Highlights
-
•
Executive dysfunction may be a transdiagnostic cognitive deficit of psychopathology in youth.
-
•
Poorer executive function prospectively predicted two-year change in general psychopathology.
-
•
Greater general psychopathology prospectively predicted two-year change in executive function.
-
•
Executive function is both a risk marker and consequence of youth transdiagnostic psychopathology.
Keywords: General psychopathology, p Factor, Executive function, Longitudinal, Risk factor, Transdiagnostic
Abstract
A general psychopathology (‘p’) factor captures shared variation across mental disorders. One hypothesis is that poor executive function (EF) contributes to p. Although EF is related to p concurrently, it is unclear whether EF predicts or is a consequence of p. For the first time, we examined prospective relations between EF and p in 9845 preadolescents (aged 9–12) from the Adolescent Brain Cognitive Development Study® longitudinally over two years. We identified higher-order factor models of psychopathology at baseline and one- and two-year follow-up waves. Consistent with previous research, a cross-sectional inverse relationship between EF and p emerged. Using residualized-change models, baseline EF prospectively predicted p factor scores two years later, controlling for prior p, sex, age, race/ethnicity, parental education, and family income. Baseline p factor scores also prospectively predicted change in EF two years later. Tests of specificity revealed that bi-directional prospective relations between EF and p were largely generalizable across externalizing, internalizing, neurodevelopmental, somatization, and detachment symptoms. EF consistently predicted change in externalizing and neurodevelopmental symptoms. These novel results suggest that executive dysfunction is both a risk marker and consequence of general psychopathology. EF may be a promising transdiagnostic intervention target to prevent the onset and maintenance of psychopathology.
1. Introduction
Executive functions (EF) consist of related, but separable, cognitive abilities that subserve goal-directed behavior, including inhibitory control, cognitive flexibility, and working memory (Miyake et al., 2000). EF skills are necessary for top-down regulation of thoughts, feelings, and actions and are associated with many aspects of daily functioning (Diamond, 2013). Not surprisingly, EF deficits are present in a wide range of mental disorders (Abramovitch et al., 2021; Snyder et al., 2015). Indeed, poor EF has been posited as a transdiagnostic cognitive deficit linked to psychopathology broadly (McTeague et al., 2016; Snyder et al., 2015; Zelazo, 2020).
Given the high rates of comorbidity across mental disorder categories (Kessler et al., 2005b), accumulating research has focused on transdiagnostic models of psychopathology. A general psychopathology (‘p’) factor (Caspi et al., 2014; Lahey et al., 2012) capturing shared variation across mental disorders has been identified in diverse samples (Caspi and Moffitt, 2018; Lahey et al., 2017) across the lifespan (Caspi et al., 2020). However, the meaning of the p factor is not year clear. One hypothesis is that p broadly reflects a general liability to psychopathology; however, others have wondered whether the p factor reflects response biases or statistical artifact (e.g., Watts et al., 2020). Consistent with the evidence showing EF deficits in many forms of psychopathology, one conceptualization of the p factor is that it may partly reflect executive dysfunction as a common cause of all mental disorders. Accordingly, poorer EF has been associated with higher levels of the p factor in multiple studies of children (Cardenas-Iniguez et al., 2020; Huang-Pollock et al., 2017; Martel et al., 2017), adolescents (Bloemen et al., 2018; Castellanos-Ryan et al., 2016; Snyder et al., 2019a; Wade et al., 2019; White et al., 2017), and adults (Caspi et al., 2014; Romer and Pizzagalli, 2021).
The hypothesis that poor EF may contribute to the p factor is supported by neuroimaging and genetics research as well. Specifically, structural and functional neural abnormalities in frontoparietal, default mode, visual association, and cerebello-thalamo-cerebro-cortical circuits involved in cognitive control have been identified in transdiagnostic meta-analyses (Goodkind et al., 2015; McTeague et al., 2017; Sha et al., 2019; Shanmugan et al., 2016) and in studies of the neural correlates of the p factor (Elliott et al., 2018; Karcher et al., 2020; Moberget et al., 2019; Romer et al., 2019, 2018; Sato et al., 2016; Snyder et al., 2017). Shared genetic influences on EF, cognitive function, and transdiagnostic psychopathology in youth twins also has been identified (Alnæs et al., 2018; Grotzinger et al., 2019; Harden et al., 2020).
However, much of this research was conducted using cross-sectional data, which by design, cannot establish temporal precedence between EF and psychopathology. As EF skills are necessary for the regulation of thoughts, emotions, and behaviors, theory would suggest that EF deficits precede the onset of psychopathology. Although few in number, some longitudinal studies find poorer executive/cognitive functioning predicts subsequent symptoms (Campbell and von Stauffenberg, 2009; Cannon et al., 2006; Caspi et al., 2020; Kenny et al., 2019; Letkiewicz et al., 2014; Mac Giollabhui et al., 2019; Niendam et al., 2003; Stange et al., 2016). Also, executive dysfunction has been found during remission (Snyder, 2013) and in unaffected family members of individuals with mental disorders (e.g., Bora et al., 2009), suggesting that EF may be an intermediate phenotype of psychopathology.
Alternatively, it is plausible that executive dysfunctions emerge as a consequence of psychopathology. This could occur because psychopathology during early development could impede the growth of EF (Brieant et al., 2020). Or EF and p may be concurrently related because mental disorder symptoms (e.g., anhedonia, poor sleep, ruminations, obsessions, psychosis, etc.) interfere with neurocognitive test performance. Recent cross-lagged panel studies found that after controlling for cross-sectional associations, EF did not predict later adolescent internalizing and externalizing psychopathology (Brieant et al., 2020; Donati et al., 2021). However, these studies did not examine relations with the p factor.
Of course, some combination of these hypothesized relations between EF and psychopathology may be true. EF may serve both as a risk marker and consequence of psychopathology, and contemporaneous associations between EF and psychopathology may be inflated by symptom-related interference on test performance. To test the hypothesis that EF is an early antecedent risk marker for the p factor, it is thus critical to establish the temporal order of relations between EF and psychopathology using a longitudinal design, and to control for prior symptomatology. Indeed, to the best of our knowledge, no study has directly tested whether EF prospectively predicts future levels or are a consequence of the p factor in preadolescents. It also is unclear whether EF is a non-specific risk marker or consequence of p or if it is only related to specific families of disorders. Moreover, testing these hypotheses in preadolescents may be particularly advantageous for identifying early risk markers during a period of greater neural plasticity (Giedd et al., 1999) prior to the onset of most forms of psychopathology in adolescence.
Therefore, in the current study, we used three waves of clinical and neurocognitive data from 9845 preadolescents (aged 9–12) from the Adolescent Brain Cognitive Development (ABCD) study to begin to tease apart whether EF is a prospective predictor or consequence of general psychopathology. To this end, we identified higher-order and one-factor models of the structure of psychopathology and EF, respectively, at the three waves using confirmatory factor analysis (CFA). We extracted factor scores from these models and tested the cross-sectional relations between EF and p to determine replicability of prior concurrent findings. Using residualized change models, we tested whether baseline EF factor scores prospectively predicted p factor scores one and two years later, controlling for prior p scores. We also tested whether baseline p factor scores prospectively predicted EF scores two years later, controlling for baseline EF. Finally, we conducted tests of specificity to determine whether relations between EF and psychopathology were generalizable across externalizing, internalizing, neurodevelopmental, somatization, and detachment symptoms. We hypothesized that 1) EF and p would be inversely related cross-sectionally, 2) lower baseline EF would prospectively predict one- and two-year increases in p, 3) lower baseline p would prospectively predict two-year increases in EF, and 4) relations between EF and psychopathology would be generalizable across families of disorders.
2. Materials and methods
2.1. Participants
The ABCD sample consists of 11,875 children who participated in a major collaboration between 22 sites across the U.S. to investigate psychological and neurobiological development from preadolescence to early adulthood. Full recruitment details can be found elsewhere (Garavan et al., 2018). Exclusion criteria for children were limited to not being fluent in English, having a parent not fluent in English or Spanish, major medical or neurological conditions, gestational age <28 weeks or birthweight <1200 g, contraindications to MRI scanning, a history of traumatic brain injury, a current diagnosis of schizophrenia, moderate/severe autism spectrum disorder, intellectual disability, or alcohol/substance use disorder. Institutional review board approval was obtained for each site before data collection. All parents provided written informed consent and all children provided assent.
Demographic, clinical, and neurocognitive data were accessed from the National Institutes of Mental Health Data Archive (see Acknowledgments). The current study is based on 9856 unrelated children (randomly selecting one child per family when more than one participated; mean age in months = 118.85, SD = 7.40; 47.5 % females) from the ABCD 3.0 data release (DOI 10.15154/1519007), which included data collected between September 1, 2016 and February 15, 2020. In the 3.0 release, data were available from 100 %, 95 %, and 55 % of the baseline and one- and two-year follow-up samples, respectively, as data collection is ongoing. Participants with complete nonresponse on clinical and neurocognitive test data from each wave were excluded from analyses (Inclusion Ns: baseline N = 9845; one-year follow-up N = 9244; two-year follow-up N = 5332).
2.2. Measures
Full details on measures are presented in Supplementary Methods. Children and their parent/guardian completed assessments during in-person visits at the three waves.
2.2.1. Psychopathology
Child psychopathology at each wave was assessed with the Child Behavior Checklist (CBCL; age 6–18 form) (Achenbach, 2009). The CBCL is a parent rating scale consisting of 119 items describing child behaviors and emotions. Parents rate the extent to which that behavior was characteristic of their child over the past six months on a scale of 0 (“Not True (as far as you know)”), 1 (“Somewhat or Sometimes True”), or 2 (“Very True or Often True”).
2.2.2. Executive function
The NIH Toolbox Cognition measures (http://www.nihtoolbox.org) (Weintraub et al., 2013) were administered to children on computer tablets monitored by an experimenter at baseline (Luciana et al., 2018). Uncorrected scores from tests of EF and processing speed were used including the Flanker Task (Fan et al., 2002), Dimensional Change Card Sort Task (Zelazo, 2006), List Sorting Working Memory Test (Tulsky et al., 2014), and Pattern Comparison Processing Speed Test (Carlozzi et al., 2015). None of the tests were administered at the one-year follow-up wave; the Flanker and Pattern Comparison Processing Speed tests were administered to participants at the two-year follow-up wave.
2.2.3. Demographic variables
Parents/guardians reported their child’s sex assigned at birth, age (in months), race, and ethnicity (Hispanic/Latino/a) at baseline. Race and ethnicity were dummy-coded into Black, Asian, Hispanic, and Other variables with Non-Hispanic Caucasian as the reference group. Parental education and total combined family income over the past twelve months were included as measures of socioeconomic status.
2.3. Statistical analysis
Our analyses were conducted in the following steps. First, we used CFA to fit measurement models of the structure of psychopathology and EF. Second, we examined the longitudinal measurement invariance of the psychopathology factors over the three waves to determine the stability of the factors over time. Third, factor scores were extracted from the measurement models using the standard regression method in Mplus. We tested factor score intercorrelations over the three waves to determine their reliability over time. Fourth, we used ordinary least squares (OLS) regression to examine the cross-sectional relations between EF and p factor scores at the baseline and two-year follow-up waves. Fifth, residualized change models were used to examine the prospective relations between EF and p factor scores over the two-year time period. Each of these analyses is described in detail below.
2.3.1. Confirmatory factor analyses
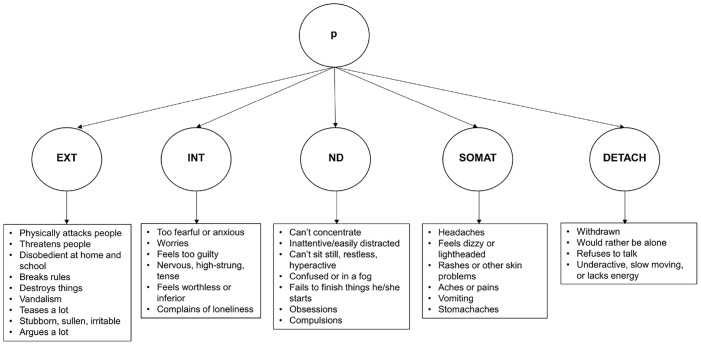
Higher-order and correlated factors models of the structure of psychopathology were fit at each of the three waves using CBCL items. We chose to test a higher-order over a bi-factor model because it has been identified in prior work using ABCD release 2.0 baseline data (Michelini et al., 2019) and recent research has shown that there are no meaningful differences between higher-order and bi-factor models in the ABCD baseline sample (Clark et al., 2020). We used the higher-order factor structure from Michelini et al. (2019), which identified five lower-order factors (externalizing, internalizing, neurodevelopmental, somatization, and detachment) and a higher-order p factor (Fig. 1). We also tested a correlated factors model of these five factors to examine specificity of relations with EF. Michelini et al. (2019) found that 31 CBCL items either did not load on any factor or were low frequency items (<0.5 % rated as 1 or 2). We excluded these items from our CFAs. They also found 10 CBCL items that cross-loaded on more than one factor, which we also excluded to create a more stringent criterion for identifying p. They created six composite scores of items that were highly correlated (r>0.75), which we retained in our analyses (see Supplementary Methods for details).
Fig. 1.
Higher-Order Model of the Structure of Psychopathology.
Note. The higher-order structure of psychopathology is shown with a higher-order p factor and five lower-order factors (Michelini et al., 2019). This model was tested at each of the three waves. Example CBCL items loading on the lower-order factors are shown in bullets (loadings >0.6). EXT = Externalizing; INT = Internalizing; ND = Neurodevelopmental; SOMAT = Somatization; DETACH = Detachment.
For the CFA of EF, we tested a one-factor model using the four baseline neurocognitive tests. As only the Flanker and Pattern Comparison Processing Speed tests were administered at the two-year follow-up wave, uncorrected scores from these tests were z-scored and averaged to obtain a measure of EF at baseline and two-year follow-up waves (Flanker and Pattern test scores are correlated: r = 0.377 at baseline; r = 0.424 at two-year follow-up). These mean EF test scores were used in regression and residualized change models including two-year follow-up data.
The CFAs were performed in Mplus version 8.4 (Muthen and Muthen, 1998) using the weighted least squares means and variance adjusted (WLSMV) algorithm. The WLSMV estimator is appropriate for categorical and nonmultivariate normal data and provides consistent estimates when data are missing at random with respect to covariates (Asparouhov and Muthén, 2010). Maximum likelihood estimation with robust standard errors (MLR) was used for the one-factor EF model. We assessed each model’s fit to the data using the chi-square value, comparative fit index (CFI), Tucker-Lewis index (TLI), and root-mean square error of approximation (RMSEA). Nonsignificant chi-square tests indicate good model fit; nonetheless, this test generally is overpowered in large sample sizes. CFI and TLI > 0.90 indicate adequate fit; RMSEA < 0.08 is considered acceptable (Kline, 2015). All CFAs were conducted with the full samples at each wave. CFAs also were conducted using only CBCL and EF test data from participants with data available in the two-year follow-up wave (N = 5332).
2.3.2. Measurement invariance and factor score intercorrelations over time
We examined the longitudinal measurement invariance of the psychopathology factors over the three waves to determine whether the factors are equivalently measured over time. We tested models of configural, metric, and scalar invariance. Configural invariance tests whether the same factor structure can adequately fit the data over time. To do this, we tested a model in which the CBCL items loaded on the same factor at each wave. Metric invariance tests whether the factor loadings are equivalent over time. If metric invariance holds, we can examine change in relative status of participants over time using residualized change models. We tested a model in which the factor loadings were equivalent across the three waves. Scalar invariance tests whether the factor loadings and intercepts are equivalent over time. As CBCL items were treated as ordinal and WLSMV estimation was employed, scalar invariance tests equivalence of response category thresholds rather than intercepts. Therefore, for this test, we equated both the factor loadings and thresholds of each CBCL item over the three waves. We used likelihood ratio testing to compare the fit of these three models (using “DIFFTEST” function in Mplus for WLSMV estimation), which tested whether adding additional equality constraints resulted in a significant decrement to model fit. We expected that scalar invariance would not hold given that research shows significant growth in psychopathology throughout youth development (Copeland et al., 2011; Kessler et al., 2005a; Solmi et al., 2021). In addition to the tests of longitudinal measurement invariance of the latent factors, we also examined correlations between the factor scores derived from these latent models as a test of reliability over the three waves.
2.3.3. Regression and residualized change models
First, we used OLS regression to examine cross-sectional relations between EF and p. P factor scores were regressed on EF factor scores at baseline and two-year follow-up waves. Second, we conducted two residualized change regression models of one- and two-year change in p factor scores. In the one-year change model, one-year follow-up p factor scores were regressed on baseline EF factor scores controlling for baseline p factor scores. In the two-year change model, two-year follow-up p factor scores were regressed on baseline EF factor scores controlling for baseline and one-year follow-up p factor scores. Third, we conducted a two-year residualized change model in which two-year follow-up mean EF test scores (i.e., mean of Flanker and Pattern Comparison tests) were regressed on baseline p factor scores controlling for baseline mean EF test scores.
We also tested putative specificity of relations between EF and psychopathology by examining relations between EF and the lower-order factors. Using the regression and residualized change models as described above, relations between EF factor scores and externalizing, internalizing, neurodevelopmental, somatization, and detachment factor scores from the correlated factors model were tested separately.
These analyses were conducted in Mplus using robust estimates (MLR). A random intercept for site was included in all models using the TYPE = TWOLEVEL command to account for nesting within site. Covariates included sex, age, and dummy-coded race/ethnicity1 . We corrected for multiple comparisons by using a false discovery rate (FDR) procedure (q<0.01) (Benjamini and Hochberg, 1995).
2.3.4. Sensitivity analyses
Parental education and family income are markers of socioeconomic status, which may influence both EF and psychopathology. Therefore, we conducted sensitivity analyses with parental education and total combined family income included as additional covariates in the regression and residualized change models. We also wanted to ensure that results were not driven by differences between the EF factor scores used in cross-sectional and one-year change models and the mean of the Flanker and Pattern test scores used in two-year change models. We tested all regression and residualized change models using the mean of these EF test scores as additional sensitivity analyses.
3. Results
3.1. Descriptive statistics
Descriptive statistics for all study variables for the full baseline (N = 9845) and one- (N = 9244) and two-year follow-up samples (N = 5332) are summarized in Table 1. Baseline differences in all study variables between participants with available two-year follow-up data (N = 5332) versus those who did not have data available (N = 4513) were tested. The sample of participants with two-year follow-up data were significantly older, had greater proportion of Non-Hispanic White participants, smaller proportion of Black participants, had higher parental education and family income, and better performance on the four EF tests than participants without two-year follow-up data. To account for these differences, age and race/ethnicity (and sex) were included as covariates in all analyses; parental education and family income were included in sensitivity analyses.
Table 1.
Baseline Descriptive Statistics of All Study Variables and Differences between Participants with and without Two-Year Follow-Up Data.
| Baseline |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Measures | Full Baseline Sample |
Two-Year Wave Data Available |
Two-Year Wave Data Unavailable |
X2/t | P-value | |||||
| N | Min | Max | Mean (SD) or % | N | Mean (SD) or % | N | Mean (SD) or % | |||
| Age (months) | 9845 | 107 | 132 | 118.85 (7.40) | 5332 | 119.34 (7.34) | 4513 | 118.27 (7.44) | 7.15 | <0.001 |
| Sex (% Female) | 9845 | 47.5 | 5332 | 46.8 | 4513 | 48.4 | 2.71 | 0.100 | ||
| Non-Hispanic White (%) | 9845 | 50.7 | 5332 | 55.3 | 4513 | 45.3 | 97.67 | <0.001 | ||
| Black (%) | 9845 | 18.1 | 5332 | 14.0 | 4513 | 22.9 | 128.92 | <0.001 | ||
| Asian (%) | 9845 | 5.0 | 5332 | 5.1 | 4513 | 4.9 | 0.30 | 0.584 | ||
| Hispanic (%) | 9845 | 11.9 | 5332 | 12.1 | 4513 | 11.8 | 0.19 | 0.660 | ||
| Other (%) | 9845 | 14.2 | 5332 | 13.5 | 4513 | 15.2 | 5.72 | 0.017 | ||
| Parent Education (%) | 9831 | 5324 | 4507 | 84.45 | <0.001 | |||||
| No HS | 1.5 | 1.2 | 1.8 | |||||||
| Some HS | 5.4 | 4.4 | 6.5 | |||||||
| HS Grad | 10.8 | 9.2 | 12.7 | |||||||
| Some College | 16.5 | 16.1 | 16.9 | |||||||
| Associate’s | 12.9 | 12.7 | 13.1 | |||||||
| Bachelor’s | 27.5 | 30.0 | 24.7 | |||||||
| Master’s | 22.1 | 22.9 | 21.0 | |||||||
| Doctoral | 3.3 | 3.4 | 3.2 | |||||||
| Family Income (%) | 8980 | 4930 | 4050 | 86.43 | <0.001 | |||||
| <$5000 | 4.0 | 2.8 | 5.4 | |||||||
| $5000−11,999 | 4.1 | 3.4 | 4.8 | |||||||
| $12,000-$15,999 | 2.7 | 2.3 | 3.2 | |||||||
| $16,000-$24,999 | 5.0 | 4.2 | 5.9 | |||||||
| $25,000-$34,999 | 6.3 | 6.2 | 6.3 | |||||||
| $35,000-$49,999 | 8.6 | 8.7 | 8.5 | |||||||
| $50,000-$74,999 | 13.6 | 13.9 | 13.3 | |||||||
| $75,000-$99,999 | 14.5 | 15.7 | 13.0 | |||||||
| $100,000-$199,999 | 30.0 | 30.7 | 29.0 | |||||||
| >$200,000 | 11.3 | 12.0 | 10.5 | |||||||
| Mean CBCL items (z) | 9845 | −48.92 | 2.92 | −0.02 (1.07) | 5332 | 0.00 (0.39) | 4513 | −0.03 (1.51) | 1.24 | 0.217 |
| Mean Flanker (z) | 9721 | −4.46 | 2.40 | 0.00 (1.00) | 5282 | 0.07 (0.96) | 4439 | −0.08 (1.04) | 7.18 | <0.001 |
| Mean List (z) | 9691 | −5.00 | 3.24 | 0.00 (1.00) | 5272 | 0.08 (0.95) | 4419 | −0.09 (1.05) | 8.44 | <0.001 |
| Mean Card Sort (z) | 9722 | −4.44 | 2.88 | 0.00 (1.00) | 5284 | 0.07 (0.97) | 4438 | −0.08 (1.03) | 7.66 | <0.001 |
| Mean Processing Speed (z) | 9704 | −3.55 | 3.56 | 0.00 (1.00) | 5276 | 0.04 (0.99) | 4428 | −0.05 (1.01) | 4.19 | <0.001 |
Note. Comparisons were made by chi-square tests for categorical variables and independent samples t-tests for continuous variables. P-values are unadjusted; p-values that survived FDR correction for the 14 tests (q<0.01) are indicated in bold. Slight variations in Ns reflect missing data in some variables. HS=High School.
3.2. Confirmatory factor analyses
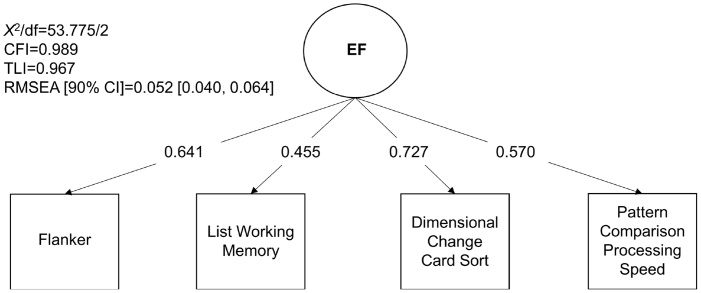
The one-factor model of EF fit the data well (Fig. 2). For the higher-order and correlated factors models, we removed eight CBCL items that were either cross-loading, did not have enough variability, or resulted in a non-positive definite solution at one or more wave (60 items and composites remained). The models fit the data adequately at each wave (Table 2). Loadings were positive and statistically significant (p < 0.001). Goodness of fit and factor loadings were highly similar between models including the full (N=9845 for baseline; N=9244 for one-year follow-up) compared to the reduced sample (N=5332) (see Supplementary Tables 1–4). Factor scores extracted from baseline models with the full and reduced samples were entirely correlated (r = 1.0); therefore, we used factor scores estimated from models using the full sample at each wave. Descriptive statistics of the factor scores and bivariate correlations with covariates are shown in Supplementary Tables 5 and 6, respectively (see Supplementary Figs. 1–3 for factor score histograms and Supplementary Results for details).
Fig. 2.
One-Factor Model of Executive Function.
Note. Model fit statistics and standardized loadings are shown of a baseline one-factor model of executive function (EF) with loadings from the Flanker, List Sorting Working Memory, Dimensional Change Card Sort, and Pattern Comparison Processing Speed tests. X2=chi-square; df = degrees of freedom; CFI = Comparative Fit Index; TLI = Tucker Lewis Index; RMSEA = Root Mean Square Error of Approximation; CI = confidence interval.
Table 2.
Model Fit Statistics, Standardized Loadings on p, and Factor Correlations from Confirmatory Factor Analysis Models of the Structure of Psychopathology at the Three Waves.
| Baseline | One-Year Follow-Up | Two-Year Follow-Up | |
|---|---|---|---|
| Higher-Order Model | |||
| X2/df | 18571.662/1705 | 17907.856/1705 | 10623.222/1705 |
| CFI | 0.926 | 0.922 | 0.917 |
| TLI | 0.923 | 0.919 | 0.914 |
| RMSEA [90 % CI] | 0.032 [0.031, 0.032] | 0.032 [0.032, 0.032] | 0.031 [0.031, 0.032] |
| Standardized Loadings on p | |||
| EXT | 0.838 | 0.832 | 0.831 |
| INT | 0.797 | 0.776 | 0.775 |
| ND | 0.934 | 0.937 | 0.935 |
| SOMAT | 0.571 | 0.541 | 0.579 |
| DETACH | 0.867 | 0.872 | 0.843 |
| Correlated Factors Model | |||
| X2/df | 18208.881/1700 | 17688.757/1700 | 10327.043/1700 |
| CFI | 0.927 | 0.923 | 0.920 |
| TLI | 0.925 | 0.920 | 0.916 |
| RMSEA [90 % CI] | 0.031 [0.031, 0.032] | 0.032 [0.031, 0.032] | 0.031 [0.030, 0.031] |
| Factor Correlations | |||
| EXT ↔ INT | 0.663 | 0.640 | 0.634 |
| EXT ↔ ND | 0.798 | 0.796 | 0.801 |
| EXT ↔ SOMAT | 0.457 | 0.427 | 0.461 |
| EXT ↔ DETACH | 0.708 | 0.703 | 0.662 |
| INT ↔ ND | 0.719 | 0.698 | 0.685 |
| INT ↔ SOMAT | 0.563 | 0.523 | 0.557 |
| INT ↔ DETACH | 0.713 | 0.715 | 0.721 |
| ND ↔ SOMAT | 0.478 | 0.462 | 0.476 |
| ND ↔ DETACH | 0.825 | 0.825 | 0.791 |
| SOMAT ↔ DETACH | 0.516 | 0.485 | 0.517 |
Note. Model fit statistics, standardized loadings on the p factor, and factor intercorrelations are shown for the higher-order and correlated factors models, respectively (all p < 0.001). Ns=9845, 9244, and 5332 for baseline and one- and two-year follow-up waves, respectively. X2=chi-square; df = degrees of freedom; CFI = Comparative Fit Index; TLI = Tucker Lewis Index; RMSEA = Root Mean Square Error of Approximation; CI = confidence interval; EXT = Externalizing; INT = Internalizing; ND = Neurodevelopmental; SOMAT = Somatization; DETACH = Detachment; ↔=“correlates with.”.
3.3. Measurement invariance and factor score reliability over time
Conducting a direct test of longitudinal measurement invariance of the higher-order model did not converge using WLSMV estimation. Therefore, we tested the invariance of the lower-order psychopathology factors in separate models (Table 3). Likelihood ratio testing showed that imposing metric invariance did not result in a significant decrement in model fit relative to configural invariance. These findings support the use of residualized change models to examine change in relative status of participants over time. At the same time, imposing scalar invariance did result in significantly worse fit relative to metric invariance, suggestive of changes in threshold intercepts over time.
Table 3.
Test of Longitudinal Measurement Invariance of the Lower-Order Psychopathology Factors.
| Model | Test of Overall Fit |
LRT Relative to Prior Model |
Fit Indices |
||||||
|---|---|---|---|---|---|---|---|---|---|
| X2 | df | P-value | ΔX2 | Δdf | P-value | RMSEA | CFI | TLI | |
| EXT | |||||||||
| Configural | 9763.85 | 2622 | <0.001 | 0.023 | 0.962 | 0.960 | |||
| Metric | 8012.53 | 2670 | <0.001 | 47.50 | 48 | 0.493 | 0.020 | 0.972 | 0.971 |
| Scalar | 8267.28 | 2718 | <0.001 | 668.59 | 48 | <0.001 | 0.020 | 0.971 | 0.970 |
| INT | |||||||||
| Configural | 2369.19 | 372 | <0.001 | 0.032 | 0.970 | 0.965 | |||
| Metric | 1917.67 | 390 | <0.001 | 13.41 | 18 | 0.767 | 0.027 | 0.977 | 0.974 |
| Scalar | 2027.78 | 408 | <0.001 | 158.50 | 18 | <0.001 | 0.028 | 0.976 | 0.974 |
| ND | |||||||||
| Configural | 4921.45 | 555 | <0.001 | 0.039 | 0.960 | 0.954 | |||
| Metric | 4081.27 | 577 | <0.001 | 30.00 | 22 | 0.119 | 0.034 | 0.968 | 0.965 |
| Scalar | 4232.75 | 599 | <0.001 | 216.97 | 22 | <0.001 | 0.034 | 0.966 | 0.965 |
| SOMAT | |||||||||
| Configural | 808.36 | 225 | <0.001 | 0.022 | 0.983 | 0.979 | |||
| Metric | 730.86 | 239 | <0.001 | 17.46 | 14 | 0.233 | 0.020 | 0.985 | 0.983 |
| Scalar | 778.72 | 253 | <0.001 | 59.55 | 14 | <0.001 | 0.020 | 0.984 | 0.983 |
| DETACH | |||||||||
| Configural | 463.00 | 72 | <0.001 | 0.032 | 0.985 | 0.978 | |||
| Metric | 403.55 | 80 | <0.001 | 7.63 | 8 | 0.470 | 0.028 | 0.987 | 0.983 |
| Scalar | 551.64 | 88 | <0.001 | 224.27 | 8 | <0.001 | 0.032 | 0.982 | 0.978 |
Note. Likelihood ratio testing (LRT) was used to compare the fit of models testing configural, metric, and scalar longitudinal invariance across the three waves. Tests of overall model fit, LRT relative to the prior model, and fit indices are shown for each model. X2=chi-square; df = degrees of freedom; ΔX2=change in chi-square; Δdf = change in degrees of freedom; RMSEA = Root Mean Square Error of Approximation; CFI = Comparative Fit Index; TLI = Tucker Lewis Index; EXT = Externalizing; INT = Internalizing; ND = Neurodevelopmental; SOMAT = Somatization; DETACH = Detachment.
In terms of measurement invariance of the p factor, Table 2 shows that the p factor loadings are highly similar across waves. To provide a test of metric invariance of p, we fixed p factor loadings in the one- and two-year follow-up models to be equivalent to the loadings from the baseline model and we compared model fit. Although not a formal test of metric invariance, fit statistics were highly similar between these models (Supplementary Table 7), suggesting that equating the baseline p factor loadings to be the same in the follow-up waves did not worsen model fit. P factor scores were strongly correlated over time, suggesting high reliability over the waves (Table 4). The lower-order factors also were moderately to strongly correlated over time, with externalizing and neurodevelopmental factors showing the strongest correlations. The mean of the Flanker and Pattern Comparison tests at baseline and two-year follow-up waves also were moderately correlated (r = 0.519, p < 0.001).
Table 4.
Factor Intercorrelations Across the Three Waves.
 |
Note. Factor score intercorrelations are shown between the baseline (denoted as 1) and one- and two-year follow-up waves (denoted as 2 and 3, respectively). EXT = Externalizing; INT = Internalizing; ND = Neurodevelopmental; SOMAT = Somatization; DETACH = Detachment.
The shaded cells refer to correlations between the same factor scores over time (i.e., P1 with P2 and P3; EXT1 with EXT2 and EXT3, etc.).
3.4. Cross-sectional relations between EF and psychopathology
Consistent with prior research and our first hypothesis, EF factor scores were negatively concurrently related to p factor scores at both baseline and two-year follow-up waves (Table 5). EF factor scores also were inversely related to the lower-order psychopathology factor scores at both waves, apart from somatization at the two-year follow-up wave, which was marginally inversely related to EF.
Table 5.
Cross-sectional Relationships between Executive Functioning and Psychopathology Factors.
| Std. B | S.E. | 95 % CI | P-Value | |
|---|---|---|---|---|
| Baseline (N = 9725) | ||||
| EF1→p1 | −0.129 | 0.011 | [−0.150, −0.107] | <0.001 |
| EF1→EXT1 | −0.118 | 0.011 | [−0.140, −0.096] | <0.001 |
| EF1→INT1 | −0.080 | 0.012 | [−0.104, −0.056] | <0.001 |
| EF1→ND1 | −0.144 | 0.010 | [−0.164, −0.124] | <0.001 |
| EF1→SOMAT1 | −0.046 | 0.011 | [−0.068, −0.024] | <0.001 |
| EF1→DETACH1 | −0.120 | 0.012 | [−0.143, −0.097] | <0.001 |
| Two-Year Follow-Up (N = 5238) | ||||
| EF3→p3 | −0.103 | 0.011 | [−0.124, −0.081] | <0.001 |
| EF3→EXT3 | −0.083 | 0.012 | [−0.107, −0.058] | <0.001 |
| EF3→INT3 | −0.055 | 0.015 | [−0.084, −0.026] | 0.001 |
| EF3→ND3 | −0.120 | 0.010 | [−0.141, −0.100] | <0.001 |
| EF3→SOMAT3 | −0.039 | 0.016 | [−0.069, −0.008] | 0.013 |
| EF3→DETACH3 | −0.087 | 0.010 | [−0.107, −0.068] | <0.001 |
Note. P-values are unadjusted; p-values that survived FDR correction for the 12 tests (q<0.01) are indicated in bold. Standardized betas <0.2 were considered weak, betas >0.2 - <0.5 were considered moderate, and betas >0.5 were considered strong (following guidelines for interpreting standardized betas as effect sizes) (Acock, 2014). 1=baseline; EF1=baseline executive function factor scores; EF3=Mean of two-year follow-up Flanker and Pattern Comparison Processing Speed tests; EXT = Externalizing; INT = Internalizing; ND = Neurodevelopmental; SOMAT = Somatization; DETACH = Detachment; →=“predicts;” S.E.=standard error; CI = confidence interval.
3.5. Does EF prospectively predict psychopathology?
The prospective relationship between baseline EF factor scores and one-year follow-up p factor scores did not reach the statistical significance threshold (FDR correction q<0.01) (Table 6). However, baseline EF factor scores significantly prospectively predicted two-year follow-up p factor scores controlling for baseline and one-year follow-up p factor scores and covariates. Analyses of specificity showed that baseline EF factor scores negatively prospectively predicted one- and two-year change in externalizing and neurodevelopmental factor scores. Baseline EF factor scores negatively prospectively predicted two-year, but not one-year change in somatization and detachment factor scores. Neither one- or two-year change in internalizing factor scores were significantly predicted by baseline EF.
Table 6.
One- and Two-Year Residualized Change in Psychopathology Factor Scores as Predicted by Executive Function.
| Std. B | S.E. | 95 % CI | P-Value | |
|---|---|---|---|---|
| One-Year Change (N = 9120) | ||||
| EF1→p2 | −0.017 | 0.009 | [−0.034, 0.001] | 0.058 |
| p1→p2 | 0.745 | 0.009 | [0.727, 0.764] | <0.001 |
| EF1→EXT2 | −0.035 | 0.009 | [−0.052, −0.018] | <0.001 |
| EXT1→EXT2 | 0.686 | 0.009 | [0.668, 0.704] | <0.001 |
| EF1→INT2 | −0.008 | 0.012 | [−0.031, 0.015] | 0.514 |
| INT1→INT2 | 0.624 | 0.008 | [0.608, 0.639] | <0.001 |
| EF1→ND2 | −0.044 | 0.009 | [−0.063, −0.026] | <0.001 |
| ND1→ND2 | 0.677 | 0.009 | [0.660, 0.694] | <0.001 |
| EF1→SOMAT2 | −0.003 | 0.012 | [−0.026, 0.021] | 0.831 |
| SOMAT1→SOMAT2 | 0.532 | 0.008 | [0.517, 0.547] | <0.001 |
| EF1→DETACH2 | −0.026 | 0.011 | [−0.049, −0.004] | 0.020 |
| DETACH1→DETACH2 | 0.643 | 0.010 | [0.642, 0.662] | <0.001 |
| Two-Year Change (N = 5152) | ||||
| EF1→p3 | −0.028 | 0.009 | [−0.045, −0.011] | 0.002 |
| p1→p3 | 0.316 | 0.018 | [0.280, 0.352] | <0.001 |
| p2→p3 | 0.519 | 0.019 | [0.482, 0.555] | <0.001 |
| EF1→EXT3 | −0.032 | 0.009 | [−0.050, −0.015] | <0.001 |
| EXT1→EXT3 | 0.348 | 0.009 | [0.330, 0.366] | <0.001 |
| EXT2→EXT3 | 0.449 | 0.011 | [0.427, 0.470] | <0.001 |
| EF1→INT3 | −0.023 | 0.010 | [−0.043, −0.002] | 0.030 |
| INT1→INT3 | 0.316 | 0.010 | [0.297, 0.336] | <0.001 |
| INT2→INT3 | 0.434 | 0.008 | [0.418, 0.450] | <0.001 |
| EF1→ND3 | −0.047 | 0.010 | [−0.066, −0.028] | <0.001 |
| ND1→ND3 | 0.340 | 0.011 | [0.318, 0.362] | <0.001 |
| ND2→ND3 | 0.456 | 0.010 | [0.436, 0.476] | <0.001 |
| EF1→SOMAT3 | −0.027 | 0.009 | [−0.044, −0.010] | 0.002 |
| SOMAT1→SOMAT3 | 0.297 | 0.010 | [0.279, 0.316] | <0.001 |
| SOMAT2→SOMAT3 | 0.385 | 0.014 | [0.358, 0.412] | <0.001 |
| EF1→DETACH3 | −0.029 | 0.010 | [−0.048, −0.009] | 0.005 |
| DETACH1→DETACH3 | 0.323 | 0.013 | [0.298, 0.348] | <0.001 |
| DETACH2→DETACH3 | 0.439 | 0.014 | [0.412, 0.465] | <0.001 |
Note. P-values are unadjusted; p-values that survived FDR correction for the 30 tests (q<0.01) are indicated in bold. Standardized betas <0.2 were considered weak, betas >0.2 - <0.5 were considered moderate, and betas >0.5 were considered strong (following guidelines for interpreting standardized betas as effect sizes) (Acock, 2014). 1=baseline; 2=one-year follow-up; 3=two-year follow-up wave; EF = Executive Function factor scores; EXT = Externalizing; INT = Internalizing; ND = Neurodevelopmental; SOMAT = Somatization; DETACH = Detachment; → = “predicts;” S.E.=standard error; CI = confidence interval.
3.6. Does psychopathology prospectively predict EF?
Baseline p factor scores were significantly prospectively related to EF factor scores two years later, controlling for baseline EF factor scores and covariates (Table 7). Each of the lower-order psychopathology factors at baseline also were significant prospective predictors of change in EF factor scores.
Table 7.
Two-Year Residualized Change in Executive Function as Predicted by Psychopathology Factors.
| Std. B | S.E. | 95 % CI | P-Value | |
|---|---|---|---|---|
| p1→EF3 | −0.066 | 0.013 | [−0.092, −0.040] | <0.001 |
| EXT1→EF3 | −0.055 | 0.013 | [−0.080, −0.029] | <0.001 |
| INT1→EF3 | −0.036 | 0.013 | [−0.062, −0.010] | 0.007 |
| ND1→EF3 | −0.076 | 0.013 | [−0.101, −0.052] | <0.001 |
| SOMAT1→EF3 | −0.034 | 0.012 | [−0.058, −0.009] | 0.007 |
| DETACH1→EF3 | −0.060 | 0.014 | [−0.088, −0.033] | <0.001 |
| EF1→EF3 | 0.487 | 0.013 | [0.461, 0.513] | <0.001 |
Note. P-values are unadjusted; p-values that survived FDR correction for the 7 tests (q<0.01) are indicated in bold. N = 5193. Standardized betas <0.2 were considered weak, betas >0.2 - <0.5 were considered moderate, and betas >0.5 were considered strong (following guidelines for interpreting standardized betas as effect sizes) (Acock, 2014). 1=baseline; 3=two-year follow-up; EF1=Mean of baseline Flanker and Pattern Comparison Processing Speed tests; EF3=Mean of two-year follow-up Flanker and Pattern Comparison Processing Speed tests; EXT = Externalizing; INT = Internalizing; ND = Neurodevelopmental; SOMAT = Somatization; DETACH = Detachment; → = “predicts;” S.E.=standard error; CI = confidence interval.
3.7. Sensitivity analyses
Cross-sectional and prospective relations between EF and p factor scores persisted after controlling for parental education and family income (Supplementary Table 8). Relations between EF and the lower-order psychopathology factors also were unaffected by the inclusion of these variables. We found that results were similar when using the mean of the Flanker and Pattern Comparison Processing Speed tests instead of the EF factor scores in the regression and residualized change models (Supplementary Table 9).
4. Discussion
In the ABCD study of preadolescents aged 9–12, we examined cross-sectional and prospective relations between EF and p over a two-year period. Consistent with prior research (Bloemen et al., 2018; Cardenas-Iniguez et al., 2020; Caspi et al., 2014; Castellanos-Ryan et al., 2016; Huang-Pollock et al., 2017; Martel et al., 2017; Snyder et al., 2019a; Wade et al., 2019; White et al., 2017), we found negative cross-sectional relationships between EF and p at the baseline (ages 9–10) and two-year follow-up waves (ages 11–12). Poorer EF was a prospective predictor of increases in general psychopathology over a two-year period. Higher p factor scores at baseline also prospectively predicted two-year decreases in EF performance. These results suggest that executive dysfunction is both a risk marker and consequence of general psychopathology, signifying a potentially complex interplay between EF and psychopathology over time.
How might executive dysfunction lead to the development of future psychopathology in youth? One theory (i.e., iterative reprocessing theory (IR)) (Cunningham and Zelazo, 2007; Zelazo, 2015) posits that situations involving novel, uncertain, or conflicting information require individuals to pause and reflect on their course of action. This reflection necessitates EF skills such as holding information in mind while simultaneously updating such information, inhibitory control over a pre-potent response, and the creation of new rules or switching between rules. Failure to pause and reflect on new information using EF skills leads to less flexibility in adapting to new situations, poorer conflict resolution, and engagement in maladaptive behaviors, each of which are common features of mental disorders. Indeed, twin studies suggest that there are shared genetic influences on EF and p (Alnæs et al., 2018; Grotzinger et al., 2019; Harden et al., 2020). Stress and early life adversity, common predictors of many forms of psychopathology (McLaughlin et al., 2020) and the p factor (Schaefer et al., 2018; Snyder et al., 2019b), also influence EF (Pechtel and Pizzagalli, 2011). Zelazo (2020) proposes a theory of transdiagnostic risk for general psychopathology in which early life adversity impacts the development of EF skills, which in turn predicts the development of future psychopathology. In the current study, we did not consider the role of early life stress on EF and p, although we did find that relations persisted after controlling for parental education and family income, two indicators of socioeconomic status. Nevertheless, it will be important to examine the role of early life stress on EF and psychopathology over time in future longitudinal research.
Why might higher levels of childhood p lead to future executive dysfunction? One explanation for this finding is that higher levels of p might hinder children’s ability to practice and develop EF skills. For example, mental disorder symptoms could reduce opportunities to develop EF skills (e.g., greater school absenteeism, less engagement in hobbies/activities) (Egger et al., 2003; Wood et al., 2012) or maladaptive cognitions (e.g., rumination, worries, obsessions, negative automatic thoughts), poorer attentional control, and greater symptom-related distress could overly tax cognitive resources leading to reduced ability to engage EF skills in daily life (i.e., the resource allocation hypothesis; Levens et al., 2009). We cannot rule out the possibility that p predicts future reductions in EF due to concurrent mental disorder symptoms interfering with neurocognitive test performance. However, our results are more consistent with the hypothesis that preadolescent p may impede growth of EF given that we controlled for the cross-sectional relationship between p and EF at baseline, which could control for the disruptive effects of p on EF testing. Recent longitudinal cross-lagged panel studies also found support for the prospective relation between childhood psychopathology and later decreases in adolescent EF (Brieant et al., 2020; Donati et al., 2021).
The current findings also are consistent with recent neuroimaging research, which has found structural and functional alterations in frontoparietal, default mode, visual association cortex, and cerebello-thalamo-cerebro-cortical neural circuits involved in executive control in those high in p (Elliott et al., 2018; Karcher et al., 2020; Moberget et al., 2019; Romer et al., 2019, 2018; Romer and Pizzagalli, 2021; Sato et al., 2016). An important next step will be to determine whether structural and functional alterations in these neural circuits prospectively predict future general psychopathology and whether those relations are mediated by executive dysfunction.
Although the effect sizes of relations between EF and psychopathology factors were small, which could indicate low practical significance, we would argue that this is not the case. We examined the prospective relations between EF and p over a short time frame of two years. EF was found to be a marginal prospective predictor of change in p factor scores over a one-year period but a reliable predictor of change in p over two years, which suggests that the influence of executive dysfunction on future psychopathology may take longer than one year to manifest. We identified these EF deficits in children, prior to the onset of most mental disorders and during a period of extensive neurodevelopmental changes, particularly in neural circuits involved in cognitive control (Giedd et al., 1999), which could explain the small effect sizes in the ABCD sample of preadolescents.
One hypothesis from this work is that the effects of these early risk factors may compound on each other over time to ultimately increase comorbidity and severity of psychiatric symptoms throughout the lifespan. This hypothesis would be consistent with a dynamic mutualism theory, which posits that symptom comorbidity as captured by the p factor may increase over time, and was supported by a recent longitudinal study in children (McElroy et al., 2018). Further, our work would suggest a possible mechanism (i.e., executive dysfunction) through which this dynamic mutualism occurs. Indeed, using longitudinal data from a population-representative birth cohort, Caspi et al. (2020) found that poorer age 3 brain health prospectively predicted higher p factor scores in adulthood, and that higher p factor scores were associated with greater cognitive decline from childhood to adulthood and older midlife brain age. Future research should examine the dynamic interplay between EF and general psychopathology over longer periods of time throughout youth development.
Tests of specificity revealed that the prospective relations of psychopathology predicting change in EF were generalizable across externalizing, internalizing, neurodevelopmental, somatization, and detachment symptoms. This suggests that executive dysfunction is a non-specific consequence of all forms of childhood psychopathology. This finding is consistent with recent research showing that signs of accelerated aging (Wertz et al., 2021) and microstructural abnormalities (Romer et al., 2020) related to the p factor are not specific to any particular disorder family. Alternatively, some families of disorders were more consistently prospectively predicted by poorer EF than others. Specifically, baseline EF only consistently prospectively predicted both one- and two-year change in externalizing and neurodevelopmental symptoms. Baseline EF predicted two-year change in detachment and somatization symptoms but did not significantly predict change in internalizing symptoms (although this effect size was similar to the size of relations with the other factors). One possibility is that rates of depression and social anxiety, core internalizing symptoms, typically onset in and rise markedly from adolescence through early adulthood (Avenevoli et al., 2015; Kessler et al., 2005a). EF may be more predictive of internalizing disorders as these youth are followed into adolescence.
In terms of the longitudinal measurement invariance of the psychopathology factors, we found that the externalizing, internalizing, neurodevelopmental, somatization, and detachment factors demonstrated metric (equal factor loadings over time) invariance over the three waves. The loadings on the p factor also were highly similar across waves, consistent with prior adult studies demonstrating longitudinal metric invariance of the p factor (Forbes et al., 2021; Gluschkoff et al., 2019). Alternatively, one study did not find evidence of metric invariance of p over time in a community sample of children aged 3–6 (Olino et al., 2018). Correlations between factor scores over time generally were high (except for the somatization factor, for which correlations were moderate), suggesting that the transdiagnostic factors were reliable and relatively stable over time, consistent with prior research (Forbes et al., 2021; Gluschkoff et al., 2019; Murray et al., 2016).
Our study was limited in the following ways. First, there were only three waves of data available at the time of this analysis, and the third wave only had 55 % of the sample data available (owing to the fact that data collection is ongoing). This limited our analysis approach to tests of linear changes in EF and psychopathology over time, as nonlinear models require at least four timepoints. Second, we also had to restrict our sample to youth with data available for all models examining two-year change, and there were significant differences in study variables between those with and without two-year follow-up data that could have influenced the results. However, fit statistics, factor loadings, and factor scores were highly similar between models identified using the full versus reduced samples. Third, our measures of EF were not comprehensive and were not measured at all three waves, which prevented from examining relations between psychopathology factors and specific facets of EF (i.e., shifting, working memory, inhibition) or from testing associations with EF at one-year follow-up. Fourth, our CFAs relied on parent reports of child symptoms, which could be subject to reporting biases. However, recent ABCD research showed that maternal psychopathology did not bias parent reporting of child psychopathology (Olino et al., 2021).
Ultimately, this research points to EF as a potential transdiagnostic intervention target to prevent the onset and maintenance of psychopathology in youth, especially externalizing and neurodevelopmental disorders. Although EF interventions have improved EF task performance in children, particularly in those with marked EF deficits (Diamond, 2013), these improvements have not transferred to improved daily functioning or clinical symptoms (Rabipour and Raz, 2012). Many evidence-based psychotherapeutic interventions rely on EF skills to identify thoughts, feelings, and behaviors related to symptoms and engage in cognitive restructuring (e.g., cognitive behavioral therapy). Therefore, children with relatively poorer EF skills may benefit from additional EF skills-focused interventions prior to enrollment in psychotherapy. Further, given the prospective bidirectional relationship between EF and p, our findings suggest that interventions targeting mental disorder symptoms also could lead to improvements in EF.
5. Conclusions
This study’s results suggest that executive dysfunction is both a risk factor for and consequence of general psychopathology independent of sex, age, race/ethnicity, parental education, and family income. As this research was conducted in children, our findings indicate that EF may be an early risk marker for future psychopathology prior to the onset of most mental disorders in adolescence. EF may be a promising early transdiagnostic treatment target to prevent the onset and maintenance of youth psychopathology. Interventions targeting early psychopathology also may improve subsequent EF performance.
Disclosures
Over the past 3 years, Dr. Pizzagalli has received consulting fees from Albright Stonebridge Group, BlackThorn Therapeutics, Boehringer Ingelheim, Compass Pathway, Concert Pharmaceuticals, Engrail Therapeutics, Neurocrine Biosciences, Otsuka Pharmaceuticals, and Takeda Pharmaceuticals; one honorarium from Alkermes, and research funding from NIMH, Dana Foundation, Brain and Behavior Research Foundation, and Millennium Pharmaceuticals. In addition, he has received stock options from BlackThorn Therapeutics. Dr. Romer has no financial disclosures, and there are no conflicts of interest with the work conducted in this study. All views expressed are solely those of the authors.
Data statement
The data used in this manuscript is available through the National Data Archives Database (DOI 10.15154/1521137).
CRediT authorship contribution statement
Adrienne L. Romer: Conceptualization, Formal analysis, Data curation, Writing - original draft, Visualization. Diego A. Pizzagalli: Resources, Writing - review & editing, Supervision.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Over the past 3 years, Dr. Pizzagalli has received consulting fees from Albright Stonebridge Group, BlackThorn Therapeutics, Boehringer Ingelheim, Compass Pathway, Concert Pharmaceuticals, Engrail Therapeutics, Neurocrine Biosciences, Otsuka Pharmaceuticals, and Takeda Pharmaceuticals; one honorarium from Alkermes, and research funding from NIMH, Dana Foundation, Brain and Behavior Research Foundation, and Millennium Pharmaceuticals. In addition, he has received stock options from BlackThorn Therapeutics. Dr. Romer has no financial disclosures, and there are no conflicts of interest with the work conducted in this study. All views expressed are solely those of the authors.
Acknowledgments
Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive DevelopmentSM (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10,000 children age 9-10 and follow them over 10 years into early adulthood. The ABCD Study® is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041048, U01DA050989, U01DA051016, U01DA041022, U01DA051018, U01DA051037, U01DA050987, U01DA041174, U01DA041106, U01DA041117, U01DA041028, U01DA041134, U01DA050988, U01DA051039, U01DA041156, U01DA041025, U01DA041120, U01DA051038, U01DA041148, U01DA041093, U01DA041089, U24DA041123, U24DA041147. A full list of supporters is available at https://abcdstudy.org/federal-partners.html. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/consortium_members/. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators. The ABCD data repository grows and changes over time. The ABCD data used in this report came from DOI 10.15154/1521137. DOIs can be found at https://doi.org/10.15154/1521137. Authors received funding support from the National Institutes of Health: Dr. Pizzagalli (grants R01 MH101521 and R37 MH068376) and Dr. Romer (grant F32 MH124409).
Footnotes
There were a few instances in which there was no within-site variation for the dummy-coded Black (site 22) and Asian variables (site 7) in regression or residualized change models. In these instances, either site 22 was removed from the analysis (N=34) or the Asian participants were included in the Other race dummy-coded variable.
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2021.100994.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Abramovitch A., Short T., Schweiger A. The c factor: cognitive dysfunction as a transdiagnostic dimension in psychopathology. Clin. Psychol. Rev. 2021 doi: 10.1016/j.cpr.2021.102007. [DOI] [PubMed] [Google Scholar]
- Achenbach T.M. University of Vermont Research Center for Children, Youth, and Families; Burlington, VT: 2009. The Achenbach System of Emprically Based Assessment (ASEBA): Development, Findings, Theory and Applications. [Google Scholar]
- Acock A.C. 4th ed. ed. Stata Press; 2014. A Gentle Introduction to Stata. [Google Scholar]
- Alnæs D., Kaufmann T., Doan N.T., Córdova-Palomera A., Wang Y., Bettella F., Moberget T., Andreassen O.A., Westlye L.T. Association of heritable cognitive ability and psychopathology with white matter properties in children and adolescents. JAMA Psychiatry. 2018;75:287–295. doi: 10.1001/jamapsychiatry.2017.4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asparouhov T., Muthén B. 2010. Weighted Least Squares Estimation With Missing Data. [Google Scholar]
- Avenevoli S., Swendsen J., He J.-P., Burstein M., Merikangas K. Major depression in the national comorbidity survey- adolescent supplement: prevalence, correlates, and treatment. J. Am. Acad. Child Adolesc. Psychiatry. 2015;54:37–44.e2. doi: 10.1016/j.jaac.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995;57:289–300. [Google Scholar]
- Bloemen A.J.P., Oldehinkel A.J., Laceulle O.M., Ormel J., Rommelse N.N.J., Hartman C.A. The association between executive functioning and psychopathology: general or specific? Psychol. Med. 2018;48:1787–1794. doi: 10.1017/S0033291717003269. [DOI] [PubMed] [Google Scholar]
- Bora E., Yucel M., Pantelis C. Cognitive endophenotypes of bipolar disorder: a meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. J. Affect. Disord. 2009;113:1–20. doi: 10.1016/j.jad.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Brieant A., King-Casas B., Kim-Spoon J. Transactional relations between developmental trajectories of executive functioning and internalizing and externalizing symptomatology in adolescence. Dev. Psychopathol. 2020:1–12. doi: 10.1017/S0954579420001054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S.B., von Stauffenberg C. Delay and inhibition as early predictors of ADHD symptoms in third grade. J. Abnorm. Child Psychol. 2009;37:1–15. doi: 10.1007/s10802-008-9270-4. [DOI] [PubMed] [Google Scholar]
- Cannon M., Moffitt T.E., Caspi A., Murray R.M., Harrington H., Poulton R. Neuropsychological performance at the age of 13 years and adult schizophreniform disorder: prospective birth cohort study. Br. J. Psychiatry. 2006;189:463–464. doi: 10.1192/bjp.bp.105.020552. [DOI] [PubMed] [Google Scholar]
- Cardenas-Iniguez C., Moore T.M., Kaczkurkin A.N., Meyer F.A.C., Satterthwaite T.D., Fair D.A., White T., Blok E., Applegate B., Thompson L.M., Rosenberg M.D., Hedeker D., Berman M.G., Lahey B.B. Direct and indirect associations of widespread individual differences in brain white matter microstructure with executive functioning and general and specific dimensions of psychopathology in children. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2020 doi: 10.1016/j.bpsc.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlozzi N.E., Beaumont J.L., Tulsky D.S., Gershon R.C. The NIH toolbox pattern comparison processing speed test: normative data. Arch. Clin. Neuropsychol. 2015;30:359–368. doi: 10.1093/arclin/acv031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A., Moffitt T.E. All for one and one for all: mental disorders in one dimension. Am. J. Psychiatry. 2018;175:831–844. doi: 10.1176/appi.ajp.2018.17121383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A., Houts R.M., Belsky D.W., Goldman-Mellor S.J., Harrington H., Israel S., Meier M.H., Ramrakha S., Shalev I., Poulton R., Moffitt T.E. The p factor: one general psychopathology factor in the structure of psychiatric disorders? Clin. Psychol. Sci. 2014;2:119–137. doi: 10.1177/2167702613497473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A., Houts R.M., Ambler A., Danese A., Elliott M.L., Hariri A., Harrington H., Hogan S., Poulton R., Ramrakha S., Rasmussen L.J.H., Reuben A., Richmond-Rakerd L., Sugden K., Wertz J., Williams B.S., Moffitt T.E. Longitudinal assessment of mental health disorders and comorbidities across 4 decades among participants in the Dunedin Birth Cohort Study. JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.3221. e203221–e203221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos-Ryan N., Brière F.N., O’Leary-Barrett M., Banaschewski T., Bokde A., Bromberg U., Büchel C., Flor H., Frouin V., Gallinat J., Garavan H., Martinot J.-L., Nees F., Paus T., Pausova Z., Rietschel M., Smolka M.N., Robbins T.W., Whelan R., Schumann G., Conrod P. The structure of psychopathology in adolescence and its common personality and cognitive correlates. J. Abnorm. Psychol. 2016;125:1039–1052. doi: 10.1037/abn0000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D.A., Hicks B.M., Angstadt M., Rutherford S., Taxali A., Hyde L.W., Weigard A.S., Heitzeg M., Sripada C. The general factor of psychopathology in the adolescent brain cognitive development (ABCD) study: a comparison of alternative modeling approaches (preprint) Clin. Psychol. Sci. 2020;9(2):169–182. doi: 10.1177/2167702620959317. https://doi-org.ezp-prod1.hul.harvard.edu/10.1177/2167702620959317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland W., Shanahan L., Costello E.J., Angold A. Cumulative prevalence of psychiatric disorders by young adulthood: a prospective cohort analysis from the Great Smoky Mountains Study. J. Am. Acad. Child Adolesc. Psychiatry. 2011;50:252–261. doi: 10.1016/j.jaac.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham W.A., Zelazo P.D. Attitudes and evaluations: a social cognitive neuroscience perspective. Trends Cogn. Sci. 2007;11:97–104. doi: 10.1016/j.tics.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Diamond A. Executive functions. Annu. Rev. Psychol. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donati G., Meaburn E., Dumontheil I. Internalising and externalising in early adolescence predict later executive function, not the other way around: a cross-lagged panel analysis. Cogn. Emot. 2021:1–13. doi: 10.1080/02699931.2021.1918644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger H.L., Costello J.E., Angold A. School refusal and psychiatric disorders: a community study. J. Am. Acad. Child Adolesc. Psychiatry. 2003;42:797–807. doi: 10.1097/01.CHI.0000046865.56865.79. [DOI] [PubMed] [Google Scholar]
- Elliott, Romer A., Knodt A.R., Hariri A.R. A connectome wide functional signature of transdiagnostic risk for mental illness. Biol. Psychiatry. 2018;84:452–459. doi: 10.1016/j.biopsych.2018.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J., Mcc B.D., Sommer T., Raz A., Posner M.I. Testing the efficiency and independence of attentional networks. J. Cogn. Neurosci. 2002:340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- Forbes M.K., Greene A.L., Levin-Aspenson H.F., Watts A.L., Hallquist M., Lahey B.B., Markon K.E., Patrick C.J., Tackett J.L., Waldman I.D., Wright A.G.C., Caspi A., Ivanova M., Kotov R., Samuel D.B., Eaton N.R., Krueger R.F. Three recommendations based on a comparison of the reliability and validity of the predominant models used in research on the empirical structure of psychopathology. J. Abnorm. Psychol. 2021;130:297–317. doi: 10.1037/abn0000533. [DOI] [PubMed] [Google Scholar]
- Garavan H., Bartsch H., Conway K., Decastro A., Goldstein R.Z., Heeringa S., Jernigan T., Potter A., Thompson W., Zahs D. Recruiting the ABCD sample: design considerations and procedures. Dev. Cogn. Neurosci. 2018;32:16–22. doi: 10.1016/j.dcn.2018.04.004. The Adolescent Brain Cognitive Development (ABCD) Consortium: Rationale, Aims, and Assessment Strategy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd J.N., Blumenthal J., Jeffries N.O., Castellanos F.X., Liu H., Zijdenbos A., Paus T., Evans A.C., Rapoport J.L. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gluschkoff K., Jokela M., Rosenström T. The general psychopathology factor: structural stability and generalizability to within-individual changes. Front. Psychiatry. 2019;10 doi: 10.3389/fpsyt.2019.00594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkind M., Eickhoff S.B., Oathes D.J., Jiang Y., Chang A., Jones-Hagata L.B., Ortega B.N., Zaiko Y.V., Roach E.L., Korgaonkar M.S., Grieve S.M., Galatzer-Levy I., Fox P.T., Etkin A. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72:305–315. doi: 10.1001/jamapsychiatry.2014.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotzinger A.D., Cheung A.K., Patterson M.W., Harden K.P., Tucker-Drob E.M. Genetic and environmental links between general factors of psychopathology and cognitive ability in early childhood. Clin. Psychol. Sci. 2019;7:430–444. doi: 10.1177/2167702618820018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden K.P., Engelhardt L.E., Mann F.D., Patterson M.W., Grotzinger A.D., Savicki S.L., Thibodeaux M.L., Freis S.M., Tackett J.L., Church J.A., Tucker-Drob E.M. Genetic associations between executive functions and a general factor of psychopathology. J. Am. Acad. Child Adolesc. Psychiatry. 2020;59:749–758. doi: 10.1016/j.jaac.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang-Pollock C., Shapiro Z., Galloway-Long H., Weigard A. Is poor working memory a transdiagnostic risk factor for psychopathology? J. Abnorm. Child Psychol. 2017;45:1477–1490. doi: 10.1007/s10802-016-0219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karcher N.R., Michelini G., Kotov R., Barch D.M. Associations between resting-state functional connectivity and a hierarchical dimensional structure of psychopathology in middle childhood. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2020 doi: 10.1016/j.bpsc.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny L., Cribb S.J., Pellicano E. Childhood executive function predicts later autistic features and adaptive behavior in young autistic people: a 12-Year prospective study. J. Abnorm. Child Psychol. 2019;47:1089–1099. doi: 10.1007/s10802-018-0493-8. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Berglund P., Demler O., Jin R., Merikangas K.R., Walters E.E. Lifetime prevalence and age-of-Onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch. Gen. Psychiatry. 2005;62:593. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Chiu W.T., Demler O., Walters E.E. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey replication. Arch. Gen. Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline R.B. fourth edition. Guilford Publications; 2015. Principles and Practice of Structural Equation Modeling. [Google Scholar]
- Lahey B.B., Applegate B., Hakes J.K., Zald D.H., Hariri A.R., Rathouz P.J. Is there a general factor of prevalent psychopathology during adulthood? J. Abnorm. Psychol. 2012;121:971–977. doi: 10.1037/a0028355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey B.B., Krueger R.F., Rathouz P.J., Waldman I.D., Zald D.H. A hierarchical causal taxonomy of psychopathology across the life span. Psychol. Bull. 2017;143:142–186. doi: 10.1037/bul0000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letkiewicz A.M., Miller G.A., Crocker L.D., Warren S.L., Infantolino Z.P., Mimnaugh K.J., Heller W. Executive function deficits in daily life prospectively predict increases in depressive symptoms. Cogn. Ther. Res. 2014;38:612–620. doi: 10.1007/s10608-014-9629-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levens S.M., Muhtadie L., Gotlib I.H. Rumination and impaired resource allocation in depression. J. Abnorm. Psychol. 2009;118:757–766. doi: 10.1037/a0017206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M., Bjork J.M., Nagel B.J., Barch D.M., Gonzalez R., Nixon S.J., Banich M.T. Adolescent neurocognitive development and impacts of substance use: overview of the adolescent brain cognitive development (ABCD) baseline neurocognition battery. Dev. Cogn. Neurosci. 2018;32:67–79. doi: 10.1016/j.dcn.2018.02.006. The Adolescent Brain Cognitive Development (ABCD) Consortium: Rationale, Aims, and Assessment Strategy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Giollabhui N., Olino T.M., Nielsen J., Abramson L.Y., Alloy L.B. Is worse attention a risk factor for or a consequence of depression, or are worse attention and depression better accounted for by stress? A prospective test of three hypotheses. Clin. Psychol. Sci. 2019;7:93–109. doi: 10.1177/2167702618794920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel M.M., Pan P.M., Hoffmann M.S., Gadelha A., Rosário M.Cdo, Mari Jde J., Manfro G.G., Miguel E.C., Paus T., Bressan R.A., Rohde L.A., Salum G.A. A general psychopathology factor (P factor) in children: structural model analysis and external validation through familial risk and child global executive function. J. Abnorm. Psychol. 2017;126:137–148. doi: 10.1037/abn0000205. [DOI] [PubMed] [Google Scholar]
- McElroy E., Belsky J., Carragher N., Fearon P., Patalay P. Developmental stability of general and specific factors of psychopathology from early childhood to adolescence: dynamic mutualism or p-differentiation? J. Child Psychol. Psychiatry. 2018;59:667–675. doi: 10.1111/jcpp.12849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Colich N.L., Rodman A.M., Weissman D.G. Mechanisms linking childhood trauma exposure and psychopathology: a transdiagnostic model of risk and resilience. BMC Med. 2020;18:96. doi: 10.1186/s12916-020-01561-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague L.M., Goodkind M.S., Etkin A. Transdiagnostic impairment of cognitive control in mental illness. J. Psychiatr. Res. 2016;83:37–46. doi: 10.1016/j.jpsychires.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague L.M., Huemer J., Carreon D.M., Jiang Y., Eickhoff S.B., Etkin A. Identification of common neural circuit disruptions in cognitive control across psychiatric disorders. Am. J. Psychiatry. 2017;174:676–685. doi: 10.1176/appi.ajp.2017.16040400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelini G., Barch D.M., Tian Y., Watson D., Klein D.N., Kotov R. Delineating and validating higher-order dimensions of psychopathology in the Adolescent Brain Cognitive Development (ABCD) study. Transl. Psychiatry. 2019;9:1–15. doi: 10.1038/s41398-019-0593-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A., Friedman N.P., Emerson M.J., Witzki A.H., Howerter A., Wager T.D. The unity and diversity of executive functions and their contributions to complex “Frontal lobe” tasks: a latent variable analysis. Cognit. Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Moberget T., Alnæs D., Kaufmann T., Doan N.T., Córdova-Palomera A., Norbom L.B., Rokicki J., van der Meer D., Andreassen O.A., Westlye L.T. Cerebellar gray matter volume is associated with cognitive function and psychopathology in adolescence. Biol. Psychiatry Clin. Impact Psychosis Risk Mechanisms. 2019;86:65–75. doi: 10.1016/j.biopsych.2019.01.019. [DOI] [PubMed] [Google Scholar]
- Murray A.L., Eisner M., Ribeaud D. The development of the general factor of psychopathology ‘p factor’ through childhood and adolescence. J. Abnorm. Child Psychol. 2016;44:1573–1586. doi: 10.1007/s10802-016-0132-1. [DOI] [PubMed] [Google Scholar]
- Muthen L.K., Muthen B.O. eight ed. Muthen & Muthen; Los Angeles, CA: 1998. Mplus User’s Guide. [Google Scholar]
- Niendam T.A., Bearden C.E., Rosso I.M., Sanchez L.E., Hadley T., Nuechterlein K.H., Cannon T.D. A prospective study of childhood neurocognitive functioning in schizophrenic patients and their siblings. Am. J. Psychiatry. 2003;160:2060–2062. doi: 10.1176/appi.ajp.160.11.2060. [DOI] [PubMed] [Google Scholar]
- Olino T.M., Bufferd S.J., Dougherty L.R., Dyson M.W., Carlson G.A., Klein D.N. The development of latent dimensions of psychopathology across early childhood: stability of dimensions and moderators of change. J. Abnorm. Child Psychol. 2018;46:1373–1383. doi: 10.1007/s10802-018-0398-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olino T.M., Michelini G., Mennies R.J., Kotov R., Klein D.N. Does maternal psychopathology bias reports of offspring symptoms? A study using moderated non-linear factor analysis. J. Child Psychol. Psychiatry. 2021 doi: 10.1111/jcpp.13394. n/a. [DOI] [PubMed] [Google Scholar]
- Pechtel P., Pizzagalli D.A. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology (Berl.) 2011;214:55–70. doi: 10.1007/s00213-010-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabipour S., Raz A. Training the brain: fact and fad in cognitive and behavioral remediation. Brain Cogn. 2012;79:159–179. doi: 10.1016/j.bandc.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Romer A.L., Pizzagalli D.A. Associations between brain structural alterations, executive dysfunction, and general psychopathology in a healthy and cross-diagnostic adult patient sample. Biol. Psychiatry Glob. Open Sci. 2021 doi: 10.1016/j.bpsgos.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer, Knodt A.R., Houts R., Brigidi B.D., Moffitt T.E., Caspi A., Hariri A.R. Structural alterations within cerebellar circuitry are associated with general liability for common mental disorders. Mol. Psychiatry. 2018;23:1084–1090. doi: 10.1038/mp.2017.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer, Knodt A.R., Sison M.L., Ireland D., Houts R., Ramrakha S., Poulton R., Keenan R., Melzer T.R., Moffitt T.E., Caspi A., Hariri A.R. Replicability of structural brain alterations associated with general psychopathology: evidence from a population-representative birth cohort. Mol. Psychiatry. 2019:1–8. doi: 10.1038/s41380-019-0621-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer A.L., Elliott M.L., Knodt A.R., Sison M.L., Ireland D., Houts R., Ramrakha S., Poulton R., Keenan R., Melzer T.R., Moffitt T.E., Caspi A., Hariri A.R. Pervasively thinner neocortex as a transdiagnostic feature of general psychopathology. Am. J. Psychiatry. 2020;178:174–182. doi: 10.1176/appi.ajp.2020.19090934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato J.R., Salum G.A., Gadelha A., Crossley N., Vieira G., Manfro G.G., Zugman A., Picon F.A., Pan P.M., Hoexter M.Q., Anés M., Moura L.M., Del’Aquilla M.A.G., Jr E.A., McGuire P., Lacerda A.L.T., Rohde L.A., Miguel E.C., Jackowski A.P., Bressan R.A. Default mode network maturation and psychopathology in children and adolescents. J. Child Psychol. Psychiatry. 2016;57:55–64. doi: 10.1111/jcpp.12444. [DOI] [PubMed] [Google Scholar]
- Schaefer J.D., Moffitt T.E., Arseneault L., Danese A., Fisher H.L., Houts R., Sheridan M.A., Wertz J., Caspi A. Adolescent victimization and early-adult psychopathology: approaching causal inference using a longitudinal twin study to rule out noncausal explanations. Clin. Psychol. Sci. 2018;6:352–371. doi: 10.1177/2167702617741381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha Z., Wager T.D., Mechelli A., He Y. Common dysfunction of large-scale neurocognitive networks across psychiatric disorders. Biol. Psychiatry. 2019;85(5):379–388. doi: 10.1016/j.biopsych.2018.11.011. [DOI] [PubMed] [Google Scholar]
- Shanmugan S., Wolf D.H., Calkins M.E., Moore T.M., Ruparel K., Hopson R.D., Vandekar S.N., Roalf D.R., Elliott M.A., Jackson C., Gennatas E.D., Leibenluft E., Pine D.S., Shinohara R.T., Hakonarson H., Gur R.C., Gur R.E., Satterthwaite T.D. Common and dissociable mechanisms of executive system dysfunction across psychiatric disorders in youth. Am. J. Psychiatry. 2016;173:517–526. doi: 10.1176/appi.ajp.2015.15060725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder H.R. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychol. Bull. 2013;139:81–132. doi: 10.1037/a0028727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder H.R., Miyake A., Hankin B.L. Advancing understanding of executive function impairments and psychopathology: bridging the gap between clinical and cognitive approaches. Front. Psychol. 2015;6 doi: 10.3389/fpsyg.2015.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder H.R., Hankin B.L., Sandman C.A., Head K., Davis E.P. Distinct patterns of reduced prefrontal and limbic grey matter volume in childhood general and internalizing psychopathology. Clin. Psychol. Sci. 2017;5:1001–1013. doi: 10.1177/2167702617714563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder H.R., Friedman N.P., Hankin B.L. Transdiagnostic mechanisms of psychopathology in youth: executive functions, dependent stress, and rumination. Cogn. Ther. Res. 2019;43:834–851. doi: 10.1007/s10608-019-10016-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder H.R., Young J.F., Hankin B.L. Chronic stress exposure and generation are related to the P-Factor and externalizing specific psychopathology in youth. J. Clin. Child Adolesc. Psychol. 2019;48:306–315. doi: 10.1080/15374416.2017.1321002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solmi M., Radua J., Olivola M., Croce E., Soardo L., Salazar de Pablo G., Il Shin J., Kirkbride J.B., Jones P., Kim J.H., Kim J.Y., Carvalho A.F., Seeman M.V., Correll C.U., Fusar-Poli P. Age at onset of mental disorders worldwide: large-scale meta-analysis of 192 epidemiological studies. Mol. Psychiatry. 2021:1–15. doi: 10.1038/s41380-021-01161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange J.P., Connolly S.L., Burke T.A., Hamilton J.L., Hamlat E.J., Abramson L.Y., Alloy L.B. Inflexible cognition predicts first onset of major depressive episodes in adolescence. Depress. Anxiety. 2016;33:1005–1012. doi: 10.1002/da.22513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulsky D.S., Carlozzi N., Chiaravalloti N.D., Beaumont J.L., Kisala P.A., Mungas D., Conway K., Gershon R. NIH toolbox cognition battery (NIHTB-CB): the list sorting test to measure working memory. J. Int. Neuropsychol. Soc. JINS. 2014;20:599–610. doi: 10.1017/S135561771400040X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade M., Zeanah C.H., Fox N.A., Nelson C.A. Global deficits in executive functioning are transdiagnostic mediators between severe childhood neglect and psychopathology in adolescence. Psychol. Med. 2019:1–8. doi: 10.1017/S0033291719001764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts A.L., Lane S.P., Bonifay W., Steinley D., Meyer F.A.C. Building theories on top of, and not independent of, statistical models: the case of the p-factor. Psychol. Inq. 2020;31:310–320. doi: 10.1080/1047840x.2020.1853476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S., Dikmen S.S., Heaton R.K., Tulsky D.S., Zelazo P.D., Bauer P.J., Carlozzi N.E., Slotkin J., Blitz D., Wallner-Allen K., Fox N.A., Beaumont J.L., Mungas D., Nowinski C.J., Richler J., Deocampo J.A., Anderson J.E., Manly J.J., Borosh B., Havlik R., Conway K., Edwards E., Freund L., King J.W., Moy C., Witt E., Gershon R.C. Cognition assessment using the NIH toolbox. Neurology. 2013;80:S54–S64. doi: 10.1212/WNL.0b013e3182872ded. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz J., Caspi A., Ambler A., Broadbent J., Hancox R.J., Harrington H., Hogan S., Houts R.M., Leung J.H., Poulton R., Purdy S.C., Ramrakha S., Rasmussen L.J.H., Richmond-Rakerd L.S., Thorne P.R., Wilson G.A., Moffitt T.E. Association of history of psychopathology with accelerated aging at Midlife. JAMA Psychiatry. 2021 doi: 10.1001/jamapsychiatry.2020.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White L.K., Moore T.M., Calkins M.E., Wolf D.H., Satterthwaite T.D., Leibenluft E., Pine D.S., Gur R.C., Gur R.E. An evaluation of the specificity of executive function impairment in developmental psychopathology. J. Am. Acad. Child Adolesc. Psychiatry. 2017;56:975–982.e3. doi: 10.1016/j.jaac.2017.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J.J., Lynne S.D., Langer D.A., Wood P.A., Clark S.L., Eddy J.M., Ialongo N. School attendance problems and youth psychopathology: structural cross-lagged regression models in three longitudinal datasets. Child Dev. 2012;83:351–366. doi: 10.1111/j.1467-8624.2011.01677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazo P. The Dimensional Change Card Sort (DCCS): a method of assessing executive function in children. Nat. Protoc. 2006;1:297–301. doi: 10.1038/nprot.2006.46. [DOI] [PubMed] [Google Scholar]
- Zelazo P.D. Executive function: reflection, iterative reprocessing, complexity, and the developing brain. Dev. Rev., Theories of development. 2015;38:55–68. doi: 10.1016/j.dr.2015.07.001. [DOI] [Google Scholar]
- Zelazo P.D. Executive function and psychopathology: a neurodevelopmental perspective. Annu. Rev. Clin. Psychol. 2020;16:431–454. doi: 10.1146/annurev-clinpsy-072319-024242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.