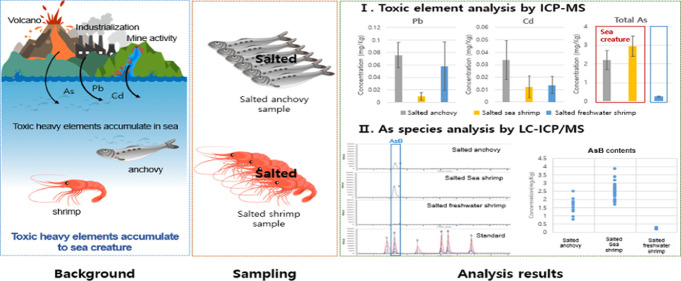
Abstract
Toxic elements (Cd, Pb, and As) accumulate into the environment by industrialization and natural phenomena and then pass to organisms. Analysis of toxic elements in food must be accurately carried out on a regular basis so as to avoid any adverse impact. Salted foods are difficult samples and accurate analysis of As is not easy due to salt interference. In this study, analysis of As was carried without influence of salts in three types of salted foods via an analytical method, which was validated using spiking recovery experiments and by analyzing certified reference materials. As a result, toxic elements were detected in all samples but none of these exceeded the World Health Organization recommended limits. Among the As species, arsenobetaine (AsB) was the most abundant, while inorganic As was below the detection limit in all samples. All the analyzed salted food samples appeared to be safe for consumption. In addition, the analysis of sea shrimp, freshwater shrimp, and seawater verified As bioaccumulation in these organisms from the environment.
1. Introduction
Environmental concentrations of toxic elements such as lead (Pb), cadmium (Cd), and arsenic (As) have augmented recently due to increased agricultural, industrial, and mining activities. These toxic elements accumulate in the environment without decomposition and eventually concentrating in the sea. The toxic elements pass into the living organisms and thus to the food chain, causing not only environmental problems but also posing serious health risks to humans.1,2 Around the world, various recommendation levels for these toxic elements in foods have been established and are considered as standards.3
Pb is widespread in the earth’s crust and humans, and exposure to Pb can damage the nervous system.4 Cd is also of particular concern because of its known skeletal toxicity, nephrotoxicity, and carcinogenicity.5 As has been classified as carcinogenic to humans by the International Agency for Research on Cancer; however, its toxicological effects depend on its chemical form and oxidation state.6
Arsenobetaine (AsB) is the major As species in seafood and is considered nontoxic due to its extremely low toxicity.7 However, its other organic species also exist, such as methylarsonic acid (MMA) and dimethylarsinic acid (DMA). Inorganic As species such as arsenite (As3+) and arsenate (As5+) are considered to have extreme toxicological effects on humans.8 A chronic exposure to inorganic As has been associated with cancer, skin lesions, cardiovascular disease, and neurotoxicity.9 Hence, it is very important to measure each As species content while investigating As toxicity from foods.10
Salted foods are traditional Korean fermented foods made with shrimp and small fishes. They have a distinctive flavor and a long shelf life. However, it is difficult to analyze their As content due to the complex matrix of high salt concentration. When conducting As analysis by inductively coupled plasma mass spectrometry (ICP–MS), the interferences must be removed before analysis as the salt (NaCl) has the same mass-to-charge ratio (m/z value) as As.11
For trace element analysis, it is always important to select a more sensitive analytical method and validate its application to subject samples.12 ICP–MS has been reported in the literature as the most effective quantitative analytical method to measure trace elements in food samples due to its high sensitivity and selectivity. This technique efficiently measures concentrations up to microgram/liter (μg/L) in foods through efficient ionization on plasma.13,14 A high-performance liquid chromatography (HPLC) system using a column for the separation of specific substances has also been reported to display excellent efficiency.15 The coupling of HPLC and ICP–MS thus ensures a suitable separation of As species, as well as good sensitivity and selectivity for quantification even at trace levels in food samples.16,17 It has already been reported in the literature that sea water is contaminated with arsenic; in relatively higher concentrations than surface water.18 Therefore, the main hypothesis of this study was to measure the arsenic concentration in sea salts. In addition, the degree of arsenic contamination of living organisms in the sea and living organisms in surface water was compared by analyzing freshwater prawns and sea prawns that are living in large numbers in the sea.
This study aimed to (i) validate and apply ICP–MS for the analysis of As, Pb, and Cd, and HPLC–ICP–MS for As species determination in salted food and sea salt samples. Also, (ii) to evaluate arsenic accumulation in sea and sea creatures. Additionally, (iii) the concentrations of toxic elements in the salted food samples were compared to the recommended standards of the World Health Organization (WHO) to assess any possible toxicity to consumers.
2. Results and Discussion
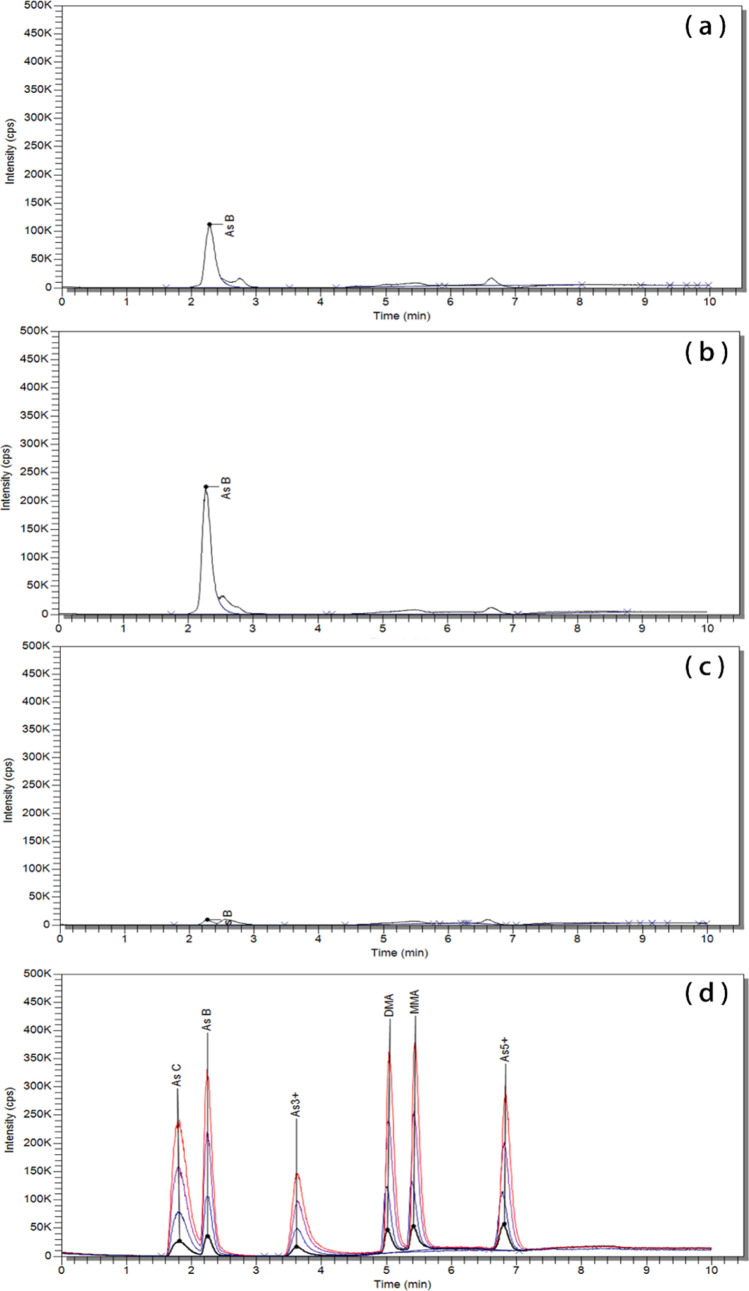
The quality assurance parameters for the validation of the analytical techniques applied for the quantification of toxic elements and arsenic species contents in salted foods are given in Table 1, and the accuracies, determined via standard reference material (SRM) analyses, are given in Table 2. The total toxic element contents of the salted food samples are reported in Table 3, and the concentrations of the six As species are given in Table 4. Additionally, the As species contents of sea salts from five countries are reported in Table 5. HPLC–ICP–MS chromatograms for six As species obtained from three types of salted food samples and a standard solution are shown in Figure 1.
Table 1. Analytical Method Validation Parameters for Toxic Elements and Arsenic Species.
| spike
recovery (%) |
|||||||
|---|---|---|---|---|---|---|---|
| analyte | linearity | LOD (μg/kg) | LOQ (μg/kg) | CV (%) | lowb | mediumb | highc |
| Total Toxic Elements | |||||||
| Cd | 0.99998 | 0.0830 | 0.274 | 1.09 | 90.6 | 104 | 97.6 |
| Pb | 0.99992 | 0.0521 | 0.172 | 0.882 | 94.7 | 93.5 | 104 |
| tAs | 0.99998 | 0.0669 | 0.221 | 1.40 | 103 | 92.1 | 98.4 |
| Inorganic As Species | |||||||
| As3+ | 0.9995 | 1.12 | 3.72 | 2.83 | 91.2 | 96.3 | 92.4 |
| As5+ | 0.9991 | 2.38 | 7.94 | 3.37 | 93.2 | 91.6 | 90.5 |
| Organic As Species | |||||||
| AsC | 0.9994 | 1.08 | 3.57 | 2.92 | 92.7 | 91.5 | 93.1 |
| AsB | 0.9993 | 1.00 | 3.34 | 2.64 | 101 | 92.6 | 104 |
| DMA | 0.9996 | 0.732 | 2.44 | 2.11 | 103 | 96.0 | 97.7 |
| MMA | 0.9994 | 0.978 | 3.26 | 2.76 | 103 | 98.2 | 107 |
aAdded 10 μg/L each of Cd and Pb and 500 μg/L of tAs and each As species to the sample for three (n = 3) measurements.
Added 50 μg/L each of Cd and Pb and 250 μg/L of tAs and each As species to the sample for three (n = 3) measurements.
Added 150 μg/L each of Cd and Pb and 5000 μg/L of tAs and each As species to the sample for three (n = 3) measurements.
Table 2. Analysis of Standard Reference Materials (Spinach Leaves, NIST SRM-1570a).
| analyte | certified concentration (mg/kg) | measured concentration (mg/kg) | recovery (%) |
|---|---|---|---|
| Spinach Leaves, NIST SRM-1570a | |||
| Cd | 2.89 ± 0.07 | 2.66 | 92.0a |
| Pb | 0.200 | 0.183 | 91.7 |
| Rice Powder, NIST SRM-1568b | |||
| tAs | 0.285 ± 0.014 | 0.259 | 91.2 |
| Inorganic arsenicb | 0.092 ± 0.010 | 0.088 | 95.7 |
| DMA | 0.180 ± 0.012 | 0.172 | 95.6 |
| MMA | 0.0116 ± 0.0035 | 0.0105 | 90.5 |
Values are recovery of three (n = 3) measurements.
Inorganic arsenic: sum of As3+ and A5+.
Table 3. Concentrations (mg/kg) of Toxic Elements in Salted Food.
| salted anchovy | salted sea shrimp | salted fresh water shrimp | |
|---|---|---|---|
| Cd | 0.0341ba± 0.0150b(0.00712–0.053) | 0.0121a ± 0.00902 (0.00317–0.0360) | 0.0141a ± 0.00703 (0.00616–0.0251) |
| Pb | 0.0750c ± 0.0201 (0.0501–0.077) | 0.00921a ± 0.00610 (0.00201–0.0243) | 0.0581b ± 0.0392 (0.0291–0.125) |
| tAs | 2.21b ± 0.503 (1.60–3.23) | 2.93c ± 0.532 (2.17–4.61) | 0.250a ± 0.0292 (0.211–0.328) |
a–c Values with different superscript letters within a row differ significantly (p < 0.05).
Values are mean ± standard deviations of three (n = 3) measurements.
Table 4. Concentrations (mg/kg) of Arsenic Species in Salted Food.
| salted anchovy | salted sea shrimp | salted fresh water shrimp | |
|---|---|---|---|
| Organic Arsenic | |||
| AsC | <DLa | <DL | <DL |
| AsB | 1.55bb± 0.514c(0.755–2.49) | 2.50c ± 0.455 (1.68–3.85) | 0.218a ± 0.0280 (0.169–0.272) |
| DMA | <DL | <DL | <DL |
| MMA | <DL | <DL | <DL |
| Inorganic Arsenic | |||
| As3+ | <DL | <DL | <DL |
| As5+ | <DL | <DL | <DL |
DL: below the detection limit.
a–c Values with different superscript letters within a row differ significantly (p < 0.05).
Values are mean ± standard deviations of three (n = 3) measurements.
Table 5. Concentrations (mg/kg) of Arsenic Species in Sea Salt of Different Countries.
| Korea | Vietnam | China | Australia | New Zealand | |
|---|---|---|---|---|---|
| Organic Arsenic | |||||
| AsC | <DLa | <DL | <DL | <DL | <DL |
| AsB | 0.0621nsb± 0.0131c(0.0410–0.0772) | 0.0421ns ± 0.00302 (0.0382–0.0451) | 0.0442ns ± 0.00413 (0.0411–0.0492) | 0.0482ns ± 0.00610 (0.0411–0.0552) | 0.0482ns ± 0.00402 (0.0443–0.0551) |
| DMA | <DL | <DL | <DL | <DL | <DL |
| MMA | <DL | <DL | <DL | <DL | <DL |
| Inorganic Arsenic | |||||
| AsIII | <DL | <DL | <DL | <DL | <DL |
| AsV | <DL | <DL | <DL | <DL | <DL |
DL: below detection limit.
ns: nonsignificantly differ within a row (p < 0.05).
Values are mean ± standard deviations of three (n = 3) measurements.
Figure 1.
Arsenic speciation chromatograms obtained by LC–ICP–MS. (a) Salted anchovy, (b) salted sea shrimp, (c) salted fresh water shrimp, and (d) standard solution of six arsenic species.
2.1. Validation of the Analytical Method
The linearity of each standard curve was at least 0.9992 for the toxic elements, and the LOD and LOQ values ranged from 0.0521 to 0.0830 and 0.172 to 0.274 μg/kg, respectively. The CV % values of the analytes ranged from 0.882 (Pb) to 1.40% (As), and the spiked recovery percentage ranged from 90.6 (Cd) to 106.8% (MMA) (Table 1). For the SRM(NIST SRM-1570a) analysis, the recovery percentages of Cd and Pb were 91.7 and 92.0%, respectively, and that for As the analysis of SRM (NIST SRM-1568b) was 91.2% (Table 2).
A number of studies have reported the good separation capabilities of HPLC and the highly sensitive ICP–MS detection, identification, and quantification in food samples.19,20 In the current study, six As species were detected by HPLC–ICP–MS. The validation results of As species are given in Table 2. The linearity was at least 0.9991, and the LOD and LOQ values ranged from 0.732 μg/kg (DMA) to 2.38 μg/kg (As5+) and 2.44 μg/kg (DMA) to 7.94 μg/kg (As5+), respectively. The CV % values ranged from 2.11% (DMA) to 3.37% (As5+). Recovery experiments were performed by adding three different concentrations of standard solutions to salted sea shrimp. As a result, the obtained recoveries were 90.6 to 104% for Cd, 94.7 to 104% for Pb, and 92.1 to 103% for tAs. For As species, recoveries were 91.5% (AsC) to 107% (MMA) (Table 1). SRM (NIST SRM-1568b) was used for determination of the recovery percentage of each As species. It is certified for DMA, MMA, and inorganic As only. In the case of inorganic As, it is defined as total arsenic without distinguishing As3+ and As5+. Therefore, in this study, inorganic As was calculated as the sum of As3+ and As5+, and the recovery values ranged from 90.5 to 95.7% (Table 2).
The abovementioned parameters for the validation of the applied analytical method subject foods fulfilled the requirements of the Association of Official Analytical Chemists,21 thus confirming their successful application for the determination of concentrations of toxic elements and As species.
2.2. Toxic Element Concentrations in Salted Foods
The presence of toxic elements in the food chain is harmful and tackling this issue requires knowledge of their sources and contamination potential. This study analyzed three different types of salted foods (54 samples) for three toxic elements. The obtained means ± standard deviations and the range of toxic element concentrations are shown in Table 3.
Cd concentrations ranged from 0.00341 to 0.053 mg/kg and were the highest in salted anchovy (0.0341 ± 0.0150 mg/kg). Salted sea and freshwater shrimp showed similar Cd concentrations of 0.0121 ± 0.00902 and 0.0141 ± 0.00703 mg/kg, respectively. Pb was detected in all samples but was the highest in salted anchovy (0.0750 ± 0.0201 mg/kg). Salted sea and freshwater shrimp showed concentrations of 0.00921 ± 0.00610 and 0.0581 ± 0.0392 mg/kg, respectively. The average concentration of total arsenic (tAs) ranged from 0.250 to 2.21 mg/kg. Importantly, the concentrations of tAs in salted anchovy (2.21 ± 0.503 mg/kg) and salted sea shrimp (2.93 ± 0.532 mg/kg) were 10 to 12 times higher than that in salted freshwater shrimp (0.250 ± 0.0292 mg/kg). According to the literature, naturally occurring As on earth crust flows into the sea and becomes concentrated since the earth crust was formed. Thus, As accumulation is expected to be high in sea creatures,22 which are also confirmed by the current results.
According to the World Health Organization, the Provisional Tolerable Weekly Intake Levels (PTWI) are 15 μg/kg body weight for As, 25 μg/kg body weight Cd, and 7 μg/kg body weight Pb.23 Therefore, our results suggest that the salted foods analyzed in the present study do not pose any health concern to consumers under normal diet.
2.3. Concentrations of As Species in Salted Foods
As is classified as a group 1 carcinogen and of the three toxic elements analyzed in this study, it had the highest concentrations. However, chemical toxicity varies with As species, and therefore species analysis is required to determine the actual toxicity of As. Inorganic As (As3+ and As5+) is more harmful than organic As (AsC, AsB, DMA, and MMA). In particular, AsB is considered nontoxic. Therefore, speciation of As is required to clarify the health risks of As from food.
The As species were clearly separated and identified in the standard (Figure 1), and their quantification in the samples is shown in Table 4. Inorganic As was not detected, only AsB was detected in all samples. This indicates no toxicity or health threat from consumption of the subject salted food samples. Additionally, a significant difference between AsB concentrations in sea (shrimp and anchovy) and freshwater creatures (shrimp) was observed. The average concentrations of AsB in salted sea shrimp and salted anchovy were 2.50 ± 0.455 and 1.55 ± 0.514 mg/kg, respectively. However, salted fresh water shrimp had a comparatively low concentration of 0.218 ± 0.0280 mg/kg. The tAs contents of 13.1–62.6 mg/kg dry weight (d.w.) have been reported in anchovy in the literature, with AsB accounting for 80–97% of tAs.24 For shrimp, the tAs content reported in the literature is 34 mg/kg d.w., with AsB accounting for 80% of tAs and inorganic As for 0.21%.25 In another study, an As content of 1.3 mg/kg d.w. has been reported for freshwater shrimp,26 10–48 times lower than that in sea creatures. These results are consistent with our present research findings and indicate that AsB is the main As species in sea creatures. This may be due to the reason that AsB is concentrated in sea creatures during growth.
In this study, sea salt was also analyzed to evidence that since the earth’s crust was formed it is affecting As concentrations in sea creatures. Sea salt is produced by evaporation of seawater; therefore, it can be a concentrated form of seawater. Sea salt was collected from five areas and analyzed; the results are shown in Table 5. Only AsB was detected in the sea salt samples, with average concentrations ranging from 0.042 to 0.062 mg/kg. The As content was the highest in Korean sea salt but no significant difference was observed. The As concentration of seawater was calculated using the salinity of the salt samples and the fact that the average salinity of seawater was 34.9% (Table S3, Supporting Information). It was confirmed that the AsB concentration of sea ranged from 0.0421 to 0.0621 mg/kg, which was thought to be closely related to the accumulation of AsB in sea creatures.
3. Conclusions
This study investigated concentrations of total toxic elements (As, Pb, and Cd) and As species in three types of salted food and sea salts. The analytical method developed in this study was less time-consuming and expensive than conventional analysis. Moreover, its effectiveness has been verified for salted foods and sea salt samples. All toxic element contents were below the limits established for foods by the WHO.
The accumulation of toxic heavy elements in the body is directly affected by the environment. In this study, the concentrations of toxic elements in sea water were calculated through solar salt analysis. This method facilitated effective analysis of arsenic species with trace levels of contamination. The results formed a strong basis for analysis of As accumulations that have different measured concentrations depending on the growth environment despite being the same species. AsB was detected in the salt, which is the predominant species in sea shrimp. This is important evidence to clearly prove the accumulation of arsenic species in sea creatures.
4. Materials and Methods
4.1. Reagents
Ultrapure (18 mΩ) water (Millipore, Bedford, MA, USA) was used for all the experiments. Analytical reagent grade HNO3 (70%) and H2O2 (30%) were purchased from Dong Woo Fine Chem Co., Ltd., Korea. The toxic element standard solutions were obtained from AnApure Co, Ltd. Daejeon, Korea. SRMs (SRM 1570a spinach leaves and SRM 1568b rice flour) were obtained from the National Institute of Standards and Technology (Gaithersburg, MD, USA). Arsenocholine (AsC; 95%), arsenobetaine (AsB; 97%), dimethylarsinic acid (DMA; 98%), and monosodium acid methane arsonate sesquihydrate (MMA; 99.0%) were purchased from Wako Pure Chemical Industries (Osaka, Japan), Fluka (Buchs, Switzerland), Strem Chemicals (Newburyport, MA, USA), and Chem Service (West Chester, PA, USA), respectively. Sodium (meta) arsenite (As3+; 99.0%), sodium arsenate dibasic heptahydrate (As5+; 99.9%), ammonium bicarbonate (≥99.5%), ammonium phosphate monobasic (≥99.99%), and ammonium nitrate (≥99.99%) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
4.2. Instrumentation
A Multiwave 3000 microwave sample preparation system (Anton Paar, Graz, Austria) equipped with high-pressure polytetrafluoroethylene vessels (MF 100) was used for sample digestion. An ICP–MS system (300D, PerkinElmer Sciex, CT, USA) was used to measure total toxic metal concentrations. The HPLC system (Flexar, PerkinElmer Sciex, CT, USA) was coupled to the ICP–MS to determine six types of As species. The operational parameters of these instruments are shown in S1 Table (Supporting Information).
4.3. Sampling
Fifty-four samples belonging to three food types were purchased from supermarkets all around South Korea. These included salted anchovy (10), salted freshwater shrimp (14), and salted sea shrimp (30). Additionally, 30 samples of sea salt from five countries including South Korea (10), Vietnam (5), China (5), Australia (5), and New Zealand (5) were obtained. The sea salts were analyzed for As species and to determine the correlation between salted anchovy, sea shrimp, and salted freshwater shrimp of As species and other toxic elements contents. All the samples were purchased in triplicate at different time intervals from March to September 2017. The samples were homogenized using a food blender (MR 350CA, Braun, Spain), properly labeled, and stored in a freezer at −20 °C prior to analysis.
4.4. Sample Preparation
4.4.1. Digestion of Samples for Analysis of Toxic Elements
Approximately 2.0 g of each homogenized sample was directly weighed into polytetrafluoroethylene digestion vessels. Then, 7.0 mL of concentrated HNO3 (70%) and 2.0 mL of H2O2 were added as catalysts for the digestion. The digestion procedure was as follows: (1) 1000 W at 80 °C for 5 min, (2) 1000 W at 150 °C for 5 min, (3) 1000 W at 190 °C for 15 min, (4) 1000 W at 190 °C for 20 min, and (5) 0 W for 30 min for cooling. After cooling, the contents of the tubes were transferred to a volumetric flask, augmented to 50.0 mL with ultrapure deionized water, and analyzed for Pb, Cd, and total As by ICP–MS.28
4.4.2. Extraction for Analysis of As Species
Approximately 2.0 g of each homogenized sample was weighed into a 50.0 mL volumetric flask, augmented with 50% (v/v) methanol in 1% HNO3, and extracted by ultrasonic extraction for 30 min at 30 °C. After extraction, the samples were centrifuged at 3500×g for 15 min. The supernatant layer was filtered through a 0.45 μm syringe-type polyvinylidene difluoride membrane filter (BDH, Leicester, UK), and the filtrate was analyzed by HPLC–ICP–MS. Here, filtration was modified from the cartridge purification method used by Khan et al.27 by using a membrane filter syringe. This modification/simplification helped reduce experimental time and cost.
4.5. Analyses of Toxic Elements and As Species
4.5.1. Determination of Total Toxic Elements
Cd, Pb, and As were analyzed by ICP–MS. The drift of the instrument was corrected by running a multielement standard for every 10 analyses. The operating parameters and conditions are listed in Table S1 (Supporting Information). Analytical isotopes of toxic elements of Cd, Pb, and As, were respectively, 111, 208, and 91. For As analysis, chlorine (35Cl) combines with argon (40Ar) to form 40Ar35Cl, which has the same mass as As (75As), and therefore causes interference during the analysis of As. Thus, in this study, As was converted to 75As16O using oxygen as a cell gas, with a measured m/z of 91, to avoid interference from 40Ar35Cl.29
The quantitative values were obtained using the following equation
where A is the final volume (mL) after the pretreatment process, B is the sample amount (g), C is the dilution factor, and D is the instrument measurement result (ug/L).
4.5.2. Determination of As Species
External calibration was performed for the quantitative analyses of the samples, and standard solutions were prepared by diluting stock solutions. Six types of As species were separated by an ion exchange column (Hamilton PRP X-100, 4.1 250, 10 mm), and the gradient conditions for mobile phases A and B were 2 mM ammonium bicarbonate in 1% methanol with a pH of 8.0 and a mixture of 20 mM ammonium nitrate and 20 mM ammonium phosphate in 1% methanol with a pH of 9.2, respectively. The injection volume was 50.0 μL and the column temperature was room temperature.27 All necessary operating parameters were as given in Table S2 (Supporting Information).
4.6. Quality Assurance
The analytical methods applied were validated using linearity, limits of detection (LOD), limits of quantification (LOQ), precision, recovery of spiking tests, and analyzing SRMs for accuracy. Linearity was evaluated from the calibration curves of each analyte and the precision was obtained as a percentage coefficient of variation (CV %) from the relative standard deviation of 10 repeated determination. LOD and LOQ were determined as three and ten times the standard deviation of the blank divided by the slope of the analytical curve, respectively.27 The pretreatment process of inorganic analysis completely decomposes organic matter through acid hydrolysis, so the effect of the matrix has relatively small impact compared to other physicochemical experiments. Therefore, the accuracy of the analysis at the corresponding concentration was confirmed using two food matrices, whose concentration was confirmed. And also, toxic elements’ standard material spiking recovery in the sample was performed. Therefore, three different concentration spiking recovery tests on salted food (homogenized salted sea shrimp) were conducted for all analyte elements and recovery percentages (%) were reported. The low concentrations were 10 μg/L each of Cd and Pb and 500 μg/L of tAs and each As species, the medium concentrations were 50 μg/L each of Cd and Pb and 250 μg/L of tAs and each As species, and the high concentrations were 150 μg/L each of Cd and Pb and 5000 μg/L of tAs and each As species. In order to ensure the accuracy of the method, two types of food SRMs (1570a, spinach leaves and 568b, and rice flour) were also analyzed.
4.7. Statistical Analysis
The data are reported as the mean ± standard deviation of triplicate measurements. Significant differences (p < 0.05) measured among the means were reported via one-way analysis of variance (ANOVA) and Tukey’s honest significant difference test. SPSS Statistical Package for Social Sciences Software Version 20 was used for the statistical analyses (IBM, New York, USA).
Glossary
Abbreviations
- arsenobetaine
AsB
- methylarsonic acid
MMA
- dimethylarsinic acid
DMA
- arsenocholine
AsC
- arsenite
As3+
- arsenate
As5+
- inductively coupled plasma mass spectrometry
ICP–MS
- high performance liquid chromatography
HPLC
- World Health Organization
WHO
- limits of detection
LOD
- limits of quantification
LOQ
- coefficient of variation
CV
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c01273.
ICP–MS operating conditions for determination of toxic elements; HPLC–ICP–MS operating conditions for determination of arsenic species; and concentrations (mg/kg) of arsenic species in seawater based on sea salt (PDF)
This work was supported by World Institute of Kimchi (grant number KE2102-2), funded by the Ministry of Science and ICT, Republic of Korea.
The authors declare no competing financial interest.
Supplementary Material
References
- Shahbaz M.; Hashmi M. Z.; Malik R. N.; Yasmin A. Relationship between heavy metals concentrations in egret species, their environment and food chain differences from two headworks of Pakistan. Chemosphere 2013, 93, 274–282. 10.1016/j.chemosphere.2013.04.078. [DOI] [PubMed] [Google Scholar]
- Da Sacco L.; Baldassarre A.; Masotti A. Diet’s role in the toxicity of inorganic arsenic (iAs): A journey from soil to children’s mouth. J. Geochem. Explor. 2013, 131, 45–51. 10.1016/j.gexplo.2012.11.014. [DOI] [Google Scholar]
- Melø R.; Gellein K.; Evje L.; Syversen T. Minerals and trace elements in commercial infant food. Food Chem. Toxicol. 2008, 46, 3339–3342. 10.1016/j.fct.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Flora G.; Gupta D.; Tiwari A. Toxicity of lead: a review with recent updates. Interdiscip. Toxicol. 2012, 5, 47–58. 10.2478/v10102-012-0009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J.; Plant J. A.; Voulvoulis N.; Oates C. J.; Ihlenfeld C. Cadmium levels in Europe: implications for human health. Environ. Geochem. Health 2010, 32, 1–12. 10.1007/s10653-009-9273-2. [DOI] [PubMed] [Google Scholar]
- Mohammed Abdul K. S.; Jayasinghe S. S.; Chandana E. P. S.; Jayasumana C.; De Silva P. M. C. S. Arsenic and human health effects: A review. Environ. Toxicol. Pharmacol. 2015, 40, 828–846. 10.1016/j.etap.2015.09.016. [DOI] [PubMed] [Google Scholar]
- Duarte F. A.; Pereira J. S. F.; Barin J. S.; Mesko M. F.; Dressler V. L.; de Moraes Flores É. M.; Knapp G. Seafood digestion by microwave-induced combustion for total arsenic determination by atomic spectrometry techniques with hydride generation. J. Anal. At. Spectrom. 2009, 24, 224–227. 10.1039/b810952d. [DOI] [Google Scholar]
- Francesconi K. A. Arsenic species in seafood: origin and human health implications. Pure Appl. Chem. 2010, 82, 373–381. 10.1351/pac-con-09-07-01. [DOI] [Google Scholar]
- Straif K.; Benbrahim-Tallaa L.; Baan R.; Grosse Y.; Secretan B.; El Ghissassi F.; Bouvard V.; Guha N.; Freeman C.; Galichet L.; Cogliano V. A review of human carcinogens-part C: metals, arsenic, dusts, and fibres. Lancet Oncol. 2009, 10, 453–454. 10.1016/s1470-2045(09)70134-2. [DOI] [PubMed] [Google Scholar]
- Rahman M. A.; Hogan B.; Duncan E.; Doyle C.; Krassoi R.; Rahman M. M.; Naidu R.; Lim R. P.; Maher W.; Hassler C. Toxicity of arsenic species to three freshwater organisms and biotransformation of inorganic arsenic by freshwater phytoplankton (Chlorella sp. CE-35). Ecotoxicol. Environ. Saf. 2014, 106, 126–135. 10.1016/j.ecoenv.2014.03.004. [DOI] [PubMed] [Google Scholar]
- Jackson B. P.; Liba A.; Nelson J. Advantages of reaction cell ICP-MS on doubly charged interferences for arsenic and selenium analysis in foods. J. Anal. At. Spectrom. 2015, 30, 1179–1183. 10.1039/c4ja00310a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao C. R. M.; Sahuquillo A.; Lopez Sanchez J. F. A review of the different methods applied in environmental geochemistry for single and sequential extraction of trace elements in soils and related materials. Water Air Soil Pollut. 2008, 189, 291–333. 10.1007/s11270-007-9564-0. [DOI] [Google Scholar]
- Tokalıoğlu Ş. Determination of trace elements in commonly consumed medicinal herbs by ICP-MS and multivariate analysis. Food Chem. 2012, 134, 2504–2508. 10.1016/j.foodchem.2012.04.093. [DOI] [PubMed] [Google Scholar]
- Khan N.; Jeong I. S.; Hwang I. M.; Kim J. S.; Choi S. H.; Nho E. Y.; Choi J. Y.; Park K. S.; Kim K. S. Analysis of minor and trace elements in milk and yogurts by inductively coupled plasma-mass spectrometry (ICP-MS). Food Chem. 2014, 147, 220–224. 10.1016/j.foodchem.2013.09.147. [DOI] [PubMed] [Google Scholar]
- B’hymer C.; Caruso J. A. Arsenic and its speciation analysis using high-performance liquid chromatography and inductively coupled plasma mass spectrometry. J. Chromatogr. A 2004, 1045, 1–13. 10.1016/j.chroma.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Bolea-Fernandez E.; Balcaen L.; Resano M.; Vanhaecke F. Overcoming spectral overlap via inductively coupled plasma-tandem mass spectrometry (ICP-MS/MS). A tutorial review. J. Anal. At. Spectrom. 2017, 32, 1660–1679. 10.1039/c7ja00010c. [DOI] [Google Scholar]
- Schmidt L.; Landero J. A.; La Rosa Novo D.; Duarte F. A.; Mesko M. F.; Caruso J. A.; Flores E. M. M. Feasible method for As speciation in several types of seafood by LC-ICP-MS-MS. Food Chem. 2018, 255, 340–347. 10.1016/j.foodchem.2018.02.079. [DOI] [PubMed] [Google Scholar]
- Gao Y.; Baisch P.; Mirlean N.; Rodrigues da Silva Júnior F. M.; Van Larebeke N.; Baeyens W.; Leermakers M. Arsenic speciation in fish and shellfish from the North Sea (Southern bight) and Açu Port area (Brazil) and health risks related to seafood consumption. Chemosphere 2018, 191, 89–96. 10.1016/j.chemosphere.2017.10.031. [DOI] [PubMed] [Google Scholar]
- Zmozinski A. V.; Llorente-Mirandes T.; López-Sánchez J. F.; da Silva M. M. Establishment of a method for determination of arsenic species in seafood by LC-ICP-MS. Food Chem. 2015, 173, 1073–1082. 10.1016/j.foodchem.2014.10.102. [DOI] [PubMed] [Google Scholar]
- Schmidt L.; Landero J. A.; Santos R. F.; Mesko M. F.; Mello P. A.; Flores E. M. M.; Caruso J. A. Arsenic speciation in seafood by LC-ICP-MS/MS: method development and influence of culinary treatment. J. Anal. At. Spectrom. 2017, 32, 1490–1499. 10.1039/c7ja00052a. [DOI] [Google Scholar]
- Standard method performance requirements (AOAC SMPR 211.009) for Cr, Mo and Se in infant formula and adult/pediatric nutritional formula. J. AOAC Int. 2012, 95, 297. [PubMed] [Google Scholar]
- Turkmen M.; Turkmen A.; Tepe Y. Metal contaminations in five fish species from Black, Marmara, Aegean and Mediterranean seas, Turkey. J. Chil. Chem. Soc. 2008, 53, 1424–1428. 10.4067/s0717-97072008000100021. [DOI] [PubMed] [Google Scholar]
- Hwang I. M.; Yang J. S.; Jung J. H.; Lee H. W.; Lee H. M.; Seo H. Y.; Khan N.; Jamila N.; Kim K. S.; Kim S. H. Dietary intake assessment of macro, trace, and toxic elements via consumption of kimchi in South Korea. J. Sci. Food Agric. 2019, 99, 6474–6481. 10.1002/jsfa.9926. [DOI] [PubMed] [Google Scholar]
- Kalantzi I.; Mylona K.; Sofoulaki K.; Tsapakis M.; Pergantis S. A. Arsenic speciation in fish from Greek coastal areas. J. Environ. Sci. 2017, 56, 300–312. 10.1016/j.jes.2017.03.033. [DOI] [PubMed] [Google Scholar]
- Krishnakumar P. K.; Qurban M. A.; Stiboller M.; Nachman K. E.; Joydas T. V.; Manikandan K. P.; Mushir S. A.; Francesconi K. A. Arsenic and arsenic species in shellfish and finfish from the western Arabian Gulf and consumer health risk assessment. Sci. Total Environ. 2016, 566–567, 1235–1244. 10.1016/j.scitotenv.2016.05.180. [DOI] [PubMed] [Google Scholar]
- Prapaiwong N.; Boyd C. E. Trace elements in waters of inland, low-salinity shrimp ponds in Alabama. Aquacult. Res. 2014, 45, 327–333. 10.1111/j.1365-2109.2012.03230.x. [DOI] [Google Scholar]
- Khan N.; Jeong I. S.; Hwang I. M.; Kim J. S.; Choi S. H.; Nho E. Y.; Choi J. Y.; Kwak B.-M.; Ahn J.-H.; Yoon T.; Kim K. S. Method validation for simultaneous determination of chromium, molybdenum and selenium in infant formulas by ICP-OES and ICP-MS. Food Chem. 2013, 141, 3566–3570. 10.1016/j.foodchem.2013.06.034. [DOI] [PubMed] [Google Scholar]
- Khan N.; Ryu K. Y.; Choi J. Y.; Nho E. Y.; Habte G.; Choi H.; Kim M. H.; Park K. S.; Kim K. S. Determination of toxic heavy metals and speciation of arsenic in seaweeds from South Korea. Food Chem. 2015, 169, 464–470. 10.1016/j.foodchem.2014.08.020. [DOI] [PubMed] [Google Scholar]
- Balcaen L.; Bolea-Fernandez E.; Resano M.; Vanhaecke F. Inductively coupled plasma–tandem mass spectrometry (ICP-MS/MS): A powerful and universal tool for the interference-free determination of (ultra) trace elements – A tutorial review. Anal. Chim. Acta 2015, 894, 7–19. 10.1016/j.aca.2015.08.053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.