Abstract
Background: Staphylococcus aureus is a common human pathogen capable of causing diverse illnesses with possible recurrent infections. Although recent studies have highlighted the role of cellular immunity in recurrent infections, the mechanism by which S. aureus evades host responses remains largely unexplored.
Methods: This study utilizes in vitro and in vivo infection experiments to investigate difference of pro-inflammatory responses and subsequent adaptive immune responses between adsA mutant and WT S. aureus strain infection.
Findings: We demonstrated that adenosine synthase A (AdsA), a potent S. aureus virulence factor, can alter Th17 responses by interfering with NLRP3 inflammasome-mediated IL-1β production. Specifically, S. aureus virulence factor AdsA dampens Th1/Th17 immunity by limiting the release of IL-1β and other Th polarizing cytokines. In particular, AdsA obstructs the release of IL-1β via the adenosine/A2aR/NLRP3 axis. Using a murine infection model, pharmacological inhibition of A2a receptor enhanced S. aureus-specific Th17 responses, whereas inhibition of NLRP3 and caspase-1 downregulated these responses. Our results showed that AdsA contributes to recurrent S. aureus infection by restraining protective Th1/Th17 responses.
Interpretation: Our study provides important mechanistic insights for therapeutic and vaccination strategies against S. aureus infections.
Keywords: Staphylococcus aureus, T cell responses, NLRP3 inflammasome, Adenosine synthase A
Research in context.
Evidence before this study
Staphylococcus aureusis becoming one of the most common bacterial infections across the world, posing a major threat to current health care system. The difficulty in developing efficient treatments lies in lack of understanding of its pathogenic mechanisms. Previous works conducted by other groups have identified several roles of AdsA in the pathogenesis of S. aureus by suppressing innate immune responses. These include impediment of phagocytosis of neutrophils, initiating apoptosis of macrophages, downregulating the secretion of type IIA secretory phospholipase A2. However, there is no studies reporting the influence of AdsA on adaptive immunity, especially on T cell responses. This work used in vitro and in vivo infection experiments to investigate how AdsA dampen the development of host anti-S. aureus adaptive immunity.
Added value of this study
This work demonstrated that AdsA can inhibit the development of protective T cell responses by reducing production of relevant pro-inflammatory cytokines during S. aureus infection. Moreover, we further identify that AdsA evades the protective Th17 immunity by constraining the activation of NLRP3 inflammasome and IL-1β production. Consequently, this work provides new evidence that S. aureus dampens host adaptive immunity via AdsA, contributing to recurrent infection.
Implications of all the available evidence
Understanding of how AdsA interferes with the establishment of long-term protective immunity facilitates the drugs and vaccines development which aim to improve specific cellular immunity, ameliorating immunosuppressive environment elicited by S. aureus.
Alt-text: Unlabelled box
1. Introduction
Staphylococcus aureus (S. aureus) is one of the most common causes of community-acquired and healthcare-associated bacterial infections [1]. S. aureus infections lead to a variety of clinical manifestations ranging from skin and soft-tissue infections to invasive diseases including blood stream infections, endocarditis, and sepsis [2]. Moreover, the emergence of methicillin-resistant S. aureus (MRSA) further highlights the severe threat of S. aureus infections as a major global public health concern [3]. Notably, prior exposure to S. aureus does not always confer protection against subsequent S. aureus infection, particularly in some situations such as recurrent skin infection [4,5]. Our lack of understanding of how S. aureus constrains protective immunity has impeded the development of efficient treatments against S. aureus infections.
Using different infection models, several host factors have been implicated in the protection against S. aureus. These protective responses include the complement system [6], neutrophils [7], macrophages [8], IL-17A producing γδ+ T cells [9], humoral responses [10], and Th1 and Th17 immune responses [8,11]. Although humoral responses have long been recognized as a critical indicator of anti-S. aureus immunity, individuals with robust S. aureus-specific antibody responses are not always protected from the next infection, indicating additional host factors may also play essential roles in establishing efficient immunity against S. aureus [12]. Recently, accumulating evidence has shed light on the role of cellular immunity in preventing the course of S. aureus infection. Patients with diseases that cause defects in Th17 differentiation display increased susceptibility toward S. aureus infection [13]. Th17 cells can potentiate bacterial killing by enhancing phagocytosis by neutrophils via the secretion of IL-17 family cytokines (IL-17A and IL-17F) [14]. Memory Th1 cells have also been reported to accelerate the clearance of S. aureus in blood stream infections [8]. Recurrent infections in a large number of patients implies the inability to establish protective T cell responses during S. aureus infection. So far, only O-acetyltransferase (OatA) has been shown to suppress the development of protective Th17 immune responses by interfering with cytokine milieu involved in Th development [15]. Consequently, it is of significant importance to investigate the mechanisms by which S. aureus counteracts host cellular immunity that contribute to recurrent infections.
The inflammasome is another crucial host factor involved in anti-S. aureus immunity [16]. The inflammasome is an intracellular multiprotein complex that is assembled upon detection of specific pathogenic signals or danger signals such as flagellin, toxins, and silica particles [17,18]. In S. aureus infection, inflammasomes are widely activated, which triggers a specific form of cell death termed pyroptosis, as well as the secretion of potent inflammatory cytokines such as IL-1β [18,19] that are involved in Th17 development [20]. Importantly, one of the most studied inflammasomes, NACHT, LRR and PYD domains-containing protein 3 (NLRP3) inflammasome has been shown to be triggered by extracellular pore-forming toxins (PFTs) [21,22] or peptidoglycan (PGN) [23] from S. aureus. In a skin infection model, NLRP3/IL-1β/IL-1R signaling was demonstrated to recruit neutrophils to restrict infection [24].
Adenosine synthase A (AdsA) is an important immune evasion factor by which S. aureus modulates host pro-inflammatory responses, resulting in a prolonged infection [25]. The well-defined enzymatic activity of AdsA facilitates the degradation of ATP, ADP, and AMP to adenosine [26], and the conversion of neutrophil extracellular traps (NETs) to deoxyadenosine [27]. In our previous work, our bioinformatic analysis showed that AdsA was prevalent in most of the S. aureus isolates [28]. Previous studies have shown that AdsA can inhibit phagocytic clearance [29] and secretion of antibacterial peptide sPLA2-IIA [30], and induce apoptosis of macrophages [27] via adenosine signaling or deoxyadenosine signaling. However, the interaction of AdsA with the host adaptive immunity remains unclear. In this study, we provide evidence that AdsA contributes to recurrent S. aureus infection by restraining protective Th1 and Th17 responses. Specifically, AdsA interferes with Th17 development by attenuating NLRP3-mediated IL-1β production through the adenosine/A2AR pathway. Overall, our study revealed a novel mechanism that contributes to S. aureus recurrent infection, which provides potential mechanistic insights for therapeutic and vaccination strategies.
2. Materials and methods
2.1. Reagents and primers
Reagents and primer sequences are detailed in Table S1 in Supplementary materials.
2.2. Cell culture
THP-1 monocytic cell line (American Type Culture Collection Cat# TIB-202, RRID:CVCL_0006) was validated by the ATCC using short tandem repeat analysis. THP-1 cells were cultured in RPMI-1640 supplemented with 10% heat-inactivated fetal bovine serum (HI-FBS), 100 U/mL penicillin, and 0.1 mg/mL streptomycin. For infection experiment, THP1 were firstly differentiated into macrophages using 50 nM Phorbol 12-myristate 13-acetate (PMA) for 24 h. After stimulation, cells were washed with 1640-RPMI medium and cultured with medium without PMA for 24 h. Cells were tested for mycoplasma contamination by The University of Hong Kong, LKS Faculty of Medicine core facility.
2.3. Human primary cell culture
Human peripheral blood mononuclear cells (PBMC) were isolated from human buffy coat (provided by Department of Microbiology, The University of Hong Kong) by Ficoll-Paque gradient protocol. In brief, 30 mL of 1:1 PBS diluted buffy coat from healthy donors was layered on Ficoll-Paque Plus (GE Healthcare, Life Sciences) and centrifuged at 450 x g for 30 min at room temperature. Separated layers of PBMC were collected and washed two times with RPMI-1640 medium. After washing, cells were resuspended in 4 mL red blood cell lysing buffer (Biolegend, RBC Lysis Buffer) and incubated for 5 min at room temperature. Following two subsequent washes, the cell pellet was resuspended in RPMI-1640 medium supplemented with 10% FBS, 100 U/mL penicillin, and 0.1 mg/mL streptomycin for use in further infection experiments. To differentiate human monocyte-derived macrophages (HMDM), isolated PBMC were seeded on poly-L-lysine-coated coverslips in 24-well plate and cultured in RPMI-1640 medium supplemented with L-glutamine, 10% FBS, 1 × penicillin/streptomycin, 10 mM HEPES, and 50 ng/mL hGM-CSF (PeproTech) for up to 7 days differentiation.
2.4. Mice dendritic cell culture
Bone marrow cells extracted from femur of 8 to 12-week-old female BALB/c mice were cultured in RPMI-1640 medium supplemented with L-glutamine, 10% heat inactivated-FBS, 1 × penicillin/streptomycin, 10 mM HEPES, 50 μM β-mercaptoethanol, and 20 ng/mL mGM-CSF (PeproTech) for up to 7 days differentiation.
2.5. Bacterial strains
S. aureus USA300 strain (provided by Professor Richard Yi-Tsun Kao, USA300 FPR3757 MRSA) and its isogenic adsA variant were grown in Brain Heart Infusion (BHI) at 37 °C. Unmarked, non-polar deletion of adsA was constructed using plasmid pKOR1 as described previously [31]. Briefly, 5’- and 3’-flanking regions of adsA were PCR amplified from chromosomal DNA of S. aureus USA300 strain with primers AdsA-UF (5’ CGGAATTCTGCGGCTCATGCAATGAC 3’), AdsA-UR (5’ GGCACTGACATGTTCGAGACTTGCCATAATC 3’), AdsA-DF (5’ AGTCTCGAACATGTCAGTGCCTAAAGGTAG 3’), and AdsA-DR (5’ GGGGTACCTCCCTACAGCTAAAATGG 3’). Individual PCR products were then mixed to generate an in-frame deletion pattern of adsA. The overlapping amplicon containing the in-frame deletion pattern was sub-cloned into pKOR1, to generate pKOR1-ΔadsA. The recombinant plasmid pKOR1-ΔadsA was introduced into DH5a, followed by electro-transformation into S. aureus RN4220 and subsequently into USA300. The selection of allelic replacement was performed as described previously. The deletion of AdsA was further confirmed by PCR using primers AdsA-UF/AdsA-DR and inner primers AdsA-IF (5’ TATCCATGGCCGACTAGC 3’)/AdsA-IR (5’ ACCTGTTTGTGCCAATGC 3’) specific for the deleted sequence and DNA sequencing. The adsA complemented plasmid padsA was constructed from pALC2084 by replacing the tetracycline-inducible promoter with adsA promoter (700 bp upstream of the start site) and replacing the GFP reporter gene with the full length sequence of adsA. The complemented plasmid padsA was electro-transformed into S. aureus RN4220 and subsequently into USA300.
2.6. Animals
Animal care and experimental protocols were performed in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care guidelines (www.aaalac.org) and with approval from our institutional animal care and use committee at The University of Hong Kong (CULATR5163-19). BALB/c and C57BL/6 mice were provided by the Laboratory Animal Unit, The University of Hong Kong. Nlrp3−/− and Casp1−/− transgenic mice were provided by Dr. Pamaela Pui Wai Lee. Mice were housed in specific pathogen-free facilities and 8 to 12-week-old female mice were utilized for all in vitro and in vivo experiments.
2.7. Cell infection experiments
One day before the bacterial infection experiments, S. aureus strains were inoculated and cultured with BHI broth overnight. Next day, the overnight culture of bacteria were sub-cultured in fresh BHI broth at a dilution of 1:100 and grown at 37°C. Following 3 h of culture, S. aureus was harvested and washed two to three times in cold PBS with centrifugation. Finally, S. aureus strains were diluted in PBS to the desired volume, yielding an OD600 of 0.5 (1 × 108 CFU/mL), followed by further centrifugation and resuspension to the required bacterial concentration. The number of bacteria was determined by serial dilution and colony formation on BHI agar plates. Mammalian cells were plated in 24-well plates at 4 × 105 cells/well and infected with S. aureus strains in antibiotic-free medium at the indicated MOI.
2.8. Animal infection experiments
The protocol for harvesting and calculating WT and variant S. aureus strains was the same as above. Before in vivo infection, age-matched mice were weighed and randomly allocated into each group. To induce the systemic blood infection model of S. aureus, 200 µL of bacterial suspension (1 × 107 CFU) was administered intravenously into naïve 6-week-old female BALB/c mice. To establish the reinfection model, mice were intraperitoneally infected with WT S. aureus USA300 strain, adsA mutant strain, or WT/adsA mutant strains (4 × 107 CFU) for a total of three times at 7-day intervals. After 14 days convalescent period, mice were rechallenged with a high dose of WT (4 × 107 CFU) or low dose of WT (2 × 107 CFU). After bacterial infection, the health condition of mice were frequently monitored in compliance with the humane end points (HEP) form. To measure staphylococcal burden in the blood after 2 h of i.v. infection, mice were anesthetized by intraperitoneal injection of 80–120 mg ketamine and 3–6 mg xylazine per kilogram of body weight and blood was collected via tail vein. Blood samples were incubated on ice in 0.5% saponin/PBS for host cell lysis. Serial dilutions were performed on BHI agar plates for colony formation. To enumerate bacteria in tissues, mice were euthanized by CO2 inhalation, and organs including lungs, spleens and kidneys were harvested and homogenized in 1% Triton X-100/PBS. Aliquots of homogenates were serially diluted and spread on BHI agar plates for colony formation. For histopathology, kidneys were incubated in 4% paraformaldehyde (PFA) at room temperature for 24 h. Tissues were embedded in paraffin, thin-sectioned, and stained with hematoxylin-eosin for examination by microscopy.
2.9. In vitro restimulation of splenocytes
Animals were sacrificed at indicated time points in the reinfection model. Spleens were harvested and grinded into a cell suspension. After centrifugation, splenocytes were subject to red blood cell lysing, washing, and filtering. The single-cell suspension was cultured in RPMI-1640 media supplemented with 10% FBS, 100 U/mL penicillin and 0.1 mg/mL streptomycin. For restimulation, splenocytes were seeded on 24-well plates at 4 × 105 cells/well and stimulated with heat-killed S. aureus at a MOI of 5 for 4 days. Cultured supernatants were collected for measurement of cytokines by ELISA. For intracellular cytokine analysis, splenocytes were seeded on 24-well plates at 4 × 105 cells/well and stimulated with heat-killed S. aureus at a MOI of 5 for 1 day in the presence of brefeldin A. The following antibodies were used for flow cytometry analysis: anti-Mouse IL-17A APC (cat. 506915; Biolegend), anti-Mouse IFN-ɣ Brilliant Violet 421™ (cat. 505830; Biolegend), anti-Mouse CD3 FITC (Thermo Fisher Scientific Cat# 11-0032-82, RRID:AB_2572431), and anti-Mouse CD4 PE-Cyanine7 (Thermo Fisher Scientific Cat# 25-0041-82, RRID:AB_469576).
2.10. BMDC stimulation and flow cytometry analysis
After differentiation, BMDC were plated in 24-well plates at 4 × 105 cells/well and infected with S. aureus strains at the indicated MOI. For surface marker analysis, cells were detached with PBS containing 5 mM EDTA and then incubated in FACS buffer (PBS containing 3% FBS and 0.1% sodium azide). Cells were incubated with purified neutralizing monoclonal antibodies against CD16:CD32 (Fc Block; Biolegend) for 15 min at 4 °C. Next, cells were stained with specific antibodies for 30 min at 4 °C in the dark. The following antibody were used for flow cytometry analysis: anti-Mouse I-A/I-E FITC (BD Biosciences Cat# 553623, RRID:AB_394958), anti-Mouse CD86 PE (BD Biosciences Cat# 553692, RRID:AB_394994), and anti-Mouse CD40 PE (BD Biosciences Cat# 553791, RRID:AB_395055). The stained cells were analyzed by flow cytometry (ACEA NovoCyte Quanteon) with FlowJo 10.4.0 software (TreeStar, Co).
2.11. Cell viability assay and ELISA
Cultured supernatants of relevant cells were collected and centrifuged at 13,000 rpm for 4 min to remove cell debris and bacteria. Levels of LDH in cultured supernatants were measured by CytoTox 96 Non-Radioactive Cytotoxicity Assay kit (Promega). Cell viability was measured by CellTiter Glo Luminescent Cell Viability Assay (Promega). ELISA assay was performed according to the manufacturer's instructions.
2.12. Generation of CRISPR-Cas9 knockout cell lines
All THP1 knockout cell lines used in this study were generated using Cas9-encoding lentiCRISPRv2 vector from Zhang Feng lab (Addgene plasmid #52961). Single guide RNAs (sgRNAs) targeting human AIM2, NLRP3, PYCARD and caspase-1 were designed using the online sgRNA Designer from Broad Institute. All sgRNAs were annealed and cloned into plasmid lentiCRISPRv2 according to Zhang Feng's protocol. The lentiviral particles were produced from HEK293T cells transfected with lentiCRISPRv2 vector and two packaging plasmids pMD2.G and psPAX2 (Addgene plasmids #12259 and #12260) using PEI-MAX (Polysciences), followed by further concentration by ultracentrifugation. THP-1 cells were transduced by spinoculation in the presence of 8 μg/mL polybrene. A polyclonal population was selected using 1 mg/mL puromycin for at least 1 week. Genetic ablation was verified by Western blot analysis.
2.13. RNA interference
Sequences of siRNAs are listed in Table S1. All siRNAs were designed according to previously published studies and synthesized by GenePharma (Shanghai, China). The control siRNA (negative control) was provided by GenePharma. Lipofectamine® RNAiMAX Reagent (Invitrogen) was used for transient transfection of siRNAs into BMDC. At 48-72 h after transfection, BMDC were prepared for the bacterial infection experiment.
2.14. Western blotting
For detection of the cleaved form of caspase-1, cell culture supernatants were precipitated by methanol-chloroform method. The supernatant was mixed with an equal volume of methanol and 0.25 volumes of chloroform, vortexed, and then centrifuged for 15 min at 20,000 x g. The upper phase was discarded and the interphase was mixed with methanol. After centrifugation for 5 min at 20,000 x g, the pellet was resuspended in 2x SDS-PAGE sample buffer and boiled for 5 min at 100 °C. Protein samples were separated by 15% SDS-PAGE and then transferred onto PVDF membranes. Total cell lysates were prepared in RIPA buffer (50 mM Tris-Cl, pH 7.4, 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 1% sodium deoxycholate and 0.5 mM EDTA) supplemented with 1x protease inhibitor cocktail (Roche) and boiled for 5 min at 100 °C. Lysate aliquots were separated by SDS-PAGE and transferred onto PVDF membranes. Blots were probed with primary antibody: rabbit anti-caspase-1 (1:1000, Abcam, Cat# ab179515, RRID:AB_2884954), mouse anti-β-actin (1:5000, Sigma-Aldrich, Cat# A5316, RRID:AB_476743) and rabbit anti-NLRP3 (1:1000, Cell Signaling Technology, Cat# 15101, RRID:AB_2722591). Anti-rabbit/mouse antibodies conjugated to HRP were used as secondary reagents. All the antibodies are authenticated by companies and relevant documentations were provided in Supplemental Data.
2.15. RNA isolation and quantitative reverse transcription PCR
Total RNA was extracted from cells using TRIzol Reagent (Invitrogen) and 1 μg total RNA was used for reverse transcription (Takara) according to manufacturer's instructions. The cDNA was used for the quantitative RT-PCR analysis using Applied Biosystems StepOnePlus™ Real-Time PCR System and SYBR Premix Ex Taq kit (Takara) according to the manufacturer's instructions. Primers of the genes of interest are listed in Table S1. The data were normalized to GAPDH and fold change in gene expression was calculated using the comparative CT method (2−ΔΔCT).
2.16. Statistical analysis
Data were presented as mean ± SD. Data from in vitro experiments were assumed to follow a normal distribution. Unpaired Student's t test was used to compare means from two groups. One-way analysis of variance (ANOVA) with Bonferroni correction was used to compare means among multiple groups. For data not following normal distribution, non-parametric Mann-Whitney U test was used. Log-rank test was used for the survival analysis. All statistical tests were performed in GraphPad Prism 8.0 and Microsoft Excel. A P value less than 0.05 was considered to be statistically significant.
2.17. Ethics statement
All the animal experiments were done in compliance with the NIH Guide for the Care and Use of Laboratory Animals (National Academies Press, 2011) and were approved by the Animal Ethics committees of The University of Hong Kong.
2.18. Role of funding source
Funders providing financial support for this study do not participate in study design, data collection, data analyses, interpretation, or writing of report.
3. Results
3.1. S. aureus adsA mutant strain elicits potent inflammatory responses
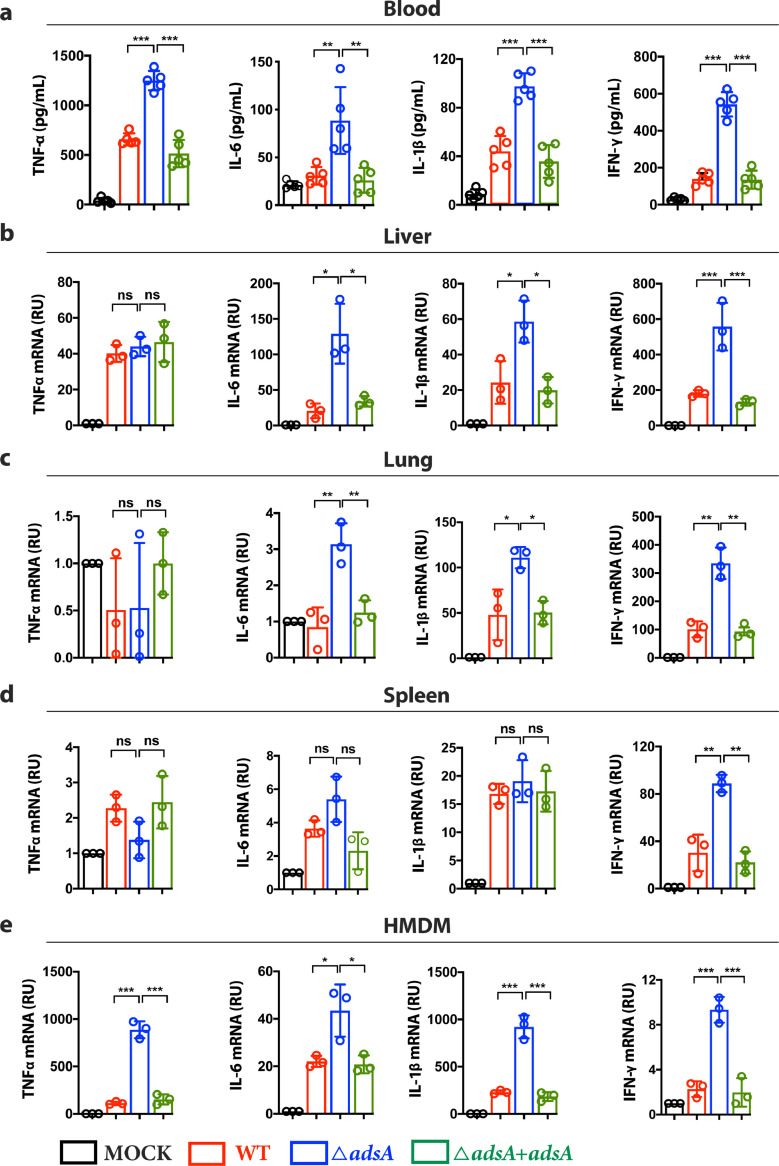
To explore the role of AdsA in modulating host immune responses, we first generated an adsA mutant strain based on USA300 background by allelic replacement [31]. BALB/c mice were infected with intravenous (i.v.) injection of 107 CFU S. aureus USA300 adsA mutant, adsA-complemented strain, or wild type (WT) USA300 strain. The level of representative pro-inflammatory cytokines in the blood was quantified by enzyme-linked immunosorbent assay (ELISA). Our results showed that mice infected with adsA mutant strain had significantly higher levels of TNF-α, IL-6, IL-1β, and IFN-ɣ compared with mice infected with WT or adsA-complemented strains (Fig. 1a) Consistent with previous work [29], mice infected with adsA mutant displayed enhanced bacterial clearance in the blood (Fig. S1a, b). Quantitative real-time polymerase chain reaction (qRT-PCR) analysis of tissue samples from infected mice showed significantly upregulated mRNA expressions of IL-6 and IL-1β in the liver and lung, and IFN-ɣ in the liver, lung, and spleen in response to adsA variant infection (Fig. 1b, c, d). These effects were not caused by differences in the bacterial load in these tissues, as colony-forming units (CFUs) recovered from the two groups were comparable (Fig. S1c, d). In agreement with the observations in mice, primary human monocyte-derived macrophages (HMDM) infected with adsA mutant showed upregulated mRNA levels of TNF-α, IL-6, IL-1β, and IFN-ɣ compared with cells treated with WT or complemented strains (Fig. 1e). Collectively, these findings suggest that AdsA suppresses the production of pro-inflammatory cytokines during systemic S. aureus infection.
Figure 1.
- S. aureusadsA mutant strain elicits potent inflammatory responses. (a) ELISA analysis of TNF-α, IL-6, IL-1β, and IFN-ɣ in blood collected from BALB/c mice infected with wild type (WT) USA300 strain, adsA mutant strain, or adsA complemented strain (107 CFU, i.v., 3 h post-infection) (n = 5 mice per group; ⁎⁎P < 0.005,⁎⁎⁎P < 0.001, Mann-Whitney U test, Data are shown as mean ± SD). (b) qPCR analysis of TNF-α, IL-6, IL-1β, and IFN-ɣ mRNA expression in liver harvested from BALB/c mice infected with WT, adsA mutant, or adsA complemented strains (107 CFU, iv, 24 h post-infection) (n = 3; *P < 0.05, ***P < 0.001, n.s. P > 0.05, Student's t test, Data are shown as mean ± SD). (c) qPCR analysis of TNF-α, IL-6, IL-1β, and IFN-ɣ mRNA expression in lung harvested from BALB/c mice infected with WT, adsA mutant, or adsA complemented strains (107 CFU, i.v., 24 h post-infection) (n = 3; *P < 0.05, ⁎⁎P < 0.005, n.s. P > 0.05, Student's t test, Data are shown as mean ± SD). (d) qPCR analysis of TNF-α, IL-6, IL-1β, and IFN-ɣ mRNA expression in spleen collected from BALB/c mice infected with WT, adsA mutant, or adsA complemented strains (107 CFU, i.v., 24 h post-infection) (n = 3; ⁎⁎P < 0.005, n.s. P > 0.05, Student's t test, Data are shown as mean ± SD). (e) qPCR analysis of TNF-α, IL-6, IL-1β and IFN-ɣ mRNA expression in HMDM infected by WT strain or adsA mutant strain (MOI=100; 8 h) (n = 3; *P < 0.05, ⁎⁎⁎P < 0.001, Student's t test, Data are shown as mean ± SD). Data are representative of three independent experiments.
3.2. S. aureus inhibits inflammasome activation via AdsA and adenosine production
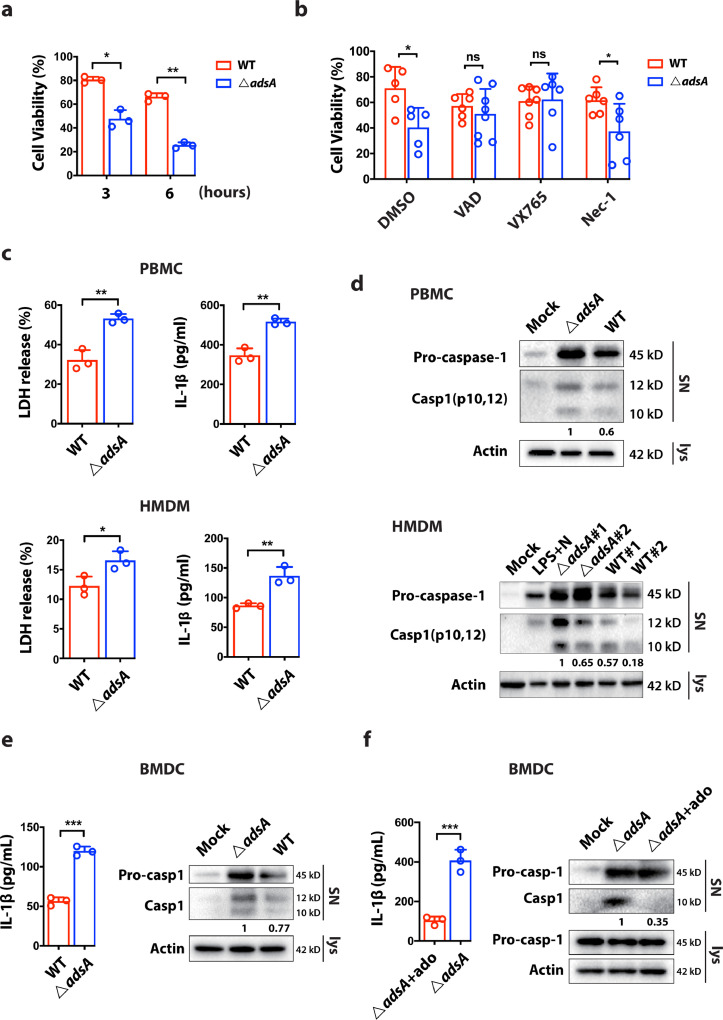
Upon infection by S. aureus, host immune responses are widely activated including the production of pro-inflammatory cytokines and the recruitment of neutrophils to the site of infection. Many of these responses are involved in the activation of inflammasomes. Inflammasome have two major biological roles: (i) the maturation and secretion of potent inflammatory cytokine IL-1β and (ii) the induction of pyroptosis [19]. We demonstrated that mice intravenously infected with adsA mutant strain had significantly higher IL-1β level in the blood compared with mice infected with the WT strain (Fig. 1a), implying that S. aureus AdsA might suppress inflammasome activation. To further examine the activation of inflammasome in responses to S. aureus infection, we examined the viability of HMDM after infection with either WT or the adsA mutant strains. The cell viability assay showed that adsA mutant killed 75% of HMDM at 6 h post-infection, whereas the wild type strain only induced cell death in 34% of infected HMDM at the same time point (Fig. 2a). To further dissect the mode of cell death (i.e., apoptosis, pyroptosis, or necroptosis) induced by the mutant strain, specific pharmacological inhibitors were used to block the corresponding cell death executor. Our data demonstrated that treatment with pan-caspase inhibitor Z-VAD-FMK or caspase-1 inhibitor VX765 rescued adsA mutant-induced cell death, indicating AdsA might attenuate caspase-1 activity during S. aureus infection (Fig. 2b). We further examined the effect of AdsA on caspase-1-dependent inflammasome activation by lactate dehydrogenase (LDH) release assay, ELISA for detection of IL-1β, and immunoblot analysis of caspase-1 activation. The LDH release assay showed that AdsA prevented pyroptosis, and ELISA detected IL-1β production in HMDM and peripheral blood mononuclear cells (PBMC) (Fig. 2c). In line with above findings, the immunoblot analysis demonstrated the wild type strain impeded caspase-1 activation (cleaved p10 and p12 band) in human macrophages and monocytes from different donors compared with the adsA mutant (Fig. 2d). Consistently, we obtained similar results from THP-1-derived macrophages (Fig. S2a). Activation of inflammasomes in different cell types can initiate different immune responses. As dendritic cells (DC) serve as a central bridge for adaptive immunity, we next explored the role of AdsA in regulating IL-1β release from DC. Our results showed that AdsA suppressed IL-1β release and caspase-1 cleavage in mouse bone marrow-derived dendritic cells (BMDC) (Fig. 2e). A previous study reported that AdsA could facilitate adenosine production by catalyzing the degradation of adenosine monophosphate (AMP) [29]. Adenosine is a physiological immune modulator, which inhibits the secretion of pro-inflammatory cytokines. However, its role in IL-1β production during S. aureus infection is unclear. We speculated that wild type S. aureus may suppress inflammasome activation by AdsA-mediated generation of adenosine. In agreement with our hypothesis, IL-1β release and immunoblot analysis in BMDC infected with adsA mutant showed impaired inflammasome activation when pretreated with adenosine (Fig. 2f). Taken together, these results demonstrate that AdsA attenuates caspase-1-dependent inflammasome activation and IL-1β secretion in immune cells, inhibiting host immunity during the S. aureus infection.
Figure 2.
- S. aureus inhibits inflammasome activation via AdsA and adenosine production. (a) Analysis of cell viability changes in HMDM after infection with wild type (WT) USA300 strain or ΔadsA mutant strain (MOI=100; 3 to 8 h) (n=3; *P < 0.05, ⁎⁎P < 0.005, Student's t test, Data are shown as mean ± SD). (b) Analysis of cell viability changes in HMDM after infection with WT strain or adsA mutant strain (MOI=100; 8 h) in the absence or presence of indicated drugs [Z-VAD-FMK, 25 μM; VX765, 20 μM; Necrostatin-1 (Nec-1), 5 μM] (n=3; *P < 0.05,⁎⁎P < 0.005, n.s. P > 0.05, Student's t test, Data are shown as mean ± SD). (c) Analysis of LDH release and IL-1β release in PBMC and HMDM after infection (MOI=100; 6 h) (n=3; *P < 0.05,⁎⁎P < 0.005, Student's t test, Data are shown as mean ± SD). The numbers under the lanes indicate the cleaved caspase-1 band intensity, which was normalized to β-actin. (d) Western blot analysis of indicated proteins in the supernatant (SN) or cell lysate in PBMC and HMDM after infection with WT strain or adsA mutant strain (MOI=100; 6 h; #1 donor 1 and #2 donor 2). The numbers under the lanes indicate the cleaved caspase-1 band intensity, which was normalized to β-actin. (e) Analysis of IL-1β release and immunoblot of indicated proteins in the supernatant (SN) or cell lysate in BMDC after infection with WT strain or adsA mutant strain (MOI=20; 6 h) (n=3; ⁎⁎⁎P < 0.0001, Student's t test, Data are shown as mean ± SD). The numbers under the lanes indicate the cleaved caspase-1 band intensity, which was normalized to β-actin. (f) Analysis of IL-1β release and immunoblot of indicated proteins in the supernatant (SN) or cell lysate in BMDC after infection with adsA mutant strain (MOI=20; 6 h) in the presence or absence of adenosine (100 μM) (n=3; ⁎⁎⁎P < 0.001, Student's t test, Data are shown as mean ± SD). The numbers under the lanes indicate the cleaved caspase-1 band intensity, which was normalized to β-actin. Data are representative of two independent experiments.
3.3. AdsA dampens NLRP3 inflammasome-mediated IL-1β release via the adenosine/A2AR axis
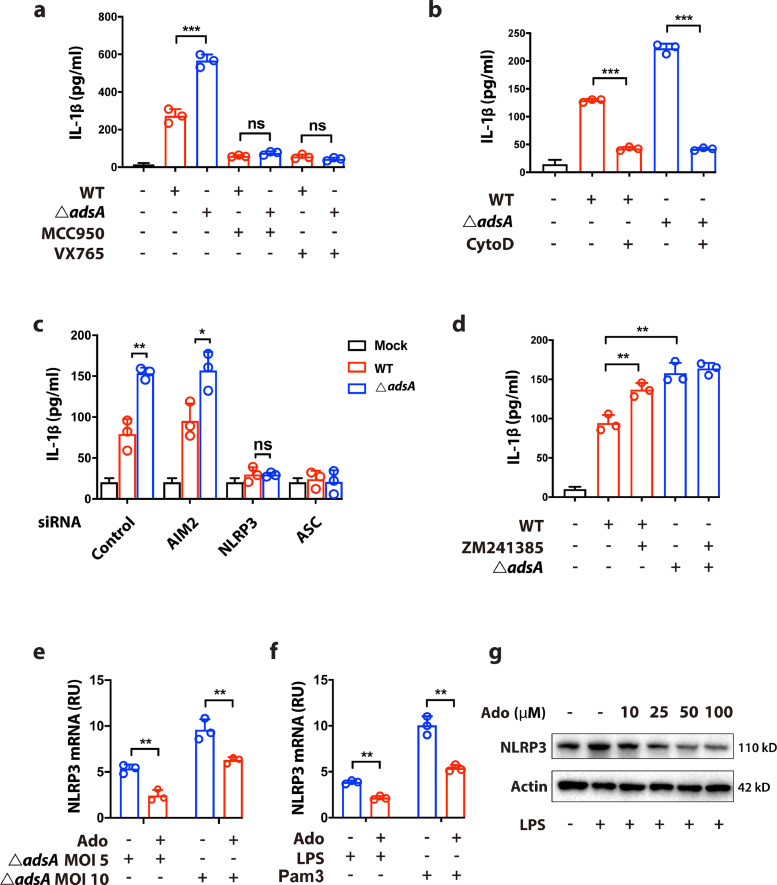
As DC are professional antigen-presenting cells (APC) and are critical mediators for initiating T lymphocytes lineage differentiation, inflammasome activation in DC could have a profound influence on cellular immunity. We, therefore, sought to further delineate the detailed mechanism by which AdsA attenuates inflammasome activation in BMDC by using pharmacological inhibitors and siRNA-mediated knockdown. Previous reports demonstrated that phagocytosis-linked PGN degradation is essential for NLRP3 inflammasome activation during S. aureus infection [23]. To determine whether NLRP3 inflammasome is affected by AdsA, BMDC infected with WT or adsA mutant were treated with NLRP3-specific inhibitor MCC950. The results showed that IL-1β release in BMDC during S. aureus infection was primarily induced through NLRP3, as NLRP3 inhibitor MCC950 largely dampened IL-1β production to similar levels after caspase-1 inhibitor VX765 treatment (Fig. 3a). Consistent with previous studies, pretreatment with Cytochalasin D, a potent inhibitor of actin polymerization and phagocytosis, potently suppressed IL-1β production in BMDC (Fig. 3b). The MCC950 treatment largely prevented the release of IL-1β in BMDC infected with either WT or adsA mutant strains, indicating NLRP3 inflammasome mostly contributes to the release of IL-1β upon S. aureus infection, implying a role of AdsA in modulating NLRP3 inflammasome. To rule out any potential off-target effects due to the pharmacological inhibitors, we conducted infection experiments in BMDC with siRNA-mediated knockdown of key genes involved in inflammasome signaling, including AIM2 (absent in melanoma 2, an intracellular DNA sensing inflammasome), NLRP3, and ASC (PYD and CARD domain containing protein, a common downstream molecule of AIM2 and NLRP3) (Fig. S3a). The results showed that IL-1β production was significantly decreased in BMDC with knockdown of NLRP3 and ASC, but not AIM2, which further confirms that only NLRP3 inflammasome is activated in S. aureus infection (Fig. 3c). Similar results were observed in THP-1-derived macrophages with individually ablated AIM2, NLRP3, ASC, and caspase-1 by CRISPR-Cas9 editing (Fig. S3b).
Figure 3.
- Adenosine synthase A dampens NLRP3 inflammasome-mediated IL-1β release via A2a receptor. (a) Analysis of IL-1β release in BMDC infected with wild type (WT) USA300 strain or adsA mutant strain (MOI=20; 6 h) in the absence or presence of indicated drugs (MCC950, 10 μM; VX765, 10 μM) (n=3; n.s. P > 0.05, ⁎⁎⁎P < 0.001, Student's t test, Data are shown as mean ± SD). (b) Analysis of IL-1β release in BMDC infected with WT strain or ΔadsA mutant strain (MOI=20; 6 h) in the absence or presence of indicated drug (Cytochalasin D, 5 μM) (n=3; ⁎⁎⁎P < 0.001, Student's t test, Data are shown as mean ± SD). (c) Analysis of IL-1β release after siRNA-mediated knockdown of Aim2, Nlrp3 and Asc in BMDC infected with WT strain or ΔadsA mutant strain (MOI=20; 6 h) (n=3; n.s. P > 0.05, *P < 0.05, ⁎⁎P < 0.005, Student's t test, Data are shown as mean ± SD). (d) Analysis of IL-1β release in BMDC infected with WT strain or ΔadsA mutant strain (MOI=20; 6 h) in the absence or presence of indicated drug (ZM241385, 10 μM) (n=3; ⁎⁎⁎P < 0.001, Student's t test, Data are shown as mean ± SD). (e) qPCR analysis of NLRP3 expression in indicated BMDC infected with adsA mutant strain (MOI=5 or 10; 4 h) in the absence or presence of adenosine (100 μM) (n=3; ⁎⁎P < 0.005, Student's t test, Data are shown as mean ± SD). (f) qPCR analysis of NLRP3 expression in indicated BMDC treated with LPS (100 ng/mL) or Pam3CSK4 (100 ng/mL) for 4 h in the absence or presence of adenosine (100 μM) (n=3; ⁎⁎P < 0.005, Student's t test, Data are shown as mean ± SD). (g) Immunoblot analysis of NLRP3 protein level in BMDC pretreated with a series of doses of adenosine and stimulated with LPS (100 ng/mL) for 4 h. Data are representative of two independent experiments.
The immune modulatory characteristics of adenosine are attributed to its four trans-membrane receptors: A1, A2A, A2B, and A3 [32]. Activation of these receptors can induce pro-inflammatory or anti-inflammatory effects. The abundance and distribution of these four receptors vary in different cell types and tissues. Among them, A2A receptor (A2AR) is known for its anti-inflammatory role in immune cells. Hence, we evaluated whether the AdsA/adenosine/A2AR signaling pathway is implicated in NLRP3 inflammasomes in dendritic cells. Our result showed that addition of ZM241385, a selective pharmacological inhibitor of A2AR in BMDC infected with WT strain increased IL-1β production to a level comparable to adsA mutant infection (Fig. 3d). We examined whether the attenuated production of IL-1β mediated by the adenosine/A2AR signaling was due to the perturbation of the priming signal of NLRP3 inflammasome. Our results showed that adenosine treatment in BMDC decreased the mRNA expression level of NLRP3 when stimulated by live S. aureus, TLR2 agonist (Pam3CSK4), or TLR4 agonist (LPS), respectively (Fig. 3e, f). Moreover, adenosine also inhibited NLRP3 expression at the protein level in a dose-dependent manner (Fig. 3g). Taken together, our results suggest that AdsA specifically inhibits NLRP3 inflammasome activation via adenosine/A2AR axis in dendritic cells.
3.4. AdsA inhibits DC maturation and perturbs cytokine milieu for optimal T cell immunity
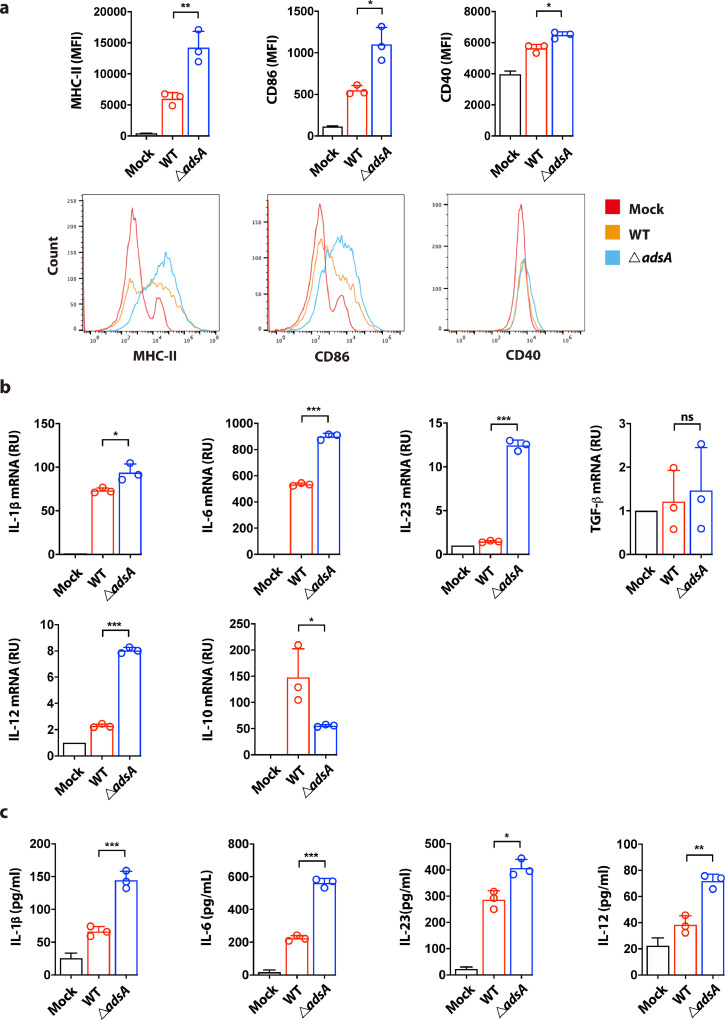
Increasing evidence shows that inflammasomes help establish adaptive immunity through promoting the production of danger signals or bioactive cytokines [33]. The pro-inflammatory cytokine IL-1β can manipulate extensive immune responses through IL-1R signaling in a paracrine or autocrine manner. As AdsA can inhibit IL-1β production in BMDC, we next sought to characterize the role of AdsA in modulating dendritic cell function, particularly the cytokine milieu for developing protective immunity. We first evaluated the activation and maturation of BMDC under in vitro settings. Flow cytometry analysis showed the BMDC infected with adsA variant displayed higher expressions of DC maturation markers including CD40, CD86, and major histocompatibility complex (MHC) II in comparison to WT strain infection (Fig. 4a). Furthermore, mRNA expression and ELISA analysis showed that AdsA inhibited production of Th17-polarizing cytokines including IL-1β, IL-6, and IL-23 (with the exception of TGF-β) in S. aureus-infected BMDC (Fig. 4b & c). In contrast, IL-10, an anti-inflammatory cytokine reported to inhibit Th17 development, was upregulated in BMDC infected with the WT strain compared with the mutant strain (Fig. 4b). Intriguingly, we also found that Th1-polarizing cytokine IL-12 was significantly induced at both mRNA and protein levels in BMDC infected with adsA mutant (Fig. 4b & c). Overall, our data indicate that Adenosine synthase A can hinder maturation of dendritic cells and suppress production of Th polarizing cytokines, implying a role of AdsA in coordinating subsequent Th1/Th17 immunity.
Figure 4.
- Adenosine synthase A inhibits DC maturation and perturbs cytokine milieu for optimal T cell immunity. (a) Flow cytometric analysis of the expression of maturation markers including MHC-II, CD86, and CD40 in BMDC infected with wild type (WT) USA300 strain or ΔadsA mutant strain (MOI=10; 16 h) (n=3; *P < 0.05, ⁎⁎P < 0.005, Student's t test, Data are shown as mean ± SD). (b) qPCR analysis of IL-1β, IL-6, IL-23, TGF-β, IL-12, and IL-10 mRNA expression in BMDC infected with WT strain or adsA mutant strain (MOI=10; 8 h) (n=3; *P < 0.05, ⁎⁎⁎P < 0.001, n.s. P > 0.05, Student's t test, Data are shown as mean ± SD). (c) ELISA analysis of IL-1β, IL-6, IL-23, and IL-12 in culture supernatant collected from BMDC infected with WT strain or adsA mutant strain (MOI=10; 8 h) (n=3; *P < 0.05, ⁎⁎P < 0.005, ⁎⁎⁎P < 0.001, Student's t test, Data are shown as mean ± SD). Data are representative of two independent experiments.
3.5. AdsA suppresses Th17 responses via NLRP3 inflammasome and A2AR pathway in vivo
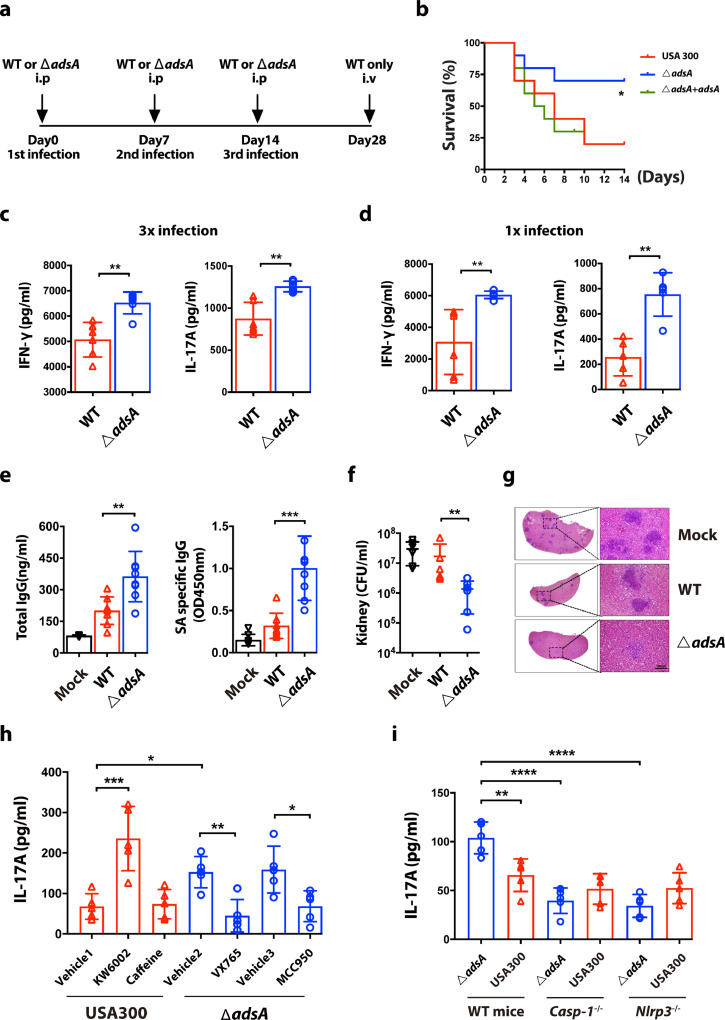
To assess the impact of AdsA on adaptive immunity, we adopted a murine reinfection model described elsewhere [8]. In this model, BALB/c mice were infected with intraperitoneal (i.p.) injection of WT or adsA mutant strains for a total of three times. At day 28 after the last infection, mice in both groups were rechallenged with a high or low dose of WT S. aureus USA300 (Fig. 5a). Importantly, the survival rate of mice repeatedly infected with adsA mutant (70%) was significantly higher (p < 0.05, Log rank test) than in mice infected with the WT strain (20%) or complemented strain (30%) (Fig. 5b). This result indicates that prior exposure to adsA mutant confers better protection to the host against subsequent S. aureus infections, which reveals that AdsA is a key immune evasion factor of S. aureus in suppressing the establishment of protective immunity. To examine whether AdsA has an impact on Th1/Th17 responses, spleens were harvested from infected mice on day 7 and 20 (1st and 3rd i.p. infection, respectively). Extracted splenocytes were stimulated with heat-killed S. aureus and cultured for ELISA detection of IL-17A and IFN-ɣ production. ELISA and intracellular cytokine staining analysis of splenocytes showed that mice challenged with adsA mutant had increased production of IL-17A and IFN-ɣ, indicating enhanced Th17 and Th1 responses in these mice compared with mice infected with WT (Fig. 5c & d, Fig. S4). In regard to humoral responses, adsA variant immunization induced higher levels of total IgG and S. aureus-specific IgG in the serum compared with the WT immunization group (Fig. 5e). Similar results for antibody responses were also observed in mice with first i.p. infection (Fig. S5). The adsA mutant immunization elicited a better immune response and conferred improved protection compared with WT immunization. When challenged with low dose of S. aureus, the bacterial load in the kidneys of mice repeatedly treated with adsA mutant was significantly lower than in mice treated with the WT strain (Fig. 5f). Histological analysis showed the kidneys of mice in mock and WT treatment groups developed more and larger abscesses compared with the adsA mutant immunization group (Fig. 5g).
Figure 5.
- Adenosine synthase A restrains Th17 response via NLRP3 inflammasome and A2aR pathway. (a) Schematic diagram for S. aureus intraperitoneal reinfection model. (b) Survival of 3x reinfected BALB/c mice rechallenged with wild type (WT) USA300 strain [4 × 107 CFU, i.v. (intravenously)] for 14 days (n = 10 mice per group; *P < 0.05, Kaplan-Meier survival curves were generated, Log rank test was performed). (c) ELISA analysis of IL-17A and IFN-ɣ in culture supernatant from splenocytes (harvested from 3x infection mice) restimulated by heat-killed S. aureus USA300 (MOI=5, 4 days) (n = 6 mice per group; ⁎⁎P < 0.005, Mann-Whitney U test, Data are shown as mean ± SD). (d) ELISA analysis of IL-17A and IFN-ɣ in culture supernatant from splenocytes (harvested from 1x infection mice) restimulated by heat-killed S. aureus USA300 (MOI=5, 4 days) (n = 6 mice per group; ⁎⁎P < 0.005, Mann-Whitney U test, Data are shown as mean ± SD). (e) ELISA analysis of mean total and S. aureus-specific IgG levels in the serum from mice reinfected with WT strain or adsA mutant strain (4 × 107 CFU, i.p.) at day 17 (n = 8 mice per group; ⁎⁎P < 0.005, ⁎⁎⁎P < 0.001, Mann-Whitney U test, Data are shown as mean ± SD). (f) CFU analysis of staphylococcal burden in kidneys from 3x reinfected BALB/c mice rechallenged with WT strain for 3 days (2 × 107 CFU, iv) (n = 6 mice per group; ⁎⁎P < 0.005, Mann-Whitney U test, Data are shown as mean ± SD). (g) Representative kidney H&E-stained histologic sections at 3 days in the reinfection model, Scale bars, 100 µm. (h) ELISA analysis of IL-17A in culture supernatant from splenocytes (harvested from 3x infection mice treated with indicated inhibitors and vehicle control) restimulated by heat-killed S. aureus USA300 (MOI=5, 4 days) (n = 5 mice per group; *P<0.05, ⁎⁎P < 0.005, ⁎⁎⁎P<0.001, ANOVA followed by Bonferroni correction, Data are shown as mean ± SD). (i) ELISA analysis of IL-17A in culture supernatant from splenocytes (harvested from 3x infection wild type C57BL/6 mice, Casp1−/− and Nlrp3−/− mice) restimulated by heat-killed S. aureus USA300 (MOI=5, 4 days) (n = 5 mice per group; ⁎⁎P < 0.005, ⁎⁎⁎P<0.001, ANOVA followed by Bonferroni correction, Data are shown as mean ± SD). Data are representative of two independent experiments.
The role of inflammasome/IL-1β/IL-1R signaling in the development of antigen-specific Th17 responses is well defined [20,34]. Our in vitro experiments using BMDC indicated that AdsA dampens NLRP3 inflammasome-mediated IL-1β release via A2A receptor (Fig. 3e). We next evaluated whether the adenosine/A2AR/NLRP3 axis contributes to Th17 development during the course of S. aureus infection using an in vivo model. In line with in vitro infection experiments, wild type S. aureus challenge in mice pretreated with A2AR-specific antagonist KW6002 developed stronger Th17 responses than in mice pretreated with the vehicle control. However, treatment with caffeine, a pan adenosine receptor antagonist, did not influence Th17 immunity (Fig. 5h). Furthermore, upon wild type S. aureus challenge, mice pretreated with caspase-1 inhibitor VX765 or NLRP3 inhibitor MCC950 showed decreased IL-17A production compared with mice pretreated with vehicle controls, indicating a role of NLRP3 inflammasome in the differentiation of S. aureus-specific Th17 immunity. In addition, Casp1−/− and Nlrp3−/− mice showed no differences in the development of Th17 responses when immunized with WT S. aureus USA300 or its isogenic adsA variant strains (Fig. 5i). Taken together, we demonstrated that AdsA suppresses Th17 immunity in vivovia the adenosine/A2AR/NLRP3 axis, contributing to recurrent S. aureus infection.
4. Discussion
A characteristic of S. aureus infection is that it can impair and evade the host immunity, leading to persistent and recurrent infections [4]. In particular, it was reported that a failure to establish T cell responses is critical in recurring S. aureus infections [8,15]. In this study, we demonstrated that AdsA can suppress the production of pro-inflammatory cytokines, which are important in the development of protective T cell responses. In addition, we also revealed a mechanism of AdsA in the evasion of protective host Th17 immunity through downregulating NLRP3 inflammasome-mediated IL-1β release. Overall, our findings extend our understanding of host-pathogen interactions during S. aureus infection.
As the inflammasome is crucial for innate immunity signaling in host-pathogen interactions, it stand to reason that it also plays an essential role in the pathogenesis of S. aureus infections [16]. Mice with impaired inflammasome activity have decreased neutrophil recruitment, resulting in impaired bacterial clearance at the site of the infection [24]. It is well established in several murine models that NLRP3 inflammasomes are activated in S. aureus infection. The underlying mechanisms involved in S. aureus-mediated NLRP3 inflammasome activation can be divided into two pathways: [1] pore forming toxins (hemolysin, leukocidin and Panton-Valentine leukocidin) secreted by S. aureus rupture the cellular membrane, leading to potassium efflux-dependent NLRP3 inflammasome activation; or [2] phagocytosis and lysosomal degradation of S. aureus peptidoglycan contribute to NLRP3 inflammasome-mediated IL-1β release [23,35]. In our study, we stimulated BMDC with live S. aureus instead of bacterial culture filtrates containing large amount of PFTs. We found that BMDC pretreated with MCC950 or cytochalasin D showed little IL-1β production, implying that phagocytosis-dependent NLRP3 activation was predominant in our in vitro infection assay. The production of IL-1β is reported to be regulated by RIP1/RIP3/MLKL mediated necroptosis, which constrains excessive inflammation [36]. However, in our study, use of a necroptosis inhibitor did not apparently prevent S. aureus-induced cytotoxicity. The in vitro infection assay in our study also highlighted a role of adenosine signaling in S. aureus-mediated IL-1β production, as A2AR antagonist suppressed IL-1β release. However, our results do not preclude the possibility that AdsA can suppress inflammasomes in vivo by other mechanisms. In a murine model, bacterial infection increased extracellular ATP levels and NLRP3 inflammasome activation, thereby promoting anti-bacterial immunity [37]. Given that AdsA is capable of degrading extracellular ATP, it is possible that AdsA could suppress IL-1β production by decreasing ATP levels in vivo.
Mechanistically, we demonstrated that the AdsA/adenosine/A2AR axis can affect S. aureus-induced IL-1β release by interfering with the priming signal of NLRP3 inflammasome. In general, adenosine directly acts on the A2a receptor and inhibits NF-κB/p38 MAPK signaling activity, thereby downregulating the expression of pro-inflammatory cytokines including TNF-α, IL-6, and IL-1β [15,38]. In contrast to our findings, another study reported that adenosine and A2a receptor signaling enhanced NLRP3 inflammasome activation by amplifying the priming signal [39]. Different from the infection conditions in our study, they treated bone marrow-derived macrophages (BMDM) with adenosine after a long period of LPS priming. Nevertheless, these findings indicate adenosine plays a complex role in the regulation of inflammasome at different stages of bacterial infection. The detailed mechanism of adenosine/A2AR axis in the modulation of NLRP3 inflammasome during S. aureus infection merits further investigation.
To date, several lines of evidence have shown that inflammasome plays a critical role in shaping adaptive immunity. Inflammasome-inducing IL-1 family of cytokines instruct the differentiation of naïve T cells into various subtypes [33]. Among these cytokines, IL-1β promotes the differentiation of Th17 cells via IL-1R signaling [20]. For example, adenylate cyclase toxin (CyaA) from Bordetella pertussis was reported to induce antigen-specific Th17 immune responses through the NLRP3/IL-1β/IL-1R axis [34]. Similar results were also observed using a common adjuvant, complete Freund's adjuvant, which was able to promote IL-1β/IL-1R signaling-dependent Th17 immunity [40]. In addition, some cancer chemotherapeutic agents can activate NLRP3 inflammasome in myeloid-derived suppressor cells, resulting in impaired anti-tumor activity through enhanced pro-angiogenic Th17 responses [41]. In line with these observations, our results showed that mice treated with VX765 or MCC950 had decreased IL-17A production upon restimulation, suggesting a role of NLRP3 inflammasome in establishing S. aureus-specific Th17 responses. These IL-1β-mediated Th17 responses can protect against S. aureus infection, as reported by other groups [15,42]. We highlighted a role of AdsA in suppressing the protective Th17 responses by impairing the NLRP3/IL-1β/IL-1R axis. In our study, we found AdsA also disrupted the Th1 immune response. Although Th1 immunity is orchestrated by another critical inflammasome-mediated IL-1 family of cytokines, IL-18, we did not observe an apparent decrease in IFN-ɣ production in mice treated with VX765, suggesting that AdsA may inhibit Th1 responses via a caspase-1 independent mechanism.
Since Thammavongsa et al first identified that AdsA can interfere with host immune responses, several mechanisms of how AdsA interferes with anti-S. aureus immunity has been revealed. AdsA can improve bacterial survival in the blood by hijacking phagocytic killing of neutrophils via adenosine signaling [29]; AdsA can induce caspase-3 dependent apoptosis of phagocytic macrophages in tissue abscesses, leading to persistent infection [27]; and AdsA can inhibit the expression of an antimicrobial peptide, type IIA secretory phospholipase A2, in respiratory infections [30]. However, the exact role of AdsA in adaptive immunity remains elusive. Our study is first to show that AdsA inhibits the development of optimal humoral responses and Th1/Th17 immunity during S. aureus infection. Consistently, our previous work also demonstrated that AdsA is a potential vaccine target [43]. Mice immunized with aluminum hydroxide–formulated recombinant AdsA (rAdsA) exhibited resistance to S. aureus skin and invasive infection in respective murine models. Besides, the current study also demonstrated a role of adenosine/A2AR/NLRP3 axis in dampening Th17 immunity. Moreover, a recent study showed that preliminary anti-S. aureus immune responses could be inhibited after the initial exposure to S. aureus, making it difficult to induce efficient T cell immunity in subsequent vaccinations in human [44]. Besides, adenosine was reported to enhance the immunosuppressive activities of regulatory T cells (Tregs) [45], indicating a potential approach of AdsA in modulating host immune responses and meriting further investigation in the future work. Our findings add to our understanding of how S. aureus virulence factors constrain host protective immunity and expand the scope of therapeutic approaches.
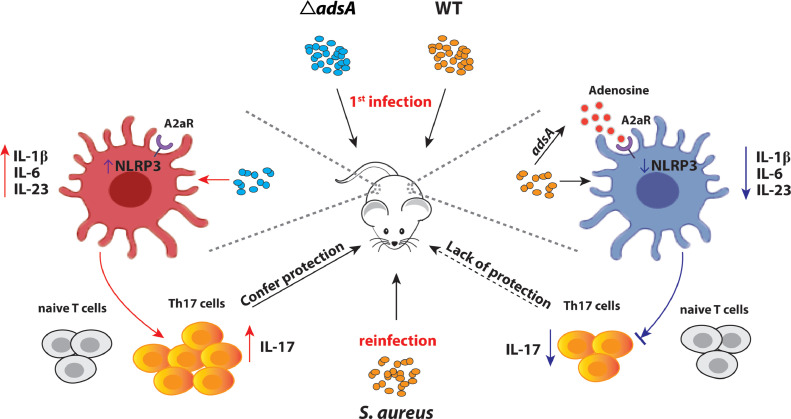
In summary, our work (illustrated in Fig. 6) highlights that S. aureus can subvert protective immune responses through a cell-wall anchored immune evasion factor AdsA. The results further demonstrate that AdsA restricts Th17 immune responses via the A2AR/NLRP3/IL-1β axis that promotes the survival of S. aureus in subsequent infections. A better understanding of how S. aureus evades host immunity will facilitate the development of treatment strategies against S. aureus infection.
Figure 6.
- Schematic summary of AdsA in the modulation of NLRP3-mediated IL-1β release and Th17 differentiation.
Contributors
Jian Deng, Bao-zhong Zhang, Hin Chu, Jian-Dong Huang, and K.Y. Yuen designed the experiments. Jian Deng, Bao-zhong Zhang, and Hin Chu performed the experiments. Xiao-lei Wang, Yi-xin Wang, Dong Yang, Hua-Rui Gong, Renhao Li, Cun Li, Ying Dou, Peng Gao, Jasper Fuk-Woo Chan, and Richard Yi-Tsun Kao participated in the study. Jian Deng, Bao-zhong Zhang, Hin Chu, Jian-Dong Huang and K.Y. Yuen analyzed the data. Jian Deng, Bao-zhong Zhang, Hin Chu, and Jian-Dong Huang wrote the manuscript. Hin Chu and Xiao-lei Wang have verified the underlying data. All authors have read and approved the final version of the manuscript.
Declaration of Competing Interest
The authors declare no competing interests.
Acknowledgments
Funding
This work was supported by grants from Shenzhen Peacock project (KQTD2015033-117210153), and Guangdong Science and Technology Department (2020B1212030004) to J.H. and China Postdoctoral Science Foundation (2019M663167) to BZZ. We also thank the L & T Charitable Foundation, the Guangdong Science and Technology Department (2020B1212030004), and the Program for Guangdong Introducing Innovative and Entrepreneurial Teams (2019BT02Y198) for their support.
Acknowledgments
We thank the technicians in the laboratory of Jian-Dong Huang for their help and for providing resources used in this study. This work was supported by grants from Shenzhen Peacock project (KQTD2015033-117210153), and Guangdong Science and Technology Department (2020B1212030004) to J.H. and China Postdoctoral Science Foundation (2019M663167) to BZZ. We also thank the L & T Charitable Foundation, the Guangdong Science and Technology Department (2020B1212030004), and the Program for Guangdong Introducing Innovative and Entrepreneurial Teams (2019BT02Y198) for their support.
Data sharing statement
All data used to draw the conclusions in this paper are provided in the paper and/or in the supplementary materials.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2021.103505.
Appendix. Supplementary materials
Full blots
ANTI-b-ACTIN datasheet
Anti-mouse IFN-gamma Brilliant Violet 421 datasheet
Anti-mouse IL-17A APC datasheet
Anti-pro Caspase-1 + p10 + p12 antibody datasheet
CD3 Monoclonal Antibody (17A2), FITC, eBioscience™ datasheet
CD4 Antibody (GK1.5), PE-Cyanine7 datasheet
FITC Rat Anti-Mouse I-A/I-E datasheet
NLRP3 (D4D8T) Rabbit mAb (#15101) Datasheet
PE Rat Anti-Mouse CD40 datasheet
PE Rat Anti-Mouse CD86 datasheet
TIB-202 Product Sheet - THP-1
ARRIVE Author Checklist
References
- 1.Klevens RM, Edwards JR, Gaynes RP. National Nosocomial Infections Surveillance S. The impact of antimicrobial-resistant, health care-associated infections on mortality in the United States. Clin Infect Dis. 2008;47(7):927–930. doi: 10.1086/591698. [DOI] [PubMed] [Google Scholar]
- 2.David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus–Epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. 2010;23(3):616–687. doi: 10.1128/CMR.00081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yilmaz M, Elaldi N, Balkan II, Arslan F, Batirel AA, Bakici MZ. Mortality predictors of Staphylococcus aureus bacteremia–A prospective multicenter study. Ann Clin Microbiol. 2016;15:7. doi: 10.1186/s12941-016-0122-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kallen AJ, Mu Y, Bulens S, Reingold A, Petit S, Gershman K. Health care-associated invasive MRSA infections, 2005-2008. JAMA. 2010;304(6):641–648. doi: 10.1001/jama.2010.1115. [DOI] [PubMed] [Google Scholar]
- 5.Wertheim HF, Vos MC, Ott A, van Belkum A, Voss A, Kluytmans JA. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet. 2004;364(9435):703–705. doi: 10.1016/S0140-6736(04)16897-9. [DOI] [PubMed] [Google Scholar]
- 6.Cunnion KM, Frank MM. Complement activation influences Staphylococcus aureus adherence to endothelial cells. Infect Immun. 2003;71(3):1321–1327. doi: 10.1128/IAI.71.3.1321-1327.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spaan AN, Surewaard BG, Nijland R, van Strijp JA. Neutrophils versus Staphylococcus aureus–A biological tug of war. Annu Rev Microbiol. 2013;67:629–650. doi: 10.1146/annurev-micro-092412-155746. [DOI] [PubMed] [Google Scholar]
- 8.Brown AF, Murphy AG, Lalor SJ, Leech JM, O’Keeffe KM, Mac Aogain M. Memory Th1 cells are protective in invasive Staphylococcus aureus infection. PLoS pathog. 2015;11(11) doi: 10.1371/journal.ppat.1005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy AG, O’Keeffe KM, Lalor SJ, Maher BM, Mills KH, McLoughlin RM. Staphylococcus aureus infection of mice expands a population of memory gammadelta T cells that are protective against subsequent infection. J Immunol. 2014;192(8):3697–3708. doi: 10.4049/jimmunol.1303420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montgomery CP, Daniels M, Zhao F, Alegre ML, Chong AS, Daum RS. Protective immunity against recurrent Staphylococcus aureus skin infection requires antibody and interleukin-17A. Infect Immun. 2014;82(5):2125–2134. doi: 10.1128/IAI.01491-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin L, Ibrahim AS, Xu X, Farber JM, Avanesian V, Baquir B. Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS pathog. 2009;5(12) doi: 10.1371/journal.ppat.1000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fowler VG, Jr., Proctor RA. Where does a Staphylococcus aureus vaccine stand? Clin Microbiol Infect. 2014;20:66–75. doi: 10.1111/1469-0691.12570. Suppl 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM. Impaired T (H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nat. 2008;452(7188):773–776. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007;19(3):281–286. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Sanchez M, Kolar SL, Muller S, Reyes CN, Wolf AJ, Ogawa C. O-Acetylation of Peptidoglycan limits helper T cell priming and permits Staphylococcus aureus reinfection. Cell Host Microbe. 2017;22(4):543–551. doi: 10.1016/j.chom.2017.08.008. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melehani JH, Duncan JA. Inflammasome activation can mediate tissue-specific pathogenesis or protection in Staphylococcus aureus infection. Curr Top Microbiol Immunol. 2016;397:257–282. doi: 10.1007/978-3-319-41171-2_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Sci (NY) 2008;320(5876):674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M. Cryopyrin activates the inflammasome in response to toxins and ATP. Nat. 2006;440(7081):228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 19.Martinon F, Burns K, Tschopp J. The inflammasome–A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10(2):417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 20.Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immun. 2009;30(4):576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holzinger D, Gieldon L, Mysore V, Nippe N, Taxman DJ, Duncan JA. Staphylococcus aureus Panton-Valentine leukocidin induces an inflammatory response in human phagocytes via the NLRP3 inflammasome. Journal Leukoc Biol. 2012;92(5):1069–1081. doi: 10.1189/jlb.0112014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melehani JH, James DB, DuMont AL, Torres VJ, Duncan JA. Staphylococcus aureus leukocidin A/B (LukAB) kills human monocytes via host NLRP3 and ASC when extracellular, but not intracellular. PLoS pathog. 2015;11(6) doi: 10.1371/journal.ppat.1004970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimada T, Park BG, Wolf AJ, Brikos C, Goodridge HS, Becker CA. Staphylococcus aureus evades lysozyme-based peptidoglycan digestion that links phagocytosis, inflammasome activation, and IL-1beta secretion. Cell Host Microbe. 2010;7(1):38–49. doi: 10.1016/j.chom.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho JS, Guo Y, Ramos RI, Hebroni F, Plaisier SB, Xuan C. Neutrophil-derived IL-1beta is sufficient for abscess formation in immunity against Staphylococcus aureus in mice. PLoS Pathog. 2012;8(11) doi: 10.1371/journal.ppat.1003047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thammavongsa V, Kim HK, Missiakas D, Schneewind O. Staphylococcal manipulation of host immune responses. Nat Rev Microbiol. 2015;13(9):529–543. doi: 10.1038/nrmicro3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thammavongsa V, Schneewind O, Missiakas DM. Enzymatic properties of Staphylococcus aureus Adenosine synthase (AdsA) BMC Biochem. 2011;12:56. doi: 10.1186/1471-2091-12-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thammavongsa V, Missiakas DM, Schneewind O. Staphylococcus aureus degrades neutrophil extracellular traps to promote immune cell death. Sci. 2013;342(6160):863–866. doi: 10.1126/science.1242255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deng J, Wang X, Zhang BZ, Gao P, Lin Q, Kao RY. Broad and effective protection against Staphylococcus aureus is elicited by a multivalent vaccine formulated with novel antigens. mSphere. 2019;4(5):e00362–19. doi: 10.1128/mSphere.00362-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thammavongsa V, Kern JW, Missiakas DM, Schneewind O. Staphylococcus aureus synthesizes adenosine to escape host immune responses. J Exp Med. 2009;206(11):2417–2427. doi: 10.1084/jem.20090097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pernet E, Brunet J, Guillemot L, Chignard M, Touqui L, Wu Y. Staphylococcus aureus adenosine inhibits sPLA2-IIA-mediated host killing in the airways. J Immunol. 2015;194(11):5312–5319. doi: 10.4049/jimmunol.1402665. [DOI] [PubMed] [Google Scholar]
- 31.Bae T, Schneewind O. Allelic replacement in Staphylococcus aureus with inducible counter-selection. Plasmid. 2006;55(1):58–63. doi: 10.1016/j.plasmid.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 32.Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5(3):247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evavold CL, Kagan JC. How inflammasomes inform adaptive immunity. J Mol Biol. 2018;430(2):217–237. doi: 10.1016/j.jmb.2017.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dunne A, Ross PJ, Pospisilova E, Masin J, Meaney A, Sutton CE. Inflammasome activation by adenylate cyclase toxin directs Th17 responses and protection against Bordetella pertussis. J Immunol. 2010;185(3):1711–1719. doi: 10.4049/jimmunol.1000105. [DOI] [PubMed] [Google Scholar]
- 35.Wolf AJ, Reyes CN, Liang W, Becker C, Shimada K, Wheeler ML. Hexokinase is an innate immune receptor for the detection of bacterial peptidoglycan. Cell. 2016;166(3):624–636. doi: 10.1016/j.cell.2016.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kitur K, Wachtel S, Brown A, Wickersham M, Paulino F, Penaloza HF. Necroptosis promotes Staphylococcus aureus clearance by inhibiting excessive inflammatory signaling. Cell Rep. 2016;16(8):2219–2230. doi: 10.1016/j.celrep.2016.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiang Y, Wang X, Yan C, Gao Q, Li SA, Liu J. Adenosine-5′-triphosphate (ATP) protects mice against bacterial infection by activation of the NLRP3 inflammasome. PLoS One. 2013;8(5):e63759. doi: 10.1371/journal.pone.0063759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D. Cutting edge–NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183(2):787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ouyang X, Ghani A, Malik A, Wilder T, Colegio OR, Flavell RA. Adenosine is required for sustained inflammasome activation via the A (2)A receptor and the HIF-1alpha pathway. Nat Commun. 2013;4:2909. doi: 10.1038/ncomms3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shenderov K, Barber DL, Mayer-Barber KD, Gurcha SS, Jankovic D, Feng CG. Cord factor and peptidoglycan recapitulate the Th17-promoting adjuvant activity of mycobacteria through mincle/CARD9 signaling and the inflammasome. J Immunol. 2013;190(11):5722–5730. doi: 10.4049/jimmunol.1203343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bruchard M, Mignot G, Derangere V, Chalmin F, Chevriaux A, Vegran F. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat Med. 2013;19(1):57–64. doi: 10.1038/nm.2999. [DOI] [PubMed] [Google Scholar]
- 42.Zielinski CE, Mele F, Aschenbrenner D, Jarrossay D, Ronchi F, Gattorno M. Pathogen-induced human TH17 cells produce IFN-gamma or IL-10 and are regulated by IL-1beta. Nat. 2012;484(7395):514–518. doi: 10.1038/nature10957. [DOI] [PubMed] [Google Scholar]
- 43.Zhang BZ, Cai J, Yu B, Xiong L, Lin Q, Yang XY. Immunotherapy targeting Adenosine synthase A decreases severity of Staphylococcus aureus infection in mouse model. J Infect Dis. 2017;216(2):245–253. doi: 10.1093/infdis/jix290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee B, Olaniyi R, Kwiecinski JM, Wardenburg JB. Staphylococcus aureus toxin suppresses antigen-specific T cell responses. J Clin Invest. 2020;130(3):1122–1127. doi: 10.1172/JCI130728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han KL, Thomas SV, Koontz SM, Changpriroa CM, Ha SK, Malech HL. Adenosine A (2)A receptor agonist-mediated increase in donor-derived regulatory T cells suppresses development of graft-versus-host disease. J Immunol. 2013;190(1):458–468. doi: 10.4049/jimmunol.1201325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.