Abstract

Adsorption is one of the commonly used methods in wastewater treatment, but it has the problem of high cost and a complicated production process. In this paper, a low-cost and efficient decolorizing adsorbent was successfully prepared based on waste polyacrylonitrile fiber (PANF). The waste PANF was ammoniated by propylene diamine derivates (PANAMF), and benzylamine (PANABMF) and quaternary ammonium ions (PANQMF) were introduced for PANAMF to regulate hydrophilicity and hydrophobicity. With acidic red 249 as the model anionic dye, influences of the adsorption center structure, the degree of modification, the concentration of acid, the dye structure, and the auxiliary agent in the solution on the dye adsorption performance were studied. Isothermal models, kinetic models, reusability, and continuous application ability of the fiber adsorbent were discussed. PANAMF, PANABMF, and PANAQF exhibit excellent adsorption performance compared to the common adsorbent. After protonation, the saturation adsorption value can reach 2051.3 mg/g for PANAMF. PANAMF also exhibited excellent reusability, and the adsorption capacity after being reused eight times still can keep 72.7% of that for the first time. The adsorption of the anionic dye for PANAMF is a chemisorption process, and the rate-determining step is changed from the diffuse step to the adsorption on the surface with the adsorption time. PANAMF can also be used in the continuous flow process, and the absorption amount is similar to that in the batch adsorption, which shows excellent commercial application potential.
1. Introduction
Water pollution has become one of the most pressing health crises in the world. One of the crucial sources of water pollution is industrial wastewater. Dye is one of the most severe sources of pollution to water.1,2 The nature of most dyes is precarious and does not have the function of self-degradation, which seriously affects the light intake and the chromaticity of the water. Thus, the organisms carrying out normal life activities in the water are greatly threatened, and dyes even cause severe carcinogenicity to life.3−7 Up to now, various technologies have been used to remove dyes from wastewater, such as physical adsorption, biological degradation, oxidation, and electrolysis.8−13 Among them, the adsorption method is a universal treatment method with the advantages of simple operation and high treatment capacity.14,15
Common adsorbents include activated carbon adsorbents, metallic and non-metallic oxide adsorbents (silica gel, alumina, molecular sieve, natural clay, etc.), and other natural adsorbents (sawdust, straw, chitosan, cellulose, etc.).16−19 The structure of the adsorbent is the key factor to influencing the adsorbent properties. In general, adsorbents have porous structures or a variety of active functional groups such as hydroxyl, carboxyl, amino, and sulfhydryl groups.20−22 Dye molecules can diffuse and enter into the adsorbent by various interaction forces between the adsorbent and the dye, such as van der Waals forces, hydrogen bonds, coordination bonds, covalent bonds, and interionic forces.23−27 For example, Davarnejad et al. recently reported the adsorption of methyl blue dye using grape-stem powder mixed with activated carbon.28 And He et al. successfully synthesized a WSS-based hydrochar (WSH) adsorbent with a higher specific surface area to remove methyl orange in aqueous solutions.29 However, adsorption methods still have problems such as the high adsorbent cost, poor recovery and reuse rate, and secondary pollution now.30,31 It is urgent to develop a new adsorbent with a lower cost, higher recovery rate, and no secondary pollution.
In all decolorizing adsorbents, the fiber adsorbent is a low-cost, readily available material, which is one of the development directions of functional textile materials. The fiber adsorbent not only has the adsorption property of active carbon but also has better regeneration stability and selective adsorption ability than many adsorbents.32−35 The fiber adsorbents can be prepared from natural fibers, such as cotton,19 wool,36 piaçava fibers, and so on;37 electrospinning after polymer modification;38 and chemical modification. Polyacrylonitrile fiber (PANF) is widely used as a water treatment adsorbent because of its comprehensive strength, resistance to organic solvents, and excellent heat resistance. More importantly, a large number of cyano-groups in PANF can be easily converted into amide, carboxylic acid, amidoxime, hydrazide, and other groups.34,39−41 Phosphate, polydopamine, and polyethylenimine have been grafted on the surface of PAN fiber. The modified PANF could achieve excellent adsorption through ion exchange, electrostatic attraction, and complexing force,42−44 such as the adsorption of palladium ions, copper and silver ions,45 chromium ions,46 oil, diesel, and Microcystis aeruginosa cells.47 It can be seen that these modified PANFs exhibit good adsorption capacity and stability. However, there is no specific paper that discusses the adsorption of different anionic dyes by modified PANFs. In theory, because of the particular structure of the dye and fiber, the cationic PANF should have a good adsorption effect on the anionic dye.41
In the production process and the consumption of daily life, tens of thousands of discarded PANFs are produced. The treatment of these discarded PANFs is the common interesting point in both the industrial and academic fields. It is a good choice to turn PANF into a fiber absorbent for water treatment.48 Herein, using waste PANF as the raw material, amination and quaternary amination are performed. The prepared fiber adsorbents are used to remove anionic dye, and the mechanism and practicability are studied. An environmentally friendly, low-cost, and readily available adsorbent is prepared and can be used to remove organic dyes from wastewater in a large scale.
2. Experimental Section
2.1. Materials
PANF was obtained from the waste acrylic clothing. N,N-Dimethyl-1,3-malondiamine (DMPDA), benzylamine, n-butane bromide, sulfuric acid, sodium chloride, sodium sulfate, sodium acetate, sodium lauryl sulfate, Peregal O-25, and sodium hydroxide anhydrous ethanol were purchased from Shanghai McLean Biochemical Technology Co., Ltd. Acidic Brilliant Red B (C.I. Acid red 249, AR249), Reactive Red 3BS (C.I. Reactive red 195, RR195), Neutral Dark Yellow GL (C.I. Acid yellow 128, AY128), and Direct Pink 12B (C.I. Direct red 31, DR31) were obtained from Hangzhou Huashi Xiasha Textile Technology Co., Ltd. (Hangzhou, China).
2.2. Preparation of the Fiber Adsorbent
Synthesis of PANAMF: PAN fibers (30 g) were boiled in 300 mL of deionized water for 30 min to remove impurities and then dried for 12 h. Five grams of pre-PAN fiber, 70 mL of DMPDA, and 30 mL of water were added to a 250 mL three-neck flask and stirred mechanically in 90 °C for 2–6 h. The fiber was filtered and washed to neutral with sulfuric acid solution and water. PANAMF was dried in a vacuum drier at 60 °C.
Synthesis of PANABMF: PANAMF, n-butyl bromide, and ethanol (30 mL) were added into 250 mL three-neck flask and reacted at 80 °C for 0.2–2 h. The fiber was filtered, washed to neutral, and dried (Scheme 1).
Scheme 1. Preparation Process for the PAN Fiber Adsorbent.
Synthesis of PANAQF: Pre-PAN fiber, benzylamine (40 mL), and water (20 mL) were added to a 250 mL three-neck flask and stirred mechanically in 90 °C for 2–6 h. Then, 5 g of the obtained fiber, 70 mL of DMPDA, and 30 mL of water were placed into a 250 mL three-neck flask and stirred mechanically in 90 °C for 4 h. The fiber was filtered, washed to neutral, and dried.
The weight gain rate of the modified fiber (Ga) can be calculated from the following equation:
where W1 is the weight of the unmodified fiber and W2 is the weight of the modified fiber.
2.3. Characterization of the Fiber Adsorbent
The adsorbent samples were tracked by Fourier transform infrared (FTIR) spectroscopy. The surface elements of different fiber adsorbents were measured and analyzed by X-ray photoelectron spectroscopy (XPS). The morphology and surface structure of the sample were studied by a Philips XL30-E scanning electron microscope (SEM). An ultraviolet spectrophotometer was used to measure the absorbance of dye in the process of adsorption. The corresponding absorbance and the standard curve of the dye solution concentration were used to calculate the concentration.
2.4. Adsorption Experiments
Adsorption experiments were carried out in a constant-temperature oscillating water bath, and the shaking speed was set at 200 rpm. The modified fiber and the dye solution were added in a 100 mL conical flask and shaken at 50 °C. At regular intervals, the solution was withdrawn and the absorbance was measured. The dye concentration was calculated according to the standard curve. In the adsorption isotherm experiment, the initial concentration of AR249 was set at 9000–12,000 mg/L.
The flow adsorption properties of fibers were investigated by the continuous flow device. The flow device contains a column with a length of 150 mm and diameter of 20 mm, two beakers, and a peristaltic pump. The column was filled with PANAMF (Ga = 42.6%, 10 g). The concentration of AR249 dye in the wastewater tank was 8 g/L, and the flow rate was 2 mL/min. The effluent was collected, and the absorbance was measured.
The dye was purified with the following procedures. Two grams of the dye was dissolved in 20 mL of DMF. The insoluble salt was filtrated, and the filtrate was added into 200 mL of acetone. The filter cake was collected and dried in the vacuum oven at 60 °C.
Adsorption capacity is expressed by adsorption quantity Qe (mg/g) in batch experiment and removal efficiency R (%) in flow adsorption, which can be calculated from the following equations:
where V0 is the initial volume of the dye wastewater, Vt is the volume of the dye solution at time t (h), C0 and C1 (g/L) are the initial concentration and equilibrium concentration of the dye, and m (g) is the mass of the adsorbent.
2.5. Desorption Experiment
PANAMF (0.2 g) with the saturation adsorption of ARB was put into 40 mL of NaOH (1 g/L) solution and shaken at 250 rpm and 25 °C. This fiber was desorbed twice, and the desorption solution was gathered. The absorbance of the desorption solution was measured. The regenerated PANAMF was then washed to neutral for reuse.
3. Results and Discussion
3.1. Characterization of the Fiber Adsorbent
3.1.1. FTIR Analysis
The FTIR spectra of PAN fiber before and after the modification are shown in Figure 1. The infrared characteristic absorption peak at 2243 cm–1 corresponds to the stretching vibration absorption peak of C≡N on the polyacrylonitrile fiber,49 and the peak at 2940 cm–1 is due to the C–H stretching vibration of CH, CH2, and CH3, respectively.41 The strength of the C≡N peak for the modified fiber is significantly reduced, demonstrating that the cyano-group reacts with amino-group. The amino fibers (b–d) have a wide absorption peak at 3700–3300 cm–1, which is attributed to the N–H stretching vibration absorption peak of the amide bond. In addition, a strong and wide absorption peak appears at 1650–1455 cm–1, which is due to the superposition of C=O stretching vibration, N–H bending vibration, and C–N stretching vibration.50 All these results indicate the formation of amide bonds and grafting of amino groups after modification; that is, cyano-groups on PANF have been successfully modified.
Figure 1.

FTIR spectra of (a) PANF, (b) PANAMF, (c) PANABF, and (d) PANABMF.
3.1.2. XPS Characterization
The XPS analysis method was used to study the changes of surface element composition (Figure 2 and Table S1). After modification, the content of N in the surface decreases, while that of O and C increases. The corresponding decrease of N/C molar ratio is due to the presence of a large number of carbon chains on the fiber surface. The increase in the O/C molar ratio can be attributed to the conversion of the cyano-group to the amide group. Moreover, the peak of Br in PANQMF appears at 265 eV, which confirms the reaction between n-butyl bromide and PANAMF. The conversion of the cyano-group to amide bond after modification is also further confirmed according to the result on the high-resolution N 1s peak (Figure 2b–d). As shown in Figure 2b, these is one peak at 398.2 eV, which corresponds to the nitrile group. Compared with PAN, the N 1s spectra (Figure 3d) have peaks at 401.4 and 399.2 eV that are attributed to the O=C–N and C–N bonds in PANAMF. A new peak (Figure 2e) appears at 402.2 eV for PANQMF, which is due to the prolongation of the N element in PANQMF and the formation of the quaternary ammonium group. Therefore, tertiary amine functional groups and quaternary amine functional groups are grafted on the surface of the PAN fiber.
Figure 2.
(a) The typical XPS survey of PANF, PANAMF, PANABMF, and PANQMF; (b) N 1s spectra for PANF; (c) N 1s spectra for PANAMF; and (d) N 1s spectra for PANQMF.
Figure 3.

The SEM images of (a) PANF, (b) PANAMF, (c) PANABMF, and (d) PANQMF.
3.1.3. SEM Study
SEM figures of PANF, PANAMF, PANABMF, and PANQMF are shown in Figure 3. Compared with the modified fiber, PANF has a smoother surface and a smaller diameter. The surfaces of PANABMF and PANQMF are rougher and the wrinkle is deeper than that of PANAMF. Due to the strong alkalinity of amine modifiers, the ordered arrangement of polymer chains on the polyacrylonitrile surface is destroyed, resulting in the surface etching for PANAMF and PANABMF. As shown in Figure 3b–c, the fiber diameter increases after the modification step, which is attributed to the fact that PANF is not alkali-resistant and the swelling of the fiber occurs. However, the integrity of the fiber adsorbent is maintained and the fiber after modification can be used in the adsorption experiment. The specific surface areas of PANF, PANAMF, PANABMF, and PANQMF were also increased (Table S2). The higher specific surface area for the modified fiber also indicated that surface etching exists.
3.2. Adsorption Property
3.2.1. Effect of the Amine Modifier on Adsorption Property
The fiber adsorbents with different weight gains can be obtained by changing the amination reaction time (Table S3), and the influence of weight gain on the adsorption of AR249 is shown in Figure 4.
Figure 4.

Adsorption capacity of different amine modified fiber adsorbents.
Firstly, the adsorption capacity of each fiber adsorbent increases with the increase of tertiary amine degree. The linear relationship between adsorption degree and adsorption effect can be observed, and the slope has no significant change. The increase of tertiary amine functional degree indicates the increase of the number of dimethylamine in a unit, where the number of N(CH3)2 functional groups denotes the number of adsorption sites. PANABMF has a better adsorption capacity than PANAMF, indicating that the introduction of phenyl increases the adsorption capacity of the fiber adsorbent. The instantaneous dipoles and Π–Π conjugation effect are produced between the benzene ring on the dye and fiber. When the dyes are close to the surface of the fiber adsorbent (PANABMF), the dyes diffuse more easily into the fiber. Therefore, the adsorption capacity of PANABMF to the dye is improved.
3.2.2. Effect of the Quaternization Degree on Adsorption Property
Tertiary amine functional groups can be further quaternized, and the quaternary amine modification rate was controlled by the reaction time. PANAMF with a weight gain of 43.6% was used. As shown in Figure 5, the adsorption fiber after quaternization has a higher capacity, and the adsorption capacity of quaternary amine increases with the increase of the quaternization degree. In particular, the highest adsorption capacity (1748.7 mg/g) is gained when the tertiary amine functional group on PANAMF is almost completely modified. The quaternary amine group is more polar and has a more stable environment compared to the tertiary amine group. Dye molecule can be adsorbed by the cation group more easily than by the quaternary amine group. In fact, the unmodified tertiary amine functional group also can adsorb the dye molecule.
Figure 5.

Adsorption capacity of different amine modified fiber adsorbents.
3.2.3. Effect of the Acid Amount in Solution on Adsorption Property
The influence of the acid amount on the saturated adsorption capacity is shown in Figure 6. For PANAMF and PANABMF, with the increase in the acid concentration, the adsorption capacity to AR249 firstly increases and then decreases. Interestingly, the saturated adsorption capacity for PANAMF is 1253.5 mg/g in the nonacidic environment, and the adsorption capacity was significantly increased to 2051.3 mg/g with the addition of 0.05 mol/L of HCl. Although PANABMF exhibits a higher saturated adsorption capacity than PANAMF in the nonacidic environment, PANAMF has a higher saturated adsorption capacity when the acid amount ranged from 0.005 to 0.25 mol/L. This is due to the fact that there are more tertiary amine functional groups on PANAMF. With the introduction of H+, the dimethylamine group on PANAMF is protonated to NH(CH3)2+. Therefore, electrostatic interaction makes negatively charged AR249 dyes more easily accessible to fiber adsorbents, which improves the adsorption effect.51 However, when the acid concentration is too high in the solution, the dissociation of acid dyes will be inhibited. The formation of the ion pair in the surface is too difficult, and the adsorption effect is reduced sharply.
Figure 6.
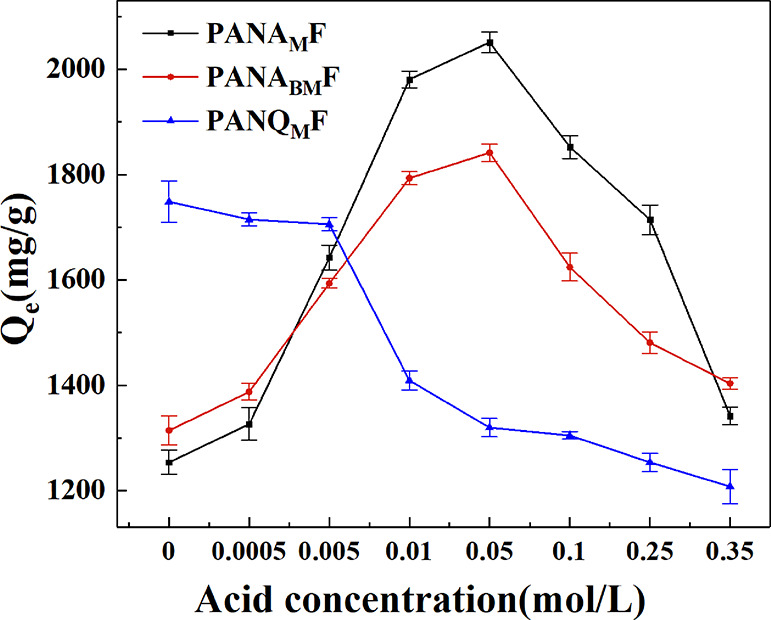
Adsorption capacity at different acid concentrations.
The increase of acid amount also has an inhibitory effect on the adsorption capacity of PANQMF. After the acid amount in the solution reaches 0.005 mol/L, the adsorption capacity of PANQMF begins to decrease. With the same modification degree, the saturated adsorption capacity for PANAMF is higher than that for PANQMF. The volume of NH(CH3)2+ is smaller than that of the quaternary ammonium group, and the charge density is higher. Therefore, more dye ion can be adsorbed.
3.2.4. Effect of Different Kinds of Anionic Dyes on Adsorption Properties
Anionic dyes commonly used in dyeing experiments include acid dyes, neutral dyes, reactive dyes, and so on. To further investigate the adsorption capacity of the modified fiber, AR249 (acid dye), DR31 (direct dye), AY128 (metal complexing dye), and RR195 (reactive dye) were used. The structure of the dyes is shown in Figure S1. It can be seen that PANAMF shows good adsorption properties for all anionic dyes of different structures and the saturated adsorption capacity is higher than 1700 mg/g (Figure 7). For AR249 dyes, the highest adsorption property is reached. This is because the anionic dyes with a small molecular structure have a high ionic diffusion rate in water and are more likely to contact with the adsorption sites of fiber. For AY128 and DR31, the large molecular weight and special structure easily cause the aggregation, resulting in a poor adsorption effect. For reactive dyes, the affinity between the dye and the fiber is increased after hydrolysis. Although the amount of the negative charge decreases, the water solubility decreases and the dye is prone to be adsorbed and diffuse into the fiber. The anionic dye adsorption capacity of the fibrous adsorbents developed in this work is compared with various other typical adsorbents reported in the literature (Table 1). As expected, this fiber adsorbent shows a higher adsorption capacity of different anionic dyes.
Figure 7.

Adsorption capacity of different dyes to PANAMF.
Table 1. Comparison of Anionic Dye Adsorption Performance of Fiber Adsorbents Reported in the Literature.
| adsorbents | dye | adsorption capacity (mg/g) | reference |
|---|---|---|---|
| PMSAE-TTDD/PMSAE-TAD | MBa | 1652.12 | (23) |
| polyaspartate-montmorillonite composite with quaternary phosphonium salt (Mt/IPS2) | MB | 7958 | (52) |
| PAN(QAS-12)F | MOb | 595.7 | (53) |
| fibrous chitosan/sodium alginate composite foams | AB172c | 817 | (54) |
| Juncus effusus (JE)-based adsorbent | AY11d | 526.3 | (55) |
| RR195e | 452.5 | ||
| DB15f | 255.1 | ||
| carbon-dot modified polyacrylonitrile fibers (PANF-g-CDs) | MO | 422 | (56) |
| layer-by-layer self-assembled dopamine/PEI fibers (KF-DOPA-PEI/GA) | MO | 85 | (57) |
| sisal fiber | RB5g | 310.2 | (58) |
Methyl blue.
Methyl orange.
Acid black 172 B.
Acid yellow 11.
Reactive red 195.
Direct blue 15.
Reactive black 5.
3.2.5. Effect of Inorganic Salt and the Surfactant in Solution on Adsorption Property
In the industrial printing and dyeing wastewater, there are not only a lot of remaining dyes but also a lot of surfactants and salts. Considering the effect of additives on the adsorption capacity, 0.02 mol/L of NaCl, Na2SO4, and NaAc; 0.2 g/L of sodium dodecyl sulfate (SDS); and 0.2 g/L of Peregal O-25 were added to the AR249 solution, respectively.
As shown in Figure 8, the addition of NaCl, NaAc, and Na2SO4 causes the decrease in the saturated adsorption capacity. It is attributed to competitive adsorption between the anions of the electrolyte and the dye anions. Sodium acetate has the most obvious inhibitory effect. This is because the ion pair of Ac– and the adsorption site is more stable and not easy to desorb. With the addition of Peregal O-25, the dye and the nonionic surfactant can form a complex, and the long ethylene oxide chain in Peregal O-25 increases the solubility of dye, resulting in the desorption of the dye. Notably, the adsorption capacity increases with the addition of SDS. SDS plays the role of buffer to open the channel of adsorptive sites owing to the small fraction and long chain segment.59,60 The binding force of SDS and adsorbent is weaker than that of dye molecules and adsorbent, while the SDS molecules can be quickly adsorbed in the surface of the fiber. The long alkane chain on the SDS can attract the dye to diffuse onto the fiber, and slowly, the SDS on the dye adsorption site is replaced by the dye.61−63
Figure 8.

Adsorption capacity of PANAMF under different additions.
3.3. Adsorption Isotherm
The adsorption isotherm was investigated according to the Langmuir, Freundlich, and Dubinin–Radushkevich models. The fitting curves of the three isothermal models are shown in Figure S2, and related parameters are listed in Table 2. For PANAMF, isotherms’ absorption curves can be fitted very well to both the Langmuir and Freundlich models, and the correlation coefficients are 0.999 and 0.979, respectively. According to the Langmuir model, the maximum adsorption capacity is 2050.3 mg/g, indicating that PANAMF’s adsorption of the AR249 dye belongs to the monolayer adsorption process. The large value for KL indicates that there is a strong acting force between the dye anion and the fiber adsorbent, and the adsorption reaction is easy to proceed. For the Freundlich model, the value of 1/n is less than 1, indicating that the adsorption process is the favorable chemical adsorption. The above results show that the adsorption is a comprehensive process of chemical adsorption and physical adsorption. The chemical adsorption based on the formation of ionic bonds is the main adsorption driving force, and the van der Waals force between the polymer chain segment and dye molecules is the secondary adsorption driving force.
Table 2. Adsorption Isotherm Parameters Obtained from Isotherm Models Fitting to the Experimental Data.
| Langmuir | Freundlich | D–R | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Qm (mg/g) | KL (L/mg) | R2 | KF (mg(1 – 1/i)·L1/n·g–1) | 1/n | R2 | Qm (mg/g) | β | E (kJ/mol) | R2 |
| 2050.3 | 0.018 | 0.999 | 939.286 | 0.115 | 0.979 | 2034.63 | 1.38 | 19.389 | 0.893 |
3.4. Kinetic Study
The kinetics of the adsorption process was studied to investigate the adsorption mechanism.64,65 The pseudo-first-order kinetics, pseudo-second-order kinetics, and particle diffusion model were used.
-
(1)The pseudo-first-order model can be expressed by the following equation:

where K1 (min–1) is the pseudo-first-order kinetic rate constant and Qe (mg/g) and Qt (mg/g) are the adsorption capacities of the adsorbent on the dye ions at the time of equilibrium and time t (h), respectively.
-
(2)The pseudo-second-order model is expressed as follows:

where K2 (min–1) is the pseudo-second-order kinetic rate constant.
-
(3)The intraparticle diffusion model is expressed as follows:

were Ki (g·mg–1·min-0.5) is the diffusion rate constant within the particle and Ci is the constant related to boundary layer thickness.
The fitting curves of different dynamics models are shown in Figure 9. The pseudo-first-order model is not fitted to describe the adsorption process. For the pseudo-second-order kinetics model, a high correlation coefficient (R2 = 0.995) is observed. The result indicated that the adsorption process was related to the surface activity points of the adsorbent. It can be seen from Figure 9c that the kinetic curve from 0 to 13 h also can be fitted well by the intraparticle diffusion model and the correlation coefficient is 0.971. For the intraparticle diffusion model, the curve does not pass through the origin, which proves that AR249 anions have a slow diffusion process and a dynamic equilibrium state is reached after adsorption saturation. Therefore, for this absorption process, the rate-determining step is the diffusion of the dye in the initial stage. With the increase in the amount of dye diffused into the fiber, the polymer chain is looser than that in the initial stage. The rate-determining step is the adsorption of the dye from the solution to the surface of the fiber.
Figure 9.
(a) Effect of contact time on AR249 adsorption by PANAMF. Linear fitting of (b) pseudo-first-order kinetics, (c) pseudo-second-order kinetics, and (d) intraparticle diffusion for adsorption of AR249 on PANAMF.
3.5. Adsorption Mechanism
The above adsorption isotherm verifies that the adsorption process is chemical adsorption. Combined with the kinetic study, as shown in Figure 10, the adsorption is initiated mainly by the electrostatic force. The dye constantly diffuses from the outside to the inside and quickly adheres to the adsorption site. When the adsorption site is filled, van der Waals force, hydrogen bonding, and dispersion force will still indicate that the dye diffuses into the fiber.66,67 The dye in the inner layer of the fiber can loosen the polymer chain, and the diffuse rate is increased.
Figure 10.
(a) The adsorption mechanism of PANAMF for AR249. (b) The binding of AR249 to PANAMF.
Notably, the degree of tertiary amine functionality is an important factor to the adsorption capacity. The high adsorption capacity of PANMF is attributed to its high degree of modification. Meanwhile, the large molecular chain of the raw PANF is arranged in a neat way, and the chain segment is hydrophobic. Therefore, the great repulsion would prevent the dye molecules from entering the fiber. The introduction of cationic amine groups weakens the binding force between the main chains in the fiber, and the degree of regularity is decreased. A diffusion pathway is opened, and the dye can diffuse into the inner layer quickly.
3.6. The Recyclability of the Fiber Absorber
The reusability of the adsorbent is the premise for the large-scale use. In this study, PANAMF was regenerated eight times in the alkaline environment. The adsorption and desorption capacities for AR249 are shown in Figure 11. Both adsorption capacity and desorption capacity firstly decrease with the cycle times and then keep steady after the fifth cycle. NaOH can react with NH(CH3)+, and the positive charge density is decreased dramatically. However, the strong combination of the dye ions and amine functional groups existed in the inner layer of the fiber. Therefore, the adsorption sites were occupied and dye anion cannot fall off. It is worth noting that PANAMF’s adsorption capacity was 1492.4 mg/g after eight cycles of adsorption, which was 72.7% of that for the first adsorption. And the structure of the fiber has no significant change after the cycles (Figure S4). Therefore, PANAMF shows good reuse performance.
Figure 11.

Reusability of PANAMF for the adsorption of AR249.
3.7. The Application of the Fiber Absorber in Continuous Adsorption
To further explore the applicability of the adsorbent, the flow adsorption system was constructed and PANAMF was used as the filler (Figure 12). It can be seen that the effluent is still in a clear state at 6 h and the pH value is 7. The solution is still clarified at 18.5 h, and the absorbance measured by the UV spectrophotometer is always less than 0.01 (Figure 13, Table S5). The adsorption value for PANAMF in this continuous system for 18.5 h is 1776 mg/g. Therefore, PANAMF has an excellent and reliable adsorption effect, which also implies that it has the potential for large-scale industrial production applications.
Figure 12.

Magnified flow adsorption system of PANAMF for the adsorption of AR249.
Figure 13.

The removal efficiency of PANAMF for AR249 in magnified flow adsorption system and absorbance of reservoir.
4. Conclusions
In conclusion, a kind of low-cost and efficient adsorbent was successfully prepared by using waste PANF as raw material. The cyanide groups on the fibers were ammoniated, and benzylamine (PANABMF) and quaternary ammonium ions were introduced. Three kinds of fiber adsorbents, PANAMF, PANABMF, and PANQMF, were obtained. The modified fiber with the higher functional group degree shows better adsorption capacity. The introduction of benzylamine and quaternary ammonium ions can decrease the polarity and increase the amount of effective active center, respectively. PANQMF showed the highest saturation adsorption value (2051.3 mg/g) for AR249 when the acid concentration was 0.05 mol/L. PANAMF also showed better reusability, and the saturation adsorption value was 1492.4 mg/g after being reused eight times. The addition of salt and additives in the solution had no significant effect on the adsorption. The adsorption thermodynamics and dynamics indicated that the adsorption process was mainly based on the formation of ionic bonds, supplemented by the van der Waals force interaction and hydrogen bond. PANAMF can be used as the adsorption filler in continuous adsorption, and the adsorption capacity reaches 1776 mg/g. In general, an environmentally friendly, low-cost, and readily available adsorbent was prepared and exhibited great potential in industrial applications.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (21606206).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c01780.
Surface element content and atomic ratio (Table S1); specific surface area (Table S2); weight gain and functional degree (Table S3); absorbance–concentration curve equation (Table S4); different dye structures (Figure S1); linear fitting of models (Figure S2); optical microscopic diameter of cross-section distribution (Figure S3); SEM of PANAMF (Figure S4); flow adsorption system of PANAMF (Figure S5); and pH value and absorbance of the reservoir (Table S5) (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Bhatia D.; Sharma N. R.; Singh J.; Kanwar R. S. Biological methods for textile dye removal from wastewater: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1836–1876. 10.1080/10643389.2017.1393263. [DOI] [Google Scholar]
- Ikehata K.; Gamal El-Din M.; Snyder S. A. Ozonation and advanced oxidation treatment of emerging organic pollutants in water and wastewater. Ozone: Sci. Eng. 2008, 30, 21–26. 10.1080/01919510701728970. [DOI] [Google Scholar]
- Tkaczyk A.; Mitrowska K.; Posyniak A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Sci. Total Environ. 2020, 717, 137222. 10.1016/j.scitotenv.2020.137222. [DOI] [PubMed] [Google Scholar]
- Jeon Y. S.; Lei J.; Kim J. H. Dye adsorption characteristics of alginate/polyaspartate hydrogels. J. Ind. Eng. Chem. 2008, 14, 726–731. 10.1016/j.jiec.2008.07.007. [DOI] [Google Scholar]
- Mosoarca G.; Vancea C.; Popa S.; Gheju M.; Boran S. Syringa vulgaris leaves powder a novel low-cost adsorbent for methylene blue removal: isotherms, kinetics, thermodynamic and optimization by Taguchi method. Sci. Rep. 2020, 10, 17676. 10.1038/s41598-020-74819-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T.; Li Y.; Du Q.; Sun J.; Jiao Y.; Yang G.; Wang Z.; Xia Y.; Zhang W.; Wang K.; Zhu H.; Wu D. Adsorption of methylene blue from aqueous solution by graphene. Colloids Surf., B 2012, 90, 197–203. 10.1016/j.colsurfb.2011.10.019. [DOI] [PubMed] [Google Scholar]
- Liu B.; Wang D.; Yu G.; Meng X. Adsorption of heavy metal ions, dyes and proteins by chitosan composites and derivatives - A review. J. Ocean Univ. China 2013, 12, 500–508. 10.1007/s11802-013-2113-0. [DOI] [Google Scholar]
- Shon H. K.; Vigneswaran S.; Kim J.-H.; Ngo H. H. Application of hybrid photocatalysis systems coupled with flocculation and adsorption to biologically treated sewage effluent for organic removal. Korean J. Chem. Eng. 2007, 24, 618–623. 10.1007/s11814-007-0013-y. [DOI] [Google Scholar]
- Abbasi M.; Asl N. R. Sonochemical degradation of Basic Blue 41 dye assisted by nanoTiO2 and H2O2. J. Hazard. Mater. 2008, 153, 942–947. 10.1016/j.jhazmat.2007.09.045. [DOI] [PubMed] [Google Scholar]
- Khodabandehloo A.; Rahbar-Kelishami A.; Shayesteh H. Methylene blue removal using Salix babylonica (Weeping willow) leaves powder as a low-cost biosorbent in batch mode: Kinetic, equilibrium, and thermodynamic studies. J. Mol. Liq. 2017, 244, 540–548. 10.1016/j.molliq.2017.08.108. [DOI] [Google Scholar]
- Buonomenna M. G.; Gordano A.; Golemme G.; Drioli E. Preparation, characterization and use of PEEKWC nanofiltration membranes for removal of Azur B dye from aqueous media. React. Funct. Polym. 2009, 69, 259–263. 10.1016/j.reactfunctpolym.2009.01.004. [DOI] [Google Scholar]
- Iqbal J.; Shah N. S.; Sayed M.; Niazi N. K.; Imran M.; Khan J. A.; Khan Z. U. H.; Hussien A. G. S.; Polychronopoulou K.; Howari F. Nano-zerovalent manganese/biochar composite for the adsorptive and oxidative removal of Congo-red dye from aqueous solutions. J. Hazard. Mater. 2021, 403, 123854. 10.1016/j.jhazmat.2020.123854. [DOI] [PubMed] [Google Scholar]
- Abebe B.; Murthy H. C. A.; Zerefa E.; Abdisa E. Porous PVA/Zn-Fe-Mn oxide nanocomposites: methylene blue dye adsorption studies. Mater. Res. Express 2020, 7, 065002 10.1088/2053-1591/ab94fc. [DOI] [Google Scholar]
- Rafatullah M.; Sulaiman O.; Hashim R.; Ahmad A. Adsorption of methylene blue on low-cost adsorbents: a review. J. Hazard. Mater. 2010, 177, 70–80. 10.1016/j.jhazmat.2009.12.047. [DOI] [PubMed] [Google Scholar]
- Mashkoor F.; Nasar A. Magsorbents: Potential candidates in wastewater treatment technology - A review on the removal of methylene blue dye. J. Magn. Magn. Mater. 2020, 500, 166408. 10.1016/j.jmmm.2020.166408. [DOI] [Google Scholar]
- Xu S.; Niu X.; Hou Z.; Gao C.; Lu J.; Pang Y.; Fang M.; Lu Y.; Chen Y.; S J. K.; Li T.; Xu J. A multifunctional gelatine-quaternary ammonium copolymer: An efficient material for reducing dye emission in leather tanning process by superior anionic dye adsorption. J. Hazard. Mater. 2020, 383, 121142. 10.1016/j.jhazmat.2019.121142. [DOI] [PubMed] [Google Scholar]
- Tony M. A. An industrial ecology approach: green cellulose-based bio-adsorbent from sugar industry residue for treating textile industry wastewater effluent. Int. J. Environ. Anal. Chem. 2021, 101, 167–183. 10.1080/03067319.2019.1661397. [DOI] [Google Scholar]
- Rathee G.; Awasthi A.; Sood D.; Tomar R.; Tomar V.; Chandra R. A new biocompatible ternary Layered Double Hydroxide Adsorbent for ultrafast removal of anionic organic dyes. Sci. Rep. 2019, 9, 16225. 10.1038/s41598-019-52849-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue X.; Huang J.; Jiang F.; Li H.; Chen Y. Synthesis and characterization of cellulose-based adsorbent for removal of anionic and cationic dyes. J. Eng. Fibers Fabr. 2019, 14, 1558925019828194. 10.1177/1558925019828194. [DOI] [Google Scholar]
- Li Z.; Chen L.; Yang Q.; Yang H.; Zhou Y. Compacted stainless steel mesh-supported Co3O4 porous nanobelts for HCHO catalytic oxidation and Co3O4@Co3S4 via in situ sulfurization as platinum-free counter electrode for flexible dye-sensitized solar cells. Appl. Surf. Sci. 2021, 536, 147815. 10.1016/j.apsusc.2020.147815. [DOI] [Google Scholar]
- Tang X.; Ran G.; Li J.; Zhang Z.; Xiang C. Extremely efficient and rapidly adsorb methylene blue using porous adsorbent prepared from waste paper: Kinetics and equilibrium studies. J. Hazard. Mater. 2021, 402, 123579. 10.1016/j.jhazmat.2020.123579. [DOI] [PubMed] [Google Scholar]
- Li L.; Ren H.; Liu Y.; Liu X.; Zhao Y.; Zhou X.; Kang W.; Zhuang X.; Cheng B. Facile construction of hierarchical porous ultrafine alumina fibers (HPAFs) and its application for dye adsorption. Microporous Mesoporous Mater. 2020, 308, 110544. 10.1016/j.micromeso.2020.110544. [DOI] [Google Scholar]
- Zhang X.; Li Z.; Lin S.; Théato P. Fibrous Materials Based on Polymeric Salicyl Active Esters as Efficient Adsorbents for Selective Removal of Anionic Dye. ACS Appl. Mater. Interfaces 2020, 12, 21100–21113. 10.1021/acsami.0c03039. [DOI] [PubMed] [Google Scholar]
- Lv Y.; Ma J.; Liu K.; Jiang Y.; Yang G.; Liu Y.; Lin C.; Ye X.; Shi Y.; Liu M.; Chen L. Rapid elimination of trace bisphenol pollutants with porous β-cyclodextrin modified cellulose nanofibrous membrane in water: adsorption behavior and mechanism. J. Hazard. Mater. 2021, 403, 123666. 10.1016/j.jhazmat.2020.123666. [DOI] [PubMed] [Google Scholar]
- Li X.; Yuan H.; Quan X.; Chen S.; You S. Effective adsorption of sulfamethoxazole, bisphenol A and methyl orange on nanoporous carbon derived from metal-organic frameworks. J. Environ. Sci. 2018, 63, 250–259. 10.1016/j.jes.2017.10.019. [DOI] [PubMed] [Google Scholar]
- Matsuiwa K.; Sugibayashi Y.; Tsubomoto Y.; Hayashi S.; Nakanishi W. Behavior of Intramolecular π-π Interactions with Doubly Degenerated Bond Paths Between Carbon Atoms in Opposite Benzene Rings of Diethenodihydronaphthalenes by QTAIM Approach. ChemistrySelect 2017, 2, 90–100. 10.1002/slct.201601494. [DOI] [Google Scholar]
- Jawad A. H.; Abdulhameed A. S.; Reghioua A.; Yaseen Z. M. Zwitterion composite chitosan-epichlorohydrin/zeolite for adsorption of methylene blue and reactive red 120 dyes. Int. J. Biol. Macromol. 2020, 163, 756–765. 10.1016/j.ijbiomac.2020.07.014. [DOI] [PubMed] [Google Scholar]
- Davarnejad R.; Afshar S.; Etehadfar P. Activated carbon blended with grape stalks powder: Properties modification and its application in a dye adsorption. Arabian J. Chem. 2020, 13, 5463–5473. 10.1016/j.arabjc.2020.03.025. [DOI] [Google Scholar]
- He C.; Lin H.; Dai L.; Qiu R.; Tang Y.; Wang Y.; Duan P.-G.; Ok Y. S. Waste shrimp shell-derived hydrochar as an emergent material for methyl orange removal in aqueous solutions. Environ. Int. 2020, 134, 105340. 10.1016/j.envint.2019.105340. [DOI] [PubMed] [Google Scholar]
- Lai K. C.; Lee L. Y.; Hiew B. Y. Z.; Thangalazhy-Gopakumar S.; Gan S. Environmental application of three-dimensional graphene materials as adsorbents for dyes and heavy metals: Review on ice-templating method and adsorption mechanisms. J. Environ. Sci. 2019, 79, 174–199. 10.1016/j.jes.2018.11.023. [DOI] [PubMed] [Google Scholar]
- Zhu Y.; Wang W.; Yu H.; Wang A. Preparation of porous adsorbent via Pickering emulsion template for water treatment: A review. J. Environ. Sci. 2020, 88, 217–236. 10.1016/j.jes.2019.09.001. [DOI] [PubMed] [Google Scholar]
- Chen C.; Kang J.; Shen J.; Zhao S.; Wang B.; Chen Z.; Chen Q. Selective and efficient removal of Hg (II) from aqueous media by a low-cost dendrimer-grafted polyacrylonitrile fiber: Performance and mechanism. Chemosphere 2021, 262, 127836. 10.1016/j.chemosphere.2020.127836. [DOI] [PubMed] [Google Scholar]
- Duan W.; Wang J.; Chang L.; Zhao L.; Tian Z.; Huang Z.; Huang W. Adsorption of mercury(II) from water by a novel sPAN fiber containing sulfhydryl, carboxyl and amino groups. RSC Adv. 2018, 8, 38259–38269. 10.1039/C8RA06998K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N.; Yang Y.; Chen S.; Zhang Q. Preparation of amine group-containing chelating fiber for thorough removal of mercury ions. J. Hazard. Mater. 2009, 171, 288–293. 10.1016/j.jhazmat.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Ji F.; Li C.; Tang B.; Xu J.; Lu G.; Liu P. Preparation of cellulose acetate/zeolite composite fiber and its adsorption behavior for heavy metal ions in aqueous solution. Chem. Eng. J. 2012, 209, 325–333. 10.1016/j.cej.2012.08.014. [DOI] [Google Scholar]
- Khamis M. I.; Ibrahim T. H.; Jumean F. H.; Sara Z. A.; Atallah B. A. Cyclic Sequential Removal of Alizarin Red S Dye and Cr(VI) Ions Using Wool as a Low-Cost Adsorbent. Processes 2020, 8, 556. 10.3390/pr8050556. [DOI] [Google Scholar]
- Marques B. S.; Frantz T. S.; Junior T. R. S. C.; de Almeida Pinto L. A.; Dotto G. L. Adsorption of a textile dye onto piacava fibers: kinetic, equilibrium, thermodynamics, and application in simulated effluents. Environ. Sci. Pollut. Res. 2019, 26, 28584–28592. 10.1007/s11356-018-3587-5. [DOI] [PubMed] [Google Scholar]
- Xu Q.; Peng J.; Zhang W.; Wang X.; Lou T. Electrospun cellulose acetate/P(DMDAAC-AM) nanofibrous membranes for dye adsorption. J. Appl. Polym. Sci. 2020, 137, 48565. 10.1002/app.48565. [DOI] [Google Scholar]
- Sun Y.; Zheng W. Polyethylenimine-functionalized polyacrylonitrile anion exchange fiber as a novel adsorbent for rapid removal of nitrate from wastewater. Chemosphere 2020, 258, 127373. 10.1016/j.chemosphere.2020.127373. [DOI] [PubMed] [Google Scholar]
- Jung Y.; Ko Y. G.; Do T.; Chun Y.; Choi U. S.; Kim C. H. Core/shell hybrid fiber with aminated PAN and Fe2O3 as a high-capacity adsorbent for phosphate ions. J. Hazard. Mater. 2019, 378, 120726. 10.1016/j.jhazmat.2019.06.003. [DOI] [PubMed] [Google Scholar]
- Du J.; Xu G.; Lin H.; Wang G.; Tao M.; Zhang W. Highly efficient reduction of carbonyls, azides, and benzyl halides by NaBH4 in water catalyzed by PANF-immobilized quaternary ammonium salts. Green Chem. 2016, 18, 2726–2735. 10.1039/C5GC02621K. [DOI] [Google Scholar]
- Lee C.-G.; Alvarez P. J. J.; Nam A.; Park S.-J.; Do T.; Choi U.-S.; Lee S.-H. Arsenic(V) removal using an amine-doped acrylic ion exchange fiber: Kinetic, equilibrium, and regeneration studies. J. Hazard. Mater. 2017, 325, 223–229. 10.1016/j.jhazmat.2016.12.003. [DOI] [PubMed] [Google Scholar]
- Zheng W.; Hu J.; Han Z.; Diesel E.; Wang Z.; Zheng Z.; Ba C.; Langer J.; Economy J. Interactions of Cr(VI) with hybrid anion exchange/porous carbon fibers in aqueous solution at natural pH. Chem. Eng. J. 2016, 287, 54–61. 10.1016/j.cej.2015.10.064. [DOI] [Google Scholar]
- Cheng Y.; He P.; Dong F.; Nie X.; Ding C.; Wang S.; Zhang Y.; Liu H.; Zhou S. Polyamine and amidoxime groups modified bifunctional polyacrylonitrile-based ion exchange fibers for highly efficient extraction of U(VI) from real uranium mine water. Chem. Eng. J. 2019, 367, 198–207. 10.1016/j.cej.2019.02.149. [DOI] [Google Scholar]
- Zhao R.; Li X.; Sun B.; Shen M.; Tan X.; Ding Y.; Jiang Z.; Wang C. Preparation of phosphorylated polyacrylonitrile-based nanofiber mat and its application for heavy metal ion removal. Chem. Eng. J. 2015, 268, 290–299. 10.1016/j.cej.2015.01.061. [DOI] [Google Scholar]
- Li X.; Li Y.; Ye Z. Preparation of macroporous bead adsorbents based on poly(vinyl alcohol)/chitosan and their adsorption properties for heavy metals from aqueous solution. Chem. Eng. J. 2011, 178, 60–68. 10.1016/j.cej.2011.10.012. [DOI] [Google Scholar]
- Kim H. S.; Park Y. H.; Kim S.; Choi Y.-E. Application of a polyethylenimine-modified polyacrylonitrile-biomass waste composite fiber sorbent for the removal of a harmful cyanobacterial species from an aqueous solution. Environ. Res. 2020, 190, 109997. 10.1016/j.envres.2020.109997. [DOI] [PubMed] [Google Scholar]
- Ma Y.; Zhang B.; Ma H.; Yu M.; Li L.; Li J. Polyethylenimine nanofibrous adsorbent for highly effective removal of anionic dyes from aqueous solution. Sci. China-Mater. 2016, 59, 38–50. 10.1007/s40843-016-0117-y. [DOI] [Google Scholar]
- Yao Y.; Liang Y.; Navik R.; Dong X.; Cai Y.; Zhang P. Modification of Polyacrylonitrile Fibers by Coupling to Thiosemicarbazones. Materials 2019, 12, 3980. 10.3390/ma12233980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P.; Liu Y.; Cao J.; Tao M.; Zhang W. Tuning the Catalytic Activity of Tertiary-Amine Functionalized Polyacrylonitrile Fibers by Adjusting the Surface Microenvironment. ChemCatChem 2017, 9, 3725–3732. 10.1002/cctc.201700515. [DOI] [Google Scholar]
- Oladipo A. A.; Gazi M.; Saber-Samandari S. Adsorption of anthraquinone dye onto eco-friendly semi-IPN biocomposite hydrogel: Equilibrium isotherms, kinetic studies and optimization. J. Taiwan Inst. Chem. Eng. 2014, 45, 653–664. 10.1016/j.jtice.2013.07.013. [DOI] [Google Scholar]
- Elsherbiny A. S.; El-Hefnawy M. E.; Gemeay A. H. Linker impact on the adsorption capacity of polyaspartate/montmorillonite composites towards methyl blue removal. Chem. Eng. J. 2017, 315, 142–151. 10.1016/j.cej.2017.01.002. [DOI] [Google Scholar]
- Zhao J.; Wang L.; Xiao J.; Tao M.; Zhang W. Removal of anionic azo dyes from aqueous solutions by quaternary ammonium salt-functionalized fibers with adjustable surface microenvironments. React. Funct. Polym. 2020, 154, 104684. 10.1016/j.reactfunctpolym.2020.104684. [DOI] [Google Scholar]
- Zhao X.; Wang X.; Lou T. Preparation of fibrous chitosan/sodium alginate composite foams for the adsorption of cationic and anionic dyes. J. Hazard. Mater. 2021, 403, 124054. 10.1016/j.jhazmat.2020.124054. [DOI] [PubMed] [Google Scholar]
- Xia L.; Zhou S.; Zhang C.; Fu Z.; Wang A.; Zhang Q.; Wang Y.; Liu X.; Wang X.; Xu W. Environment-friendly Juncus effusus-based adsorbent with a three-dimensional network structure for highly efficient removal of dyes from wastewater. J. Cleaner Prod. 2020, 259, 120812. 10.1016/j.jclepro.2020.120812. [DOI] [Google Scholar]
- Wang B.; Chen P.-Y.; Zhao R.-X.; Zhang L.; Chen Y.; Yu L.-P. Carbon-dot modified polyacrylonitrile fibers: Recyclable materials capable of selectively and reversibly adsorbing small-sized anionic dyes. Chem. Eng. J. 2020, 391, 123484. 10.1016/j.cej.2019.123484. [DOI] [Google Scholar]
- Chai F.; Wang R.; Rao P.; Zhang W.; Yan L.; Yang N.; Cai Y.; Xi C. Layer-by-layer self-assembled dopamine/PEI fibers derived from Ceiba pentandra for the anionic dye adsorption. Desalin. Water Treat. 2019, 171, 408–417. 10.5004/dwt.2019.24771. [DOI] [Google Scholar]
- Vargas V. H.; Paveglio R. R.; Pauletto P. d. S.; Salau N. P. G.; Dotto L. G. Sisal fiber as an alternative and cost-effective adsorbent for the removal of methylene blue and reactive black 5 dyes from aqueous solutions. Chem. Eng. Commun. 2020, 207, 523–536. 10.1080/00986445.2019.1605362. [DOI] [Google Scholar]
- Pan X.; Zhang M.; Liu H.; Ouyang S.; Ding N.; Zhang P. Adsorption behavior and mechanism of acid orange 7 and methylene blue on self-assembled three-dimensional MgAl layered double hydroxide: Experimental and DFT investigation. Appl. Surf. Sci. 2020, 522, 146370. 10.1016/j.apsusc.2020.146370. [DOI] [Google Scholar]
- Mathew M. L.; Gopalakrishnan A.; Aravindakumar C. T.; Aravind U. K. Low - cost multilayered green fiber for the treatment of textile industry waste water. J. Hazard. Mater. 2019, 365, 297–305. 10.1016/j.jhazmat.2018.11.014. [DOI] [PubMed] [Google Scholar]
- Sureshkumar M. V.; Namasivayam C. Adsorption behavior of Direct Red 12B and Rhodamine B from water onto surfactant-modified coconut coir pith. Colloids Surf., A 2008, 317, 277–283. 10.1016/j.colsurfa.2007.10.026. [DOI] [PubMed] [Google Scholar]
- Chatterjee S.; Lee D. S.; Lee M. W.; Woo S. H. Enhanced adsorption of congo red from aqueous solutions by chitosan hydrogel beads impregnated with cetyl trimethyl ammonium bromide. Bioresour. Technol. 2009, 100, 2803–2809. 10.1016/j.biortech.2008.12.035. [DOI] [PubMed] [Google Scholar]
- Chatterjee S.; Lee D. S.; Lee M. W.; Woo S. H. Congo red adsorption from aqueous solutions by using chitosan hydrogel beads impregnated with nonionic or anionic surfactant. Bioresour. Technol. 2009, 100, 3862–3868. 10.1016/j.biortech.2009.03.023. [DOI] [PubMed] [Google Scholar]
- Ali I.; Alothman Z. A.; Alwarthan A. Uptake of propranolol on ionic liquid iron nanocomposite adsorbent: Kinetic, thermodynamics and mechanism of adsorption. J. Mol. Liq. 2017, 236, 205–213. 10.1016/j.molliq.2017.04.028. [DOI] [Google Scholar]
- Ali I.; Alharbi O. M. L.; Alothman Z. A.; Badjah A. Y.; Alwarthan A.; Basheer A. A. Artificial neural network modelling of amido black dye sorption on iron composite nano material: Kinetics and thermodynamics studies. J. Mol. Liq. 2018, 250, 1–8. 10.1016/j.molliq.2017.11.163. [DOI] [Google Scholar]
- Qi F.-F.; Ma T.-Y.; Liu Y.; Fan Y.-M.; Li J.-Q.; Yu Y.; Chu L.-L. 3D superhydrophilic polypyrrole nanofiber mat for highly efficient adsorption of anionic azo dyes. Microchem. J. 2020, 159, 105389. 10.1016/j.microc.2020.105389. [DOI] [Google Scholar]
- Jiang Z.; Hu D. Molecular mechanism of anionic dyes adsorption on cationized rice husk cellulose from agricultural wastes. J. Mol. Liq. 2019, 276, 105–114. 10.1016/j.molliq.2018.11.153. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






