Abstract

The inhibition efficiency of cationic surfactants such as 1-ethyl-4H-benzo[d][1,3]thiazin-1-ium bromide (BTB) and N-ethyl-N,N-dioctyloctan-1-aminium bromide (DAB) for X-65 type carbon steel in oil well formation water under a H2S environment has been studied using potentiodynamic polarization and electrochemical impedance spectroscopy measurements. Fourier transform infrared and nuclear magnetic resonance spectroscopy techniques were used to confirm the chemical structures of BTB and DAB. The novelty of this work lies in modifying the long chains in the inhibitor, which leads to high efficiency. These surfactants act as good inhibitors, which inhibit both cathodic and anodic routes by adsorption on the electrode surface, which is compatible with the critical micelle concentration parameters, together with a slight positive change in the corrosion potential (Ecorr). The IE% reached 93.4% for compound BTB and 84% for compound DAB at 250 ppm. The equivalent circuit was used to analyze the model of the corrosion inhibition process. The atomic force microscopy image shows the morphology of the adsorbed layer formed on the steel alloy. Finally, a suitable inhibition mechanism was proposed.
1. Introduction
In petroleum fields, carbon steel has been used in many applications.1,2 These applications, for the most part, incite genuine destructive impacts on types of gear, tubes, and pipelines made of iron and its alloys.3,4 Subsequently, the counteractive action of metals used in the petroleum field and modern applications from consumption is indispensable, which must be managed, particularly in corrosive media. Cationic surfactants as a branch of surfactants play a vital role in many industrial processes such as food processing, oil recovery, and in petroleum additives like corrosion inhibitors.5−17
The inhibition efficacy is directly proportional to the inhibitor concentration and contact time with the metal surface. Many industrial processes generate gases like carbon dioxide, ammonia, and hydrogen disulfide. This latter gas contains sulfide ions, which react with many heavy metals. H2S is a pale gas with an offensive odor suggestive of rotten eggs. It is a weak reducing acid and is readily soluble in water with subsequent ionic dissociation.
H2S forms black metallic sulfide suspensions and/or deposits induced by corrosion. H2S enters the matrix of many alloys, resulting in brittleness in these alloys and stress corrosion cracking miscarriage particularly due to the high-strength steel.18−26 The novelty of this work lies in modifying the long chains in inhibitors, which leads to high efficiency. The current research aims to prepare novel cationic surfactants, 1-ethyl-4H-benzo[d][1,3]thiazin-1-ium bromide (BTB) and N-ethyl-N,N-dioctyloctan-1-aminium bromide (DAB), and evaluate their efficiency as corrosion inhibitors for X-65 type carbon steel in oil well formation water in a hydrogen sulfide environment using different techniques.
2. Results and Discussion
2.1. Potentiodynamic Polarization Study
Existence of hydrogen sulfide in the solution forms ferrous sulfide. The high concentration of gas results in failure of the protective layer as shown in eq 2. Corrosion potential (Ecorr), corrosion current density (icorr), cathodic and anodic Tafel slopes (βc and βa), and polarization resistance (Rp) as electrochemical parameters were calculated.
From the polarization curves shown in Figure 1a,b, it was observed that icorr reduced with increasing dose of compounds BTB and DAB compared to the blank. The following equations are used for calculating the degree of surface coverage (θ) and the inhibition efficacy ratio (η%):
| 1 |
| 2 |
where i0 and i are the corrosion current densities in the absence and presence of the inhibitor, respectively.
Figure 1.
Polarization plots of the steel electrode in formation water containing various concentrations of (a) BTB and (b) DAB inhibitors.
A good evaluation of the cathodic curve showed that the Tafel lines became more negative and so certain opportunities for both anodic and cathodic cycles, individually, comparative with the clear. This implies that the chose intensifies goes about as a blended kind inhibitor, that is, empowering block of both anodic and cathodic release responses. We notice that the slopes of both the anodic and cathodic Tafel shapes were the same. This means that the selected inhibitor does not affect the metal dissolution mechanism. icorr was reduced with the increase in the dose.27 The obtained results summarized in Table 1 indicate that the i values are significantly lower in the presence of corrosion inhibitors compared to blank solutions. The corrosion current density decreases. The data obtained from the polarization curves are plotted and listed in Table 1.
Table 1. Corrosion Parameters Obtained from Polarization Curves for BTB and DAB Inhibitors.
| inhibitor | concentration (ppm) | βa (mV dec–1) | βc (mV dec–1) | Ecorr (mV vs SCE) | icorr (μA cm–2) | θ | IE% |
|---|---|---|---|---|---|---|---|
| blank | 000 | 193.7 | –150.9 | –804.2 | 9.312 | ||
| BTB | 50 | 109.4 | –142.1 | –779.7 | 3.433 | 0.6313 | 63.13 |
| 100 | 79.7 | –156.9 | –643.5 | 2.405 | 0.7417 | 74.17 | |
| 150 | 103.9 | –192.7 | –617.6 | 1.357 | 0.8542 | 85.42 | |
| 200 | 104.9 | –153.4 | –741.8 | 0.855 | 0.9081 | 90.81 | |
| 250 | 103.4 | –193.3 | –567 | 0.638 | 0.9314 | 93.14 | |
| DAB | 50 | 75.8 | –139 | –711.4 | 2.9143 | 0.6870 | 68.70 |
| 100 | 110.6 | –124.5 | –739 | 2.87 | 0.6917 | 69.17 | |
| 150 | 109.1 | –159.1 | –745.5 | 1.942 | 0.7914 | 79.14 | |
| 200 | 102.3 | –169.7 | –728.5 | 1.854 | 0.8009 | 80.09 | |
| 250 | 71.2 | –155.7 | –742.1 | 1.499 | 0.8390 | 83.90 |
From the polarization curves, it is observed that η% of the compound BTB is more than that of the accumulated DAB. This could be attributed to the pπ–dπ connections between the inhibitor particles and the empty orbital of Fe.28−30
2.2. Electrochemical Impedance Spectroscopy (EIS)
Figures 2 and 3 show the Nyquist and bode plots of carbon steel immersed in the solution (blank) in the absence and presence of BTB and DAB, respectively.
Figure 2.

Nyquist plots for the carbon steel electrode in formation water with and without various concentrations of (a) BTB and (b) DAB inhibitors.
Figure 3.
Bode plots for the carbon steel electrode in formation water with and without various concentrations of (a) BTB and (b) DAB inhibitors.
It is clear that there is a depressed capacitive loop along the x-axis. The size of the loop increased on increasing the inhibitor concentration. This behavior indicated that the corrosion process was controlled by the polarization resistance according to the following equation23
| 3 |
Figure 4 shows the equivalent circuit Rs, film resistance Rf, charge-transfer resistance Rct, constant phase elements CPEf and CPEcd as obtained from the EIS analyzer program, and the obtained impedance data can be explained according to the following equation24
| 4 |
where Y0 represents the admittance, j = −1, and w is the angular frequency.
Figure 4.
Equivalent circuit model for the impedance data.
It is obvious from Table 2 that both Cdl and Cf decrease with an increase in the concentration of BTB and DAB.
Table 2. Impedance Parameters Obtained from the EIS Curves for BTB and DAB Inhibitors.
| inhibitor | concentration (ppm) | Rf (Ω) | QF (μF/cm2) | n1 | Qdl (μF/cm2) | Rct(KΩ/cm2) | n2 | θ | IE % |
|---|---|---|---|---|---|---|---|---|---|
| BTB | 0 | 52.6 + 2 | 132.1 + 1.1 | 0.87 + 0.02 | 538.1 | 1.659 + 0.10 | 0.83 + 0.05 | ||
| 50 | 128.9 + 3 | 87.3 + 0.9 | 0.92 + 0.03 | 40.90 | 3.112 + 0.017 | 0.88 + 0.03 | 0.4669 | 46.69 | |
| 100 | 286.2 + 5 | 63.7 + 0.8 | 0.94 + 0.01 | 56.45 | 3.995 + 0.015 | 0.91 + 0.02 | 0.5847 | 58.47 | |
| 150 | 423.3 + 6 | 52.6 + 0.7 | 0.96 + 0.04 | 23.41 | 4.905 + 0.014 | 0.92 + 0.01 | 0.6617 | 66.17 | |
| 200 | 560.7 + 4 | 46.8 + 0.8 | 0.97 + 0.01 | 4.641 | 11.03 + 0.013 | 0.93 + .01 | 0.8495 | 84.95 | |
| 250 | 592.5 + 7 | 37.2 + 0.4 | 0.98 + 0.01 | 143.9 | 25.51 + 0.012 | 0.93 + 0.01 | 0.9349 | 93.49 | |
| DAB | 50 | 117.8 + 2 | 76.8 + 0.8 | 0.88 + 0.03 | 228.8 | 2.474 + 0.016 | 0.85 + 0.05 | 0.3294 | 32.94 |
| 100 | 187.5 + 4 | 58.1 + 0.6 | 0.89 + 0.01 | 315.5 | 2.541 + 0.017 | 0.87 + 0.07 | 0.3471 | 34.71 | |
| 150 | 238.1 + 6 | 49.6 + 0.5 | 0.90 + 0.04 | 109.3 | 2.609 + 0.013 | 0.89 + 0.02 | 0.3641 | 36.41 | |
| 200 | 302.7 + 7 | 39.5 + 0.4 | 0.92 + 0.02 | 43.77 | 4.096 + 0.012 | 0.90 + 0.01 | 0.5949 | 59.49 | |
| 250 | 327.9 + 8 | 27.4 + 0.3 | 0.92 + 0.01 | 100.1 | 4.446 + 0.011 | 0.90 + 0.01 | 0.6268 | 62.68 |
This is evidence for the adsorption of the inhibitor molecules on the carbon steel surface, forming the required protective layer. According to the following two equations31
| 5 |
| 6 |
where d is the thickness of the adsorbed layer, Se is the electrode surface exposed to the aggressive solution, ∑0 is the permittivity of the vacuum, ∑ is the local dielectric constant, and F is Faraday’s constant.
Figure 3a,b shows that the phase angle increased with increasing inhibitor concentration for carbon steel (CS) immersed in media of an corrosive environment in the presence of various doses of the two chemicals used (BTB and DAB), which shows different trends compared to the blank solution. It is clear from the variation of log Z with F that the impedance values increase with increasing concentration. Also, it is clear that the variation of phase angle increases with the increasing inhibitor concentration. The shift of the phase angle from the value of 90° is evidence for the deviation from the ideal capacitive behavior. This behavior confirms the obtained data from Nyquist plots. The inhibitive ability, which is reflected by η%, markedly improved as the inhibitor concentration increased.32−34
2.3. Atomic Force Microscopy (AFM) Surface Study
An atomic force microscope is an instrument used to discriminate the shallow design of geography since it is equipped for giving pictures. AFM investigated the extent of inhibition of CS after immersion in a destructive acid solution.35Figure 5 shows the 3D AFM morphologies for the dissolution of CS in a corrosive solution of deep oil formation water in the absence and the presence of 250 ppm of the compound. The root mean square (RMS) of unpleasantness (Rq) is the normal deviance that decides the normal lines and normal harshness (Ra), clarifying the mean deviance of all harshness pictures. The AFM image of CS in a destructive medium alone in oil well formation water shows extraordinary consumption and more harshness. Interestingly, a lesser number of sites attack when using the compounds because of the shape of the adsorbing films that shield the CS surface from its current circumstance. The %IE obtained from electrochemical measurements upheld and was consistent with the deliberate harshness information. Table 3 shows the understanding of Ra and Rq esteems. In an overall perspective on harshness, the metal surface is smoothed and turns out to be delicate because of the presence of an emphatically adsorbed layer through the dynamic community position of the inhibitor.
Figure 5.
AFM images of (a) the CS samples before immersion in oil well formation water alone (blank) and (b) CS surface immersion in oil well formation water for 1 day using 250 ppm of the compound BTB.
Table 3. AFM Measurements of CS Samples in the Presence and Absence of Doses (250 ppm) of Compound BTB in Deep Oil Well Formation Water for 7 Days at 298 K.
| sample | average roughness (Ra), nm | RMS roughness (Rq), nm |
|---|---|---|
| CS alloy surface immersed in deep oil well formation water in the absence of inhibitor molecules | 260.4 | 318.2 |
| CS alloy surface immersed in 0.1 oil well formation water after immersion in 250 ppm of BTB | 95.3 | 112.6 |
2.4. Surface Tension Characteristics
The critical micelle concentration of the prepared compounds was determined at different concentrations by adjustment of the slope of the plotted information of external strain surface tension (γ) versus the natural logarithm of the solute molar concentration, ln concentration, as presented in Figure 6. Table 4 summarizes the surface tension characteristics.
Figure 6.
Surface tension vs log C of compound BTB and DAB.
Table 4. Surface Tension Characteristics of BTB and DAB.
| inhibitor | CMC, mole/dm3 | γcmc, mN/m | Γ max × 10–7, mol/m2 | Amin, n m2 | Π CMC | ΔG0mic, kJmol–1 | ΔG0ads, kJ mol–1 |
|---|---|---|---|---|---|---|---|
| BTB | 7.47 × 10–4 | 36 | 9.48 × 10–11 | 175 | 36.5 | –18.13 | –21.96 |
| DAB | 2.49 × 10–4 | 33 | 1.38 × 10–10 | 119.5 | 39.3 | –20.9 | –23.7 |
The critical micelle concentration (CMC) is a vital factor which turns out to be thermodynamically positive for surfactant atoms with respect to the structure totals (micelles) to limit the collaboration of either their head gatherings or their tail bunches with the dissolvable condition. Table 5 summarizes the standard free energy of micellization (ΔG0mic) and adsorption (ΔG0ads), which showed that compound BTB favors micellization rather than adsorption compared to inhibitor DAB with imported benzene ring and long chains.
Table 5. Standard Free Energy of Micellization (ΔG0mic) and Standard Free Energy of Adsorption (ΔG0ads).
| inhibitor | free energy of micellization ΔG0mic (kJ mol–1) | free energy of adsorption ΔG0ads (kJ mol–1) |
|---|---|---|
| BTB | –18.13 | –21.96 |
| DAB | –20.9 | –23.7 |
This demonstrates that compound BTB has the most grounded adsorption layer on the CS surface and hence the greatest inhibition efficiency, which stresses that the inhibition efficacies of the integrated compound expansion in the accompanying request as anticipated by the various methods: Tafel polarization and EIS . Using the following equation, it was found that the standard free energy of micellization (ΔG0mic) of compound DAB was lower than that of compound BTB for the synthesized surfactants:
| 7 |
The free energy of adsorption standard (ΔG0 ads) for the synthesized surfactants is shown in the following equation:
| 8 |
where Kads is the adsorption equilibrium constant. These results reveal that BTB with a longer alkyl chain possesses stronger adsorption affinity onto the CS surface and thus exhibits a better inhibition behavior.36
2.5. Inhibition Mechanism of Surfactants
The inhibition effecting for the deterioration of CS in oil well formation water was explored utilizing the examined surfactants BTB and DAB. The interference cycle depends on numerous factors like focus, the quantity of active areas and their charge densities, atomic mass, and their stability in their environments.37 In fact, the heterogeneity of surfactants with uncommon nucleophilicity, electrons and charge of heteroatoms (N, O, and S particles) will in general limit the utilization of the metal surface. The barrier depends upon the adsorption of the surfactants on the CS surface and hindering their active centers.38Figure 7 shows the advancement of the fused surfactant compounds containing heads and tails. The head hordes of the surfactants (polar part) were in rich electronically unique utilitarian social affairs, which share in the adsorption association with unfilled d-orbitals of the CS, through the relocation of adsorbed water particles from the CS by heteromolecules that can give the electron chemisorption bonding.39
Figure 7.

Corrosion inhibition mechanism of BTB.
The hydrophobicity chain (tail) of the surfactant causes the relocation of the surfactants from the game plan mass to associate, and these hydrophobic tails work as a resistance film to keep the metal away from reacting with its present situation. The adsorption collaboration depends on the tendency of the CS toward electron densities that work with better surface coverage.40
3. Experimental Section
3.1. Chemical Structure of the X-65 Type CS Electrode
An unused petroleum pipeline was cut into X-65 type CS specimens. The chemical composition (weight %) of the CS electrode was carbon, 0.09; silicon, 0.22; Mn, 1.52; P, 0.01; S, 0.05; Ni, 0.04; Cr, 0.02; Mo, 0.004; vanadium, 0.002; copper, 0.02; and aluminum, 0.04; and the rest was Fe.
3.2. Deep Oil Well Formation Water
The reservoir rock contains deep oil well formation water. This water contains different organic and inorganic salts. The elements present are Na, Ca, Mg, Cl, HCO3, and SO4. The chemical composition of this water and its physical properties are summarized in Tables 6 and 7.
Table 6. Physical Properties of the Testing Solution.
| total dissolved solids (TDS) | 9650 mg/L | density@60 F | 1.06 g/mL |
| salinity (as NaCl) | 95,556 mg/L | specific gravity | 1.06 |
| alkalinity (as CaCO3) | 320 mg/L | pH@25 °C | 6.8 |
| total hardness (as CaCO3) | 14,455 mg/L | conductivity | 12.02 × 10–2 mhos/cm@21.6 °C |
| resistivity | 0.0832 Ohm m@21.6 °C |
Table 7. Chemical Composition of the Testing Solution.
| cation | mg/L | meq/L | anion | mg/L | meq/L |
|---|---|---|---|---|---|
| lithium | 48.9 | 7.056 | fluoride | 76.71 | 4.038 |
| sodium | 30760.9 | 1337.485 | chloride | 57912.87 | 1631.405 |
| ammonium | 186.85 | 10.357 | bromide | 252.62 | 3.163 |
| potassium | 945.24 | 24.179 | nitrate | 38.17 | 0.616 |
| magnesium | 947.95 | 78.007 | nitrite | 1.84 | 0.040 |
| calcium | 4225.67 | 210.861 | phosphate | nil | nil |
| strontium | 78.08 | 1.783 | sulfate | 640.54 | 13.342 |
| barium | 1.30 | 0.019 | hydroxide | nil | nil |
| iron | nil | nil | carbonate | nil | nil |
| copper | nil | nil | bicarbonate | 390.40 | 6.399 |
3.3. Testing Solution
The testing solution is the oil well formation water containing the abovementioned specific chemical compositions. The reaction of sodium sulfide (3.53 mg L–1) with acetic acid (1.7 mg L–1) generates hydrogen sulfide gas. This is called the simulation solution, which is similar to actual media in oil wells. This work facilitates preparation inhibitors as semi-industrial or industrial of inhibitors.
3.4. Synthesis of the Inhibitors
The cationic surfactant BTB based on benzothiazole was prepared as illustrated in Scheme 1. This process was carried out using the quaternization reaction. Benzothiazole (50 mM) and 1-bromooctadecane (50 mM) were charged exclusively in a 250 mL round flask with (CH3)2CO (100 mL) as a dissolvable solvent. The resulting mixture was refluxed by mixing for 18 h, and then, the resulting mixture was maintained at room temperature. The earthy colored suspension was filtered with a filter paper, washed well twice with diethyl ether, and then recrystallized using (CH3)2CO to obtain clear product results of the synthesized compounds. The products of the earthy colored precious stone items went somewhere in the range of 78–86%.
Scheme 1. Synthesis of the Cationic Surfactant (BTB).
The cationic surfactant DAB based on tri-n-octyl amine was prepared as illustrated in Scheme 2. This process was carried out using the quaternization reaction. Tri-n-octyl amine (50 mM) and 1-bromooctadecane (50 mM) were transferred separately in a 250 mL round flask with (CH3)2CO (100 mL) as a dissolvable solvent. The resulting mixture was refluxed by mixing for 18 h, and afterward, the resulting blend was cooled to room temperature. The earthy colored suspension was filtered with a filter paper, washed well twice with diethyl ether, and afterward recrystallized from (CH3)2CO to bear the cost of the white gem results of the cationic surfactants. The products of the earthy colored precious stone items ran somewhere in the range of 80–90%.
Scheme 2. Synthesis of the Cationic Surfactant (DAB).
3.4.1. Fourier Transform Infrared (FTIR) Spectroscopy Analysis
FTIR spectra of the prepared inhibitors (BTB and DAB) contain two peaks at 3365 and 3280 cm–1, which are ascribed to N–H in both inhibitors, and peaks at 2925 and 2856 cm–1 corresponding to CH3 and CH2, in addition to a peak at 1057 cm–1 and a fingerprint peak at 724 cm–1 assigned to the asymmetric and symmetric stretching quaternary nitrogen atom (N+–C) as shown in Figure 8a,b.
Figure 8.
(a, b) FTIR spectrum of the synthesized inhibitors: (a) BTB and (b) DAB.
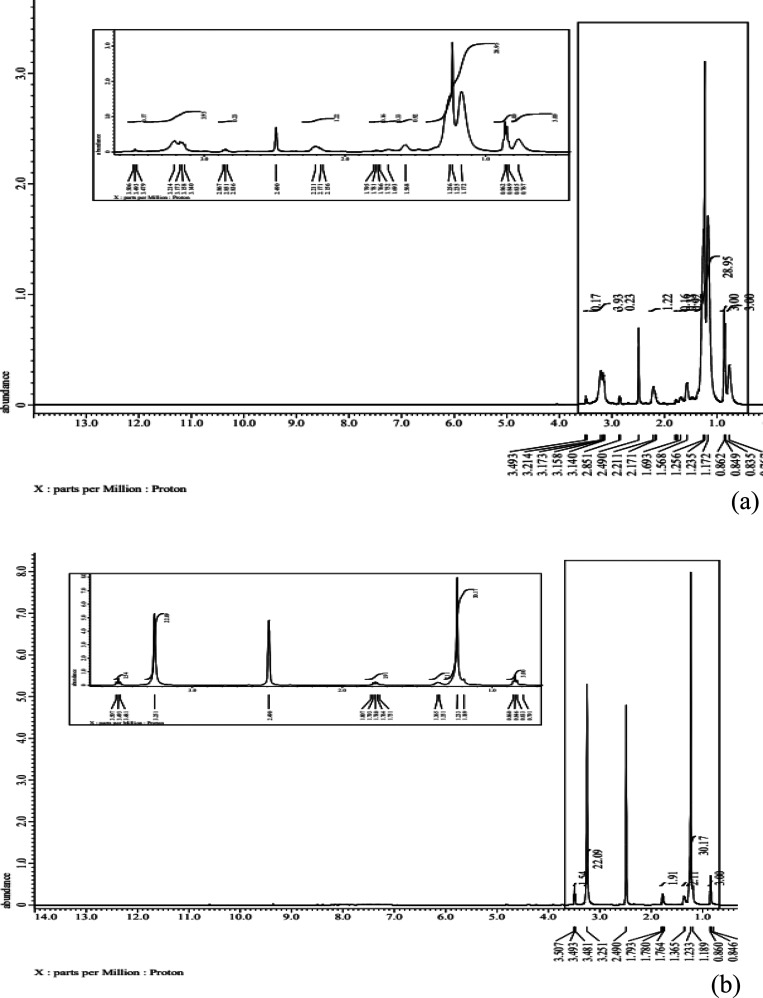
3.4.2. 1H NMR Spectroscopy
The chemical change at ∂ (1.41) for 1H proton (a) −CH3, the substance shift ∂ (4.07) for 1H protons (b) −CH2 related to alkyl collection, this compound shift at ∂ (4.13) for 1H protons (c) −CH2 of alkyl group alpha. The substance moves between ∂ (8.9) for 1H protons (g) and (h) of aryl ring. All the overhead substance changes affirm that compound BTB was effectively synthesized.
The chemical shift ∂ (0.88) to 1H proton (a) −CH3, the synthetic ∂ (3.22) for 1H Protons (b) −CH2 related to alkyl group, the compound shift at ∂ (3.28) for 1H Protons (c) −CH2 of alkyl group neer, N–C. inhibitor moves between ∂ (1.26–1.7) for 1H protons (g) and (h) of the alkyl bunch. The above substance shifts confirm that DAB was effectively synthesized. The information of 1H NMR spectra confirmed the normal hydrogen proton dispersion in the combined surfactant as shown in Figure 9a,b.
Figure 9.
Chemical structure characterization of the inhibitor, (a) 1H-NMR BTB and (b) 1H-NMR DAB.
3.5. Electrochemical Measurements
3.5.1. Potentiodynamic Polarization Measurement
Potentiodynamic polarization was carried out in an electrochemical glass cell containing a platinum electrode as an auxiliary electrode and saturated calomel electrode (SCE) as a reference electrode in addition to a working electrode with 1 cm2 surface area. The used instrument was VoltaLab 80 (Tacussel radiometer PGZ402) with Voltamaster 4 as software for the instrument, and the results were recorded at a scan rate of 1 mVs–1 after immersion of the test solution in a three-electrode system for 60 min in the absence and presence of a fixed inhibitor concentration.
3.5.2. Electrochemical Impedance Spectroscopy
Electrochemical impedance spectroscopy (EIS) measurements were carried out in the same electrochemical cell at OCP in the frequency range from 100 kHz to 20 mHz at an amplitude of 10 mV.
3.6. Surface Tension Measurements
The surface tension (γ) was measured using a Krüss K6 tensiometer type, a direct surface tension measurement, using the ring method for various concentrations of the investigated surfactants.
3.7. Surface Examination
In order to record the surface micrographs, the designed samples were immersed in the test solution in the absence and presence of 250 ppm of the inhibitor; after 24 h, the samples were removed from the test solution, cleaned, washed with distilled water, and dried, and the samples were analyzed by AFM conducted using an AFM Nanosurf-Flex-Axiom FlexAFM 5 scan head specifications with a C3000 controller operating in the dynamic force mode at 25 °C.
4. Conclusions
Newly synthesized surfactants BTB and DAB can be used as effective inhibitors for the dissolution of CS in deep oil well formation water. These surfactants were characterized using FTIR and 1HNMR spectroscopy techniques. The values of the parameters from potentiodynamic polarization measurements of the prepared surfactants show that they act as dual-type inhibitors. AFM micrographs confirm the formation of a good protective film that isolates the surface from the corrosive environment. The synthesized surfactants showed high inhibition efficiency against CS in the formation water.
Acknowledgments
The authors acknowledge the support of Petroleum Applications Department, Egyptian Petroleum Research Institute (EPRI), Egypt.
The authors declare no competing financial interest.
References
- Marín-Cruz J.; Cabrera-Sierra R.; Pech-Canul M. A.; González I. EIS study on corrosion and scale processes and their inhibition in cooling system media. Electrochim. Acta 2006, 51, 1847–1854. 10.1016/j.electacta.2005.02.104. [DOI] [Google Scholar]
- Li X.; Wang H.; Hu C.; Yang M.; Hu H.; Niu J. Characteristics of biofilms and iron corrosion scales with ground and surface waters in drinking water distribution systems. Corros. Sci. 2015, 90, 331–339. 10.1016/j.corsci.2014.10.028. [DOI] [Google Scholar]
- Hernández-Espejel A.; Domínguez-Crespo M. A.; Cabrera-Sierra R.; Rodríguez-Meneses C.; Arce-Estrada E. M. Investigations of corrosion films formed on API-X52 pipeline steel in acid sour media. Corros. Sci. 2010, 52, 2258–2267. 10.1016/j.corsci.2010.04.003. [DOI] [Google Scholar]
- Migahed M. A.; Al-Sabagh A. M.; Zaki E. G.; Mostafa H. A.; Fouda A. S. Synthesis of some novel cationic surfactants and evaluation of their performance as corrosion inhibitors for X-65 type carbon steel under H2S environment. Int. J. Electrochem. Sci. 2014, 9, 7693–7711. [Google Scholar]
- Migahed M. A.; Zaki E. G.; Shaban M. M. Corrosion control in the tubing steel of oil wells during matrix acidizing operations. RSC Adv. 2016, 6, 71384. 10.1039/c6ra12835a. [DOI] [Google Scholar]
- Dickinson E.; Miller R.. Food Colloids: Fundamentals of Formulation, Vol 258. Royal Society of Chemistry, 2001. [DOI] [PubMed] [Google Scholar]
- Singh A.; Ansari K. R.; Quraishi M. A.; Kaya S.; Banerjee P. The effect of an N-heterocyclic compound on corrosion inhibition of J55 steel in sweet corrosive medium. New J. Chem. 2019, 43, 6303–6313. 10.1039/C9NJ00356H. [DOI] [Google Scholar]
- Tripathy D. B.; Murmu M.; Banerjee P.; Quraishi M. A. Palmitic acid based environmentally benign corrosion inhibiting formulation useful during acid cleansing process in MSF desalination plants. Desalination 2019, 472, 114128 10.1016/j.desal.2019.114128. [DOI] [Google Scholar]
- Verma C.; Haque J.; Quraishi M. A.; Ebenso E. E. Aqueous phase environmental friendly organic corrosion inhibitors derived from one step multicomponent reactions: a review. J. Mol. Liq. 2019, 275, 18–40. 10.1016/j.molliq.2018.11.040. [DOI] [Google Scholar]
- Cao C.; Zhang L.; Zhang X.-X.; Du F.-P. Effect of gum arabic on the surface tension and surface dilational rheology of trisiloxane surfactant. Food Hydrocolloids 2013, 30, 456–462. 10.1016/j.foodhyd.2012.07.006. [DOI] [Google Scholar]
- Pietralik Z.; Kołodziejska Ż.; Weiss M.; Kozak M. Gemini surfactants based on bis-imidazolium alkoxy derivatives as effective agents for delivery of nucleic acids: A structural and spectroscopic study. PLoS One 2015, 10, e0144373 10.1371/journal.pone.0144373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R.; Kamal A.; Abdinejad M.; Mahajan R. K.; Kraatz H.-B. Advances in the synthesis, molecular architectures and potential applications of gemini surfactants. Adv. Colloid Interface Sci. 2017, 248, 35–68. 10.1016/j.cis.2017.07.032. [DOI] [PubMed] [Google Scholar]
- Nandwani S. K.; Malek N. I.; Lad V. N.; Chakraborty M.; Gupta S. Study on interfacial properties of Imidazolium ionic liquids as surfactant and their application in enhanced oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 2017, 516, 383–393. 10.1016/j.colsurfa.2016.12.037. [DOI] [Google Scholar]
- Fink J. K.Drilling muds. In Pet Eng Guid to oil F Chem fluids, 2nd ed. Gulf Prof Publ, Published online, 2015, pp. 1–61. [Google Scholar]
- Ziembowicz F. I.; Bender C. R.; Frizzo C. P.; Martins M. A. P.; de Souza T. D.; Kloster C. L.; Santos Garcia I. T.; Villetti M. A. Thermodynamic Insights into the Binding of Mono- and Dicationic Imidazolium Surfactant Ionic Liquids with Methylcellulose in the Diluted Regime. J. Phys. Chem. B 2017, 121, 8385–8398. 10.1021/acs.jpcb.7b03525. [DOI] [PubMed] [Google Scholar]
- Shalabi K.; Helmy A. M.; El-Askalany A. H.; Shahba M. M. New pyridinium bromide mono-cationic surfactant as corrosion inhibitor for carbon steel during chemical cleaning: Experimental and theoretical studies. J. Mol. Liq. 2019, 293, 111480 10.1016/j.molliq.2019.111480. [DOI] [Google Scholar]
- Sengupta S.; Murmu M.; Murmu N. C.; Banerjee P. Adsorption of redox-active Schiff bases and corrosion inhibiting property for mild steel in 1 molL–1 H2SO4: Experimental analysis supported by ab initio DFT, DFTB and molecular dynamics simulation approach. J. Mol. Liq. 2021, 326, 115215 10.1016/j.molliq.2020.115215. [DOI] [Google Scholar]
- Hanna F.; Sherbini G. M.; Barakat Y. Commercial fatty acid ethoxylates as corrosion inhibitors for steel in pickling acids. Br. Corros. J. 1989, 24, 269–272. 10.1179/000705989798269948. [DOI] [Google Scholar]
- Osman M. M.; Shalaby M. N. Some ethoxylated fatty acids as corrosion inhibitors for low carbon steel in formation water. Mater. Chem. Phys. 2003, 77, 261–269. 10.1016/S0254-0584(01)00580-6. [DOI] [Google Scholar]
- Al-sabagh A. M.; Migahed M. A.; Awad H. S. Reactivity of polyester aliphatic amine surfactants as corrosion inhibitors for carbon steel in formation water (deep well water). Corros. Sci. 2006, 48, 813–828. 10.1016/j.corsci.2005.04.009. [DOI] [Google Scholar]
- Abd-Elaal A. A.; Elbasiony N. M.; Shaban S. M.; Zaki E. G. Studying the corrosion inhibition of some prepared nonionic surfactants based on 3-(4-hydroxyphenyl) propanoic acid and estimating the influence of silver nanoparticles on the surface parameters. J. Mol. Liq. 2018, 249, 304–317. 10.1016/j.molliq.2017.11.052. [DOI] [Google Scholar]
- Migahed M. A.; Abd-El-Raouf M.; Al-Sabagh A. M.; Abd-El-Bary H. M. Effectiveness of some non ionic surfactants as corrosion inhibitors for carbon steel pipelines in oil fields. Electrochim. Acta 2005, 50, 4683–4689. 10.1016/j.electacta.2005.02.021. [DOI] [Google Scholar]
- el-Hajjaji F.; Messali M.; Aljuhani A.; Aouad M. R.; Hammouti B.; Belghiti M. E.; Chauhan D. S.; Quraishi M. A. Pyridazinium-based ionic liquids as novel and green corrosion inhibitors of carbon steel in acid medium: electrochemical and molecular dynamics simulation studies. J. Mol. Liq. 2018, 249, 997–1008. 10.1016/j.molliq.2017.11.111. [DOI] [Google Scholar]
- Qiang Y.; Zhang S.; Yan S.; Zou X.; Chen S. Three indazole derivatives as corrosion inhibitors of copper in a neutral chloride solution. Corros. Sci. 2017, 126, 295–304. 10.1016/j.corsci.2017.07.012. [DOI] [Google Scholar]
- Yilong Z.; Dean Z.; Daoliang L. Electrochemical and other methods for detection and determination of dissolved nitrite: A review. Int. J. Electrochem. Sci. 2015, 10, 1144–1168. [Google Scholar]
- Tüken T.; Demir F.; Kıcır N.; Sığırcık G.; Erbil M. Inhibition effect of 1-ethyl-3-methylimidazolium dicyanamide against steel corrosion. Corros. Sci. 2012, 59, 110–118. 10.1016/j.corsci.2012.02.021. [DOI] [Google Scholar]
- Sengupta S.; Murmu M.; Mandal S.; Hirani H.; Banerjee P. Competitive corrosion inhibition performance of alkyl/acyl substituted 2-(2-hydroxybenzylideneamino) phenol protecting mild steel used in adverse acidic medium: A dual approach analysis using FMOs/molecular dynamics simulation corroborated experimental findings. Colloids Surf. A Physicochem. Eng. Asp. 2021, 617, 126314 10.1016/j.colsurfa.2021.126314. [DOI] [Google Scholar]
- Saha S. K.; Dutta A.; Ghosh P.; Sukul D.; Banerjee P. Adsorption and corrosion inhibition effect of schiff base molecules on the mild steel surface in 1 M HCL medium: A combined experimental and theoretical approach. Phys. Chem. Chem. Phys. 2015, 17, 5679–5690. 10.1039/c4cp05614k. [DOI] [PubMed] [Google Scholar]
- Singh A. K.; Thakur S.; Pani B.; Singh G. Green synthesis and corrosion inhibition study of 2-amino-: N ′-((thiophen-2-yl)methylene)benzohydrazide. New J. Chem. 2018, 42, 2113–2124. 10.1039/c7nj04162d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S. K.; Murmu M.; Murmu N. C.; Banerjee P. Evaluating electronic structure of quinazolinone and pyrimidinone molecules for its corrosion inhibition effectiveness on target specific mild steel in the acidic medium: A combined DFT and MD simulation study. J. Mol. Liq. 2016, 224, 629–638. 10.1016/j.molliq.2016.09.110. [DOI] [Google Scholar]
- Yadav M.; Kumar S.; Kumari N.; Bahadur I.; Ebenso E. E. Experimental and theoretical studies on corrosion inhibition effect of synthesized benzothiazole derivatives on mild steel in 15% HCl solution. Int. J. Electrochem. Sci. 2015, 10, 602–624. [Google Scholar]
- Qiang Y.; Guo L.; Li H.; Lan X. Fabrication of environmentally friendly Losartan potassium film for corrosion inhibition of mild steel in HCl medium. Chem. Eng. J. 2021, 406, 126863 10.1016/j.cej.2020.126863. [DOI] [Google Scholar]
- Qiang Y.; Li H.; Lan X. Self-assembling anchored film basing on two tetrazole derivatives for application to protect copper in sulfuric acid environment. J. Mater. Sci. Technol. 2020, 52, 63–71. 10.1016/j.jmst.2020.04.005. [DOI] [Google Scholar]
- Qiang Y.; Zhang S.; Tan B.; Chen S. Evaluation of Ginkgo leaf extract as an eco-friendly corrosion inhibitor of X70 steel in HCl solution. Corros. Sci. 2018, 133, 6–16. 10.1016/j.corsci.2018.01.008. [DOI] [Google Scholar]
- Tang Y.; Yang X.; Yang W.; Chen Y.; Wan R. Experimental and molecular dynamics studies on corrosion inhibition of mild steel by 2-amino-5-phenyl-1, 3, 4-thiadiazole. Corros. Sci. 2010, 52, 242–249. 10.1016/j.corsci.2009.09.010. [DOI] [Google Scholar]
- Qiang Y.; Zhang S.; Guo L.; Zheng X.; Xiang B.; Chen S. Experimental and theoretical studies of four allyl imidazolium-based ionic liquids as green inhibitors for copper corrosion in sulfuric acid. Corros. Sci. 2017, 119, 68–78. 10.1016/j.corsci.2017.02.021. [DOI] [Google Scholar]
- Ashassi-Sorkhabi H.; Shaabani B.; Seifzadeh D. Effect of some pyrimidinic Shciff bases on the corrosion of mild steel in hydrochloric acid solution. Electrochim. Acta 2005, 50, 3446–3452. 10.1016/j.electacta.2004.12.019. [DOI] [Google Scholar]
- Shaban S. M. Studying the effect of newly synthesized cationic surfactant on silver nanoparticles formation and their biological activity. J. Mol. Liq. 2016, 216, 137–145. 10.1016/j.molliq.2015.12.098. [DOI] [Google Scholar]
- Negm N. A.; Morsy S. M. I. Corrosion inhibition of triethanolammonium bromide mono-and dibenzoate as cationic inhibitors in an acidic medium. J. Surfactants Deterg. 2005, 8, 283–287. 10.1007/s11743-005-0359-x. [DOI] [Google Scholar]
- Sastri V. S.; Perumareddi J. R. Molecular orbital theoretical studies of some organic corrosion inhibitors. Corrosion 1997, 53, 617–622. 10.5006/1.3290294. [DOI] [Google Scholar]