Abstract
The bioaccessibility of vitamin B12 (B12) in plant-based products fortified using wheat bran extract fermented with B12-producing food-grade Propionibacterium freudenreichii was studied by applying a standard static in vitro model. At first, a culture of P. freudenreichii, fresh or heat-treated, was subjected to in vitro assays. Then, food ingredients or products were evaluated for their in vitro bioaccessibility: spray-dried wheat bran extract powder, pasta made with an extruder using fermented bran extract and breads made with spray-dried powder or with added cyanocobalamin. B12 bioaccessibility from the fresh P. freudenreichii culture was only ca. 53%, which, when heated, increased to 73%. The bioaccessibility of B12 from the food products varied from 75% (spray-dried powder) to 95% (breads). B12 from the fortified bread was as bioaccessible as from the bread made with added cyanocobalamin (99%). The in vitro results suggest that B12 synthesized by P. freudenreichii, when fortified in the studied cereal-based products, is largely bioaccessible and could be available for absorption. Plant-based products fortified using fermentation with P. freudenreichii could thus be considered excellent sources of bioaccessible B12.
Keywords: Bioaccessibility, Vitamin B12, Fermentation, Wheat bran, Baking
Graphical abstract
Highlights
-
•
Fermented wheat bran extract or its dried form was used in the preparation of the products
-
•
Processing increased vitamin B12 bioaccessibility
-
•
Bioaccessibility of vitamin B12 from the products was >80%
-
•
Cereal products fortified using fermentation can be excellent sources of bioaccessible vitamin B12
1. Introduction
The recommendation to include more plant-based proteins in the diet (Willett et al., 2019) may increase the risk of vitamin B12 (hereafter called B12) deficiency (Green et al., 2017). Microorganisms are the primary source of B12 in nature (Watanabe and Bito, 2018) and this vitamin only exists naturally in foods of animal origin (Ball, 2006). A limited number of plant-based food sources of B12 are available, either through fortification or microbial contamination (Watanabe, 2007; Watanabe et al., 2013).
Fortification of B12 in plant-based foods using B12-producing microorganisms is an opportunity to introduce B12 into plant-based foods (Chamlagain et al., 2018; Edelmann et al., 2016). One of the proven producers of the active form of B12 with a GRAS status is Propionibacterium freudenreichii (Chamlagain et al., 2016; Deptula et al., 2017). The intracellularly biosynthesized B12 from this bacterium remains in cells unless the cell integrity is compromised, which could either be an advantage, through protecting the vitamin during food processing (Edelmann et al., 2016), or it could pose a challenge for its release during digestion. To the best of our knowledge, B12 bioaccessibility from food products fortified using P. freudenreichii has not been reported.
Food materials can be fermented for direct consumption (e.g. yoghurt like) or they can be further processed to produce final products (e.g. bread, pasta). The underlying heat treatment in the production process could affect the cell integrity, and some autolysis of the cells could be possible even during fermentation. Therefore, estimating the B12 bioaccessibility from these new B12 food sources is required.
Several static in vitro models are available for the measurement of the bioaccessibility of nutrients and food components. One of the standardized models increasingly used in bioaccessibility studies is the Minekus model (Minekus et al., 2014), which has recently been further standardized (Brodkorb et al., 2019). Dynamic in vitro models are also available, but the complexity of these models hinders their usage in routine bioaccessibility studies and static models are mostly preferred (Brodkorb et al., 2019).
In this short communication, we report on an in vitro bioaccessibility study of vitamin B12 from cell cultures of P. freudenreichii and a few B12-fortified cereal-based food products made using a wheat bran extract fermented with P. freudenreichii.
2. Materials and methods
2.1. Propionibacterium freudenreichii strain and pre-culturing
Throughout this study, the Propionibacterium freudenreichii DSM 20271 strain of dairy origin obtained from DSMZ (Braunschweig, Germany) was used as a producer of B12. The strain was pre-cultured first for three days on YEL agar plates and then in YEL medium for four days, both at 30 °C under anaerobic conditions, as described by Xie et al. (2018). The liquid YEL contained 10 g of yeast extract (LAB), 10 g of tryptone (LAB), 3.75 g of K2HPO4, 5.6 mg of MnSO4 monohydrate and 16.7 g of sodium lactate (50% w/w). For the preparation of the YEL agar medium, 10 g/L of agar was added before autoclaving.
2.2. Preparation of samples for in vitro bioaccessibility assays
2.2.1. P. freudenreichii samples
A bottle containing 150 mL of YEL was inoculated (1% v/v) with a P. freudenreichii DSM 20271 culture that had been grown for 4 days at 30 °C and then incubated under microaerophilic conditions (4 days at 30 °C) with shaking (150 rpm; Certomat H, Sartorious, France). After 4 days, the broth was studied for in vitro bioaccessibility. In addition, for the preparation of the heat-treated samples, the tubes containing the broth were heated in a boiling water bath (100 °C) for 5 min. The aim was to compare the effect of heat treatment on the release of B12 from the broths containing P. freudenreichii DSM 20271 cells.
2.2.2. Preparation of B12-enriched wheat bran extract
B12-enriched wheat bran extract was prepared in two steps. Initially, the wheat bran was bioprocessed with a commercial starter (Florapan 4 K, Lallemand, Montreal, Canada) according to the procedure described in detail by Arte et al. (2016) with an optimized bioprocessing condition (incubation at 35 °C for 24 h) in order to prepare an extract that could support better B12 production. The supernatant was then separated from the matrix by centrifugation (18000 g; 20 min at 4 °C) and its pH was adjusted to 6.4 with 10 M NaOH. In the second step, the extract was fermented with an optimized inoculum of P. freudenreichii DSM 20271 (10% v/v) for 3 days at 30 °C under microaerophilic conditions with shaking (200 rpm). The inoculum was added as cells suspended in 10-fold less water after the cells were separated from the YEL broth by centrifugation (6000 rpm; 10 min) at room temperature. The fermented bran extract was then used in the preparation of the vitamin B12-enriched spray-dried powder, extruded pasta, and breads (also with spray-dried powder) for the in vitro bioaccessibility assays.
2.2.3. Ingredients or food samples prepared with vitamin B12-rich fermented extract
2.2.3.1. Spray-dried powder
For spray drying, the dry matter content of the fermented bran extract was increased to 30% (by weight) by adding maltodextrin (MD 0955, Cerestar) with a dextrose equivalent of 5–8. A GEA NIRO A/S Mobile Minor spray dryer (Soeborg, Denmark) with a two-fluid nozzle atomizer was used with the following drying parameters: inlet temperature (air) 180 °C, outlet temperature (air) 71–73 °C, flow rate 20–35 mL/min and fan speed 2800 rpm. During the drying process, the liquid feed was stirred, and its temperature was maintained at 40–45 °C.
2.2.3.2. Extruded pasta
The buckwheat pasta was produced with the help of xanthan gum and guar gum (each 1% of the dry matter content) using a Thermo Prism PTW24 twin-screw extruder (Thermo Haake, Polylab System, Germany) with the technical details described by Ramos-Diaz et al. (2017). The diameter of the die was 5 mm. The temperature in the six sections of the extruder and the die was maintained at 70, 70, 90, 90, 90, 100 and 100 °C, respectively. The screw speed was set at 25 rpm.
The buckwheat flour and hydrocolloids (i.e. xanthan gum and guar gum) were mixed thoroughly prior to loading into the feed hopper. The fermented bran extract containing B12 was fed to the extruder at a flow rate that allowed the water content of the pasta dough to be maintained at 55%. The dry matter content of the dough contained 12% of the fermented bran extract. The resulting pasta strands were then dried in an oven (70 °C for 4 h; Sveba Dahlen, Sweden).
2.2.3.3. Bread
Wheat bread baking was performed according to a process published by Edelmann et al. (2016) using the recipe for straight-dough baking with a few modifications. Spray-dried powder was used (40 g) in 444 g of white wheat flour, with the sugar from the recipe being excluded. In addition, the baking was also performed with cyanocobalamin (CNCbl). The amount of added CNCbl in the dough was adjusted theoretically to match the B12 content of the dough prepared with the spray-dried powder. The baked breads (n = 3) were halved, and a half from each loaf was cut into small pieces, combined, and stored at −20 °C until used in the in vitro studies.
2.3. Preparation of the food samples for in vitro assays and digestive enzymes
To prepare the samples for the in vitro assays, the dried pasta was cooked in boiling water for 16 min (optimal cooking time) and drained. The cooked pasta was then crushed in a mixer (Bamix M200, Switzerland) for 10 s. Similarly, the cut bread pieces were also crushed for 10 s before the assay.
The digestion enzymes used in this study were bought from Sigma-Aldrich (Germany) and had the following measured activities (Minekus et al., 2014): α-amylase (104 U/mg) from Aspergillus oryzae used in the oral and intestinal phase, pepsin (119 U/mg) from porcine gastric mucosa used in the gastric phase, and porcine trypsin (138 U/mg) and bovine chymotrypsin (42 U/mg) used in the intestinal phase.
2.4. In vitro experiments
The in vitro assays were performed following the standardized protocol based on an international consensus developed by the COST INFOGEST network (Brodkorb et al., 2019). The activity of all the enzymes used in the assays was measured and their concentrations adjusted to the recommended enzyme activity for each in vitro test sample (Minekus et al., 2014). The simulated salivary fluid, the simulated gastric fluid and the simulated intestinal fluid were prepared as per the recommendations (Brodkorb et al., 2019; Minekus et al., 2014). Briefly, the test sample (5 g) was diluted with simulated salivary fluid (1:1) and incubated at 37 °C for 2 min at pH 7.0 under shaking (200 rpm). If less than 5 g was used (e.g. 2 g of the spray-dried powder), MilliQ water was added to adjust the weight of the sample to 5 g before the oral phase. In the next step, the bolus was then further diluted with simulated gastric fluid (1:1) including the enzyme pepsin and incubated for 2 h at pH 3.0 and 37 °C under shaking. Finally, the gastric chyme was further diluted with simulated intestinal fluid, bile salts (Sigma-Aldrich) and pancreatic enzymes (1:1) and incubated for 2 h at pH 7.0 and 37 °C under shaking. To adjust the correct volume of the mass, MilliQ water was used in each digestion step when required. The digesta from the intestinal phase were centrifuged (10,000 rpm, 4 °C, 10 min) and the supernatants were passed through the 12–15 μm qualitative paper filters (90 mm ø; VWR International, Leuven, Belgium) and were stored at −20 °C until analysed for their B12 content.
2.5. Vitamin B12 analysis and determination of bioaccessibility
The vitamin B12 content of the samples (1–5 g) and the in vitro supernatants (10 mL) was analysed according to the extraction, purification and quantitation methods explained previously (Chamlagain et al., 2015). In the case of the in vitro supernatants, the pH of the mixture was adjusted to 4.5 by the addition of a 10-fold concentrated buffer of acetic acid and sodium hydroxide (3 mL). Sodium cyanide was added in the mixture before the heat extraction in order to convert the natural forms of B12 into CNCbl. After the heat treatment, the samples were incubated (37 °C for 40 min at 150 rpm) with α-amylase, as previously explained, and finally they were purified through immunoaffinity columns and analysed with a Waters UPLC system (Chamlagain et al., 2015). The bioaccessibility (in %) was calculated from the concentration of the B12 in the digesta against the B12 content of the original samples, while taking into consideration the concentration of the blank in vitro supernatants (MilliQ water as a sample) using the following equation:
3. Results
3.1. B12 content of samples studied for in vitro bioaccessibility
The B12 contents of the food ingredients and the products (in fw) prepared with wheat bran extract fermented with P. freudenreichii are shown in Table 1. The spray-dried powder contained ca. 210 ng/g of B12, whereas the breads made with the powder or CNCbl had a B12 content of ca. 12 ng/g. On the other hand, the B12 content of the cooked extruded pasta was ca. 25 ng/g. The YEL broth fermented with P. freudenreichii had a B12 content of ca. 250 ng/g.
Table 1.
The B12 content in the P. freudenreichii broth and in food ingredients or food products made with P. freudenreichii-fermented wheat bran extract.
| Sample | B12 content (ng/g fw) average (SD), n = 3 |
|---|---|
| YEL broth (with P. freudenreichii cells) | 248.8 (19.2) |
| Spray-dried fermented bran extract powder | 209.3 (17.4) |
| Bread made with spray-dried powder | 11.8 (1.1) |
| Bread with cyanocobalamin (CNCbl) | 12.6 (1.7) |
| Extruded pasta (cooked) | 24.7 (1.4) |
3.2. Bioaccessibility of B12 from fresh or heat-treated P. freudenreichii broths
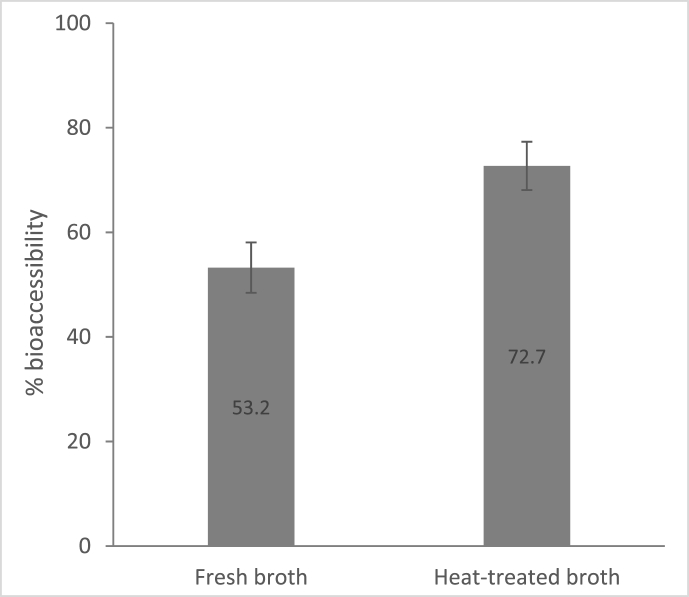
The bioaccessibility of B12 from the heat-treated (5 min at 100 °C) P. freudenreichii broths was greater than that of the fresh broths (Fig. 1). The heat-treated broths released >70% of the initial B12 content as compared to just over 50% for the fresh broths.
Fig. 1.
B12 bioaccessibility of fresh or heat-treated P. freudenreichii broths grown in YEL medium. The values are the averages of two independent experiments with three replicates. The error bars represent the standard deviation.
3.3. Bioaccessibility of B12 from fortified cereal ingredients and food products
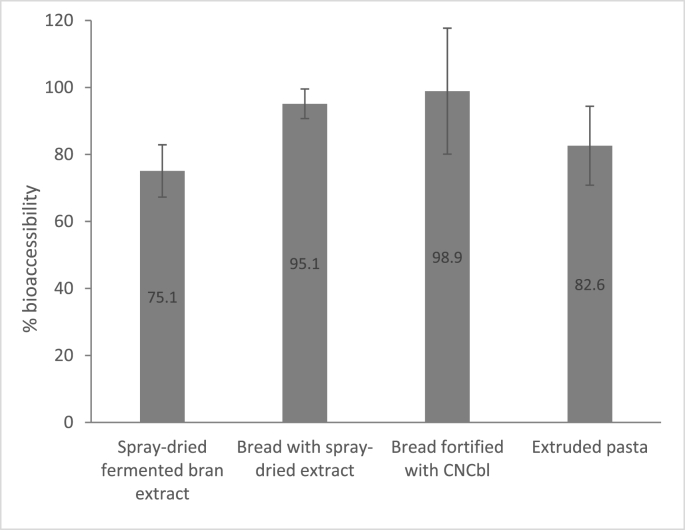
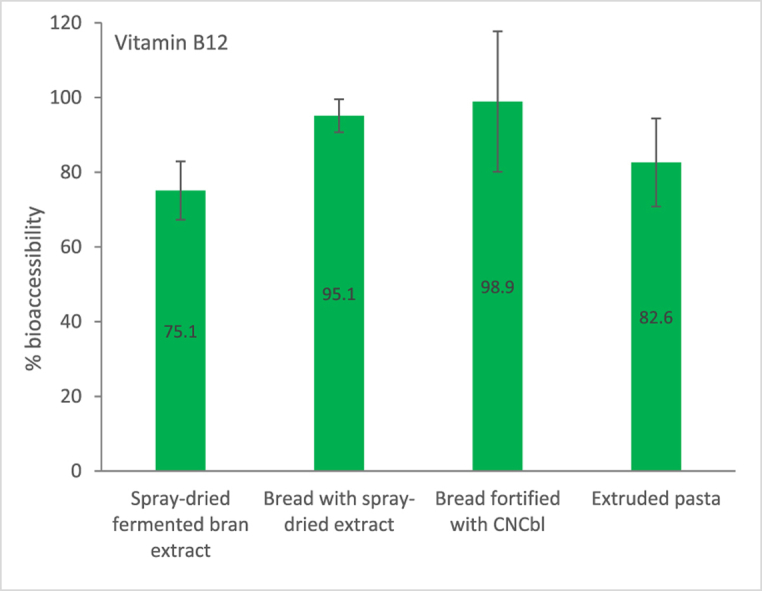
The average bioaccessibility of B12 from the spray-dried powder was 75%, while a slightly higher value was obtained for the cooked extruded pasta, with a bioaccessibility of 83% (Fig. 2). The B12 from the breads was more bioaccessible than from the spray-dried powder or the cooked pasta (Fig. 2). The breads made either with CNCbl or B12-fortified spray-dried powder were almost 100% B12 bioaccessible in the in vitro assays.
Fig. 2.
Bioaccessibility of B12 from food ingredients and products made with bran extract fermented with P. freudenreichii. The values are the averages of a single experiment with three replicates. The error bars represent the standard deviation. CNCbl = cyanocobalamin.
4. Discussion
In this study, P. freudenreichii DSM 20271 was used as a B12 producer (Chamlagain et al., 2018; Edelmann et al., 2016; Xie et al., 2018) to fortify cereal-based products with the active form of B12. B12 bioaccessibility was then determined using an in vitro method. To the best of our knowledge, this is the first study to report the bioaccessibility of B12 from plant-based products fortified using P. freudenreichii. We first assessed the bioaccessibility of B12 from P. freudenreichii cells grown in a laboratory medium and then from food ingredients and products made with a food-grade medium (wheat bran extract) fermented using this bacterium.
The results revealed that the bioaccessibility of B12 from the fresh broth was barely over 50%. This suggests that half of the B12 that was initially present in the cells was still not released, as further confirmed by the detection of the remaining B12 content in the in vitro residues (data not shown). Since B12 biosynthesized by P. freudenreichii remains inside the cells (Hugenschmidt et al., 2010), the resistance of P. freudenreichii cells to the in vitro digestion conditions used in this study could have affected the release of B12 from the cells. P. freudenreichii strains vary considerably in terms of their survival under gastrointestinal conditions (Suomalainen et al., 2008; Thierry et al., 2011) and the growth phase of the bacterium may also affect cell lysis (Lemée et al., 1994; Østlie et al., 1995) and the subsequent release of B12. However, a brief heat treatment (5 min at 100 °C) allowed for the better release of B12 from the cells during the digestion procedure, with more than 70% of the initial B12 released from the broth samples. A further heat treatment (up to 10 min) did not result in an additional increase in bioaccessibility (data not shown). Such heat treatments exist in the preparation of spray-dried powder and the production of the extruded pasta and breads included in this study. The severity of the heat treatment and other physical forces differ among the food applications, which may affect cell lysis, and the B12 bioaccessibility of the products could be significantly affected.
The bioaccessibility of B12 from the breads fortified with the spray-dried B12-enriched powder (95.1%) was as high as its bioaccessibility from the breads fortified with CNCbl (98.9%), suggesting that B12 in the fortified bread could be highly bioaccessible and available for absorption. Nonetheless, the B12 bioaccessibility from the spray-dried powder was >70%, and its bioaccessibility further improved when incorporated into the bread. Likewise, the higher bioaccessibility of B12 incorporated into pasta made with the extrusion process (>80%) shows that most of the B12 from the cooked extruded pasta was released during in vitro digestion. These bioaccessibility results for the products align with the finding of increased bioaccessibility when the cell culture was heat-treated, suggesting that heat-induced cell lysis could be one of the main factors affecting B12 bioaccessibility. For example, the bioaccessibility increased when the spray-dried powder was included in the baking process as compared to its bioaccessibility as such. Similarly, the bioaccessibility of B12 from the cooked extruded pasta where heat was applied twice (in extrusion and then in cooking) was better than that of the spray-dried powder where heat was applied just once during the drying step.
Future work could examine B12 bioaccessibility using different P. freudenreichii strains, including those strains containing a potentially protective surface layer (S-layer) (Frohnmeyer et al., 2018) to better understand cell lysis under different food-processing conditions (heat-treated or not). The bioaccessibility of B12 from different food products made using such strains needs to be evaluated.
CRediT authorship contribution statement
Bhawani Chamlagain: Experimental work, Supervision, Writing – original draft, Writing – review & editing. Liisa Peltonen: Experimental work. Minnamari Edelmann: Supervision, Writing – review & editing. Jose Martin Ramos-Diaz: Experimental work, Writing – review & editing. Asmo Kemppinen: Experimental work, Writing – review & editing. Kirsi Jouppila: Supervision, Writing – review & editing. Pekka Varmanen: Supervision, Writing – review & editing. Vieno Piironen: Resources, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The study was funded by the Academy of Finland (project number 325784) and the samples used in this study were prepared for a project funded by the Lantmännen Research Foundation.
References
- Arte E., Katina K., Holopainen-Mantila U., Nordlund E. Effect of hydrolyzing enzymes on wheat bran cell wall integrity and protein solubility. Cereal Chem. 2016;93(2):162–171. doi: 10.1094/CCHEM-03-15-0060-R. [DOI] [Google Scholar]
- Ball G.F.M. CRC Press; Boca Raton, FL: 2006. Vitamins in Foods: Analysis, Bioavailability, and Stability. [Google Scholar]
- Brodkorb A., Egger L., Alminger M., Alvito P., Assunção R., Ballance S., Recio I. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019;14(4):991–1014. doi: 10.1038/s41596-018-0119-1. [DOI] [PubMed] [Google Scholar]
- Chamlagain B., Deptula P., Edelmann M., Kariluoto S., Grattepanche F., Lacroix C., Piironen V. Effect of the lower ligand precursors on vitamin B12 production by food-grade Propionibacteria. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2016;72:117–124. doi: 10.1016/j.lwt.2016.04.023. [DOI] [Google Scholar]
- Chamlagain B., Edelmann M., Kariluoto S., Ollilainen V., Piironen V. Ultra-high performance liquid chromatographic and mass spectrometric analysis of active vitamin B12 in cells of Propionibacterium and fermented cereal matrices. Food Chem. 2015;166:630–638. doi: 10.1016/j.foodchem.2014.06.068. [DOI] [PubMed] [Google Scholar]
- Chamlagain B., Sugito T.A., Deptula P., Edelmann M., Kariluoto S., Varmanen P., Piironen V. In situ production of active vitamin B12 in cereal matrices using Propionibacterium freudenreichii. Food Sci. Nutr. 2018;6(1):67–76. doi: 10.1002/fsn3.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deptula P., Chamlagain B., Edelmann M., Sangsuwan P., Nyman T.A., Savijoki K., Varmanen P. Food-like growth conditions support production of active vitamin B12 by Propionibacterium freudenreichii 2067 without DMBI, the lower ligand base, or cobalt supplementation. Front. Microbiol. 2017;8:368. doi: 10.3389/fmicb.2017.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmann M., Chamlagain B., Santin M., Kariluoto S., Piironen V. Stability of added and in situ-produced vitamin B12 in breadmaking. Food Chem. 2016;204:21–28. doi: 10.1016/j.foodchem.2016.02.071. [DOI] [PubMed] [Google Scholar]
- Frohnmeyer E., Deptula P., Nyman T.A., Laine P.K.S., Vihinen H., Paulin L., Savijoki K. Secretome profiling of Propionibacterium freudenreichii reveals highly variable responses even among the closely related strains. Microbial Biotechnology. 2018;11(3):510–526. doi: 10.1111/1751-7915.13254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R., Allen L.H., Bjørke-Monsen A.L., Brito A., Guéant J.L., Miller J.W. Vitamin B12 deficiency. Nature Reviews Disease Primers. 2017;3:1–19. doi: 10.1038/nrdp.2017.40. [DOI] [PubMed] [Google Scholar]
- Hugenschmidt S., Schwenninger S.M., Gnehm N., Lacroix C. Screening of a natural biodiversity of lactic and propionic acid bacteria for folate and vitamin B12 production in supplemented whey permeate. Int. Dairy J. 2010;20(12):852–857. doi: 10.1016/j.idairyj.2010.05.005. [DOI] [Google Scholar]
- Lemée R., Rouault A., Guezenec S., Lortal S. Autolysis of 57 strains of dairy propionibacteria. Lait. 1994;74(4):241–251. doi: 10.1051/lait:1994420. [DOI] [Google Scholar]
- Minekus M., Alminger M., Alvito P., Ballance S., Bohn T., Bourlieu C., Brodkorb A. A standardised static in vitro digestion method suitable for food - an international consensus. Food & Function. 2014;5(6):1113–1124. doi: 10.1039/c3fo60702j. [DOI] [PubMed] [Google Scholar]
- Østlie H., Floberghagen V., Reinbold G., Hammond E.G., Vegarud G., Langsrud T. Autolysis of dairy propionibacteria: growth studies, peptidase activities, and proline production. J. Dairy Sci. 1995;78(6):1224–1237. doi: 10.3168/jds.S0022-0302(95)76742-X. [DOI] [Google Scholar]
- Ramos Diaz J.M., Sundarrajan L., Kariluoto S., Lampi A.-M., Tenitz S., Jouppila K. Partial least squares regression modeling of physical and chemical properties of corn-based snacks containing kañiwa and lupine. J. Food Process. Eng. 2017;40(2) doi: 10.1111/jfpe.12396. [DOI] [Google Scholar]
- Suomalainen T., Sigvart-Mattila P., Mättö J., Tynkkynen S. In vitro and in vivo gastrointestinal survival, antibiotic susceptibility and genetic identification of Propionibacterium freudenreichii ssp. shermanii JS. Int. Dairy J. 2008;18:271–278. doi: 10.1016/j.idairyj.2007.09.004. [DOI] [Google Scholar]
- Thierry A., Deutsch S.M., Falentin H., Dalmasso M., Cousin F.J., Jan G. New insights into physiology and metabolism of Propionibacterium freudenreichii. Int. J. Food Microbiol. 2011;149:19–27. doi: 10.1016/j.ijfoodmicro.2011.04.026. [DOI] [PubMed] [Google Scholar]
- Watanabe F. Vitamin B12 sources and bioavailability. Exp. Biol. Med. 2007;232:1266–1274. doi: 10.3181/0703-MR-67. [DOI] [PubMed] [Google Scholar]
- Watanabe F., Bito T. Vitamin B12 sources and microbial interaction. Exp. Biol. Med. 2018;243(2):148–158. doi: 10.1177/1535370217746612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe F., Yabuta Y., Tanioka Y., Bito T. Biologically active vitamin B12 compounds in foods for preventing deficiency among vegetarians and elderly subjects. J. Agric. Food Chem. 2013;61:6769–6775. doi: 10.1021/jf401545z. [DOI] [PubMed] [Google Scholar]
- Willett W., Rockström J., Loken B., Springmann M., Lang T., Vermeulen S., Murray C.J.L. Food in the Anthropocene: the EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet. 2019;393(10170):447–492. doi: 10.1016/S0140-6736(18)31788-4. [DOI] [PubMed] [Google Scholar]
- Xie C., Coda R., Chamlagain B., Edelmann M., Deptula P., Varmanen P., Katina K. In situ fortification of vitamin B12 in wheat flour and wheat bran by fermentation with Propionibacterium freudenreichii. J. Cereal. Sci. 2018;81:133–139. doi: 10.1016/j.jcs.2018.05.002. [DOI] [Google Scholar]