Abstract
Objective: This research aimed to explore the clinical significance of inflammatory reactions mediated by the IL-33/ST2 signaling pathway in heart failure (HF) patients. Methods: A total of 100 HF patients treated in the Department of Cardiology in our hospital were prospectively regarded as the observation group, and 100 healthy age and gender matched patients who were undergoing physical examination were considered as the control group. The levels of interleukin-33 (IL-33), ST2, tumor necrosis factor-α (TNF-α) and pro B-type natriuretic peptide (pro-BNP) in the peripheral blood of patients were detected. The potential correlation between IL-33 and ST2, TNF-α and pro-BNP was analyzed by Pearson. Results: The levels of IL-33, IL-10, ST2 and pro-BNP in the peripheral blood of patients in the observation group were higher than those in the control group; and they increased with the rise of cardiac function grade (all P<0.05). In addition, IL-33 was positively correlated with TNF-α, ST2 and pro-BNP (r=0.863, 0.879, 0.945; all P<0.05). Multivariate Logistic analysis revealed that the increase of IL-33 and ST2 were independent risk factors of HF. Conclusion: The IL-33 and ST2 levels in the peripheral serum of HF patients are correlated with TNF-α and BNP, the finding of which can assist in clinical diagnosis and treatment.
Keywords: Heart failure, interleukin-33, ST2, tumor necrosis factor-α, inflammatory reaction
Introduction
Epidemiological investigation shows that the morbidity of cardiovascular disease is second only to tumors; where the population morbidity approaches 10%, and the fatality rate is also close to 100/100,000. With the increase of population aging and the rising morbidity of chronic diseases, such as diabetes, hypertension and obesity, the incidence of cardiovascular diseases is increasing annually reaching 18.59% [1-3]. All kinds of cardiovascular diseases can cause damage to heart function (ventricular remodeling, myocardial dilatation), and heart failure (HF) can appear when the heart’s pumping function is seriously damaged [4-6]. Clinically, according to the type of impaired cardiac function, HF can be divided into congestive and low ejection fraction HF. Patients may have corresponding symptoms, such as chest tightness, shortness of breath, and congestion-related discomfort, which may affect the quality of life of patients, and may cause death due to malignant arrhythmia [7-9].
At the moment, HF is mainly treated by medical drugs for the adjustment of cardiac function. Active, effective and accurate markers of HF are of great significance to clinical diagnosis and treatment [10,11]. Among the currently used HF indicators, BNP and N-terminal pro-B-type natriuretic peptide (NT-proBNP) are common; however, they are easily affected by kidney function, gender, weight, etc. At the same time, it has a wide range and lacks certain characteristics and sensitivity [12,13]. The latest research has confirmed that the IL-33/ST2-mediated inflammatory reaction plays a vital role in HF occurrence. IL-33/ST2 exerts the biological efficacy of inhibiting myocardial dilatation by suppressing cardiomyocyte hypertrophy and myocardial fibrosis, and participates in cardiovascular events such as hypertension and acute coronary syndrome [14-16]. However, there are few studies on IL-33/ST2 and inflammatory factors in HF [17,18]. Based on this, this research explored the expression of IL-33/ST2 and inflammatory factors in HF patients and the potential relationship between IL-33/ST2 and heart function, in order to provide a new research direction and therapeutic intervention target for diagnosis and treatment.
General data and methods
General data
Altogether 100 HF patients treated in the Cardiology Department of our hospital from January 2019 to January 2020 were regarded as the observation group, and 100 healthy persons who were matched in age and gender, undergoing physical examination were considered as the control group. Inclusion criteria are as follows: 1. The diagnosis of all patients meets the relevant diagnostic criteria in the American Heart Association’s Guidelines for Diagnosis and Treatment of Chronic Heart Failure [19]. 2. Those whoes age was 20-70 years old. 3. The cardiac function was classified as Grades II-III. 4. HF patients seek medical advice first. Exclusion criteria were as follows: 1. Those complicated with other valve diseases or coronary atherosclerotic heart disease. 2. There was a history of malignant arrhythmia such as ventricular tachycardia and fibrillation. 3. The symptoms of HF, such as chest tightness, shortness of breath and edema, were not completely controlled. 4. Those with major organ dysfunction such as liver and kidney insufficiency. 5. Those who were complicated with immune system diseases and use of immunosuppressants. 6. No infected patients within two weeks before hospitalization. 7. Tumor patients. 8. Those who participated in other research projects. Both groups of patients knew this research and signed an informed consent form. This research was approved by the Ethics Committee of our hospital.
Methods
Treatment of HF patients
Patients were admitted to the hospital. According to the related treatment recommendations of the 2017 American Heart Association Heart Failure Guidelines [18], they were given the following measures: regular administration of ACEI drugs to improve myocardial remodeling, catecholamines to enhance cardiac function, β-blockers to reduce heart rhythm and control arrhythmia, diuretics combined with human serum albumin diuresis to decrease edema, and other measures that included routine oxygen inhalation, ECG monitoring, etc.
Detection methods of various monitoring factors
The detection methods of various factors (IL-33, ST2, TNF-α, NT-proBNP) were as follows: About 3-5 mL fasting peripheral venous blood of both groups of subjects was collected. Then, it was added into an anticoagulation tube and put into a centrifuge (Guangzhou Jidi Instrument Co., Ltd., model JIDI-20D) at 3000 r/min for 30 min. After that, the supernatant was separated and stored at -80°C for later use. The contents of IL-33, ST2 and IL-10 were detected by enzyme-linked immunosorbent assay kit (Merck, USA). The specific operation method was in line with the instructions. The NT-proBNP in the peripheral blood was examined by the fluorescence immunoassay analyzer (Getein Biotechnology Co., Ltd., model Getein1100).
Data statistics
All the data were analyzed by SPSS 22.0 statistical analysis software. The measurement data were expressed by mean ± standard deviation (x̅±sd), and those between groups were compared by variance analysis. The rates between groups were assessed by Chi-square test, and the correlation between IL-33 and IL-10, ST2 and NT-proBNP was evaluated by Pearson correlation analysis. The serum IL-33 and ST2 levels were seen as dependent variables. With the occurrence of HF as an independent variable, the variables were screened by a step-by-step forward method. Thereinto, the inclusion and exclusion criterion were 0.05 and 0.10, respectively. The relative risk of social avoidance was expressed by the calibrated odds ratio (OR value), with d=0.05 as the inspection standard. P<0.05 was considered statistically remarkable.
Results
General information comparison
This research revealed that there was no obvious difference between both groups in general data, such as sex composition, age, hypertension, dyslipidemia, diabetes, BMI, and causes of HF (P>0.05). Therefore, both groups were comparable (Table 1).
Table 1.
Comparison of general data between both groups (n; x̅±sd)
| Group | Observation group (n=100) | Control group (n=100) | t/χ2 | P |
|---|---|---|---|---|
| Gender (male/female) | 52/48 | 49/51 | 0.080 | 0.777 |
| Age (years) | 54.8±4.3 | 54.3±4.0 | 0.851 | 0.396 |
| High blood pressure (n) | 45 | 32 | 3.041 | 0.081 |
| Dyslipidemia (n) | 18 | 14 | 0.335 | 0.563 |
| Diabetes mellitus (n) | 16 | 19 | 0.139 | 0.710 |
| BMI (kg/m2) | 25.8±2.1 | 26.1±2.4 | 0.941 | 0.348 |
| Cause of heart failure (n) | 2.409 | 0.300 | ||
| Cardiomyopathy | 46 | 49 | ||
| Coronary heart disease | 32 | 31 | ||
| Valvular disease | 22 | 20 |
Comparison of IL-33/ST2 levels in peripheral blood between both groups of subjects
This research documented that the IL-33/ST2 levels in the peripheral blood of subjects in the observation group were higher than those of the healthy controls, which preliminarily indicated that the levels in HF patients were higher than those in healthy persons (P<0.05; Figures 1, 2).
Figure 1.
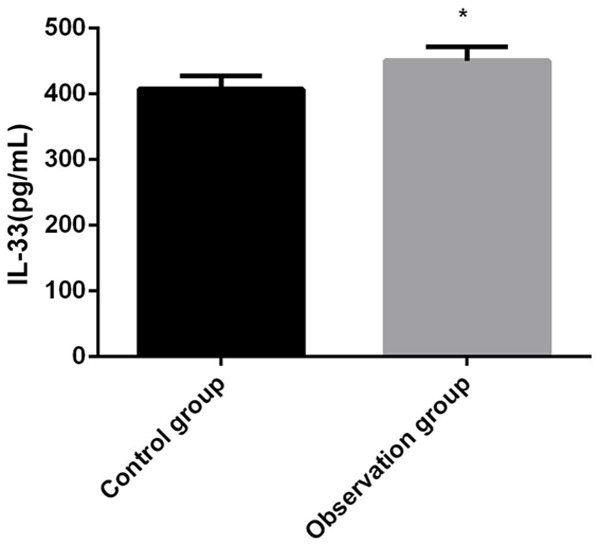
Comparison of IL-33 levels in the peripheral blood between both groups. IL-33: interleukin-33. Compared with control group, *P<0.05.
Figure 2.
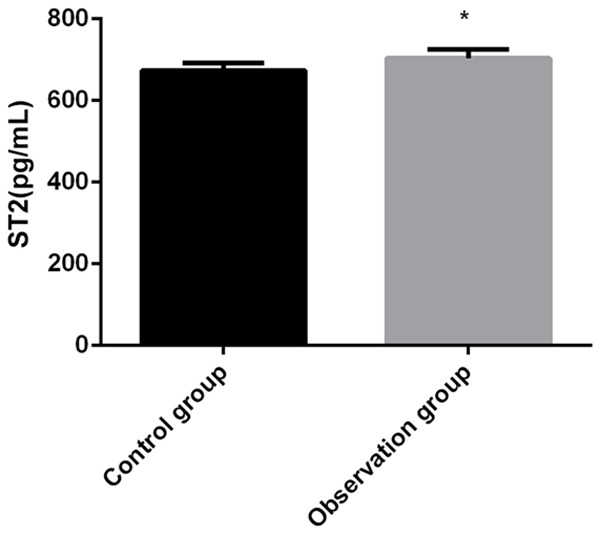
Comparison of ST2 levels in the peripheral blood between both groups. Compared with control group, *P<0.05.
Comparison of TNF-α and NT-proBNP in peripheral blood of both groups of subjects
This research found that the TNF-α and NT-proBNP levels in the peripheral blood of patients in the observation group were higher than those in the healthy control group (P<0.001), which indicated that the inflammatory levels and HF indexes of HF patients were higher than those in the control group (Table 2).
Table 2.
Comparison of inflammatory cytokines TNF-α and NT-proBNP in peripheral blood between both groups
| Group | Cases | TNF-α (μg/mL) | NT-proBNP (pg/mL) |
|---|---|---|---|
| Observation group | 100 | 278.57±19.67 | 223.46±26.46 |
| Control group | 100 | 189.87±15.59 | 136.95±20.23 |
| t/χ2 | 16.460 | 27.110 | |
| P | 0.000 | 0.000 |
Note: TNF-α: tumor necrosis factor-α; NT-proBNP: N-terminal pro-B-type natriuretic peptide.
It also revealed that in the observation group, there were 52 cases of heart function NYII grade and 48 of NYIII. Further study showed that the levels of serum IL-33, ventricular indexes (LVEDD, LVEF) and NT-proBNP in patients with NYIII grade were higher than those with NYII (P<0.001), indicating that the above indexes increased with the decrease of cardiac function (Table 3).
Table 3.
IL-33 levels and changes in ventricular indexes in patients with different cardiac function grades (x̅±sd)
| Group | IL-33 (pg/mL) | LVEDD (mm) | LVEF (%) | NT-proBNP (pg/mL) |
|---|---|---|---|---|
| Level II group | 447.36±22.68 | 51.48±5.39 | 52.65±3.28 | 189.48±35.37 |
| Level III group | 478.56±20.97 | 54.39±4.85 | 48.49±4.37 | 242.74±40.29 |
| t/χ2 | 10.101 | 4.013 | 7.613 | 9.934 |
| P | 0.000 | 0.000 | 0.000 | 0.000 |
Note: LVEDD: Left ventricular end-diastolic volume; LVEF: Left ventricular ejection fraction; NT-proBNP: N-terminal pro-B-type natriuretic peptide.
Correlation analysis between IL-33 and ST2, TNF-α, NT-proBNP and left ventricular indexes in observation group
The results manifested that IL-33 was positively correlated with ST2, TNF-α, NT-proBNP and left ventricular end-diastolic volume, but negatively correlated with left ventricular ejection fraction (Table 4).
Table 4.
Correlation analysis of IL-33 with ST2, TNF-α, NT-proBNP and left ventricular indexes
| Classification | r | P |
|---|---|---|
| ST2 | 0.879 | 0.042 |
| TNF-α | 0.863 | 0.047 |
| NT-proBNP | 0.945 | 0.003 |
| LVEDD | 0.927 | 0.021 |
| LVEF | -0.939 | 0.017 |
Note: LVEDD: Left ventricular end-diastolic volume; LVEF: Left ventricular ejection fraction; TNF-α: tumor necrosis factor-α; NT-proBNP: N-terminal pro-B-type natriuretic peptide.
Univariate Logistic regression analysis of HF patients
In this research, the serum IL-33 and ST2 levels (the average value of normal healthy controls was 407.46 pg/mL and 673.55 pg/mL, respectively, higher than that was abnormal and lower than that was normal) were considered as a dependent variable, and the occurrence of HF was regarded as an independent variable. Logistic regression analysis revealed that increased serum IL-33 and ST2 levels were independent risk factors for HF (Table 5).
Table 5.
Univariate Logistic regression analysis of heart failure patients
| Indicators | Standardizedβ | OR | 95% CI | P |
|---|---|---|---|---|
| IL-33 | 0.578 | 1.782 | 1.019~2.954 | <0.001 |
| Elevated ST2 levels | 0.524 | 1.689 | 1.14~2.430 | <0.001 |
Discussion
HF refers to irreversible damage to heart function, which ultimately affects the systolic and diastolic functions of the heart, causing a series of clinical symptoms in patients, thus affecting their quality of life [20,21]. Limited by the number of donors for surgical heart transplantation and the high cost of an artificial heart, treatment mostly depends on internal medicine. Various internal medicines can also better improve heart function, and effective hematological indexes are one of the important roles in clinical response to the efficacy [22,23]. The diagnosis of HF mainly depends on transthoracic ultrasound and pro-BNP. The former has a certain asymptomatic missed diagnosis rate and is affected by individual differences, while the latter cannot provide accurate clinical guidance because of its large fluctuation range [24]. Therefore, exploring the monitoring indicators of HF is important to improve treatment efficacy.
Previous studies tend to accept the view that HF is related to many factors, such as heredity, inflammation, eating habits, etc., among which inflammatory reactions are more common. IL-33, as a new member of IL-1 family, can trigger the inflammatory reaction by combining with downstream ST2, thus causing myocardial cell injury and even HF. This research also found that the levels of IL-33 and ST2 in the peripheral blood of HF patients were higher than those of the healthy control group. It may because this research regards the IL-33/ST2 signaling pathway as the activation system of cardiac dynamics, and excessive myocardial stretch causes excessive secretion of ST2, which activates the IL-33/ST2 signaling pathway, eventually accelerates ventricular remodeling and leads to HF. This supports previous studies that confirm reports of abnormal expression of IL-33/ST2 in the peripheral blood of patients [25].
Numerous studies have discovered that there is a negative correlation between TNF-α level and muscle strength of the myocardium and papillary muscles. A higher the content of TNF-α, leads to weaker contractility of the myocardium. This directly leads to ventricular remodeling. Meanwhile, in vitro cell experiments have proved that TNF-α is intrinsically related to cardiovascular events [26,27]. In this research, the TNF-α level in the peripheral blood of HF patients were higher than those of healthy controls, which preliminarily confirmed the relationship between TNF-α and HF occurrence. Previously, scholars also observed an excessive expression of TNF-α in the peripheral blood of HF patients [28].
The correlation between IL-33/ST2 and inflammatory factors and HF indicators was analyzed in depth. It showed that IL-33 was positively correlated with ST2, TNF-α, NT-proBNP and left ventricular related functional indicators (LVEDD and LVEF). What’s more, Logistic regression analysis also revealed that the increase of serum IL-33/ST2 levels was an independent risk factor. To some extent, IL-33/ST2 can trigger the pathophysiological process of HF by regulating the inflammatory reaction, and it has a good correlation with NT-proBNP index. Thus, it may become a potential target for treatment.
This research is still somewhat insufficient: 1. It’s a single-center study with a small sample size, which needs to be further verified by multi-center large sample experiments. 2. The IL-33/ST2 content, as a threshold for HF diagnosis and efficacy, also needs to be described in the next step. 3. The perfection of an animal model is a crucial supplement to the pathway of IL-33/ST2.
Above all, IL-33/ST2, as a key inflammatory regulation pathway, it participates in ventricular reconstruction and HF by adjusting inflammatory TNF-α. Hence, its dynamic monitoring has certain guiding significance for clinical diagnosis and treatment.
Disclosure of conflict of interest
None.
References
- 1.Frantz S. Heart failure. Dtsch Med Wochenschr. 2020;145:1353. doi: 10.1055/a-0952-9873. [DOI] [PubMed] [Google Scholar]
- 2.Sciomer S, Moscucci F, Salvioni E, Marchese G, Bussotti M, Corrà U, Piepoli MF. Role of gender, age and bmi in prognosis of heart failure. Eur J Prev Cardiol. 2020;27:46–51. doi: 10.1177/2047487320961980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aimo A, Castiglione V, Borrelli C, Saccaro LF, Franzini M, Masi S, Emdin M, Giannoni A. Oxidative stress and inflammation in the evolution of heart failure: from pathophysiology to therapeutic strategies. Eur J Prev Cardiol. 2020;27:494–510. doi: 10.1177/2047487319870344. [DOI] [PubMed] [Google Scholar]
- 4.Yan J, Pan Y, He Y, Wang R, Shao W, Dong S. The effects of serum iron level without anemia on long-term prognosis of patients with coronary heart disease complicated with chronic heart failure: a retrospective cohort study. Heart Vessels. 2020;35:1419–1428. doi: 10.1007/s00380-020-01613-0. [DOI] [PubMed] [Google Scholar]
- 5.Parikh RR, Folsom AR, Misialek JR, Rosamond WD, Chang PP, Tang W, Cushman M. Prospective study of plasma high molecular weight kininogen and prekallikrein and incidence of coronary heart disease, ischemic stroke and heart failure. Thromb Res. 2019;182:89–94. doi: 10.1016/j.thromres.2019.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lancellotti P, Dulgheru R, Marchetta S, Oury C, Garbi M. Valve disease in heart failure: secondary but not irrelevant. Heart Fail Clin. 2019;15:219–227. doi: 10.1016/j.hfc.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 7.Sethares KA, Viveiros J. Levels of illness uncertainty vary by heart failure symptom cluster group. J Cardiac Failure. 2020;26(Suppl):S90. [Google Scholar]
- 8.Abdellah AT, Mohamed AD, Hendawi HA, Omera MA. Clinical and laboratory characteristics of short-term mortality in egyptian patients with acute heart failure. Egypt Heart J. 2017;69:201–208. doi: 10.1016/j.ehj.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simmons T, Schneir A. Hypertensive crisis from a MAOI/supplement interaction leading to myocardial infarction and acute heart failure. Clin Toxicol. 2010;48:631–631. [Google Scholar]
- 10.McAlister FA, Ezekowitz JA, Armstrong PW. Heart failure treatment and the art of medical decision making. Eur J Heart Fail. 2019;21:1510–1514. doi: 10.1002/ejhf.1655. [DOI] [PubMed] [Google Scholar]
- 11.Zymliński R, Stepinska J. Looking for medications to support the treatment of acute decompensated heart failure. Cardiology. 2020;145:224–226. doi: 10.1159/000505902. [DOI] [PubMed] [Google Scholar]
- 12.Rørth R, Jhund PS, Yilmaz MB, Kristensen SL, Welsh P, Desai AS, Køber L, Prescott MF, Rouleau JL, Solomon SD, Swedberg K, Zile MR, Packer M, McMurray JJV. Comparison of BNP and NT-proBNP in patients with heart failure and reduced ejection fraction. Circ Heart Fail. 2020;13:e006541. doi: 10.1161/CIRCHEARTFAILURE.119.006541. [DOI] [PubMed] [Google Scholar]
- 13.Fringu FI, Sitar-Taut AV, Caloian B, Zdrenghea D, Comsa D, Gusetu G, Pop D. The role of Nt Pro-Bnp in the evaluation of diabetic patients with heart failure. Acta Endocrinol (Buchar) 2020;16:183–191. doi: 10.4183/aeb.2020.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tonacci A, Quattrocchi P, Gangemi S. IL33/ST2 axis in diabetic kidney disease: a literature review. Medicina (Kaunas) 2019;55:50. doi: 10.3390/medicina55020050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Umar M, Megarbane A, Shan J, Syed N, Chouery E, Aliyev E, Jithesh P, Temanni R, Mansour I, Chouchane L, Ismail Chouchane A. Genome sequencing unveils mutational landscape of the familial mediterranean fever: potential implications of IL33/ST2 signalling. J Cell Mol Med. 2020;24:11294–11306. doi: 10.1111/jcmm.15701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy S, Kakkar R, McCarthy C, Januzzi J. Inflammation in heart failure. J Am Coll Cardiol. 2020;75:1324–1340. doi: 10.1016/j.jacc.2020.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Dutka M, Bobiński R, Ulman-Włodarz I, Hajduga M, Bujok J, Pająk C, Ćwiertnia M. Various aspects of inflammation in heart failure. Heart Fail Rev. 2020;25:537–548. doi: 10.1007/s10741-019-09875-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart failure society of America. Circulation. 2017;136:e137–e161. doi: 10.1161/CIR.0000000000000509. [DOI] [PubMed] [Google Scholar]
- 19.Kalogeropoulos AP, Goulbourne C, Butler J. Diagnosis and prevention of hypertensive heart failure. Heart Fail Clin. 2019;15:435–445. doi: 10.1016/j.hfc.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Allen KE, Billingsley HE, Carbone S. Nutrition, heart failure, and quality of life: beyond dietary sodium. JACC Heart Fail. 2020;8:765–769. doi: 10.1016/j.jchf.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomes BR, Bocchi EA. Quality of life in heart failure: an important goal in treatment. Arq Bras Cardiol. 2020;114:33–34. doi: 10.36660/abc.20190741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ponz de Antonio I, de Juan Bagudá JS, Rodríguez Chaverri A, García-Cosío Carmena MD, Arribas Ynsaurriaga F, Delgado Jiménez JF. Levosimendan as bridge to transplant in patients with advanced heart failure. Rev Esp Cardiol (Engl Ed) 2020;73:422–424. doi: 10.1016/j.rec.2019.10.026. [DOI] [PubMed] [Google Scholar]
- 23.Huitema AA, Harkness K, Malik S, Suskin N, McKelvie RS. Therapies for advanced heart failure patients ineligible for heart transplantation: beyond pharmacotherapy. Can J Cardiol. 2020;36:234–243. doi: 10.1016/j.cjca.2019.11.012. [DOI] [PubMed] [Google Scholar]
- 24.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelisa A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009;10:165–193. doi: 10.1093/ejechocard/jep007. [DOI] [PubMed] [Google Scholar]
- 25.Kotsiou OS, Gourgoulianis KI, Zarogiannis SG. IL-33/ST2 axis in organ fibrosis. Front Immunol. 2018;9:2432. doi: 10.3389/fimmu.2018.02432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaur K, Sharma AK, Singal PK. Significance of changes in TNF-Alpha and IL-10 levels in the progression of heart failure subsequent to myocardial infarction. Am J Physiol Heart Circ Physiol. 2006;291:H106–H113. doi: 10.1152/ajpheart.01327.2005. [DOI] [PubMed] [Google Scholar]
- 27.Wang K, Wu SB, Cui S, Xiang SY, Wu X, Zhou MQ. Effect of electroacupuncture on hippocampal IL-6, IL-1 β, TNF-α and norepinephrine levels in acute myocardial ischemia rats. Zhen Ci Yan Jiu. 2018;43:365–369. doi: 10.13702/j.1000-0607.170972. [DOI] [PubMed] [Google Scholar]
- 28.Soyfoo MS, Nicaise C. Pathophysiologic role of interleukin-33/ST2 in Sjögren’s syndrome. Autoimmun Rev. 2021;20:102756. doi: 10.1016/j.autrev.2021.102756. [DOI] [PubMed] [Google Scholar]


