Abstract
Objective: To investigate the clinical effect of platelet-rich fibrin (PRF) combined with guided bone regeneration (GBR) in the reconstruction of peri-implantitis bone defect. Methods: This prospective study included 80 patients with peri-implantitis who underwent implant restoration in the Department of Stomatology in our hospital. The eligible patients were randomly divided into control group and observation group, with 40 cases in each group. Patients in the control group were treated with flap curettage combined with GBR, while those in the observation group received a mixture of PRF and bone powder implanted with GBR and covered with PRF biofilm. The differences of pain 24 hours after surgery, bleeding at 7 days after surgery, and the degree of bone defect between the two groups at 60 days after surgery were compared. At 60 days and 120 days after surgery, separately, the regenerated bone density of patients in the two groups was measured, analyzed and compared. The degree of regenerated bone defect in transverse and longitudinal directions after 60 days was compared between the two reconstruction procedures. Results: The pain at 24 hours after surgery and the bleeding at 7 days after surgery in the observation group were milder than those in the control group (P<0.001). There was significant difference in the degrees of bone defect at 60 days after surgery (P<0.05). Compared with the control group, the regenerated bone density of the observation group was significantly higher both at 60 days and 120 days after surgery (P<0.001). Conclusion: The combination of PRF and GBR technology has an obvious effect in repairing bone defects in patients with peri-implantitis, and can reduce the pain of patients during the repair process.
Keywords: Platelet-rich fibrin, guided bone regeneration, reconstruction of bone defect, peri-implantitis
Introduction
With the development of society and the improvement of living standards, more and more oral cavity problems occur in people. The number of patients with tooth loss and injury is also increasing year by year. Therefore, dental implant has attracted more and more attention [1-3]. Peri-implantitis is mainly caused by the progressive bone loss around the implant denture. It is also a big problem that should be dealt with during dental implant. Restoration of dental implant and reconstruction of bone defect caused by peri-implantitis have become the radical treatment for peri-implantitis [4-6].
Platelet-rich fibrin (PRF), was derived from platelet-rich plasma (PRP) and created by French physician Choukroun in 2001 [7-10]. In order to explore the effect of PRF in the reconstruction of implant bone defect, this study explored the role of PRF combined with GBR in the reconstruction of peri-implantitis bone defect.
Materials and methods
Patient information
In this study, 80 patients with peri-implantitis who underwent implant restoration in the Department of Stomatology in our hospital were selected. The general information of the patients were collected after coming to our hospital, including their age, gender, medical history and basic conditions. According to the patient’s condition, those who complied with the principle of inclusion, were randomly divided into control group and observation group, with 40 cases in each group in accordance with the random number table. Patients in the control group were treated with flap curettage, while those in the observation group were implanted with a mixture of PRF and bone powder and covered with PRF biofilm. All participants signed informed consents. The study was approved by the Academic Ethics Committee of the hospital.
Inclusion and exclusion standards
Inclusion criteria: (1) The diagnosis of peri-implantitis met the general diagnostic criteria [11]; (2) There were indications of dental implants (single) and the peripheral bone loss was ≥4 mm.
Exclusion criteria: (1) Those who could not bear implant surgery due to serious systemic diseases [12]; (2) Patients with severe diabetes and poorly controlled blood glucose; (3) Patients with bone diseases such as osteoporosis; (4) Patients with cardiopulmonary insufficiency, diabetes or other vital organ diseases; (5) Patients with mental illness.
Preparation of PRF
The process was as follows: 20 mL of venous blood was collected [13]. The venous blood was centrifuged at 3,200 rpm/12 min by TD-4M Desktop low-speed centrifuge (Shandong Biobase Scientific Instrument Co., Ltd.). After centrifugation, platelet-depleted plasma (PPP) was in the top layer, PRF was in the middle layer, and red blood cell was in the sublayer. PPP in the top layer was mixed up with bone powder FDBA (Haiao prosthodontics bone powder, XCT005149), which was used to fill up the defect area. The PRF in the middle layer was directly filled in the wound, or mixed up with bone powder FDBA, or compressed as a regenerative membrane.
Surgical procedure
Preoperative computerized tomography (CT) examination was performed to determine the patient’s condition and confirm the inflammatory bone condition after implantation [14]. The patient’s gingivitis was controlled before surgery. The main approach was as follows: The affected area was flushed, and local anti-swelling and anti-inflammatory adjuvant antibiotics (Clindamycin phosphate, HYB1064-2, Hangzhou Haoxin Biotechnology Co., Ltd.) were administered for 3-5 days. Patients in the control group was treated with flap curettage. The granulation tissue on the surface of alveolar bone and implant was removed. After repeated rinsing with gentamicin solution (Beijing Yita Biotechnology Co., Ltd., SY0024) and normal saline, tight suture was performed. Infected part of patients in the observation group were treated with flap curettage and filled up with a mixture of PRF and bone powder, then it was covered with PRF biofilm and sutured.
Outcome evaluation
Comparison of pain 24 hours after surgery
Patient’s pain was assessed 24 hours after treatment, and was classified into four levels according to their pain levels: I painless [15]; II mild pain, tolerable without medication; III moderate pain, tolerable, persistent, affecting sleep and requiring medication to reduce it; IV severe pain, intolerable, could only be improved with medication. The pain level was statistically analyzed.
Periodontal index measurement 7 days after surgery
Peri-implant sulcular fluid (PISF), modified plaque index (mPLI), modified sulcular bleeding index (mSBI) and periodontal probe depth (PPD) were measured and analyzed [16].
Comparison of postoperative regenerated bone density
Micro-CT was used to perform CT scans on the repaired part of inflammatory bone defect after dental implantation in the two groups at Day 60 and Day 120. The bone defect area was selected as the region of interest (ROI) area to determine the defect depth of teeth and the regenerated bone density [17].
Statistical analysis
All data were compared and analyzed with SPSS 22.0. The measurement data conformed to the normal distribution were expressed as the mean ± standard deviation (x̅ ± sd), and independent-samples t-test was used for comparison between groups. The count data were expressed as the number of cases (percentage) [n (%)], and chi-square (χ2) test was applied. In addition, Z-test was conducted on the frequency of pain levels in the two groups for the difference of the two independent samples. The sample sizes in the two groups were both more than 30, the probability of difference was inferred based on standard normal distribution, so as to compare whether the differences between the two groups. P<0.05 was considered statistically significant.
Results
Patient information
Patients were evaluated for the following data before surgery. The results indicated that there was no significant difference between the two groups in age, gender, bone defect before surgery, bleeding on probing and periodontal probe depth (P>0.05), see Table 1.
Table 1.
Patient information before surgery
| Items | Observation group | Control group | t/χ2 | P |
|---|---|---|---|---|
| Gender (n) | 0.220 | 0.639 | ||
| Male | 27 | 25 | ||
| Female | 13 | 15 | ||
| Age (years old) | 38.9±6.2 | 35.4±10.8 | 1.780 | 0.079 |
| Marital status (n) | 1.593 | 0.451 | ||
| Married | 32 | 30 | ||
| Unmarried | 7 | 10 | ||
| Divorced or widowed | 1 | 0 | ||
| Bone defect (mm) | ||||
| DD value | 3.93±1.57 | 3.97±1.46 | 0.118 | 0.906 |
| DW value | 4.73±1.03 | 4.97±1.21 | 0.955 | 0.342 |
| Bleeding on probing | 2.88±0.32 | 2.97±0.36 | 1.182 | 0.241 |
| Periodontal probe depth | 5.43±0.23 | 5.38±0.38 | 0.711 | 0.478 |
Note: DD: defect depth; DW: defect width.
Comparison of pain 24 hours after surgery
The pain levels of the two groups 24 hours after surgery were classified and collected. Through the comparison of the two groups, it can be seen that there were 26 patients with type I pain in the observation group while only 11 patients in the control group. There was a significant difference in pain levels in the two groups by frequency analysis (P<0.001). The pain levels in the observation group were milder than those in the control group, see Table 2.
Table 2.
Pain questionnaire 24 hours after surgery (n)
| Grouping | Control group | Observation group |
|---|---|---|
| I | 11 | 26 |
| II | 24 | 14 |
| III | 5 | 0 |
| IV | 0 | 0 |
| Z | 3.621 | |
| P | <0.001 | |
Periodontal index measurement 7 days after surgery
The basic periodontal indexes of patients in both groups were summarized. And the four indexes expressed by x̅ ± sd were summarized and analyzed. The results showed that there was significant difference in the amount of PISF, mPLI, mSBI and PPD (all P<0.001), indicating that covering with PRF biofilm in the observation group after filling with a mixture of PRF and bone powder could significantly accelerate the patient’s recovery, see Table 3.
Table 3.
Comparison of periodontal index measurement 7 days after surgery
| Items | PISF (μL) | mPLI | mSBI | PPD (mm) |
|---|---|---|---|---|
| Control group | 0.51±0.03 | 0.68±0.01 | 1.03±0.05 | 2.11±0.58 |
| Observation group | 0.35±0.05 | 0.88±0.05 | 0.71±0.03 | 1.71±0.28 |
| t | 14.103 | 24.812 | 34.714 | 3.926 |
| P | <0.001 | <0.001 | <0.001 | <0.001 |
Note: PISF: peri-implant sulcular fluid; mPLI: modified plaque index; mSBI: modified sulcular bleeding index; PPD: periodontal probe depth.
The patient’s regenerated bone density 60 days and 120 days after surgery
The results of regenerated bone density showed that there was significant difference in regenerated bone density at 60 days and 120 days after surgery (P<0.05), see Figure 1 and Table 4.
Figure 1.
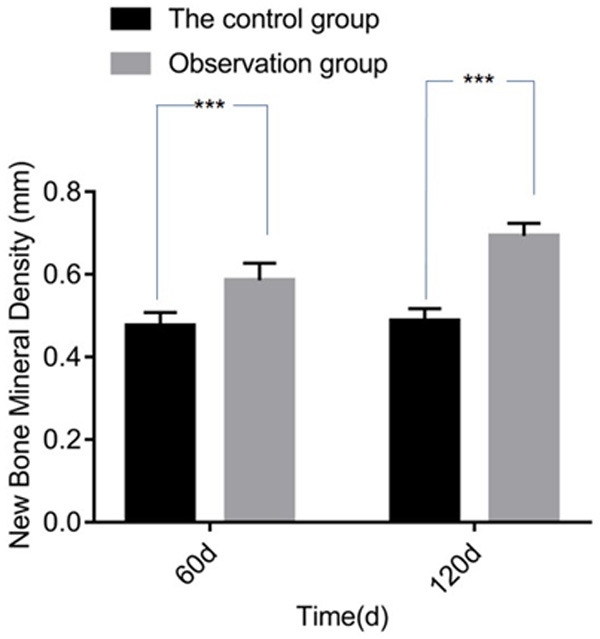
Comparison of regenerated bone density. Compared with control group, ***P<0.05.
Table 4.
Comparison of regenerated bone density
| Items | Control group | Observation group | t | P |
|---|---|---|---|---|
| Regenerated bone density (mm) | ||||
| 60 days after surgery | 0.48±0.03 | 0.59±0.04 | 11.191 | <0.001 |
| 120 days after surgery | 0.49±0.03 | 0.69±0.03 | 30.536 | <0.001 |
Comparison and analysis of bone defect depth and defect width 60 days after surgery
Dental implants would still wear out after the reconstruction for different inflammatory bone defects. There was no difference between the two groups in the baseline DD and DW values (P>0.05). There was statistically significance in DD and DW values between baseline and 60 days after surgery (P<0.001). The bone defect in the observation group was significantly different from that of the control group in both defect depth and defect width (P<0.001). The results indicated that the wear of the implants after restoration was significantly reduced by using the PRF combined with GBR technology, see Tables 5 and 6.
Table 5.
Comparison of bone defect depth 60 days after surgery
| Items | Control group | Observation group | t | P |
|---|---|---|---|---|
| DD value | ||||
| Baseline | 3.93±1.57 | 3.97±1.46 | 0.121 | 0.906 |
| 60 days after surgery | 2.27±1.20 | 1.01±0.18 | 6.568 | <0.001 |
| t | 5.311 | 12.732 | ||
| P | <0.001 | <0.001 |
Note: DD: defect depth.
Table 6.
Comparison of bone defect width 60 days after surgery
| Items | Control group | Observation group | t | P |
|---|---|---|---|---|
| DW value | ||||
| Baseline | 4.73±1.03 | 4.97±1.21 | 0.964 | 0.342 |
| 60 days after surgery | 3.52±1.22 | 1.28±0.28 | 11.318 | <0.001 |
| t | 4.788 | 18.792 | ||
| P | <0.001 | <0.001 |
Note: DW: defect width.
Discussion
Peri-implantitis bone defect is a very common phenomenon in the process of dental implant restoration. How to reduce and eliminate this inflammation has become the research focus of many scholars in this field. Although peri-implantitis has little influence on the success rate of dental implants, it still has certain influence on the treatment and postoperative quality of life [18]. Various studies have been carried out on it, including the application of GBR technology, as well as the use of GBR combined with hyperbaric oxygen (HBO) in the reconstruction and treatment of peripheral bone defects caused by peri-implantitis [19]. However, there are relatively few studies on the treatment with PRF combined with GBR technology.
It has been reported that the growth factors in PRF play an important role in the process of wound healing [20]. At the same time, it has been shown that PRF is very suitable for being used as filling materials and base materials during sinus lifting surgery. PRF is also suitable for general dental implant surgery, which can reduce inflammation, enhance osteoblasts adhesion, induce osteocyte regeneration, improve the effect of bone grafting, accelerate the repair and regeneration of soft tissue, and help with wound healing [21].
In this study, PRF combined with GBR technology was used to reconstruct peri-implantitis bone defect. The results indicated that the pain in the observation group during the reconstruction of the peri-implantitis bone defect was less than that in the control group. In the measurement of periodontal index, it can be seen that there was significant difference in the four indexes of PISF, mPLI, mSBI and PPD, indicating that patients in the observation group had better mid-term recovery effect after repair. Meanwhile, the defect degree 60 days after repair in the observation group was relatively low, and the regenerated bone density in the later period increased significantly compared with the control group. The above results support that PRF could promote the growth of soft tissues of the mucosa in the operation area of oral cavity at all stages. PRF could relieve the pain during the surgery, have a better medium-term recovery effect, reduce the reinfection of peri-implantitis and rejection, as well as accelerate the growth of regenerated bone and increase the regenerated bone density, so as to reduce wear and tear on the patient’s defect site. The results were consistent with previous report that PRF combined with GBR in the reconstruction of peri-implantitis bone defect [18].
This study was conducted by patients who came to our hospital for treatment in this region. Although there were some positive results, a comprehensive clinical study on PRF is still needed in the following process of promotion and application. At the same time, this technology has been applied to periodontal bone defects, but the follow-up time of this study was still short, and its long-term treatment effect still needed to be studied in depth with a larger sample size.
Disclosure of conflict of interest
None.
References
- 1.Degirmenci K, Atala MH. The impact of different dental implant surface properties to osseointegration success of dental implants-a systematic review. Clin Oral Implants Res. 2010;30:280–280. [Google Scholar]
- 2.Brizuela A, Herrero-Climent M, Rios-Carrasco E, Rios-Santos JV, Pérez RA, Manero JM, Gil Mur J. Influence of the elastic modulus on the osseointegration of dental implants. Materials (Basel) 2019;12:980. doi: 10.3390/ma12060980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubinstein NC, Jacobson Z, McCausland GL, Dibart S. Retrospective study of the success of dental implants placed in HIV-positive patients. Int J Implant Dent. 2019;5:30. doi: 10.1186/s40729-019-0174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duan YL, Wei CG, Yang LJ, Yan X, Liu HF, Ma H. Application value of high frequency ultrasound in the diagnosis of knee joint and surrounding soft tissue lesions. Clin Res Pract. 2019;4:158–159. [Google Scholar]
- 5.Zorina OA, Amkhadova MA, Khamukova AA, Aleskerov ES, Ajrapetov GA, Demidova AA. Osteoimmunological aspects of periodontal inflammatory destructive changes at periimplantitis, chronic periodontitis and oncological diseases of the oral cavity. Stomatologiia (Mosk) 2020;99:27–32. doi: 10.17116/stomat20209904127. [DOI] [PubMed] [Google Scholar]
- 6.Heckmann SM, Linke JJ, Graef F, Foitzik C, Wichmann MG, Weber HP. Stress and inflammation as a detrimental combination for peri-implant bone loss. J Dent Res. 2006;85:711–716. doi: 10.1177/154405910608500805. [DOI] [PubMed] [Google Scholar]
- 7.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, Gogly B. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:45–50. doi: 10.1016/j.tripleo.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Opri M, Amzoiu E, Manolea HO, Rîcă R. Computational study of physicochemical properties of the monomers used in stomatology. Key Eng Mater. 2016;695:59–64. [Google Scholar]
- 9.Zhang S, Cao D, Xie J, Li H, Chen Z, Bao Q. Platelet-rich fibrin as an alternative adjunct to tendon-exposed wound healing: a randomized controlled clinical trial. Burns. 2019;45:1152–1157. doi: 10.1016/j.burns.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Jie J, Xu YY, Xia L. Meta-analysis of platelet rich fibrin (PRF) in periodontal bone defect repair. Chin J Geriatr. 2020;18:76–80. [Google Scholar]
- 11.Blanco J, Alonso A, Sanz M. Long-term results and survival rate of implants treated with guided bone regeneration: a 5-year case series prospective study. Clin Oral Implants Res. 2005;16:294–301. doi: 10.1111/j.1600-0501.2005.01106.x. [DOI] [PubMed] [Google Scholar]
- 12.Javed F, Romanos GE. Role of implant diameter on long-term survival of dental implants placed in posterior maxilla: a systematic review. Clin Oral Investig. 2015;19:1–10. doi: 10.1007/s00784-014-1333-z. [DOI] [PubMed] [Google Scholar]
- 13.Tsujino T, Takahashi A, Yamaguchi S, Watanabe T, Isobe K, Kitamura Y, Tanaka T, Nakata K, Kawase T. Evidence for contamination of silica microparticles in advanced platelet-rich fibrin matrices prepared using silica-coated plastic tubes. Biomedicines. 2019;7:45. doi: 10.3390/biomedicines7020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Urban IA, Monje A, Lozada JL, Wang HL. Long-term evaluation of peri-implant bone level after reconstruction of severely atrophic edentulous maxilla via vertical and horizontal guided bone regeneration in combination with sinus augmentation: a case series with 1 to 15 years of loading. Clin Implant Dent Relat Res. 2017;19:46–55. doi: 10.1111/cid.12431. [DOI] [PubMed] [Google Scholar]
- 15.Yu TT, Liu J, Yin JJ, Xu XN, Yan SJ, Lan J. Effects of concentrated growth factors on relieving postoperative reaction of guided bone regeneration in the esthetic zone. Hua Xi Kou Qiang Yi Xue Za Zhi. 2019;37:398–402. doi: 10.7518/hxkq.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khader YS, Dauod AS, El-Qaderi SS, Alkafajei A, Batayha WQ. Periodontal status of diabetics compared with nondiabetics: a meta-analysis. J Diabetes Complications. 2006;20:59–68. doi: 10.1016/j.jdiacomp.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Lam YM, Chen X, Marean CW, Frey CJ. Bone density and long bone representation in archaeological faunas: comparing results from CT and photon densitometry. J Archaeol Sci. 1998;25:559–570. [Google Scholar]
- 18.Yan N, Huang T, Zhang ZY, Zhang SH, Han GL, Wang LH. Clinical application of platelet-rich fibrin combined with GBR in the reconstruction of periimplant inflammatory bone defect. J Appl Med. 2020;36:813–817. [Google Scholar]
- 19.Gao WD, Hu TQ, Zhou YQ, Cao JN, Zhao DH. A study on regeneration of the bone defects due to peri-implantitis by GBR and hyperbaric oxygen. J Clin Stomatol. 2015;31:563–565. [Google Scholar]
- 20.Zhai Z, Yang XN, Qi ZL. Progress in the application of platelet rich fibrin in fat transplantation. Chin J Plast Surg. 2019;35:205–209. [Google Scholar]
- 21.Xu FW, Liu ZH. Research and progress of Choukroun’s platelet rich fibrin in oral implant defect. Tissue Eng Res Clin Rehabil China. 2012;4:741–744. [Google Scholar]


