduction of phagosomal PtdIns4P levels compared to KO cells (Fig. 5c–d). To verify that SAC1 can hydrolyze phagosomal-associated PtdIns4P we generated a chimeric protein where the ORD domain of ORP1L was replaced by the phosphatase domain of SAC1 (Fig. S6e). Like wildtype ORP1L, this SAC1 chimera —named ORPSAC1— was exclusively recruited to RAB7-positive compartments. More importantly, ORPSAC1 robustly depleted PtdIns4P from phagosomes without affecting plasmalemmal or Golgi-associated PtdIns4P, recapitulating the effect of wildtype ORP1L overexpression (Fig. 5c–d). These observations indicate that transfer to the ER is required for hydrolysis of PtdIns4P by SAC1.
The importance of ORP1L and ER contacts in the catabolism of phagosomal PtdIns4P was validated using COS-1 cells. These cells, which are not intrinsically phagocytic, express minute amounts of ORP1L (Fig. 6a), suggesting a linkage between these functions. COS-1 cells can, however, acquire the ability to ingest IgG-opsonized targets upon transfection of FcγR2A, a phagocytic receptor. As in professional phagocytes, the resulting phagosomes accumulated PtdIns4P. Strikingly, despite their ability to mature –acquiring LAMP-1 and becoming acidic51– the phagolysosomes generated by COS-1 retained PtdIns4P for greater than 90 min (Fig. 6b–c); for comparison, the phagosomes of RAW264.7-macrophages lost most of their PtdIns4P by 60 min (Fig. 6c). We took advantage of the paucity of endogenous ORP1L to test the ability of exogenous constructs to deplete PtdIns4P. Using heterodimerization and a RAB7-based targeting construct, we recruited to the phagosomes an N-terminal truncated (ankyrin repeat-deficient) but otherwise intact ORP1L (mTq2-GAI-ORP1L in Fig. 6d), or a D478A mutant (mTq2-GAI-ORP1L-FFAT*, Fig. 6e). Recruitment of the wildtype construct caused rapid disappearance of PtdIns4P, but this was not the case when the FFAT mutant was expressed (Figs. 6d–g). Together, these findings indicate that ORP1L is required for elimination of PtdIns4P from phagosomes, and that formation of ER contacts is essential.
Figure 6. ORP1L-mediated PtdIns4P degradation is dependent on its FFAT motif.
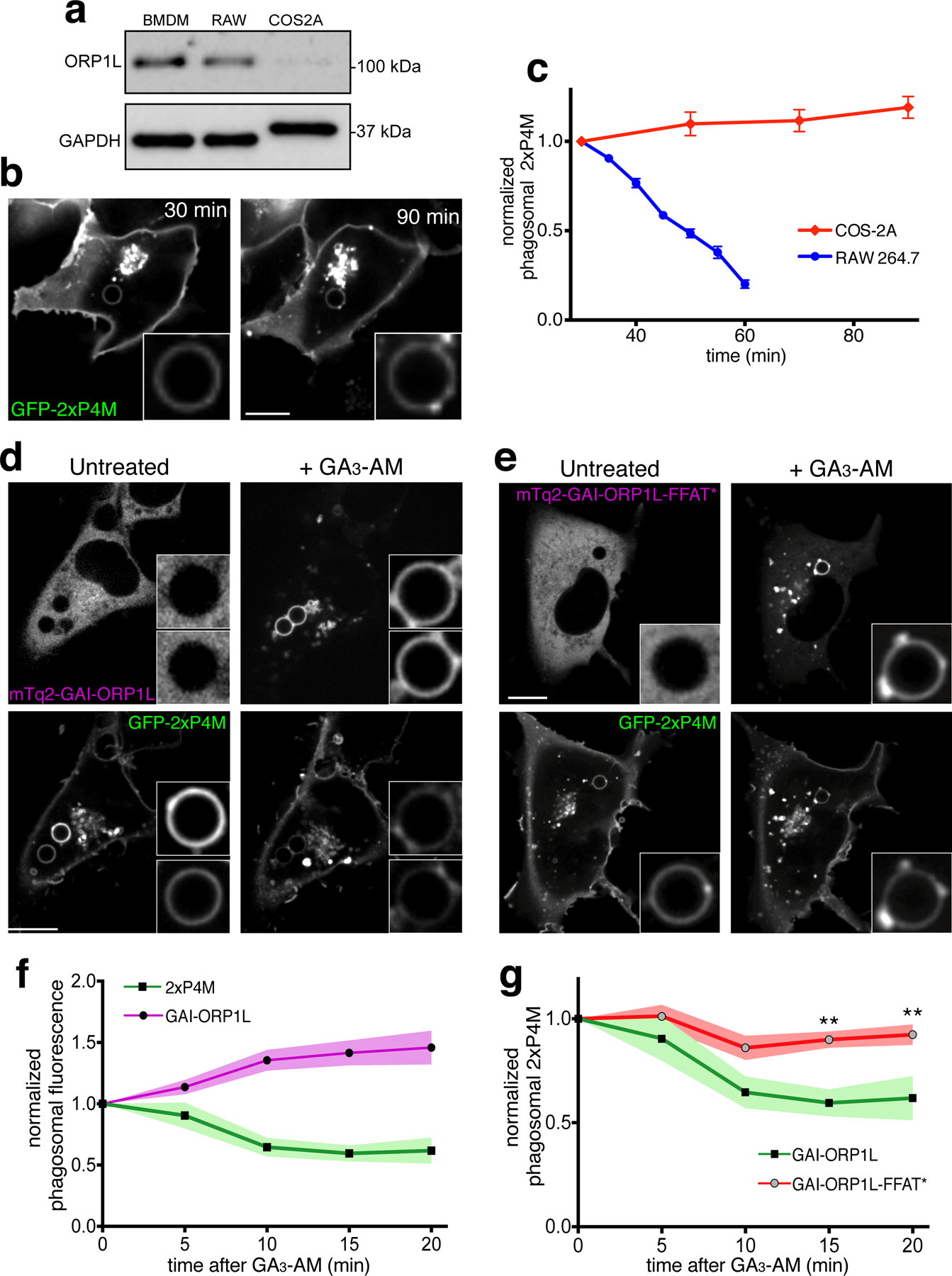
a) Representative western blot showing the expression of ORP1L in bone marrow-derived macrophages, RAW cells and COS-1-FcγR2A (COS-2A) cells. b) Confocal images of COS-2A cells expressing GFP-2xP4M during phagocytosis, acquired at the indicated times. c) Phagosomal 2xP4M content (normalized to that of the plasma membrane) of 39 phagosomes in COS-2A cells (red diamonds and line); data are means ± SE. For comparison, the blue line and circles show the 2xP4M in phagosomes of RAW macrophages (excerpted from Fig. 2b). d) Confocal images of COS-2A cells co-expressing iRFP-GID1-RAB7, mTq2-GAI92-ORP1L and GFP-2xP4M before (left) and after (right) addition of GA3-AM. e) Confocal images of COS-2A cells co-expressing iRFP-GID1-RAB7, mTq2-GAI92-ORP1L-FFAT* and GFP-2xP4M before (left) and after (right) addition of GA3-AM. f) Normalized phagosomal GFP-2xP4M (black squares) and phagosomal mTq2-GAI92-ORP1L (black circles) intensities at the indicated times after addition of GA3-AM. Data are means of 8 replicate experiments; colored zones represent area within one standard error of the mean. g) Black squares and green line and green area are re-plotted from panel f; gray circles represent means of normalized phagosomal GFP-2xP4M in cells co-expressing iRFP-GID1-RAB7, mTq2-GAI92-ORP1L-FFAT* and GFP-2xP4M at the indicated times after addition of GA3-AM; data are means of 15 replicate experiments; red colored zone represented area within standard error of the mean.
Functional implications of PtdIns4P in phagolysosomes.
As documented in Figs. 2c and S2, PtdIns4P-positive tubular structures emanate from phagosomes during the late, resolution stages, suggesting a functional role in phagolysosome resorption. This is supported by the observation that premature clearance of the phosphoinositide caused by overexpression of ORP1L inhibited tubulation and impaired resolution (Fig. 4e). We therefore wondered whether PtdIns4P might recruit or activate molecular motors required for vacuolar tubulation and scission. Whereas ORP1L forms a complex with RAB7 and RILP that recruits dynein-dynactin motors, promoting transport towards the minus-end of microtubules52, the majority of the tubules we observed using lattice light-sheet microscopy extend centrifugally. Hence, we hypothesized that PtdIns4P-rich structures recruit or activate plus-end-directed motors like kinesins. Kinesins link to lysosomes via the small GTPase ARL8B and its effector SKIP (PLEKHM2)53. In this context, ARL8B has been implicated in the clearance of cell corpses by C. elegans54. We therefore considered the possibility that PtdIns4P plays a role in the recruitment of these proteins. Co-expression experiments showed PtdIns4P to co-localize with SKIP and ARL8B on the phagosomal membrane (Figs. 7a–b, S7a–b) and in the tubular structures emanating therefrom. In sharp contrast, mutual exclusion was observed when comparing ORP1L with either ARL8B or SKIP (Fig. 7d–e). The pattern of exclusion was highly reminiscent of that observed for ORP1L vs. PtdIns4P (Fig. 4a).
Figure 7. PtdIns4P directly binds SKIP and recruits it to the phagolysosome.
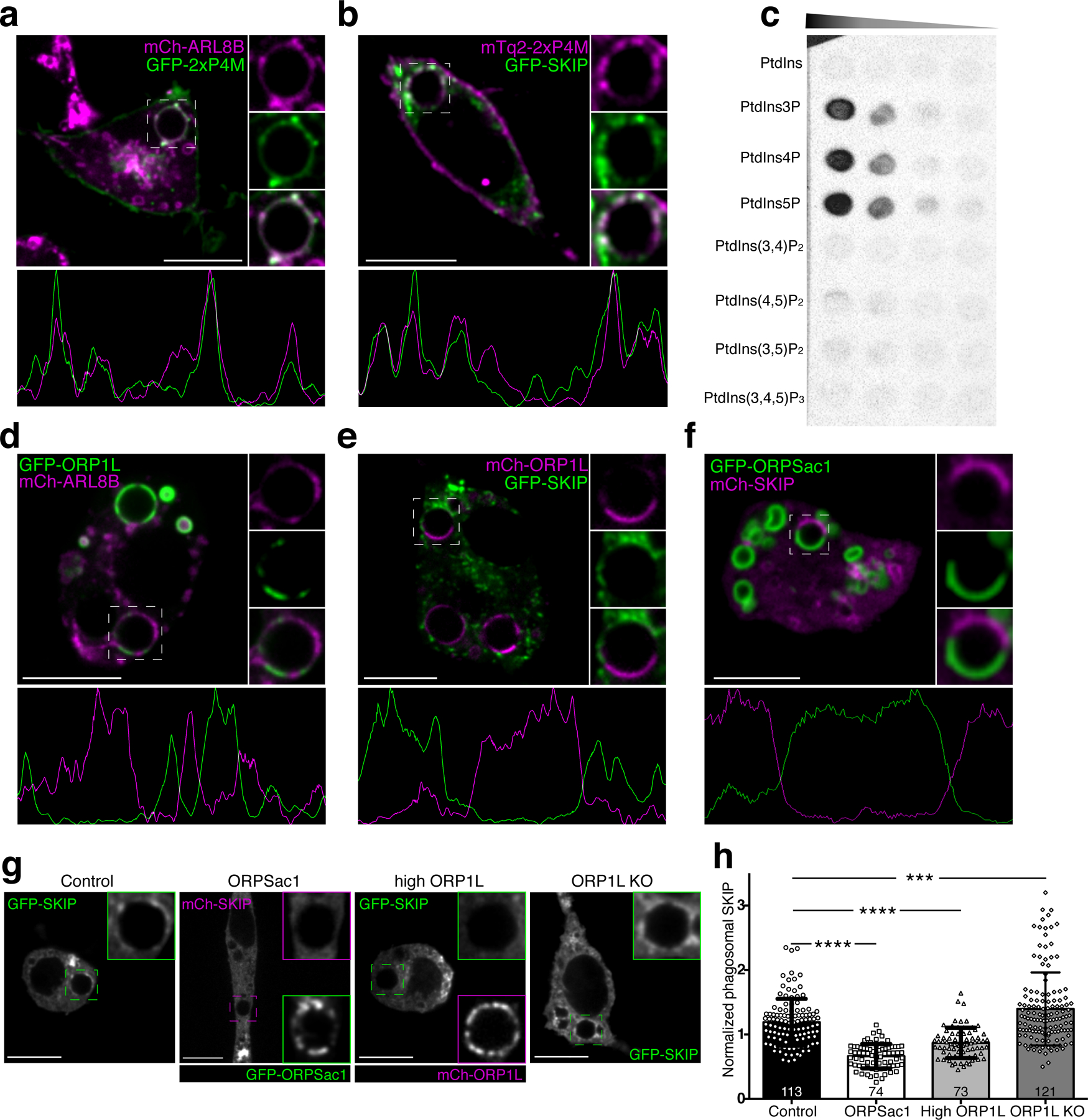
a) Representative confocal micrograph of RAW macrophages co-expressing GFP-2xP4M and mCh-ARL8b after 40 min of phagocytosis; insets show magnifications of the phagosome within the dashed box. The graph below represents a line-scan of fluorescence intensities along the periphery of the phagosome. b) Representative confocal micrograph of RAW macrophages co-expressing mCh-2xP4M and GFP-SKIP after 40 min of phagocytosis; other details as in a. c) Protein overlay assay; a hydrophobic membrane spotted with the indicated lipids was overlaid with purified GST-SKIP (0.3 µg/mL); bound protein was detected by immunoblotting. d) Representative confocal micrograph of RAW macrophages co-expressing GFP-ORP1L and mCh-ARL8b after 40 min of phagocytosis; other details as in a. e) Representative confocal micrograph of RAW macrophages co-expressing mCh-ORP1L and GFP-SKIP after 40 min of phagocytosis; other details as in a. f) Representative confocal micrograph of RAW macrophages co-expressing GFP-ORPSac1 and mCh-SKIP after 40 min of phagocytosis; other details as in a. g) RAW macrophages expressing FP-SKIP after 45 min of phagocytosis of IgG-SRBC: (from left to right) control conditions; expression of GFP-ORPSac1; overexpression of mCh-ORP1L; ORP1L KO. Scale bars = 10 µm throughout. g) Quantitation of SKIP recruitment to phagosomes under the conditions shown in f. Means ± SEM of normalized fluorescent intensities of GFP-SKIP in the number of phagosomes indicated in each bar, from 3 independent experiments. Significance calculated by two-tailed unpaired t tests.
While ARL8B does not have a lipid-binding domain, SKIP contains a PH domain near its C-terminus55; PH domains are noted for their ability to bind phosphoinositides56,57. We speculated that PtdIns4P might contribute to recruit SKIP to phagolysosomes. To assess this possibility, recombinant GST-tagged SKIP was overlaid on a membrane spotted with various phosphoinositides. GST-SKIP preferentially bound mono-phosphorylated phosphoinositides, not associating discernibly with PtdIns or with di- or tri-phosphorylated inositides (Fig. 7c). At higher protein concentrations SKIP also bound PtdIns(4,5)P2 and PtdIns(3,5)P2, albeit with lower affinity (Fig. S7c). Of note, we were unable to detect PtdIns(4,5)P2, PtdIns(3,4)P2 or PtdIns(3,4,5)P3 in resolving phagosomes.
To further explore the putative interaction between SKIP and PtdIns4P, we analyzed the fate of SKIP when altering the level of the phosphoinositide. Depletion of PtdIns4P was accomplished expressing GFP-ORPSAC1, the phagolysosomal-targeted construct possessing phosphatase activity, or by overexpressing wildtype ORP1L. Conversely, we used the ORP1L-defficient cells to increase the levels of the phosphoinositide. The distribution of SKIP was analyzed by co-transfecting SKIP tagged with a spectrally compatible fluorescent protein. As illustrated in Fig. 7g–h, SKIP failed to accumulate in regions of phagosomes that contained ORPSac1 or excess ORP1L and were hence depleted of PtdIns4P. Conversely, phagosomes of cells lacking ORP1L accumulated significantly more SKIP than those of control cells (Fig. 7g–h). We used a similar strategy to assess the dependence of ARL8B on PtdIns4P. ARL8B levels also decreased in phagosomes with excess ORP1L, although no significant difference was noted in the ORP1L-knockout cells (Fig. S7d–e).
Our data suggested a link between PtdIns4P and the tubules/vesicles that seemingly mediate phagosome resolution. To evaluate this possibility, we analyzed the effects of altering PtdIns4P on phagolysosome remodeling. As before, untreated macrophages showed extensive phagolysosome fragmentation (stage III of resolution) 4.5 h after ingestion (Fig. 8a–b). When PtdIns4P was depleted by ORPSAC1, the majority of phagosomes remained in stage II, retaining considerably larger size that in some instances exceeded the original phagosomal size (Fig. 8a–b); this was reminiscent of our observations made in cells overexpressing wildtype ORP1L (Figs. 4e, S5b), where resolution was similarly impaired. Of note, the contents of these phagosomes were clearly degraded, implying that fusion with lysosomes did occur.
Figure 8. Phagosome resolution is mediated by contacts with the ER and by PtdIns4P.
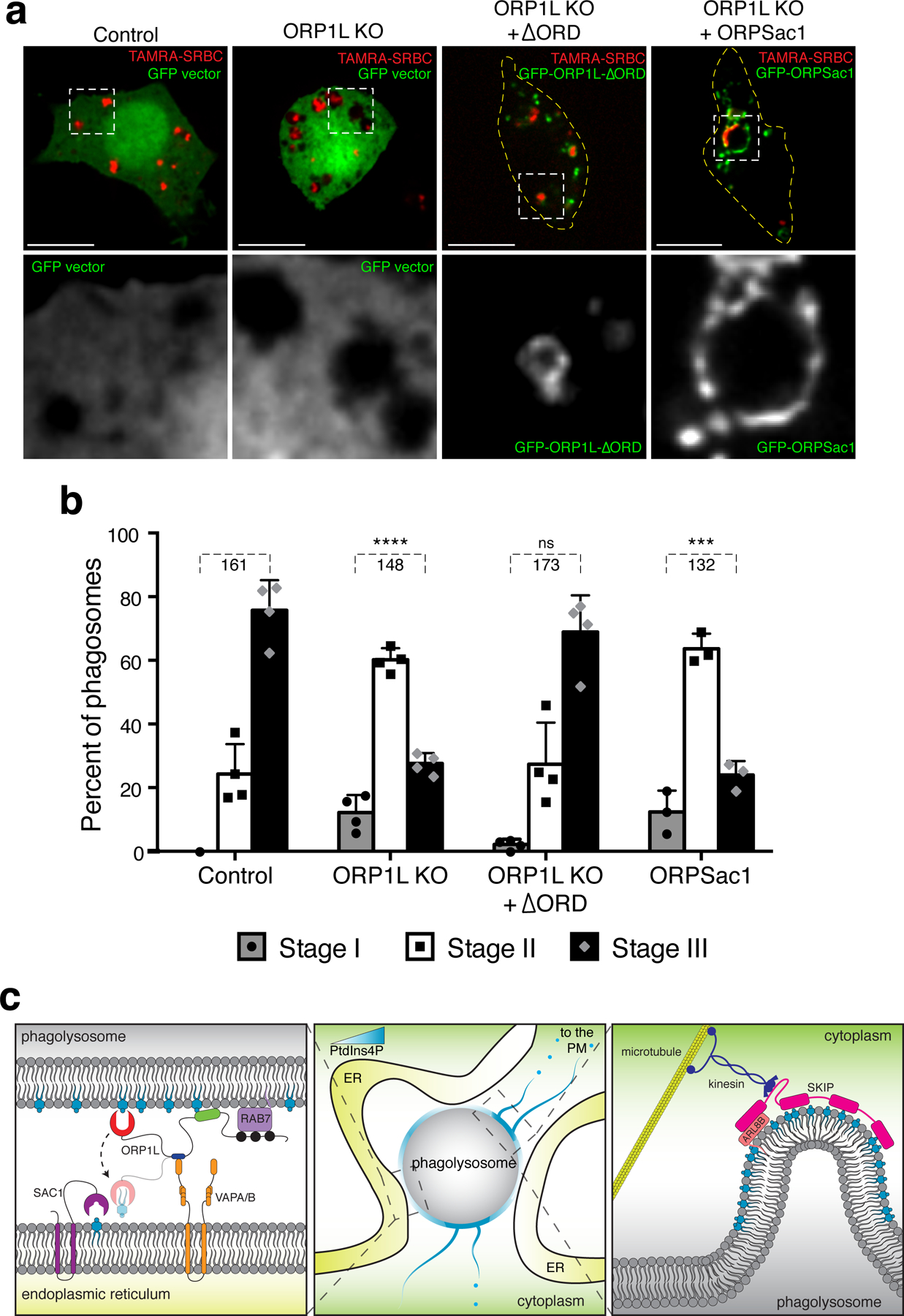
a) RAW macrophages were challenged with TAMRA-SRBC and phagosomes allowed to mature for 4.5 h: control macrophages were transfected with a control GFP vector (first column); ORP1L KO macrophages were transfected with either a GFP vector (second column); GFP-ΔORPSac1 (third column); or GFP-ORPSac1 (rightmost column). Magnifications of the green channel of the boxed area shown below. Scale bars = 10 µm. b) Assessment of phagosomal resolution by stages, defined as in Fig. 1; data are means ± SD of the number of determinations indicated below the brackets; significance assessed by two-tailed unpaired t tests. c) Hypothetical model of the regulation of phagolysosomal resolution by ORP1L-mediated contacts with the ER.
Finally, we tested the role of ORP1L-mediated ER contacts in resolution. Despite containing PtdIns4P, phagolysosomes formed by ORP1L-deficient macrophages failed to fragment normally (Fig. 8a–b). Strikingly, expression in these cells of ORP1L-ΔORD, a truncated form of ORP1L lacking the ORD domain, sufficed to restore phagosome resolution (Fig. 8a–b). This suggests that physical contacts with the ER are essential for proper phagolysosome resorption.
DISCUSSION
Following degradation of their contents, phagosomes must be resorbed to recycle their components and vacate space for additional rounds of phagocytosis. This concluding stage of phagocytosis remains largely unexplored. An mTOR-dependent step has been described, where phagosomal fission redistributes membrane and contents into the lysosomal network26. In addition, antigens are delivered from phagosomes to the membrane for presentation, a process similarly linked to formation of tubules and transport vesicles58,59.
Some information has been gained for the analogous process of autolysosome remodeling. Like phagolysosomes, autophagosomes emit tubules that are involved in the reformation of lysosomes. Clathrin and PtdIns(4,5)P2 are central to this process60,61. In macrophages, however, PtdIns(4,5)P2 is not detectable in resolving phagolysosomes (Fig. S8b), where PtdIns4P is instead the predominant phosphoinositide.
Following its abrupt disappearance shortly after phagosome closure, PtdIns4P reappears concomitantly with the elimination of PtdIns3P, marking the early-to-late phagosome transition (see24, Fig. 2b, Movie 2). Reacquisition of PtdIns4P is mediated by PI4K2A24,25. Here we report an additional, late phase of PtdIns4P depletion that coincides with the onset of phagosome resolution. At least two components contribute to the late PtdIns4P disappearance: ORP1L recruited by RAB7 transfers a fraction of the PtdIns4P to the ER, where it is hydrolyzed by SAC1. The distribution of ORP1L on the phagosomal membrane is not continuous, forming patches where PtdIns4P is preferentially depleted. The remainder of the membrane, which retains PtdIns4P, is the source of the tubules that mediate the resolution process. Detachment of such PtdIns4P-enriched tubules contributes a second component to phagolysosomal PtdIns4P depletion.
The finding that ORP1L transfers PtdIns4P to the ER is somewhat unexpected, inasmuch as ORP1L is thought to be a cholesterol sensor and transporter31–37. However, the reported ability of its ORD domain to bind PtdIns4P with even greater affinity than sterols is consistent with our findings. Whether the transport step is unidirectional is not presently clear. OSBP and other related proteins mediate counter-transport mechanisms and it is conceivable, by analogy, that another lipid is delivered from the ER to the phagolysosome by ORP1L in exchange for PtdIns4P. One obvious candidate would be cholesterol. In fact, a late phase of reacquisition of cholesterol by phagosomes has been reported62. The existence of a counter-transport mechanism remains an attractive possibility that requires additional study.
While several studies have reported essential roles of PtdIns4P at the Golgi and PM, little is known about its function in the endolysosomal network. In the recycling pathway PtdIns4P regulates retromer function and has been linked to actin nucleation via WASH63–65. WASH is necessary for scission of retromer-induced tubules from endosomes. Accordingly, the PtdIns4P-rich regions of phagolysosomal membranes were both actin- and WASH-positive (Fig. S8a). However, our evidence suggests that PtdIns4P plays a role not only in the scission of tubules, but also in their formation. We propose a mechanism whereby by binding SKIP, PtdIns4P stabilizes the ARL8b-SKIP complex on the phagolysosomal surface, enabling tubule extension towards the cell periphery.
Interestingly, resolution was impaired in ORP1L-knockout macrophages despite the higher PtdIns4P content of their phagolysosomes suggesting that, while necessary, the presence of PtdIns4P is not sufficient for resolution. An important clue was provided by the experiments using ORP1L-ΔORD. When expressed in cells lacking wildtype ORP1L, ORP1L-ΔORD rescued resolution. While incapable of binding and translocating PtdIns4P, ORP1L-ΔORD retains the ability to tether the phagosome to the ER via its FFAT motif, suggesting that this interaction is key to resolution. Anchorage to the ER may be required for kinesin to bring about tubulation, rather than cause migration of the entire phagosome to the cell periphery. Formation of the ER contact sites could also generate membrane sub-domains that exclude PtdIns4P.
In addition to affixing the phagosome to the ER, the PtdIns4P-translocating ability of ORP1L generates a discontinuous pattern of the phosphoinositide (Figs. 2a,4a,S5a). The resulting exquisite spatial segregation is probably an important contributor to effective resolution. In this regard, earlier studies showed the existence of microdomains where dynein and RAB7 cluster in cholesterol-rich patches in phagolysosomes62. These domains —which may have been generated by exchange of ER cholesterol for phagosomal PtdIns4P— are presumably also enriched in the RAB7 effectors ORP1L and RILP. Our observation that ORP1L depletes PtdIns4P from these microdomains while adjacent regions retain the phosphoinositide suggests that opposing forces are required for tubule extrusion. Specifically, while PtdIns4P-enriched domains experience centrifugal force exerted by the ARL8B/SKIP/kinesin complex, the RAB7/RILP/dynein62-containing patches likely maintain the main body of the phagolysosome in its juxtanuclear location, where it is further stabilized by tethering to the ER via VAPA/B.
MATERIALS AND METHODS
Cell culture
The murine macrophage cell line RAW 264.7 was obtained from the American Type Culture Collection (Manassas, VA) and grown as previously described24.
Primary human monocytes were isolated from peripheral blood and differentiated into macrophages as previously described66. Human monocytes were purified for immunoblotting using Pan Monocyte Isolation Kit, human (Miltenyi Biotec, Bergisch, Germany) according to the manufacturer’s protocol. Generation of COS-1-FcγR2A was previously described51
Plasmid table
| Insert | Tag | Remark |
|---|---|---|
| P4M x 2 | GFP | Gift from G. Hammond (University of Pittsburgh) |
| P4M x 2 | mCherry | Levin, R. et al. 2015 24 |
| P4M x 2 | mTq2 | This study |
| ORP1L | GFP | Gift from J. Neefjes (Leiden University) |
| ORP1L | mCherry | Gift from N. Ridgway (Dalhousie University) |
| ORP1L-D478A | GFP | Gift from J. Neefjes (Leiden University) |
| ORP1L-D478A | mCherry | This study |
| ORP1L-ΔORD | GFP | Gift from J. Neefjes (Leiden University) |
| ORP1L-HH651/652AA | GFP | This study |
| ORP1L-HH651/652AA | mCherry | This study |
| ORPSac1 | GFP | This study (Subcloned from GFP-ORP1L; and mCh-FKBP-Sac1S.c, a gift from G. Hammond |
| SAC2 | GFP | Gift from Pietro De Camilli (Yale University) |
| RAB5 | CFP | Gift from John Brumell (Hospital for Sick Children, Toronto) |
| RAB7 | CFP | Gift from John Brumell (Hospital for Sick Children, Toronto) |
| RAB7-T22N | mCherry | This study [subcloned from GFP-Rab7-T22N 68 a collaboration with C. Bucci, (University of Salento)] |
| VAPA | mCherry | This study (subcloned from GFP-VapA69) |
| VAPB | mCherry | This study (subcloned from GFP-VapA69) |
| LAMP1 | PA-GFP | This study (subcloned from pPAGFP-N1 70, a gift from J. Lippincott-Schwartz (Howard Hugues Medical Institute, Janelia Research Campus) |
| GID1-Lyn11 | YFP | This study (subcloned from YFP-GID1, addgene #37305) |
| GID1-RAB7 | iRFP | This study (subcloned from YFP-GID1, addgene #37305) and iRFP-FRB-Rab7 (gift from G. Hammond) |
| GAI92-ORP1L747 | mTq2 | This study (subcloned from CFP-GAI92, addgene #37307) |
| SKIP | GFP | This study |
| SKIP | mCherry | This study |
| PH-PLC∂ | GFP | Gift from T. Meyer (Stanford University) |
| SEC61 | mCherry | Gift from P. Kim (Hospital for Sich Children, Toronto) |
| P4C | GFP | Gift from Dr. Y. Mao (Cornell University) |
Antibodies and reagents
Rabbit monoclonal anti-ORP1L for immunofluorescence (1:100) was a kind gift from V. Olkkonen (University of Helsinki, Helsinki, Finland); rabbit polyclonal anti-ORP1L for western blots (1:2000; Abcam, ab36983, Lot: GRT56186–1); goat polyclonal anti-VAPA (K-15) (1:50; Santa Cruz Biotechnology, sc-48698, Lot: K2515); rabbit monoclonal anti-VAPB (1:200; Sigma, HPA013144, Lot: A47637); mouse monoclonal anti-GAPDH (1:5000; Millipore, MAB374, Lot: 3090497); anti-GST (1:3000; Proteintech, 660001–2-Ig); rabbit IgG fraction to SRBC (MP Biosciences, 0855806); mouse IgG to HRBC (Mybiosource, MBS8502309); washed and preserved SRBC (MP Biosciences, 0855876); PIP strips (Echelon, Salt Lake City, UT); Sheep erythrocytes (10% suspension) were purchased from MP Biomedicals. Fluorescent antibodies against mouse and rabbit were from Jackson ImmunoResearch Labs. Paraformaldehyde (16% w/v) was from Electron Microscopy Sciences. GA3-AM was a kind gift by Tasuku Ueno (University of Tokyo, Tokyo, Japan).
Transfections
RAW 264.7 macrophages were plated in 18 mm round coverslips ~36–40 h before experiments. Then, cells were transfected ~16–18 h before experiments using Fugene HD (Promega, Madison, WI) according to the manufacturer’s protocol. In brief, 1 µg of plasmid DNA was mixed with 3 µL of Fugene HD in 100 µL of serum-free RPMI. The mix was incubated for 15 min at RT. The mix was then distributed equally into two wells of a 12-well plate containing the cells on coverslips.
Phagocytosis assays
The assays have been meticulously detailed for fixed and live cells previously24,63. Cells were imaged live or fixed at the time-points indicated in either the text or the figure legends.
Gibberellin-induced dimerization system
In brief, the plant hormone gibberellin binds its receptor gibberellin insensitive dwarf1 (GID1) causing a conformational change. This alternative GID1 conformation recruits the gibberellin insensitive protein GAI47,72. To generate recruitable ORP1L variants (WT and FFAT*) we deleted the ankyrin repeats rendering a protein soluble in the cytoplasm (ORP1L747). We then linked the variants to the smallest recruitable fragment of GAI (GAI92)47 and tagged the chimeras with mTurquoise2 (Fig). A second construct consisted of YFP-tagged GID1 linked to a “membrane anchor”, for these experiments we used either the 11 amino acid tail of Lyn kinase that localizes to the plasma membrane (PM) (Fig. S4) or RAB7 (Fig. 7). We co-expressed these chimeras along with mCherry-tagged Vap proteins in HeLa cells (Fig. S4) or with GFP-2xP4M (Fig. 7). After addition of a cell permeant gibberellin analog (GA3-AM), the soluble mTq2-GAI92 chimeras are recruited to the target membrane within minutes.
Generation of CRISPR KO cell lines
ORP1L KO CRISPR cell lines were generated as follows: RAW 264.7 macrophages stably expressing EF1Alpha-Cas9-BFP were lentivirally transduced with constructs expressing a puromycin resistance cassette and an Osbpl1a targeting sgRNA. 72 hours following infection, selection was performed with puromycin (10 µg/mL) for five days. Following selection, single cells were sorted into 96-well plates and expanded for two to three weeks to generate single cell clonal knockout lines.
Gene editing measurements by Sanger sequencing
Total genomic DNA was isolated from cells using QuickExtract DNA Extraction Solution (VWR, Radnor, PA, cat# QE09050). For the PCR reaction, primers were designed approximately 250–300 basepairs upstream and 450–550 basepairs downstream of the predicted cut site. Following PCR reaction completion using a C1000 Touch Thermo Cycler (Bio-Rad), PCR products were run on an agarose gel and were gel-purified over an Econospin DNA column (Epoch, Missouri City, TX, cat# 1910–250) using a QIAquick Gel Extraction Kit (Qiagen, Hilden, Germany, cat# 28706). Sanger sequencing ab1 data were obtained from Quintara Biosciences (San Francisco facility) and the editing efficiency and indels of knockout cell lines were assessed using the online TIDE analysis tool (https://tide.deskgen.com/).
Gene silencing
The siRNA oligos (Stealth RNAi™) against mouse Osbpl1a were from ThermoFisher Scientific (Waltham, MA) with the following targeting sequences: GGCCAUGGACUUGAAGGAGUCGUUA and GCAUCCUUAGUGAGGAGGAGUUCUA
siRNA was introduced into RAW macrophages as previously described 24 with the Neon transduction system (ThermoFisher Scientific, Burlington, Canada).
Quantitative real-time PCR
Total RNA was extracted and isolated using the GeneJET RNA Purification kit (ThermoFisher Scientific). For cDNA generation, equivalent amounts of total RNA were loaded to use the SuperScript VILO cDNA kit (ThermoFisher Scientific). Then, qPCR reactions were performed in 96-well plates using TaqMan reagents (ThermoFisher Scientific). Assays for reference gene and target gene were duplexed in triplicate for every experimental replicate. The TaqMan assays were as follows: Abt1 (reference gene) Mm00803824_m1; and Osbpl1a Mm00498552_m1.
Confocal microscopy
Confocal fluorescence microscopy was performed using spinning-disk confocal microscopes (Quorum Technologies). Our systems are based on an Axiovert 200M microscope (Carl Zeiss) equipped with a ×63 oil-immersion objective (NA 1.4) and a × 1.5 magnifying lens. The microscopes carry a motorized XY stage (Applied Scientific Instrumentation), a Piezo Z-focus drive and diode-pumped solid-state lasers emitting at 440, 491, 561, 638 and 655 nm (Spectral Applied Research). Images were recorded with back-thinned, cooled charge-coupled device cameras (Hamamatsu Photonics) under command of the Volocity software (version 6.2.1; PerkinElmer). Selection of regions of interest, fluorescence intensity measurements and brightness/contrast corrections were performed with ImageJ (version 1.48; National Institutes of Health) and FIJI. Brightness and contrast parameters were adjusted across entire images and without altering the linearity of mapped pixel values.
Lattice light-sheet microscopy (LLSM)
The lattice light sheet microscope (LLSM) used in these experiments is housed in the Advanced Imaged Center (AIC) at the Howard Hughes Medical Institute Janelia research campus with the support of John Heddleston, Satya Khuon and Teng Leong-Chew. The system is configured and operated as previously described71. Briefly, RAW macrophages were grown on 5 mm round glass coverslips (Warner Instruments, Catalog # CS-5R) ~36 h before experiments. After adhesion, cells were transfected with Fugene HD 12–18 h before imaging as described above. For the assay, IgG-RBC were added to the wells containing cells on coverslips. The plates were spun down (300Xg for 10 s) in order to synchronize phagocytosis. Immediately after, coverslips were placed on a custom-made stainless-steel holder. Imaging was conducted in Hanks’ Balanced Salt Solution at 37°C. Samples were illuminated by a 2D optical lattice generated by a spatial light modulator (SLM, Fourth Dimension Displays). The sample was excited by 488 nm or 560 nm diode lasers (MPB Communications) at 25% AOTF transmittance and 50 mW initial box power through an excitation objective (Special Optics, 0.65 NA, 3.74-mm WD). Fluorescent emission was collected by detection objective (Nikon, CFI Apo LWD 25XW, 1.1 NA), and detected by a sCMOS camera (Hamamatsu Orca Flash 4.0 v2). Acquired data were deskewed as previously described71 and deconvolved using an iterative Richardson-Lucy algorithm. Point-spread functions for deconvolution were experimentally measured using 200nm tetraspeck beads adhered to 5 mm glass coverslips (Invitrogen, Catalog # T7280) for each excitation wavelength.
Transmission electron microscopy
RAW macrophages were cultured on 18-mm coverslips and challenged with IgG-SRBC as described before. Cells were incubated following phagosome maturation until pre-determined time points, fixed with 2% (vol/vol) glutaraldehyde and processed for TEM using standard methods.
Image processing
For confocal microscopy, images were acquired using Volocity (Perkin Elmin, Woodbridge, ON) and exported to ImageJ or FIJI (National Institutes of Health, Bethesda, MD) for analysis, quantification and contrast enhancement. For LLSM 3D data visualization and analysis images were exported to Imaris (Version 9.2.1, Bitplane, Concord, MA).
Lipid overlay assay
Recombinant GST tagged proteins were expressed in BL21(DE3) RP cells. 1-liter culture was grown in 37°C, induced with 1 mM IPTG (isopropyl β-D-1-thiogalactopyranoside) and further grown O.N. at 16°C. Cells were collected by centrifugation at 6000G, 15 min at 4°C. The cell pellet was re-suspended in buffer containing 50 mM TRIS PH 8, 300 mM NaCl, 5% glycerol, 5 mM DTT (Dithiothreitol) supplemented with Roche Complete EDTA-free protease inhibitors, lysozyme and DNAse. Sample was sonicated, and the soluble fraction was obtained by centrifugation (30,000 RCF, 4°C. 45 min). The soluble fraction was loaded on GSH resin (GE) pre-equilibrated with buffer. Following 2 h incubation, the resin was extensively washed, and the protein was eluted with buffer containing 50mM TRIS PH 8, 10 mM reduced glutathione. Protein concentration was evaluated by absorbance measurement at 280 nm. Sample purity was evaluated by SDS-PAGE. Lipid overlay assay was done on PIP arrays (Echelon) following manufactures protocol. Binding was detected with anti-GST Ab, anti-mouse-HRP 1:3000.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the following grants and scholarships: R.L. was funded by the Connaught International Scholarship for Doctoral Students from the University of Toronto and the National Council for Science and Technology / Consejo Nacional de Ciencia y Tecnología (CONACYT) of Mexico. FDN-143202 from the Canadian Institutes of Health Research (CIHR) to Sergio Grinstein. We thank John Heddleston, Satya Khuon and Teng Leong-Chew from the AIC for training and support for LLSM. The AIC is a jointly-funded venture of the Gordon and Betty Moore Foundation and the Howard Hughes Medical Institute.
DATA AVAILABILITY STATEMENT
The data sets generated during and/or analyzed during this study are available from the corresponding author on reasonable request
REFERENCES
- 1.Stuart LM & Ezekowitz RAB Phagocytosis. Immunity 22, 539–550 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Flannagan RS, Jaumouillé V & Grinstein S The cell biology of phagocytosis. Annu. Rev. Pathol 7, 61–98 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Levin R, Grinstein S & Canton J The life cycle of phagosomes: formation, maturation, and resolution. Immunol. Rev 273, 156–179 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Elliott MR & Ravichandran KS Clearance of apoptotic cells: implications in health and disease. J. Cell Biol 189, 1059–1070 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bianconi E et al. An estimation of the number of cells in the human body. Ann. Hum. Biol 40, 463–471 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Elliott MR & Ravichandran KS The Dynamics of Apoptotic Cell Clearance. Dev. Cell 38, 147–160 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swanson JA Shaping cups into phagosomes and macropinosomes. Nat. Rev. Mol. Cell Biol 9, 639–649 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Underhill DM Phagosome maturation: steady as she goes. Immunity 23, 343–344 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Kinchen JM & Ravichandran KS Phagosome maturation: going through the acid test. Nat. Rev. Mol. Cell Biol 9, 781–795 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fairn GD & Grinstein S How nascent phagosomes mature to become phagolysosomes. Trends Immunol 33, 397–405 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Gray M & Botelho RJ Phagocytosis: Hungry, Hungry Cells. Methods Mol. Biol. Clifton NJ 1519, 1–16 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Blander JM & Medzhitov R On regulation of phagosome maturation and antigen presentation. Nat. Immunol 7, 1029–1035 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Mantegazza AR, Magalhaes JG, Amigorena S & Marks MS Presentation of phagocytosed antigens by MHC class I and II. Traffic Cph. Den 14, 135–152 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gillooly DJ, Simonsen A & Stenmark H Phosphoinositides and phagocytosis. J. Cell Biol 155, 15–18 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deretic V et al. Phosphoinositides in phagolysosome and autophagosome biogenesis. Biochem. Soc. Symp 141–148 (2007). doi: 10.1042/BSS0740141 [DOI] [PubMed] [Google Scholar]
- 16.Levin R, Grinstein S & Schlam D Phosphoinositides in phagocytosis and macropinocytosis. Biochim. Biophys. Acta 1851, 805–823 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Araki N, Johnson MT & Swanson JA A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J. Cell Biol 135, 1249–1260 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Botelho RJ et al. Localized biphasic changes in phosphatidylinositol-4,5-bisphosphate at sites of phagocytosis. J. Cell Biol 151, 1353–1368 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marshall JG et al. Restricted accumulation of phosphatidylinositol 3-kinase products in a plasmalemmal subdomain during Fc gamma receptor-mediated phagocytosis. J. Cell Biol 153, 1369–1380 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cox D, Tseng CC, Bjekic G & Greenberg S A requirement for phosphatidylinositol 3-kinase in pseudopod extension. J. Biol. Chem 274, 1240–1247 (1999). [DOI] [PubMed] [Google Scholar]
- 21.Rohatgi R, Ho HY & Kirschner MW Mechanism of N-WASP activation by CDC42 and phosphatidylinositol 4, 5-bisphosphate. J. Cell Biol 150, 1299–1310 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fratti RA, Backer JM, Gruenberg J, Corvera S & Deretic V Role of phosphatidylinositol 3-kinase and Rab5 effectors in phagosomal biogenesis and mycobacterial phagosome maturation arrest. J. Cell Biol 154, 631–644 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vieira OV et al. Distinct roles of class I and class III phosphatidylinositol 3-kinases in phagosome formation and maturation. J. Cell Biol 155, 19–25 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levin R et al. Multiphasic dynamics of phosphatidylinositol 4-phosphate during phagocytosis. Mol. Biol. Cell 28, 128–140 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeschke A et al. Phosphatidylinositol 4-phosphate and phosphatidylinositol 3-phosphate regulate phagolysosome biogenesis. Proc. Natl. Acad. Sci. U. S. A 112, 4636–4641 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krajcovic M, Krishna S, Akkari L, Joyce JA & Overholtzer M mTOR regulates phagosome and entotic vacuole fission. Mol. Biol. Cell 24, 3736–3745 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hammond GRV, Machner MP & Balla T A novel probe for phosphatidylinositol 4-phosphate reveals multiple pools beyond the Golgi. J. Cell Biol 205, 113–126 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johansson M, Lehto M, Tanhuanpää K, Cover TL & Olkkonen VM The oxysterol-binding protein homologue ORP1L interacts with Rab7 and alters functional properties of late endocytic compartments. Mol. Biol. Cell 16, 5480–5492 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loewen CJR, Roy A & Levine TP A conserved ER targeting motif in three families of lipid binding proteins and in Opi1p binds VAP. EMBO J 22, 2025–2035 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loewen CJR & Levine TP A highly conserved binding site in vesicle-associated membrane protein-associated protein (VAP) for the FFAT motif of lipid-binding proteins. J. Biol. Chem 280, 14097–14104 (2005). [DOI] [PubMed] [Google Scholar]
- 31.Rocha N et al. Cholesterol sensor ORP1L contacts the ER protein VAP to control Rab7-RILP-p150 Glued and late endosome positioning. J. Cell Biol 185, 1209–1225 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suchanek M et al. The mammalian oxysterol-binding protein-related proteins (ORPs) bind 25-hydroxycholesterol in an evolutionarily conserved pocket. Biochem. J 405, 473–480 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vihervaara T et al. Sterol binding by OSBP-related protein 1L regulates late endosome motility and function. Cell. Mol. Life Sci. CMLS 68, 537–551 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Kant R et al. Late endosomal transport and tethering are coupled processes controlled by RILP and the cholesterol sensor ORP1L. J. Cell Sci 126, 3462–3474 (2013). [DOI] [PubMed] [Google Scholar]
- 35.Wijdeven RH et al. Cholesterol and ORP1L-mediated ER contact sites control autophagosome transport and fusion with the endocytic pathway. Nat. Commun 7, 11808 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao K & Ridgway ND Oxysterol-Binding Protein-Related Protein 1L Regulates Cholesterol Egress from the Endo-Lysosomal System. Cell Rep 19, 1807–1818 (2017). [DOI] [PubMed] [Google Scholar]
- 37.Dong J et al. Allosteric enhancement of ORP1-mediated cholesterol transport by PI(4,5)P2/PI(3,4)P2. Nat. Commun 10(1), 829 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balla T Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiol. Rev 93, 1019–1137 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rohde HM et al. The human phosphatidylinositol phosphatase SAC1 interacts with the coatomer I complex. J. Biol. Chem 278, 52689–52699 (2003). [DOI] [PubMed] [Google Scholar]
- 40.Zewe JP, Wills RC, Sangappa S, Goulden BD & Hammond GR SAC1 degrades its lipid substrate PtdIns4P in the endoplasmic reticulum to maintain a steep chemical gradient with donor membranes. eLife 7, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mesmin B et al. A Four-Step Cycle Driven by PI(4)P Hydrolysis Directs Sterol/PI(4)P Exchange by the ER-Golgi Tether OSBP. Cell 155, 830–843 (2013). [DOI] [PubMed] [Google Scholar]
- 42.von Filseck JM, Vanni S, Mesmin B, Antonny B & Drin G A phosphatidylinositol-4-phosphate powered exchange mechanism to create a lipid gradient between membranes. Nat. Commun 6, (2015). [DOI] [PubMed] [Google Scholar]
- 43.Moser von Filseck J et al. Phosphatidylserine transport by ORP/Osh proteins is driven by phosphatidylinositol 4-phosphate. Science 349, 432–436 (2015). [DOI] [PubMed] [Google Scholar]
- 44.Chung J et al. PI4P/phosphatidylserine countertransport at ORP5- and ORP8-mediated ER-plasma membrane contacts. Science 349, 428–432 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sohn M et al. PI(4,5)P2 controls plasma membrane PI4P and PS levels via ORP5/8 recruitment to ER-PM contact sites. J. Cell Biol 217, 1797–1813 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johansson M et al. The two variants of oxysterol binding protein-related protein-1 display different tissue expression patterns, have different intracellular localization, and are functionally distinct. Mol. Biol. Cell 14, 903–915 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miyamoto T et al. Rapid and orthogonal logic gating with a gibberellin-induced dimerization system. Nat. Chem. Biol 8(5), 465–70 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Saint-Jean M et al. Osh4p exchanges sterols for phosphatidylinositol 4-phosphate between lipid bilayers. J. Cell Biol 195, 965–978 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maeda K et al. Interactome map uncovers phosphatidylserine transport by oxysterol-binding proteins. Nature 501, 257–261 (2013). [DOI] [PubMed] [Google Scholar]
- 50.Tong J, Yang H, Yang H, Eom SH & Im YJ Structure of Osh3 reveals a conserved mode of phosphoinositide binding in oxysterol-binding proteins. Struct. Lond. Engl 1993 21, 1203–1213 (2013). [DOI] [PubMed] [Google Scholar]
- 51.Downey GP. et al. Phagosomal maturation, acidification and inhibition of bacterial growth in nonphagocytic cells transfected with FcgammaRIIA receptors. J. Biol. Chem 274(40), 28436–44 (1999). [DOI] [PubMed] [Google Scholar]
- 52.Johansson M et al. Activation of endosomal dynein motors by stepwise assembly of Rab7-RILP-p150Glued, ORP1L, and the receptor betalll spectrin. J. Cell Biol 176, 459–471 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosa-Ferreira C & Munro S Arl8 and SKIP act together to link lysosomes to kinesin-1. Dev. Cell 21, 1171–1178 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fazeli G, Stetter M, Lisack JN & Wehman AMC elegans Blastomeres Clear the Corpse of the Second Polar Body by LC3-Associated Phagocytosis. Cell Rep 23, 2070–2082 (2018). [DOI] [PubMed] [Google Scholar]
- 55.Boucrot E, Henry T, Borg J-P, Gorvel J-P & Méresse S The intracellular fate of Salmonella depends on the recruitment of kinesin. Science 308, 1174–1178 (2005). [DOI] [PubMed] [Google Scholar]
- 56.Lemmon MA Phosphoinositide recognition domains. Traffic Cph. Den 4, 201–213 (2003). [DOI] [PubMed] [Google Scholar]
- 57.Hammond GRV & Balla T Polyphosphoinositide binding domains: Key to inositol lipid biology. Biochim. Biophys. Acta 1851, 746–758 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mantegazza AR et al. TLR-dependent phagosome tubulation in dendritic cells promotes phagosome cross-talk to optimize MHC-II antigen presentation. Proc. Natl. Acad. Sci. U. S. A 111, 15508–15513 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saric A et al. mTOR controls lysosome tubulation and antigen presentation in macrophages and dendritic cells. Mol. Biol. Cell 27, 321–333 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rong Y et al. Clathrin and phosphatidylinositol-4,5-bisphosphate regulate autophagic lysosome reformation. Nat. Cell Biol 14, 924–934 (2012). [DOI] [PubMed] [Google Scholar]
- 61.Du W et al. Kinesin 1 Drives Autolysosome Tubulation. Dev. Cell 37, 326–336 (2016). [DOI] [PubMed] [Google Scholar]
- 62.Rai A et al. Dynein Clusters into Lipid Microdomains on Phagosomes to Drive Rapid Transport toward Lysosomes. Cell 164, 722–734 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Niu Y et al. PtdIns(4)P regulates retromer–motor interaction to facilitate dynein–cargo dissociation at the trans-Golgi network. Nat. Cell Biol 15, 417–429 (2013). [DOI] [PubMed] [Google Scholar]
- 64.Marquer C et al. Arf6 controls retromer traffic and intracellular cholesterol distribution via a phosphoinositide-based mechanism. Nat. Commun 7, 11919 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dong R et al. Endosome-ER Contacts Control Actin Nucleation and Retromer Function through VAP-Dependent Regulation of PI4P. Cell 166, 408–423 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Montaño F, Grinstein S & Levin R Quantitative Phagocytosis Assays in Primary and Cultured Macrophages. Methods Mol. Biol. Clifton NJ 1784, 151–163 (2018). [DOI] [PubMed] [Google Scholar]
- 67.Johnson DE, Ostrowski P, Jaumouillé V & Grinstein S The position of lysosomes within the cell determines their luminal pH. J. Cell Biol 212, 677–692 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harrison RE, Bucci C, Vieira OV, Schroer TA & Grinstein S Phagosomes fuse with late endosomes and/or lysosomes by extension of membrane protrusions along microtubules: role of Rab7 and RILP. Mol. Cell. Biol 23, 6494–6506 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weir ML, Xie H, Klip A & Trimble WS VAP-A binds promiscuously to both v- and tSNAREs. Biochem. Biophys. Res. Commun 286, 616–621 (2001). [DOI] [PubMed] [Google Scholar]
- 70.Patterson GH & Lippincott-Schwartz J A photoactivatable GFP for selective photolabeling of proteins and cells. Science 297, 1873–1877 (2002). [DOI] [PubMed] [Google Scholar]
- 71.Chen B-C et al. Lattice light-sheet microscopy: imaging molecules to embryos at high spatiotemporal resolution. Science 346, 1257998 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hirano K et al. GID1-mediated gibberellin signaling in plants. Trends Plant Sci 13, 192–199 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets generated during and/or analyzed during this study are available from the corresponding author on reasonable request


