Abstract
Objective: Spinal cord injury (SCI) is a common spine surgical injury that leads to loss of activities of daily living. NEUROD2, a member of the neuroD family, is newly known to play a crucial role in SCI progression. We aimed to investigate the underlying mechanism wherein miR-153 and NEUROD2 modulate the process of SCI. Methods: Expression of miR-153 and NEUROD2 in spinal cord in mice of SCI were analyzed employing western blot and qRT-PCR assays. Microglial cells were transfected with mimic of miR-153 or siRNA targeting NEUROD2 to determine the impact of miR-153 and NEUROD2 on SCI induced inflammatory reaction and oxidative stress. A luciferase reporter assay was conducted to verify the regulation of miR-153 on NERUOD2. Results: MiR-153 expression was decreased in injured spinal cord, while NERUOD2 was increased in a time-dependent manner. Addition of miR-153 mimic or silencing NERUOD2 might significantly inhibit the production of inflammation cytokines and attenuated oxidative stress in microglia cells of SCI. Luciferase reporter assay suggested that NERUOD2 was a direct target of miR-153. Conclusion: We proved that miR-153 attenuated inflammatory response and oxidative stress induced by SCI by targeting of NEUROD2, indicating a protective role in SCI progression.
Keywords: miR-153, NERUOD2, spinal cord injury, inflammatory response, oxidative stress
Introduction
Spinal cord injury (SCI) caused by high-energy injuries is a common spine injury that often leads to loss of activities of daily living, causing great physical and psychologic trauma for patients [1]. The pathological process of SCI can be divided into two phases including primary and secondary phases. It is reported that various cytokines and signal pathways mediate inflammatory response (IR), oxidative stress (OS), and apoptosis of neurons/astrocytes, which further contribute to the secondary damage after SCI. Although several therapeutic strategies have been clinically used for SCI treatment, the clinical benefit remains unsatisfactory.
microRNAs (miRNAs) are endogenous non-coding RNAs with 20-22 nucleotides, which negatively regulate gene expression through repressing the translation of target mRNAs and function importantly in biologic processes such as cellular proliferation, differentiation, apoptosis, and tumorigenesis [2,3]. An increasing number of evidence demonstrated that a variety of miRNAs were aberrantly expressed with tissue specificity in the spinal cord (SC) of mammals [4,5]. The imbalance of various miRNA expressions after SCI was recently demonstrated to be involved in the inflammatory response, apoptosis and glial scar formation in the process of SCI [6]. The participation of miRNAs in SCI provides us a novel insight to explore effective strategies for treatment of SCI.
MiR-153 was previously reported as a tumor suppresser in various cancers including glioma, gastric cancer, and non-small cell lung cancer [7,8]. Therefore, this study evaluated the role of miR-153 in process of SCI. Since the augmented inflammatory response and increased OS evidently enhanced the damage of SCI, the potential regulation of miR-153 on inflammation associated cytokines and OS related proteins were simultaneously investigated. Furthermore, the target of miR-153 was predicted and verified in this study to briefly figure out the underlying mechanism of miR-153 influencing SCI.
Materials and methods
Induction of mouse SCI
Twelve female C57bl/6J mice, aged 6-8 weeks (20-25 g of weight; from Shanghai SLAC Laboratory, China) were randomized into 4 groups of 3 mice each; 3 groups were modeled with T10 contusive SCI using a specific electromagnetic force driven impactor (10 g, 12.5 mm) [4]. The remaining group received only laminectomy as a control. The mice were anesthetized with pentobarbital sodium and perfused with cold saline to remove SCs from mice suffering SCI at 1, 3 and 5 days after injury, and from mice of control at 1 day after laminectomy. Then, the SC was exposed, and a 10 mm long SC segment containing the injury epicenter was harvested quickly for qRT-PCR. All the animal experiments complied with the ARRIVE guidelines and were carried out in line with the U.K. Animals (Scientific Procedures) Act, 1986 and associated guidelines.
Cell culture
All mice were euthanized and the vertebral columns were removed. SCs were dissociated then the cells were grown in mixed cultures for 2 d. The culture medium was refreshed with high glucose DMEM (Carlsbad, CA, USA), which contained 20% fetal bovine serum (Invitrogen, CA, USA). Ten days later, microglial cells were isolated and refreshed for further purification.
RNA extraction and qRT-PCR assay
The cells were extracted for total RNA by RNeasy Plus Micro Kit, from QIAGEN, USA, according to the manufacturer’s instructions. The total RNAs were then used to detect quality and concentration by a Nanodrop (Thermo, USA). cDNA for NEUROD2 detection was synthesized by Prime Script™ RT-PCR kit (Takara, Japan) on a thermal cycler (Bio-Rad, USA). The primers targeting human NEUROD2 and GAPDH were designed by Primer 5.0. Primer-BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi) was used to test the specificity of the primers. See Table 1 for the sequence of primers. qRT-PCR was performed in triplicate using the 2× UItraSYBR Mixture (CWBIO, China) on the CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad, USA), and the qRT-PCR program consisted of initial denaturing at 95°C for 10 min, followed by 40 cycles of 10 s at 95°C, 60 s at 60°C for NEUROD2 and GAPDH. Primer sets for miR-153 and U6 expression were purchased from RiboBio Co., Ltd. (Gangzhou, China). Relative expression level of miR-153 were calculated by 2-∆∆Ct rate method and was normalized to U6, while expression level of NEUROD2 was normalized to GAPDH.
Table 1.
Sequence information of primers
| Primer | Sequence 5’>3’ |
|---|---|
| GAPDH_forward | GGTGAAGGTCGGAGTCAACG |
| GAPDH_reverse | ACCATGTAGTTGAGGTCAATGAAGG |
| NEUROD2_forward | ACTCGGAGATTTGGGGCC |
| NEUROD2_reverse | CTCACCTCCTTCTGCCCC |
Western blot
The total cell protein was extracted with the use of RIPA lysis buffer (Cell Signaling Technology, USA) supplemented with protease and phosphatase inhibitor. The Pierce Coomassie (Bradford) Protein Assay Kit (Thermo, USA) was used to measure the concentration of total protein. Twenty micrograms of protein sample were resolved on 8% SDS-PAGE at 90 Volts and transferred to polyvinylidene difluoride membranes at 250 mA electric current. The membranes were sealed with 5% skim milk for 1 h at room temperature then incubated with primary antibodies targeting human GAPDH, NEUROD2, and iNOS (Abcam, UK) overnight at 4°C. Thereafter, the membranes were washed and incubated with goat anti-rabbit or rabbit anti-mouse IgG H&L secondary antibody conjugated with horseradish peroxidase (Abcam, USA). The signal was developed using Super Signa West Dura Extended Duration chemiluminescence substrate (Thermo, USA) and measured by ChemiDoc™ XRS + System (Bio-Rad, USA).
Elisa
Cell supernatants were collected for determination of the production of TNF-α, IL-1β, and IL-6 through ELISA kits (Thermo, USA) according to the manufacturer’s instructions. The OD value at 450 nm wavelength was recorded, and the concentration was calculated according to the standard curve.
Bioinformatic analysis
miRNA target analysis tools PicTar (http://www.pictar.org/cgi-bin/PicTar_vertebrate.cgi), TargetScan (http://www.targetscan.org/), and miRanda algorithm (http://www.microRNA.org) were used to identify the putative miRNA target.
Vector construction, luciferase reporter assays, and siRNA transfection
The wild-type (WT) 3’-untranslated regions (3’-UTR) of NEUROD2 containing the miR-153 binding sites was amplified and cloned downstream of the firefly luciferase gene in the pGL3-control vector (Promega, USA). Afterwards, the mutant (Mut) 3’-UTR plasmid was created by site-directed mutagenesis. Mimic (miR-153 mimic) and negative control (NC) oligonucleotides for miR-153 were from Gangzhou RiboBio, China). 5×104 HEK 293T cells were seeded into a 24-well dish for 24 h. Mimic or negative control for miR-153 together with pGL3-control vector carrying wild-type or mutant 3’-UTR of NEUROD2 were co-transfected into HEK 293T cells using Lipofectamine 2000 reagent following instructions. Two days after incubation, the activities of firefly and renilla luciferases were measured using the Dual Luciferase Assay Kit (Promega, USA) and normalized to those of firefly luciferase activity. All assays were designed in triplicate and repeated six times.
In order to silence NEUROD2 expression in microglial cells of SCI, small interfering RNA (siRNA) was used and siRNA against NEUROD2 was designed and synthesized by Gangzhou RiboBio, China). Microglia cells of SCI were transferred to 24-well plates at a density of 6×104 cells/well. The next day, the cells were cultured with NEUROD2 siRNA (si-NEUROD2) or control siRNA (si-NC) triplicate at a final concentration of 100-500 ng with the use of Lipofectamine 2000. Six hours after the transfection, complete growth medium was used to renew the medium, and the cells were recovered for 18 hours and cultured for one day. The supernatant was collected for ELISA and cells for western blot and qRT-PCR.
Statistical analysis
SPSS 19.0 (SPSS, USA) was employed for statistical analysis. Data were presented as mean ± standard deviation of three parallel experiments, and the differences among different groups were processed using One-way ANOVA. Differences with P<0.05 were considered significant.
Results
Expression profiles of miR-153 and NEUROD2 in mice after SCI
To investigate the potential role of miR-153 in SCI, qRT-PCR was used to analyze the level of miR-153 in adult female C57bl/6J mice after SCI. It was shown that miR-153 was decreased in SC tissues after one day from SCI, and a significant decrease was observed after 3 and 5 days from SCI, depicting a time-dependent manner (Figure 1A). We also detected the expression of NEUROD2 in SC after SCI employing qRT-PCR and western blot. As illustrated in Figure 1B-D, the expression of NEUROD2 in the SCI group was significantly higher than that in the control group (P<0.05). All these results implied that miR-153 and NEUROD2 were of great significance in SCI, so the underlying relationship among miR-153, NEUROD2 and SCI deserved advanced investigation.
Figure 1.
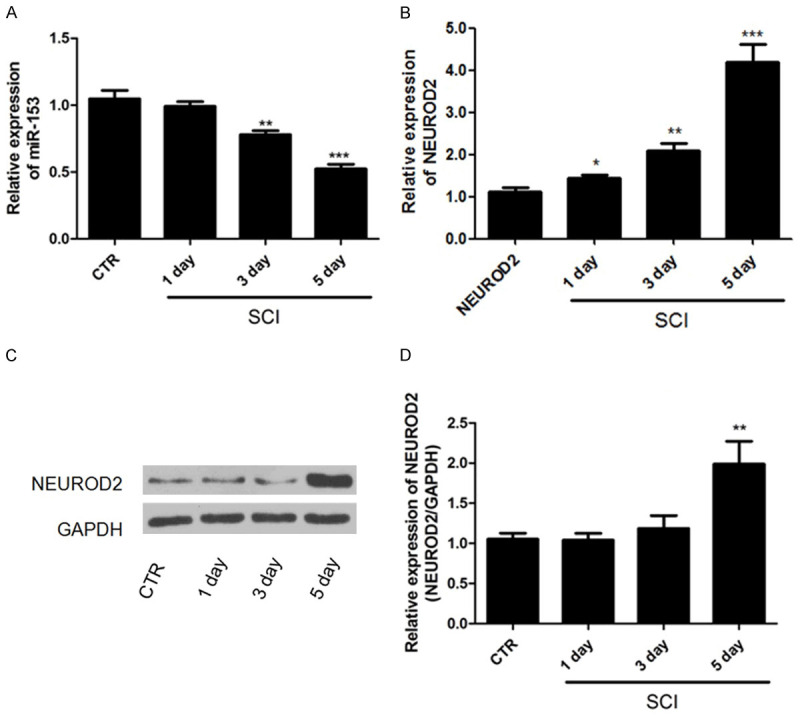
Expression profiles of miR-153 and NEUROD2 in mice after SCI. A: miR-153 was decreased in a time-dependent manner in mice after SCI. B-D: The NEUROD2 was gradually increased with time increasing. *P<0.05, **P<0.01, ***P<0.001.
Increasing miR-153 or decreasing NEUROD2 restrained IR and OS level
Enhanced inflammatory cytokine (IC) and increased OS were reported to cause secondary damage after SCI. To assess the effects of miR-153 on cytokine secretion involved in SCI, ELISA assay was performed. Microglial cells after SCI were reported as the main source of ICs; microglia cells isolated from normal and injured SC were used here. TNF-α, IL-1β and IL-6 levels were all significantly increased after SCI (P<0.01); however, increasing of the three inflammatory associated cytokines was markedly compromised by the transfection of miR-153 mimic (Figure 2A). We subsequently explored the effect of miR-153 in SCI-mediated OS. Selenoprotein type N1 (SEPN1), thioredoxin-like 1 (TXNL1), and glutathione peroxidase 1 (GPX1) expressions, all playing a vital role in reactive oxygen species scavenging, were assessed by qRT-PCR. The results indicated that SEPN1, TXNL1, and GPX1 were demonstrably downregulated after SCI (Figure 2B, P<0.01). The introduction of miR-153 mimic notably increased SEPN1, TXNL1, and GPX1 expressions. Otherwise, iNOS responsible for production of reactive oxygen species was also detected by western blot. Results demonstrated that iNOS expression was comparably higher in microglia of SCI and repressed by addition of miR-153 mimic. The above results collectively demonstrated that miR-153 probably performed a protective role in SCI by reducing ICs and promoting the expression of genes coding proteins that are in charge of oxygen species clearance.
Figure 2.
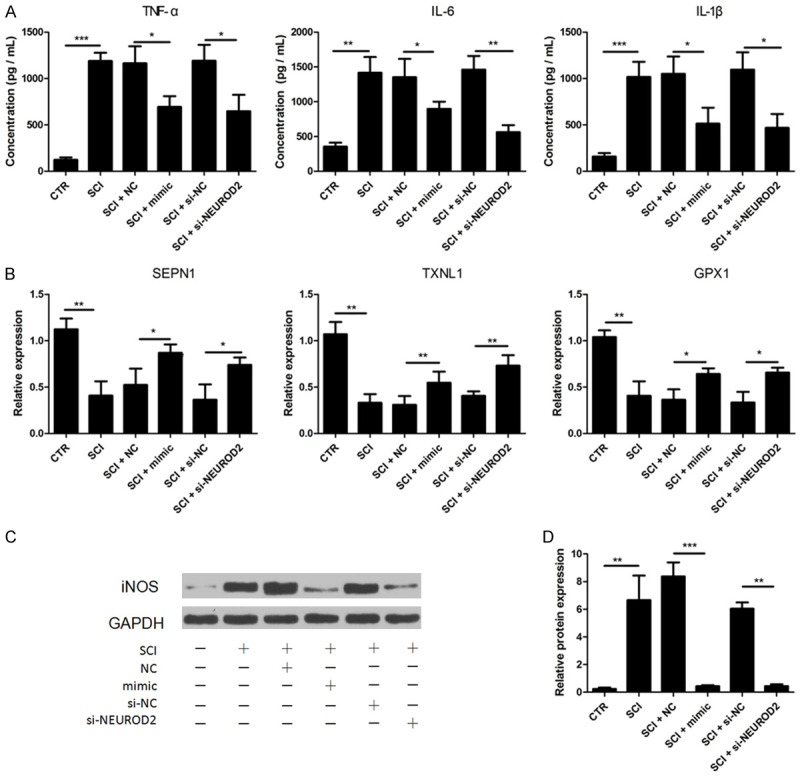
Increasing miR-153 or decreasing NEUROD2 restrained inflammatory response and oxidative stress level. A: SCI-induced forced expression of TNF-α, IL-6 and IL-1β was significantly impeded by introduction of miR-153 mimics or si-NERUOD2; B: SCI-induced decreased expression of SEPN1, TXNL1, and GPX1 was obviously enhanced by transfection of miR-153 mimics or si-NERUOD2; C and D: Overexpression of iNOS after SCI was also impaired by miR-153 mimics or si-NERUOD2 transfection. *P<0.05, **P<0.01, ***P<0.001.
In addition, we silenced NEUROD2 in microglial cells of SCI using siRNA against NEUROD2 to evaluate the direct effect of NEUROD2 on SCI processes. ELISA, western blot, and qRT-PCR results showed that production of TNF-α, IL-1β, and IL-6 was significantly dampened by NEUROD2 silencing; together, the upregulation of EPN1, TXNL1, and GPX1 and downregulation of iNOS were observed in cells transfected with si-NEUROD2 (Figure 2). These results proved that NEUROD2 played a critical role in SCI progression.
NEUROD2 is a direct target of miR-153
The level of miR-153 decreased in microglia of SCI, while the level of NEUROD2 was elevated. So we speculated that miR-153 might directly modulate NEUROD2 expression. Pearson analysis showed that the expression of NEUROD2 both in normal and injured SC was inversely correlated with that of miR-153 (Figure 3A, R=-0.959, P<0.001). Bioinformatic analysis using miRanda, TargetScan, and PicTar suggested that the 3’-UTR of NEUROD2 contained a potential binding site for miR-153 (Figure 3B). To dissect the direct regulation of NEUROD2 by miR-153, a luciferase reporter assay was performed. Co-transfection of miR-153 mimic and vector carrying wild-type 3’-UTR of NEUROD2 evidently attenuated the relative luciferase activity which was detected to have no significant difference in groups transfected with mutant 3’-UTR of NEUROD2 or negative control of miR-153 (Figure 3C). We further validated the regulation of miR-153 on NEUROD2 expression, and found that administration of miR-153 mimic markedly reduced the expression of NEUROD2 both at the mRNA and protein level in microglia of SCI (Figure 3D-F). All these results proved that NEUROD2 was directly regulated by miR-153.
Figure 3.
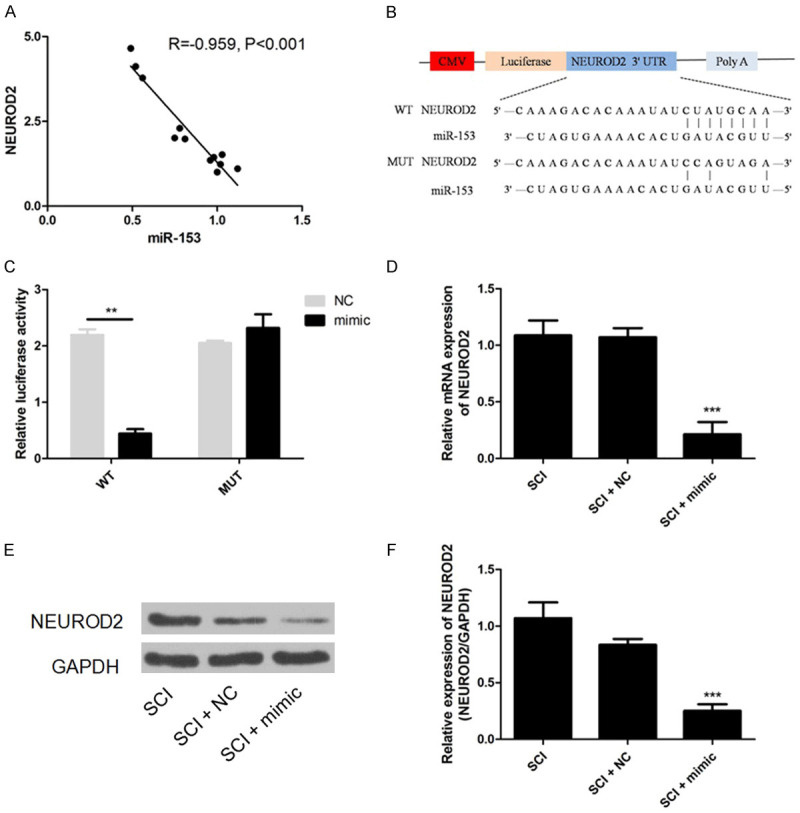
NEUROD2 is a direct target of miR-153. A: Expression of miR-153 negatively correlated with that of NEUROD2; B: Schematic diagram of NEUROD2 3’-UTR region targeted by miR-153; C: Luciferase activity of the pGL3/NEUROD2-UTR was dramatically inhibited in the miR-153 mimics group; D-F: miR-153 mimic transfection significantly decreased expression of NEUROD2. ***P<0.001.
Discussion
SCI is globally recognized as one of the most serious central nervous system injuries leading to significant neurological damage and a reduction in quality of life [5,9,10]. miRNAs, a type of endogenous RNAs, post-transcriptionally regulate gene expression and are capable of controlling certain cellular and physiologic processes. Emerging studies have reported a different expression pattern of several miRNAs between normal SC and injured SC [4,11,12], indicating that miRNAs may play an attractive role in the SCI process. Studies have confirmed that miR-153 is a tumor suppressor [7]. However, its effects on SCI remain unknown. In order to clarify the function of miR-153 in SCI progression, we detected the level of miR-153 in normal and injured SC and found a significant decrease in miR-153 in SC after injury, in a time-dependent manner. It is frequently reported that OS and associated inflammatory reaction play a major role in SCI and its secondary damage [5,13,14]. Jidong Guo and colleagues suggested that IL-19, known as an immune suppressor, may improve locomotor functional recovery caused by contusion spinal [15]. Lowering inflammatory reaction was proved to be a promising strategy for functional recovery after SCI. The increased secretion of ICs (TNF-α, IL-1β and IL-6) and imbalanced expression of OS related proteins were observed after SCI in our study, which was in line with previous studies focusing on SCI [9,14]. We, thus, subsequently assessed the influence of miR-153 on production of ICs and expression of OS related proteins. The SCI induced overproduction of TNF-α, IL-1β and IL-6 was significantly decreased by addition of miR-153 mimic. The miR-153 induced upregulation of SEPN1, TXNL1, and GPX1 and downregulation of iNOS indicated an improved OS. What we found in this study proved miR-153 has a protective role in SCI progression.
NEUROD2, a member of the neuroD family, was reported as a tumor suppressor gene in glioblastoma, while its role in SCI was still unkown [16-18]. In this article, we also detected the expression of NEUROD2 in SC after injury. We found that NEUROD2 expression was gradually increased with the increase of time. Correlation analysis revealed an inverse relationship between NEUROD2 and miR-153. Hence, it was speculated that NEUROD2 may performed as a target of miR-153. A dual luciferase assay we conducted demonstrated that miR-153 can directly bind with the 3’-UTR region of NEUROD2 and negatively regulate its expression. Addition of miR-153 mimic can markedly reduced expression of NEUROD2 in injured SC. Rahul Agrawal et al. identified that NeuroD2 was a p53-regulated gene that promotes apoptosis in hypoxic conditions and also resulted from SCI-induced blocking of angiogenesis [16]. Since we established the role of miR-153 in SCI and its regulation on NEUROD2, the impact of overexpressed NEUROD2 during SCI was still unclear. We, therefore, used siRNA to knock down the expression of NEUROD2 in microglia of SCI, and we found that the production of TNF-α, IL-1β and IL-6 was significantly reduced. Together, SEPN1, TXNL1, and GPX1 were upregulated and iNOS was downregulated by transfection of siRNA targeting NEUROD2. NEUROD1 and NEUROD4 are subtypes of neuroD family proteins and their degradation were demonstrated to relieve the SC inflammation and progression of OS [5,19,20]. MAPK/ERK signaling is related to IR and OS [21]. Further investigation indicated that NEUROD1 may induce blocking of MAPK/ERK signaling pathway by lowering levels of phosphorylated-p38 and AKT. We also assessed the effect of NEUROD2 on MAPK/ERK signaling pathway, while the silencing of NEUROD2 cannot change the levels of phosphorylated-p38 and AKT (data not shown). According to the above findings, we concluded that the attenuated inflammation and OS by miR-153 was mainly mediated by its negative regulation on NEUROD2. However, the signaling pathway potentially affected by miR-153 and NEUROD2 in processes of SCI deserved more attention. Taken together, our study revealed that miR-153 participated in the processes of SCI and demonstrated that attenuated NEUROD2 was mainly responsible for the miR-153 induced improvement after SCI. Findings of our study strengthened our knowledge of miR-153 and contributed to the basis for advanced exploration of the prognostic and diagnostic value of miR-153 in SCI.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Written informed consent was obtained from all enrolled patients, and the study was approved by the Third Affiliated Hospital of Southern Medical University.
Disclosure of conflict of interest
None.
References
- 1.Mortazavi MM, Verma K, Harmon OA, Griessenauer CJ, Adeeb N, Theodore N, Tubbs RS. The microanatomy of spinal cord injury: a review. Clin Anat. 2015;28:27–36. doi: 10.1002/ca.22432. [DOI] [PubMed] [Google Scholar]
- 2.Tigchelaar S, Streijger F, Sinha S, Flibotte S, Manouchehri N, So K, Shortt K, Okon E, Rizzuto MA, Malenica I, Courtright-Lim A, Eisen A, Keuren-Jensen KV, Nislow C, Kwon BK. Serum microRNAs reflect injury severity in a large animal model of thoracic spinal cord injury. Sci Rep. 2017;7:1376. doi: 10.1038/s41598-017-01299-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi Z, Pan B, Feng S. The emerging role of long non-coding RNA in spinal cord injury. J Cell Mol Med. 2018;22:2055–2061. doi: 10.1111/jcmm.13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J, Li L, Shen Y. Protective role of microRNA-219-5p inhibitor against spinal cord injury via liver receptor homolog-1/Wnt/beta-catenin signaling pathway regulation. Exp Ther Med. 2018;15:3563–3569. doi: 10.3892/etm.2018.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fu X, Shen Y, Wang W, Li X. MiR-30a-5p ameliorates spinal cord injury-induced inflammatory responses and oxidative stress by targeting neurod 1 through MAPK/ERK signalling. Clin Exp Pharmacol Physiol. 2018;45:68–74. doi: 10.1111/1440-1681.12856. [DOI] [PubMed] [Google Scholar]
- 6.Hu J, Zeng L, Huang J, Wang G, Lu H. miR-126 promotes angiogenesis and attenuates inflammation after contusion spinal cord injury in rats. Brain Res. 2015;1608:191–202. doi: 10.1016/j.brainres.2015.02.036. [DOI] [PubMed] [Google Scholar]
- 7.Patil KS, Basak I, Pal R, Ho HP, Alves G, Chang EJ, Larsen JP, Møller SG. A proteomics approach to investigate miR-153-3p and miR-205-5p targets in neuroblastoma cells. PLoS One. 2015;10:e0143969. doi: 10.1371/journal.pone.0143969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan Y, Du W, Wang Y, Xu C, Wang J, Zhang Y, Wang H, Ju J, Zhao L, Wang Z, Lu Y, Cai B, Pan Z. Suppression of AKT expression by miR-153 produced anti-tumor activity in lung cancer. Int J Cancer. 2015;136:1333–1340. doi: 10.1002/ijc.29103. [DOI] [PubMed] [Google Scholar]
- 9.Gao L, Dai C, Feng Z, Zhang L, Zhang Z. MiR-137 inhibited inflammatory response and apoptosis after spinal cord injury via targeting of MK2. J Cell Biochem. 2018;119:3280–3292. doi: 10.1002/jcb.26489. [DOI] [PubMed] [Google Scholar]
- 10.Hu JZ, Huang JH, Zeng L, Wang G, Cao M, Lu HB. Anti-apoptotic effect of microRNA-21 after contusion spinal cord injury in rats. J Neurotrauma. 2013;30:1349–1360. doi: 10.1089/neu.2012.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang T, Yuan W, Liu Y, Zhang Y, Wang Z, Chen X, Feng S, Xiu Y, Li W. miR-142-3p is a potential therapeutic target for sensory function recovery of spinal cord injury. Med Sci Monit. 2015;21:2553–2556. doi: 10.12659/MSM.894098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang W, Liu R, Su Y, Li H, Xie W, Ning B. MicroRNA-21-5p mediates TGF-beta-regulated fibrogenic activation of spinal fibroblasts and the formation of fibrotic scars after spinal cord injury. Int J Biol Sci. 2018;14:178–188. doi: 10.7150/ijbs.24074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhalala OG, Pan L, Sahni V, McGuire TL, Gruner K, Tourtellotte WG, Kessler JA. microRNA-21 regulates astrocytic response following spinal cord injury. J Neurosci. 2012;32:17935–47. doi: 10.1523/JNEUROSCI.3860-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dai J, Xu LJ, Han GD, Sun HL, Zhu GT, Jiang HT, Yu GY, Tang XM. MicroRNA-125b promotes the regeneration and repair of spinal cord injury through regulation of JAK/STAT pathway. Eur Rev Med Pharmacol Sci. 2018;22:582–589. doi: 10.26355/eurrev_201802_14271. [DOI] [PubMed] [Google Scholar]
- 15.Guo J, Wang H, Li L, Yuan Y, Shi X, Hou S. Treatment with IL-19 improves locomotor functional recovery after contusion trauma to the spinal cord. Br J Pharmacol. 2018;175:2611–2621. doi: 10.1111/bph.14193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agrawal R, Garg A, Benny Malgulwar P, Sharma V, Sarkar C, Kulshreshtha R. p53 and miR-210 regulated NeuroD2, a neuronal basic helix-loop-helix transcription factor, is downregulated in glioblastoma patients and functions as a tumor suppressor under hypoxic microenvironment. Int J Cancer. 2018;142:1817–1828. doi: 10.1002/ijc.31209. [DOI] [PubMed] [Google Scholar]
- 17.Bayam E, Sahin GS, Guzelsoy G, Guner G, Kabakcioglu A, Ince-Dunn G. Genome-wide target analysis of NEUROD2 provides new insights into regulation of cortical projection neuron migration and differentiation. BMC Genomics. 2015;16:681. doi: 10.1186/s12864-015-1882-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen F, Moran JT, Zhang Y, Ates KM, Yu D, Schrader LA, Das PM, Jones FE, Hall BJ. The transcription factor NeuroD2 coordinates synaptic innervation and cell intrinsic properties to control excitability of cortical pyramidal neurons. J Physiol. 2016;594:3729–3744. doi: 10.1113/JP271953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dai J, Xu LJ, Han GD, Sun HL, Zhu GT, Jiang HT, Yu GY, Tang XM. MiR-137 attenuates spinal cord injury by modulating NEUROD4 through reducing inflammation and oxidative stress. Eur Rev Med Pharmacol Sci. 2018;22:1884–1890. doi: 10.26355/eurrev_201804_14709. [DOI] [PubMed] [Google Scholar]
- 20.Brulet R, Matsuda T, Zhang L, Miranda C, Giacca M, Kaspar BK, Nakashima K, Hsieh J. NEUROD1 instructs neuronal conversion in non-reactive astrocytes. Stem Cell Reports. 2017;8:1506–1515. doi: 10.1016/j.stemcr.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paterniti I, Esposito E, Mazzon E, Bramanti P, Cuzzocrea S. Evidence for the role of PI(3)-kinase-AKT-eNOS signalling pathway in secondary inflammatory process after spinal cord compression injury in mice. Eur J Neurosci. 2011;33:1411–1420. doi: 10.1111/j.1460-9568.2011.07646.x. [DOI] [PubMed] [Google Scholar]


