Abstract
Objective: Investigate the effect of Tripterygium wilfordii polyglycoside tablets on serum inflammatory factors and T cells in patients with chronic nephritis. Methods: A total of 89 patients with chronic nephritis were randomly divided into a control group (44 cases, treated with irbesartan hydrochlorothiazide combined with dipyridamole) and an observation group (45 cases, treated with Tripterygium wilfordii polyglycoside tablets based on irbesartan hydrochlorothiazide and dipyridamole like the control group). Patients in both groups were treated for two months. The renal function, inflammatory factors, the proportion of T lymphocyte subsets, and 24 h urinary protein quantification (24 h Upro) of patients with hemodialysis were compared between the two groups before and after treatment. The occurrence of adverse reactions was recorded. Results: SCR, BUN levels, 24 h Upro, serum hs-CRP, TNF-α, and IL-8 levels in the two groups after treatment were lower than those before treatment, and those of the observation group were lower than those of the control group (all P<0.05). After treatment, the CD4+ ratio and CD4+/CD8+ ratio of the two groups of patients increased, while the CD8+ ratio decreased. The changes in the observation group were greater than those in the control group (all P<0.05). There was no significant difference in the incidence of total adverse reactions between the two groups during treatment (P>0.05). Conclusion: Treatment combined with Tripterygium wilfordii polyglycosides can significantly alleviate the inflammatory state of patients with chronic glomerulonephritis, reduce the level of proteinuria, and improve renal function. Tripterygium wilfordii polyglycosides can improve immune function of the body and has high safety.
Keywords: Chronic nephritis, Tripterygium glycosides tablets, inflammatory factors, T lymphocytes
Introduction
Chronic nephritis is a common chronic kidney disease, the most common of which is chronic glomerulonephritis. Patients with chronic nephritis can have a large amount of proteinuria and hematuria. If patients are not treated in time, edema can occur and aggravate their condition [1,2]. An immunosuppressant is a standard treatment, and this may reduce some patients’ medication compliance because of the apparent adverse reactions [3].
According to traditional Chinese medicine, chronic nephritis belongs to the category of “edema” and “turbid urine”, which should be treated mainly by removing dampness and detumescence [4,5]. Tripterygium wilfordii polyglycoside is a fat-soluble mixture extracted and refined from the root of Celastraceae plant Tripterygium wilfordii. It has the effects of dehumidification, detumescence, expelling wind, and detoxification. Modern pharmacologic studies show that it also has specific anti-inflammatory and immunomodulatory effects [6]. There are many studies on the treatment of chronic nephritis with Tripterygium Glycosides tablets. Still, most of them focus on improving renal function in patients with chronic nephritis [7,8]. Simultaneously, there are few studies on the immune function and inflammatory state of patients with chronic nephritis. Our hospital tried to use Tripterygium wilfordii polyglycosides in the adjuvant treatment of patients with chronic glomerulonephritis, which showed an excellent therapeutic effect. Adjuvant therapy with Tripterygium wilfordii polyglycosides reduced the inflammatory state and improved the body’s immune function. This study aimed to investigate the effects of Tripterygium wilfordii polyglycosides tablets on serum inflammatory factors and T cells in patients with chronic nephritis.
Materials and methods
General information
In this prospective study, about 89 patients with chronic nephritis treated in our hospital from November 2018 to January 2020 were randomly divided into a control group (44 cases, treated with irbesartan hydrochlorothiazide combined with dipyridamole) and an observation group (45 cases, treated with irbesartan hydrochlorothiazide + dipyridamole combined with Tripterygium Glycosides tablets) according to the random number table method. The medical ethics committee of our hospital approved this study.
Inclusion criteria: (1) patients aged 28-65 years old; (2) patients diagnosed with glomerulonephritis according to various clinical examinations; (3) patients with 24 h urinary protein (24 h Upro) >3.5 g, plasma albumin <30 g/L; (4) patients who signed the informed consent.
Exclusion criteria: (1) patients with acute infection; (2) patients with a history of immunosuppressant use two weeks before the enrollment; (3) patients with immune system or blood system diseases; (4) patients with a malignant tumor or severe cardiovascular and cerebrovascular diseases; (5) patients allergic to drugs in this study.
Method
The control group was treated with Irbesartan Hydrochlorothiazide combined with dipyridamole. Irbesartan Hydrochlorothiazide tablets (Nanjing Zhengda Tianqing Pharmaceutical Co., Ltd., H20057227, specification: each tablet contains irbesartan 150 mg, Hydrochlorothiazide 12.5 mg) were taken orally two tablets/time, three times/d. Tripterygium glycosides tablets were added in the observation group’s treatment compared with the treatment in the control group (Lunan Houpu Pharmaceutical Co., Ltd., Z37020344, specification: 10 mg). Tripterygium glycosides tablets are taken orally 20 mg/time, three times/d. Two groups of patients were treated continuously for two months.
Outcome measures
Main outcome measures
(1) About 5 ml of venous blood was collected before and two months after the treatment, of which 3 ml venous blood was centrifuged to separate serum for standby. The levels of serum creatinine (SCR), blood urea nitrogen (BUN), and other renal function indicators were detected by an automatic biochemical analyzer (Shandong Boke Biological Industry Co., Ltd., Model: BK-280, Origin: China).
(2) The serum levels of hs-CRP, TNF-α, and IL-8 were detected by ELISA.
(3) The remaining 2 mL venous blood was used to detect the proportion of T lymphocyte subsets (CD4+, CD8+) and calculate the ratio of CD4+/CD8+ by flow cytometry (BD company, USA, Model: FACSCalibur, Origin: USA) and its auxiliary reagents.
Secondary outcome measures
(1) The 24 h Upro levels of the two groups before and after the treatment were detected and compared by an automatic biochemical analyzer.
(2) Adverse reactions such as nausea and vomiting, dizziness and headache, elevated transaminase, and skin rash, were recorded in the two groups during the treatment. The incidence of adverse reactions = the number of adverse reactions/total cases × 100%.
Statistical analysis
SPSS 20.0 was used for statistics, and the count data were expressed as the number of cases n (%). χ2 test was used, and the measured data were represented by mean ± standard deviation (x̅ ± sd). The paired t-test was used for comparison before and after the treatment, and an independent t-test was used to compare the two groups. Statistical significance was set at P<0.05.
Results
Baseline data
There was no significant difference in the baseline data between the two groups (all P>0.05). They were comparable between the two groups. See Table 1.
Table 1.
Baseline data of the two groups (n, x̅ ± sd)
| Observation group (n=45) | Control group (n=44) | χ2/t | P | |
|---|---|---|---|---|
| Gender (n) | 0.109 | 0.741 | ||
| Male | 24 | 25 | ||
| Female | 21 | 19 | ||
| Age (years) | 44.4±5.3 | 45.2±6.8 | 0.618 | 0.538 |
| Course of the disease (n) | 4.22±1.33 | 4.76±1.94 | 1.528 | 0.130 |
| 24 h Upro (g) | 3.98±0.35 | 4.04±0.44 | 0.711 | 0.479 |
| Plasma albumin level (g/L) | 23.30±3.49 | 24.04±4.33 | 0.886 | 0.378 |
| Basic diseases (n) | 0.782 | 0.676 | ||
| Diabetes | 4 | 2 | ||
| Hypertension | 7 | 6 | ||
| Hyperlipidemia | 2 | 3 |
Note: 24 h Upro: 24 h urinary protein quantification.
Renal function
Scr and BUN levels had no significant differences between the two groups before treatment (all P>0.05). After treatment, the Scr and BUN levels in the two groups were lower than those before treatment, and those in the observation group were lower than those of the control group (all P<0.05). See Table 2.
Table 2.
Renal function in the two groups before and after treatment (x̅ ± sd)
| Groups | Scr (μmol/L) | BUN (mmol/L) |
|---|---|---|
| Observation group (n=45) | ||
| Before treatment | 95.48±9.94 | 9.05±1.77 |
| After treatment | 75.59±6.48a,b | 6.60±1.30a,b |
| Control group (n=44) | ||
| Before treatment | 94.88±7.85 | 8.88±1.64 |
| After treatment | 82.56±7.59a | 7.46±1.04a |
Note: Compared with those before treatment;
P<0.05.
Compared with the control group;
P<0.05.
Scr: serum creatinine; BUN: blood urea nitrogen.
Inflammatory factors
Before the treatment, there was no significant difference in serum hs-CRP, TNF-α, or IL-8 levels between the two groups (all P>0.05). The serum hs-CRP, TNF-α, and IL-8 levels of the two groups after treatment were lower than those before treatment, and those of the observation group were lower than those of the control group (all P<0.05). See Table 3.
Table 3.
Levels of serum inflammatory factors before and after treatment in two groups (x̅ ± sd)
| Groups | hs-CRP (mg/L) | TNF-α (ng/L) | IL-8 (ng/L) |
|---|---|---|---|
| Observation group (n=45) | |||
| Before treatment | 2.97±0.68 | 167.55±20.03 | 3.23±1.20 |
| After treatment | 1.03±0.35a,b | 103.39±13.22a,b | 1.44±0.64a,b |
| Control group (n=44) | |||
| Before treatment | 2.88±0.73 | 166.97±18.94 | 3.55±1.38 |
| After treatment | 1.84±0.40a | 124.56±14.20a | 2.30±0.79a |
Note: hs-CRP: high sensitivity C-reactive protein; TNF-α: tumor necrosis factor; IL-8: interleukin-8.
Compared with those before treatment;
P<0.05.
Compared with the control group;
P<0.05.
Percentage of T lymphocyte subsets
Before treatment, there were no significant differences in the proportion of CD4+ or CD8+ and CD4+/CD8+ ratio between the two groups (all P>0.05). After treatment, the CD4+ ratio and CD4+/CD8+ ratio of the two groups were increased, while the proportion of CD8+ was decreased. The changes in the observation group were more pronounced (all P<0.05). See Table 4.
Table 4.
The proportion of T lymphocyte subsets before and aftertreatment in the two groups (x̅ ± sd)
| Group | CD4+ (%) | CD8+ (%) | CD4+/CD8+ |
|---|---|---|---|
| Observation group (n=45) | |||
| Before treatment | 30.30±5.44 | 34.48±4.70 | 0.88±0.19 |
| After treatment | 35.89±4.30a,b | 27.46±4.57a,b | 1.30±0.32a,b |
| Control group (n=44) | |||
| Before treatment | 30.84±5.95 | 34.95±5.11 | 0.87±0.21 |
| After treatment | 32.98±4.80a | 30.04±4.38a | 1.09±0.28a |
Note: Compared with those before treatment;
P<0.05.
Compared with the control group;
P<0.05.
24 h Upro
The 24 h Upro of the observation and control groups were (3.98±0.35) g and (4.04±0.44) g respectively before the treatment. The 24 h Upro of the two groups were (1.40±0.22) g and (2.22±0.38) g respectively after the treatment. The 24 h Upro of the two groups after treatment was lower than that before treatment, and that of the observation group was lower than that of the control group (P<0.05). See Figure 1.
Figure 1.
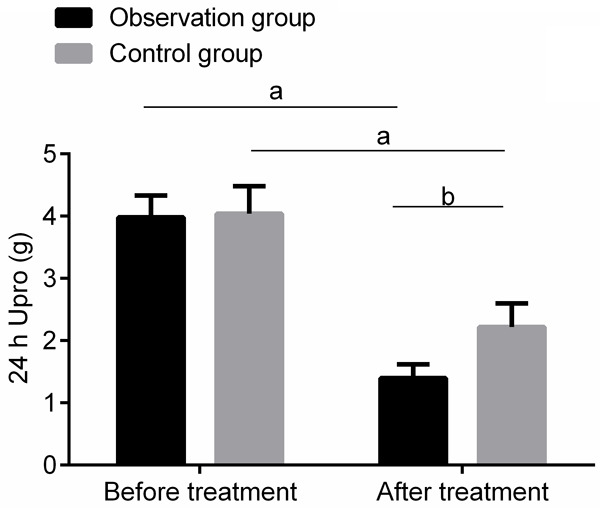
Comparison of 24 h Upro before and after treatment between the two groups. Compared with those before treatment, aP<0.05; compared with the control group, bP<0.05. 24 h Upro: 24 h urinary protein quantification.
Adverse reactions
There was no significant difference in the total incidence of adverse reactions between the two groups (P>0.05). See Table 5.
Table 5.
Incidence of adverse reactions in the two groups during the treatment (n, %)
| Groups | Nausea and vomiting | Dizziness and headache | Elevated transaminase | Rash | Total incidence |
|---|---|---|---|---|---|
| Observation group (n=45) | 2 (4.44) | 1 (2.22) | 0 (0.00) | 1 (2.22) | 4 (8.89) |
| Control group (n=44) | 2 (4.55) | 2 (4.55) | 1 (2.27) | 1 (2.27) | 6 (13.64) |
| χ 2 | 0.503 | ||||
| P | 0.478 |
Discussion
Chronic glomerulonephritis is the most common clinical type of chronic nephritis. The specific pathogenesis has not been fully elucidated, but studies have shown that an inflammatory reaction and immune response disorder are closely related to chronic glomerulonephritis occurrence [9].
Studies have shown that the serum levels of inflammatory factors in patients with chronic nephritis are abnormally higher than those in ordinary people [10,11]. Our results showed that the serum hs-CRP, TNF-α, and IL-8 levels in the two groups after treatment were lower than those before treatment. The levels of serum hs-CRP, TNF-α, and IL-8 in the observation group were lower than those of the control group, suggesting that after the treatment, serum inflammatory factors in patients with chronic glomerulonephritis are decreased. The combination of Tripterygium wilfordii polyglycosides provided definite relief of the inflammatory reaction. Wang et al. also did similar studies, which also found that Tripterygium wilfordii polyglycosides can reduce the level of serum inflammatory factors in patients with chronic glomerulonephritis and alleviate the level of inflammatory reaction [12]. Modern pharmacologic studies also showed that Tripterygium wilfordii polyglycosides have obvious anti-inflammatory effects, so the combination of Tripterygium wilfordii polyglycosides can more significantly alleviate the body’s inflammatory reaction [13,14].
T lymphocytes are the main effector cells that regulate the immune state of the body. Under normal circumstances, CD4+ and CD8+ are in a dynamic balance, and they restrict each other to maintain the normal immune function of the body [15,16]. Under pathological conditions, the balance between the two is broken, and the immune function is disordered [17]. However, the immune function of patients with chronic glomerulonephritis is disordered, which is mainly manifested by a decrease of the proportion of CD4+ T lymphocytes and an increase of the proportion of CD8+ T lymphocytes [18]. This study showed that after treatment, the proportion of CD4+ and CD4+/CD8+ in the two groups increased, and the proportion of CD8+ decreased. The changes in the observation group were more evident than those of the control group. The differences were statistically significant, suggesting that the improvement effect of Tripterygium wilfordii polyglycoside on patients’ immune function with chronic glomerulonephritis is stronger. Modern pharmacological studies show that Tripterygium wilfordii polyglycosides have a pronounced immunoregulatory effect and can enhance the immune function of model rats [19]. Zhu et al. also found that for patients with glomerulonephritis, the combination of Tripterygium wilfordii polyglycosides helped promote the body’s initially disordered immune function to return to a normal state [20].
In patients with chronic glomerulonephritis, the glomerular filtration function is impaired and a large amount of proteinuria occurred. The renal function is significantly decreased. 24 h Upro is the most objective assessment of urinary protein. The high level of 24 h Upro suggests more proteinuria and more serious renal function damage [21]. In this study, the levels of 24 h Upro, Scr, and BUN in the two groups after the treatment were lower than those before treatment, and those in the observation group were lower than those of the control group, suggesting that the combination of Tripterygium wilfordii polyglycosides can more effectively reduce the urine protein level of patients with chronic glomerulonephritis and improve renal function. Zhan et al. did a similar study, which also found that the urine protein of patients with chronic glomerulonephritis was significantly reduced, and the glomerular filtration function was also significantly improved after the combined use of Tripterygium wilfordii polyglycosides [22]. In terms of adverse reactions, no severe adverse reactions occurred in the two groups during treatment. The total incidence of adverse reactions had no significant difference between the two groups, which is reassuring for the safety of Tripterygium wilfordii polyglycosides.
However, this study is a single-center randomized controlled study with a limited sample size, and only compared the effect before and two months after the treatment. The effect of Tripterygium wilfordii polyglycosides on long-term renal function and immune function of patients with chronic nephritis still needs to be further studied.
In conclusion, the combined use of Tripterygium wilfordii polyglycosides can more significantly alleviate the inflammatory state of patients with chronic glomerulonephritis, reduce the level of proteinuria, improve renal function, and more effectively improve the body’s immune function. The combined use of Tripterygium wilfordii polyglycosides has high safety, and is worthy of clinical promotion.
Acknowledgements
This work was supported by the National Natural Science Foundation of China for Mechanism of Shensu IV intervening glomerular podocyte damage, based on hydrogen sulfide obstructing the activation of ASK1 pathway via S-sulfhydration of Txnip-Trx (82060849).
Disclosure of conflict of interest
None.
References
- 1.Floege J, Amann K. Primary glomerulonephritides. Lancet. 2016;387:2036–2048. doi: 10.1016/S0140-6736(16)00272-5. [DOI] [PubMed] [Google Scholar]
- 2.Kamyshova ES, Bobkova IN. MicroRNAs in chronic glomerulonephritis: promising biomarkers for diagnosis and prognosis estimation. Ter Arkh. 2017;89:89–96. doi: 10.17116/terarkh201789689-96. [DOI] [PubMed] [Google Scholar]
- 3.Plumb LA, Oni L, Marks SD, Tullus K. Paediatric anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis: an update on renal management. Pediatr Nephrol. 2018;33:25–39. doi: 10.1007/s00467-016-3559-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu F, Zhang PQ, Wang XQ, Nie LF, Fu XJ, Peng W, Wang Y, Li J, Bi YP, Mi XH, Ding XQ, He LQ. Multi-center randomized control study on the effects of syndrome differentiated traditional Chinese medicine therapy on CKD 1-2 with chronic nephritis proteinuria. J Sichuan Univ Med Sci. 2015;46:145–148. [PubMed] [Google Scholar]
- 5.Wei LB, Gao JR, Gao YC, Liu XC, Jiang H, Qin XJ. Effect of the traditional Chinese medicine Qi Teng Xiao Zhuo granules on chronic glomerulonephritis rats studied by using long noncoding RNAs expression profiling. Gene. 2020;728:144279–144284. doi: 10.1016/j.gene.2019.144279. [DOI] [PubMed] [Google Scholar]
- 6.Wang S, Li RM, He SH, He LG, Zhao H, Deng XH, Chen ZQ. Tripterygium wilfordii glycosides upregulate the new anti-inflammatory cytokine IL-37 through ERK1/2 and p38 MAPK signal pathways. Evid Based Complement Alternat Med. 2017;2017:9148523. doi: 10.1155/2017/9148523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang YQ, Wu YF, Xu FF, Deng JB, Wu LL, Han XD, Liang J, Guo DA, Liu B. Tripterygium glycoside fraction n2: alleviation of DSS-induced colitis by modulating immune homeostasis in mice. Phytomedicine. 2019;58:152–158. doi: 10.1016/j.phymed.2019.152855. [DOI] [PubMed] [Google Scholar]
- 8.Balasubramanian R, Marks SD. Post-infectious glomerulonephritis. Paediatr Int Child Health. 2017;37:240–247. doi: 10.1080/20469047.2017.1369642. [DOI] [PubMed] [Google Scholar]
- 9.Rossi F, Aresu L, Martini V, Trez D, Zanetti R, Coppola L, Ferri F, Zini E. Immune-complex glomerulonephritis in cats: a retrospective study based on clinico-pathological data, histopathology and ultrastructural features. BMC Vet Res. 2019;15:303–310. doi: 10.1186/s12917-019-2046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao Q, Harris DC, Wang Y. Macrophages in kidney injury, inflammation, and fibrosis. Physiology. 2015;30:183–194. doi: 10.1152/physiol.00046.2014. [DOI] [PubMed] [Google Scholar]
- 11.Lucas GNC, Leitão ACC, Alencar RL, Xavier RMF, Daher EF, Silva Junior GBD. Pathophysiological aspects of nephropathy caused by non-steroidal anti-inflammatory drugs. J Bras Nefrol. 2019;41:124–130. doi: 10.1590/2175-8239-JBN-2018-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang JW, Zou XR, Wang CJ, Wang XQ. Tripterygium glycosides in treatment of henoch-schonlein purpura nephritis: a systematic review of randomized controlled trials. Zhongguo Zhong Yao Za Zhi. 2018;43:2806–2816. doi: 10.19540/j.cnki.cjcmm.20180327.005. [DOI] [PubMed] [Google Scholar]
- 13.Zhang B, Su H, Ren XQ, Li WX, Ding Y, Zhang X, Zhai WS, Song CD. Study on mechanism of Cuscutae Semen flavonoids in improving reproductive damage of Tripterygium Glycosides Tablets in rats based on high-throughput transcriptome sequencing. Zhongguo Zhong Yao Za Zhi. 2019;44:3478–3485. doi: 10.19540/j.cnki.cjcmm.20190527.402. [DOI] [PubMed] [Google Scholar]
- 14.Wang JM, Miao MS, Qu LB, Cui Y, Zhang YY. Protective effects of geniposide against Tripterygium glycosides (TG)-induced liver injury and its mechanisms. J Toxicol Sci. 2016;41:165–173. doi: 10.2131/jts.41.165. [DOI] [PubMed] [Google Scholar]
- 15.Akbar AN, Henson SM, Lanna A. Senescence of T lymphocytes: implications for enhancing human immunity. Trends Immunol. 2016;37:866–876. doi: 10.1016/j.it.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Laidlaw BJ, Craft JE, Kaech SM. The multifaceted role of CD4(+) T cells in CD8(+) T cell memory. Nature reviews. Immunology. 2016;16:102–111. doi: 10.1038/nri.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jansen JM, Gerlach T, Elbahesh H, Rimmelzwaan GF, Saletti G. Influenza virus-specific CD4+ and CD8+ T cell-mediated immunity induced by infection and vaccination. J Clin Virol. 2019;119:44–52. doi: 10.1016/j.jcv.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Berti A, Cornec-Le Gall E, Cornec D, Casal Moura M, Matteson EL, Crowson CS, Ravindran A, Sethi S, Fervenza FC, Specks U. Incidence, prevalence, mortality and chronic renal damage of anti-neutrophil cytoplasmic antibody-associated glomerulonephritis in a 20-year population-based cohort. Nephrol Dial Transplant. 2019;34:1508–1517. doi: 10.1093/ndt/gfy250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen F, Ma YL, Ding H, Chen BP. Effects of Tripterygium wilfordii glycosides on regulatory T cells and Th17 in an IgA nephropathy rat model. Genet Mol Res. 2015;14:14900–14907. doi: 10.4238/2015.November.18.55. [DOI] [PubMed] [Google Scholar]
- 20.Zhu TF, Chu ZF, Li JH. Effect of tripterygium glycosides and Danshen injection on blood coagulation mechanism in children with allergic purpura nephritis. Zhongguo Zhong Yao Za Zhi. 2016;41:2162–2167. doi: 10.4268/cjcmm20161129. [DOI] [PubMed] [Google Scholar]
- 21.Wang F, Zhang YH. Relationship of cystatin C, fibrinogen, and 24-hour urinary protein with renal pathological grade in children with Henoch-Schönlein purpura nephritis. Zhongguo Dang Dai Er Ke Za Zhi. 2016;18:233–7. doi: 10.7499/j.issn.1008-8830.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhan HF, Jin J, Liang SK, Zhao L, Gong JG, He Q. Tripterygium glycoside protects diabetic kidney disease mouse serum-induced podocyte injury by upregulating autophagy and downregulating β-arrestin-1. Histol Histopathol. 2019;34:943–952. doi: 10.14670/HH-18-097. [DOI] [PubMed] [Google Scholar]


