Abstract
Objective: To determine the effect of different preoperative fasting time on safety and postoperative complications of painless gastrointestinal endoscopy for polyps in patients. Methods: Enrolled patients were assigned to an observation group and a control group by the random number table method (each n=68). Before operation, each patient in the observation group was fasted from solids for 6 h and from liquids for 2 h, while each one in the control group was fasted from solids for 8-12 h and from liquids for 4 h according to the conventional method. The levels of blood glucose, insulin, potassium and sodium in patients before and after operation were determined, and their hunger and thirst were recorded before anesthesia. Additionally, the incidences and degrees of vomiting and nausea among the patients after anesthesia and operation were recorded. Results: Before operation, the observation group showed higher levels of blood glucose, insulin, serum potassium and serum sodium than the control group (all P<0.001), while after operation, the observation group showed lower levels of blood glucose and insulin and higher levels of serum potassium and serum sodium than the control group (all P<0.001). In addition, the degrees and incidences of hunger and thirst in patients of the observation group were significantly lower than those in the control group before operation (P<0.01), and the degrees and incidences of nausea and vomiting in the observation group were also notably lower than those in the control group before and after operation (both P<0.05). Conclusion: For patients undergoing painless gastrointestinal endoscopy for polyps, shortening their fasting time from solids and liquids before operation can stabilize their blood glucose, insulin and electrolyte levels before and after operation, relieve their thirst and hunger before operation, and reduce the incidences of postoperative nausea and vomiting.
Keywords: Fasting time, solids and liquids, painless gastrointestinal endoscopy, polyp, postoperative recovery
Introduction
Advancement of gastrointestinal examination contributes to significantly higher detection rate of gastrointestinal polyps [1-3]. In recent years, the incidence of clinical gastrointestinal polyps is found to be in a gradual increase, which is closely related to patients’ lifestyle, treatment plan and HP infection [4-6]. Currently, the most effect method for benign non-submucosal gastrointestinal polyps <4 cm originating from epithelium tissues is surgery under painless gastrointestinal endoscopy [7,8]. Before painless gastrointestinal endoscopy, preparation should be made for gastrointestinal procedures, and the patient is required to undergo fasting from solids and liquids for a period of time, which is similar to but stricter than preoperative fasting from solids and liquids in patients undergoing elective surgery. The conventional preoperative fasting is fasting from solids for 8-12 h and from liquids for 4 h, but the actual operation time is different from the theoretical one, leading to a remarkable increase in preoperative fasting time compared with the theoretical time [9]. According to previous studies, long-term fasting will greatly increase the incidences of symptoms including hunger, thirst, and dizziness, especially among patients with weak constitution, and the insufficiency of stored hepatic glycogen will bring about adverse responses including anxiety, irritability, and hypoglycemia [10,11]. In terms of preoperative fasting, painless gastrointestinal endoscopy for polyps is different from elective surgery in that patients undergoing painless gastrointestinal endoscopy for polyps are required to orally take laxatives or receive mechanical enema for intestinal cleaning in addition to fasting from solids and liquids. During diarrhea for intestinal preparation, besides water, electrolyte will loss, and even electrolyte disorder will appear, which is more unfavorable to patients with cardio-cerebrovascular diseases, liver or kidney insufficiency, or other diseases [12]. The purpose of preoperative fasting is mainly to prevent aspiration during anesthesia, but it is found clinically that aspiration will occur only when the gastric content volume (GCV) are ≥200 mL, so the preoperative preparation plan for shortening fasting time from solids and liquids is put forward clinically [13]. One study has enrolled 3110 patients who underwent general anesthesia surgery in the premise of experiencing shorter fasting from solids and liquids, and found aspiration pneumonia in none of them [14]. Moreover, one study has pointed out that fasting from solids for 6-12 h and from liquids for 2-4 h is not bound up with aspiration pneumonia, and the fluid retained in the stomach of oral laxatives is the same as that under fasting for one day before colonoscopy. In the European Guide of 2019, it is recommended that the gastrointestinal tract should be prepared 2 h before operation, which indicates the safety and operability of shortening the preoperative fasting time [15]. At present, there is no research report on the safety and operability of shortening the fasting time from solids and liquids for patients receiving painless gastrointestinal endoscopy for polyps. This study enrolled patients undergoing painless gastrointestinal endoscopy for polyps, and evaluated the effect of shortening preoperative fasting time on the safety and postoperative recovery of those patients.
Materials and methods
General materials
A total of 136 patients with gastrointestinal polyps admitted to the center of digestive endoscopy in our hospital from July 2019 to October 2020 were enrolled for prospective study. The patients were between 20 and 70 years old, with an average age of 41.2±8.4 years. They were assigned to an observation group and a control group by the random number table method (each n=68). Before operation, each patient in the observation group (with an average age of 41.1±9.3 years) was fasted from solids for 6 h and from clear liquids for 2 h, while each one in the control group (with an average age of 41.3±7.8 years) was fasted from solids for 8-12 h and from liquids for 4 h according to the conventional method. All the above patients signed informed consent forms, and the study was approved by the ethics committee of our hospital.
Inclusion and exclusion criteria
Inclusion criteria of the study: Patients diagnosed as gastrointestinal polyps by electronic gastroscopy and colonoscopy before operation; patients between 18 and 75 years old; patients undergoing painless gastroscopy or colonoscopy electively; and those who had not taken anticoagulants within 1 week before operation. Exclusion criteria of the study: Patients unable to tolerate surgery; patients with severe comorbid heart, liver or kidney diseases; patients with malignant lesions of polyps; patients with severe coagulation disorder; patients who were difficult or inconvenient to follow up; patients unsuitable for surgery; patients with constipation or eating disorder; and those with gastric retention.
Methods
(1) The observation group: Each patient in the group was fasted from solids for 6 h and from liquids for 2 h before operation. (2) The control group: Each patient in the group was fasted from solids for 8-12 h and from liquids for 4 h before operation. All patients were required to take 1 bottle (10 mL) of lidocaine hydrochloride mucilage (Jumpcan Pharmaceutical Group Co., Ltd., Jiangsu, China) before gastroscopy, and magnesium sulfate (Pharmaceutical Group Sanchine, Harbin, China) before colonoscopy. Precautions and operation procedures were explained to them. Patients were given painless gastrointestinal endoscopy. Briefly, the patient was given 1 μg/kg fentanyl (specification: 10 mL:0.5 mg, Humanwell Pharmaceutical Co., Ltd., Yichang, China) by intravenous infusion, and then 1.5 mg/kg propofol (Libang Pharmaceutical Co., Ltd., Xi’an, China) by intravenous infusion. Painless colonoscopy was carried out on the patient after he entered the sleep state, without eyelash reflex and muscle relax of the whole body. During operation, patients with blood oxygen lower than 95% were given oxygen inhalation by nasal catheter at 2 L/min. Intraoperative hypotension means that the systolic or diastolic blood pressure of the patient decreases by 20-40% compared with the preoperative basic value, and the absolute intraoperative hypotension means that the absolute value of the average arterial pressure decreases by 50 mmHg, and the duration exceeds 5 min. If the blood pressure was still low after changing the patient’s position and reducing the intraoperative temperature, the blood pressure could be maintained with drugs for increasing blood pressure. During treatment, if the patient was restless, propofol (0.5-1.0 mg/kg) was given as appropriate to keep him in a painless state until the treatment was completely over. Then the patient was transferred to a recovery room until he was awake. No antiemetics were used during the operation.
In order to ensure the quality of operation, the following schemes were adopted: (1) All operations were carried out by a surgeon experienced in gastrointestinal endoscopy and endoscopy as the main surgeon. (2) Both groups were given the same surgical treatment plan. (3) Both groups were given the same treatment and nursing plan before and after operation. (4) Bleeding during operation was stopped by corresponding methods. (5) Both groups were given the same anesthesia plan.
Outcome measures
Primary outcome measures
(1) Venous blood sample (5 mL) was collected from each patient before operation and 1 h after operation, and serological indexes in the sample, including blood glucose, insulin, potassium, and sodium, were determined using a Beckman automatic biochemistry analyzer.
(2) The degree of hunger and thirst of each patient was understood before anesthesia and recorded. A: The degree of thirst was judged as follows: No thirst: having no sense of thirst; Mild thirst: having a slight sense of thirst; Moderate thirst: having an obvious but bearable thirst; Severe thirst: having a strong sense of thirst and unbearable lips chap. B: The degree of hunger was judged as follows: No hunger: having no sense of hunger; Mild hunger: having a slight sense of hunger; Moderate hunger: have an obvious but bearable hunger; Severe thirst: having a strong sense of hunger and unbearable dizziness, cold sweat and other discomfort. Incidence of thirst/hunger = (patients with mild thirst/hunger + patients with moderate thirst/hunger + patients with severe thirst/hunger)/the total number of patients × 100%.
(3) The incidences and degrees of vomiting and nausea among patients within 24 h after operation were recorded [16]. This study adopted relevant standards of WHO grading criteria to evaluate vomiting and nausea, which were both classified into 1-4 grades. The grading criteria for nausea: Grade 1: no symptoms; Grade 2: loss of appetite and normal eating ability; Grade 3: decrease in oral food consumption, no significant decrease in body weight, and no malnutrition and dehydration; Grade 4: insufficient oral consumption of water and energy, requirement of intravenous nutrition or hospitalization. The grading criteria for vomiting: Grade 1: no symptoms; Grade 2: 1-2 times of vomiting within 24 h; Grade 3: 3-5 times of vomiting within 24 h; Grade 4: 6 times of vomiting or more within 24 h and requirement of intravenous nutrition or hospitalization. Incidence of vomiting/nausea = (patients with grade 2 vomiting/nausea + patients with grade 3 vomiting/nausea + patients with grade 4 vomiting/nausea)/the total number of patients in the group × 100% [17].
Secondary outcome measures
The GFV was measured by bedside B ultrasound before operation. Specifically, the GFV and the ratio of GFV to body weight (GFVw) were calculated according to a formula. The cross-sectional area (CSA) of gastric antrum was measured via bedside ultrasound. Specifically, a low-frequency curved array probe was placed in the median sagittal site of upper abdomen of the patient in a right lateral position, and the probe was moved slightly to the right, or slightly rotated to scan the antrum of stomach with abdominal aorta, right lobe of liver and superior mesenteric artery as anatomical markers, thus obtaining its maximum ultrasonic image. Based on the frozen image, the maximum diameter along the anterior and posterior direction of the body (AP) and the maximum diameter in the cephalosacral direction (CC) were measured, and then the CSA was solved. The specific formula is (AP × CC × π)/4. According to latest Perlas’ formula, GFV=27 + 14.6 × right CSA - 1.28 × age [18].
Statistical analyses
SPSS 17.0 was adopted for statistical analyses. Continuous variables were expressed by the mean ± standard deviation (x̅ ± sd), and multi-group comparison of them was carried out using the one-way ANOVA. In the case of a significant difference, pairwise comparison was carried out further using the Turkey method. Enumeration data were expressed as the cases/percentage (n/%), and Pearson Chi-square test and Fisher exact probability method were adopted. P<0.05 indicates a significant difference.
Results
Comparison of general data
There was no significant difference between the two groups in general data (all P>0.05; Table 1).
Table 1.
Comparison of general information of the two groups of patients
| Item | Observation group (n=68) | Control group (n=68) | χ2/t | P |
|---|---|---|---|---|
| Gender (male/female) | 33/35 | 35/33 | 0.118 | 0.732 |
| Age (years) | 41.1±9.3 | 41.3±7.8 | 0.136 | 0.892 |
| Body mass index (kg/m2) | 23.21±2.11 | 23.23±2.02 | 0.056 | 0.955 |
| Number of polyps (n) | 1.57±0.25 | 1.59±0.27 | 0.448 | 0.655 |
| Comorbidity | ||||
| Hypertension | 22 | 25 | 0.092 | 0.821 |
| Type 2 diabetes | 11 | 12 | 0.043 | 0.943 |
| Coronary heart disease | 13 | 11 | 0.097 | 0.876 |
| ASA rating | 0.123 | 0.836 | ||
| Grade I | 56 | 54 | ||
| Grade II | 12 | 14 | ||
| Operation time (h) | 1.10±0.32 | 1.07±0.31 | 0.555 | 0.580 |
| Number of intraoperative blood pressure fluctuations (cases) | 4 | 5 | 0.119 | 0.730 |
| Hypoxemia during operation (cases) | 3 | 2 | 0.208 | 0.649 |
Comparison of relevant indexes between the two groups before and after operation
There was no aspiration during operation in both groups, and their gastrointestinal endoscopy and polypectomy were both completed. Before operation, the observation group showed higher levels of blood glucose, insulin, serum potassium and serum sodium than the control group (all P<0.001), while after operation, the observation group showed lower levels of blood glucose and insulin and higher levels of serum potassium and serum sodium than the control group (all P<0.001; Table 2).
Table 2.
Comparison of related indicators before and after operation between the two groups of patients
| Group | Observation group (n=68) | Control group (n=68) | t | P |
|---|---|---|---|---|
| Blood sugar (mmol/L) | ||||
| Preoperative | 5.82±0.92 | 4.21±0.81 | 10.831 | <0.001 |
| Postoperative | 5.62±0.88 | 6.82±1.21 | 6.614 | <0.001 |
| Insulin (mU/L) | ||||
| Preoperative | 6.75±1.11 | 3.21±1.04 | 19.191 | <0.001 |
| Postoperative | 5.19±1.29 | 9.25±1.45 | 17.251 | <0.001 |
| Potassium (mmol/L) | ||||
| Preoperative | 4.81±0.93 | 4.02±0.96 | 4.872 | <0.001 |
| Postoperative | 4.62±0.84 | 3.71±0.94 | 5.953 | <0.001 |
| Sodium (mmol/L) | ||||
| Preoperative | 141.21±7.85 | 136.24±6.24 | 4.087 | <0.001 |
| Postoperative | 139.24±6.94 | 133.65±6.14 | 4.975 | <0.001 |
Comparison of preoperative hunger and thirst between the two groups
The observation group showed significantly lower incidences of preoperative hunger and thirst than the control group (both P<0.01; Tables 3, 4).
Table 3.
Comparison of preoperative hunger between the two groups (n, %)
| Item | Control group (n=68) | Observation group (n=68) | χ2 | P |
|---|---|---|---|---|
| Hunger | 14.887 | 0.002 | ||
| No | 2 (2.94) | 17 (25.00) | ||
| Mild | 46 (67.65) | 40 (58.82) | ||
| Moderate | 16 (23.53) | 9 (13.23) | ||
| Severe | 4 (5.88) | 2 (2.94) | ||
| Total hungry | 66 (97.06) | 51 (75.00) | 13.765 | <0.001 |
Table 4.
Comparison of preoperative thirst degree between the two groups
| Item | Control group (n=68) | Observation group (n=68) | χ2 | P |
|---|---|---|---|---|
| Thirst | 55.360 | <0.001 | ||
| No | 2 (2.94) | 43 (63.24) | ||
| Mild | 38 (55.88) | 19 (27.94) | ||
| Moderate | 16 (23.53) | 4 (5.88) | ||
| Severe | 12 (17.65) | 2 (2.94) | ||
| Total number of people thirsty | 66 (97.06) | 25 (36.76) | 24.446 | <0.001 |
Comparison of degrees of postoperative nausea and vomiting between the two groups
The observation group showed notably lower incidences and degrees of nausea and vomiting than the control group before and after operation (both P<0.05; Tables 5, 6 and 7 and Figures 1, 2).
Table 5.
Comparison of postoperative nausea between the two groups
| Item | Control group (n=68) | Observation group (n=68) | χ2 | P |
|---|---|---|---|---|
| Nausea | 8.059 | 0.045 | ||
| Grade I | 57 (83.82) | 66 (97.06) | ||
| Grade II | 9 (13.24) | 1 (1.47) | ||
| Grade III | 1 (1.47) | 1 (1.47) | ||
| Grade IV | 1 (1.47) | 0 (0.00) | ||
| Total number of cases | 11 (16.18) | 2 (2.94) | 6.889 | 0.009 |
Table 6.
Comparison of the degree of postoperative vomiting between the two groups
| Item | Control group (n=68) | Observation group (n=68) | χ2 | P |
|---|---|---|---|---|
| Vomiting | 6.952 | 0.031 | ||
| Grade I | 59 (86.76) | 67 (98.53) | ||
| Grade II | 8 (11.76) | 1 (1.47) | ||
| Grade III | 1 (1.47) | 0 (0.00) | ||
| Grade IV | 0 (0.00) | 0 (0.00) | ||
| Total number of cases | 9 (13.24) | 1 (1.47) | 12.897 | <0.001 |
Table 7.
Comparison of the total number of patients with nausea and vomiting in the two groups
| Item | Control group (n=68) | Observation group (n=68) | χ2/t | P |
|---|---|---|---|---|
| Number of occurrences (n) | 12 (17.65) | 3 (4.41) | 6.069 | 0.014 |
Figure 1.
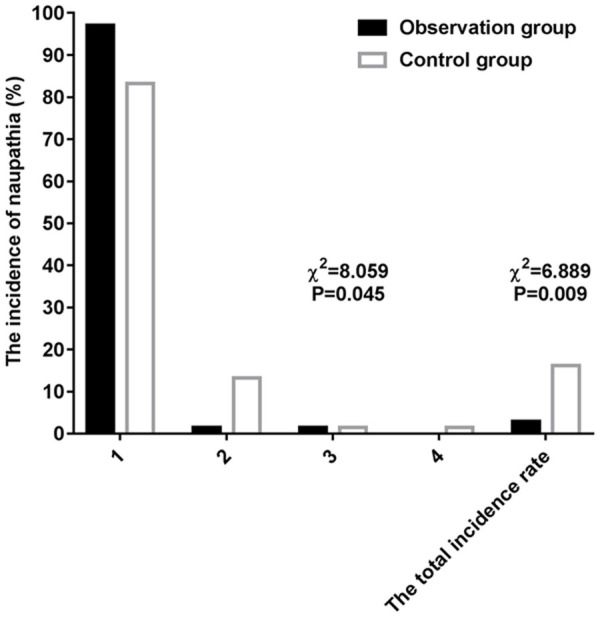
Comparison of postoperative nausea between the two groups.
Figure 2.
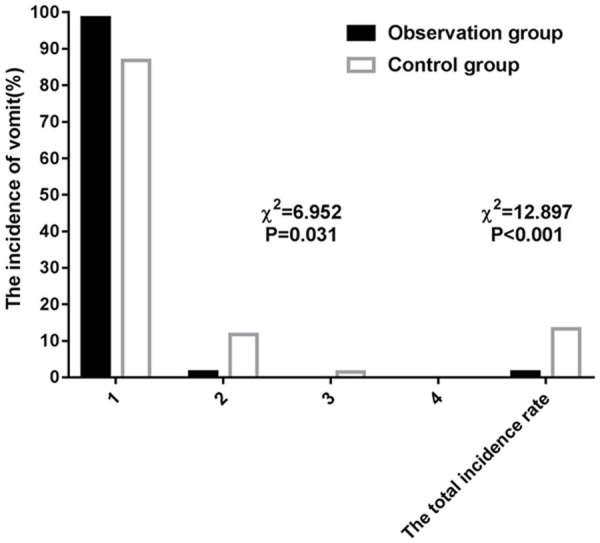
Comparison of the degree of postoperative vomiting between the two groups.
Comparison of GFV between the two groups during operation
There was no significant difference between the two groups in GFV during operation (P>0.05; Table 8).
Table 8.
Comparison of intraoperative gastric food residues between the two groups
| Item | Observation group (n=68) | Control group (n=68) | χ2/t | P |
|---|---|---|---|---|
| Stomach food residue (mL) | 51.23±6.23 | 49.35±6.12 | 1.775 | 0.078 |
Discussion
Patients undergoing surgery need fasting from solids and liquids before surgery to prevent vomiting that leads to aspiration and threatens life during surgery [19]. Conventional fasting is specified as fasting from solids for 8-12 h and from liquids for 4 h before surgery. As clinical research deepens, it is found that there is a correlation between GFV and aspiration after fasting before operation, and shortening fasting does not increase the GFV, so it is possible to shorten the preoperative fasting time [20]. Long-term fasting will give rise to a series of changes in the patient’s body, and thus result in adverse responses such as thirst, hunger, hypoglycemia and irritability [21]. The above changes are closely bound up with electrolyte disorder and blood glucose changes in the patient’s body. Additionally, after long-term fasting, a series of stresses will occur during the operation, which will make the body in a high metabolic state, causing fluctuation in blood glucose and insulin resistance [22]. In this study, the blood glucose, insulin and electrolytes of patients were monitored before and after painless gastrointestinal endoscopy for polyps. The observation group experiencing shorter fasting time from solids and liquids showed higher levels of blood glucose, insulin, serum sodium and serum potassium than the control group before operation, which implies that shortening fasting time is beneficial to the stability of patients’ body indexes before operation. After operation, the levels of blood glucose and insulin in the control group fluctuated more than those in the observation group, and the serum sodium, serum potassium and electrolyte levels in the control group decreased further to be lower than those in the observation group. It may be explained by the fact that shortening the fasting time can inhibit stress response during operation.
Our study revealed that shortening fasting from solids and liquids can relieve the hunger and thirst of patients before operation. One previous study has pointed out that fasting from solids for 2 h and from liquids for 6 h and reducing the use of purgative drugs before operation can ensure the moisture in patients, and effectively prevent thirst, dehydration, and blood concentration [23]. One other study has also revealed that hunger and thirst of patients who experienced fasting from solids and liquids for more than 12 h are significantly more serious than those who experienced it for less than 12 h, and longer fasting time gives rise to higher incidence of anxiety and insomnia [24]. Drinking water or glucose 2 h before operation can obviously relieve thirst and hunger and stabilize insulin and glucose levels, which is consistent with the results of our study [25].
Our study found that shortening fasting time from solids and liquids can reduce postoperative nausea and vomiting. One previous study has pointed out that the most common complications after anesthesia are nausea and vomiting, and the incidence of them is higher in patients undergoing painless gastrointestinal endoscopy for polyps [26]. Moreover, one study has revealed that shortening fasting time from solids and liquids will not increase the incidences of nausea and vomiting after thyroid surgery [27]. And one other study has also shown that shortening fasting from solids and liquids causes a notable decrease in the incidences of nausea and vomiting after surgery [28]. The above studies imply that shortening fasting from solids and liquids before operation can reduce postoperative nausea and vomiting.
The sample size of this study is small, so it is necessary to further expand the sample size for the future research. In addition, a multicenter randomized controlled study can be conducted to systematically evaluate the impact of intervention measures of shortening fasting time from solids and liquids on postoperative recovery of patients with polyps undergoing painless gastrointestinal endoscopy.
To sum up, for patients undergoing painless gastrointestinal endoscopy for polyps, shortening their fasting from solids and liquids before operation can stabilize their blood glucose, insulin and electrolyte levels before and after operation, relieve their thirst and hunger before operation, and reduce the incidence of postoperative nausea and vomiting among them.
Acknowledgements
This work was supported by the Medical and Health Research Project of Hainan Province (No. 2001320712A2036).
Disclosure of conflict of interest
None.
References
- 1.Prado-Núñez S, Yamamoto Kagami JM, Jeri-Yabar A, Marín Leiva J, Arévalo Suárez F, Ríos Julca N, Monge E. Gastric polyps: experience in daniel alcides carrion hospital 2014-2016. Rev Gastroenterol Peru. 2018;38:248–252. [PubMed] [Google Scholar]
- 2.Paggi S, Hassan C, Radaelli F. Predictive narrow-band imaging of colonic polyps: the optics are good. Dig Dis Sci. 2018;63:2489–2491. doi: 10.1007/s10620-018-5189-y. [DOI] [PubMed] [Google Scholar]
- 3.Berger AW, Raedler K, Langner C, Ludwig L, Dikopoulos N, Becker KF, Slotta-Huspenina J, Quante M, Schwerdel D, Perkhofer L, Kleger A, Zizer E, Oswald F, Seufferlein T, Meining A. Genetic biopsy for prediction of surveillance intervals after endoscopic resection of colonic polyps: results of the GENESIS study. United European Gastroenterol J. 2018;6:290–299. doi: 10.1177/2050640617723810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee HE, Wu TT, Chandan VS, Torbenson MS, Mounajjed T. Colonic adenomatous polyps involving submucosal lymphoglandular complexes: a diagnostic pitfall. Am J Surg Pathol. 2018;42:1083–1089. doi: 10.1097/PAS.0000000000001081. [DOI] [PubMed] [Google Scholar]
- 5.Velázquez-Dohorn ME, López-Durand CF, Gamboa-Domínguez A. Changing trends in gastric polyps. Rev Invest Clin. 2018;70:40–45. doi: 10.24875/RIC.17002430. [DOI] [PubMed] [Google Scholar]
- 6.Brito HLF, Barros C, Freire MV, Silva Filho MND, Nascimento TV. Gastric fundic gland polyps: can histology be useful to predict proton pump inhibitors use? Arq Gastroenterol. 2018;55:380–384. doi: 10.1590/S0004-2803.201800000-82. [DOI] [PubMed] [Google Scholar]
- 7.Olmez S, Sayar S, Saritas B, Savas AY, Avcioglu U, Tenlik I, Ozaslan E, Koseoglu HT, Altiparmak E. Evaluation of patients with gastric polyps. North Clin Istanb. 2018;5:41–46. doi: 10.14744/nci.2017.50480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Syed AR, Kumar U, Garg M, Dhawan M, Thakkar S. Argon plasma coagulation treatment of intraductal papillary neoplasm of biliary tract: an alternative approach. VideoGIE. 2018;3:234–235. doi: 10.1016/j.vgie.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osland E, Yunus RM, Khan S, Memon MA. Early versus traditional postoperative feeding in patients undergoing resectional gastrointestinal surgery: a meta-analysis. JPEN J Parenter Enteral Nutr. 2011;35:473–487. doi: 10.1177/0148607110385698. [DOI] [PubMed] [Google Scholar]
- 10.Xia LM, Chen LL, Li XY, Dai MZ, Huang YX. Application of reconstructed preopera tive fasting and forbidding drink process in elective surgery patients of surgical oncology. Chin J Mod Nurs. 2017;23:628–632. [Google Scholar]
- 11.Karaman Ö, Özkazanlı G, Orak MM, Mutlu S, Mutlu H, Çalışkan G, Karakuş O, Saygı B. Factors affecting postoperative mortality in patients older than 65 years undergoing surgery for hip fracture. Ulus Travma Acil Cerrahi Derg. 2015;21:44–50. doi: 10.5505/tjtes.2015.02582. [DOI] [PubMed] [Google Scholar]
- 12.Sun L, Mi Y, Li XM. Causes of hypoglycemia after gastrointestinal polypectomy and intervention measures. Chin New Clin Med. 2019;12:218–220. [Google Scholar]
- 13.Development of an enhanced recovery after surgery (ERAS) approach for lumbar spinal fusion. J Neurosurg Spine. 2017;26:411–418. doi: 10.3171/2016.9.SPINE16375. [DOI] [PubMed] [Google Scholar]
- 14.Amer MA, Smith MD, Herbison GP, Plank LD, McCall JL. Network meta-analysis of the effect of preoperative carbohydrate loading on recovery after elective surgery. Br J Surg. 2017;104:187. doi: 10.1002/bjs.10408. [DOI] [PubMed] [Google Scholar]
- 15.Hassan C, East J, Radaelli F, Spada C, Benamouzig R, Bisschops R, Bretthauer M, Dekker E, Dinis-Ribeiro M, Ferlitsch M, Fuccio L, Awadie H, Gralnek I, Jover R, Kaminski MF, Pellisé M, Triantafyllou K, Vanella G, Mangas-Sanjuan C, Frazzoni L, Van Hooft JE, Dumonceau JM. Bowel preparation colomoscopy: european society of gastrointestinal endoscopy (ESGE) guideline-update 2019. Endoscopy. 2019;51:775–794. doi: 10.1055/a-0959-0505. [DOI] [PubMed] [Google Scholar]
- 16.Reibaldi M, Fallico M, Longo A, Avitabile T, Astuto M, Murabito P, Minardi C, Bonfiglio V, Boscia F, Furino C, Rejdak R, Nowomiejska K, Toro M, Cennamo G, Cillino S, Rinaldi M, Fiore T, Cagini C, Russo A. Efficacy of three different prophylactic treatments for postoperative nausea and vomiting after vitrectomy: a randomized clinical trial. J Clin Med. 2019;8:391–312. doi: 10.3390/jcm8030391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perlas A, Mitsakakis N, Liu L, Cino M, Haldipur N, Davis L, Cubillos J, Chan V. Validation of a mathematucal model for ultrasound assessment of gastric volume by gastroscoic examination. Anesth Analg. 2013;116:357–363. doi: 10.1213/ANE.0b013e318274fc19. [DOI] [PubMed] [Google Scholar]
- 18.Ma K, Wu XX, Chen YQ, Yuan H. Effect of multimodal intervention on postoperative nausea and vomiting in patients undergoing gynecological laparoscopy. J Int Med Res. 2019;47:2026–2033. doi: 10.1177/0300060519835700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dupont G, Gavory J, Lambert P, Tsekouras N, Barbe N, Presles E, Bouvet L, Molliex S. Ultrasonographic gastric volume before unplanned surgery. Anaesthesia. 2017;72:1112–1116. doi: 10.1111/anae.13963. [DOI] [PubMed] [Google Scholar]
- 20.Kruisselbrink R, Arzola C, Endersby R, Tse C, Chan V, Perlas A. Intra- and interrater reliability of ultrasound assessment of gastric volume. Anesthesiology. 2014;121:46–51. doi: 10.1097/ALN.0000000000000193. [DOI] [PubMed] [Google Scholar]
- 21.Watson AT, Visram A. Children’s preoperative anxiety and postoperative behaviour. Paediatr Anaesth. 2003;13:188–204. doi: 10.1046/j.1460-9592.2003.00848.x. [DOI] [PubMed] [Google Scholar]
- 22.Sada F, Krasniqi A, Hamza A, Gecaj-Gashi A, Bicaj B, Kavaja F. A randomized trial of preoperative oral carbohydrates in abdominal surgery. BMC Anesthesiol. 2014;14:93. doi: 10.1186/1471-2253-14-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taniguchi H, Sasaki T, Fujita H. Preoperative management of surgical patients by “shortened fasting time”: a study on the amount of total body water by multi-frequency impedance method. Int J Med Sci. 2012;9:567–574. doi: 10.7150/ijms.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tosun B, Yava A, Açıkel C. Evaluating the effects of preoperative fasting and fluid limitation. Int J Nurs Pract. 2015;21:156–165. doi: 10.1111/ijn.12239. [DOI] [PubMed] [Google Scholar]
- 25.Yagmurdur H, Gunal S, Yildiz H, Gulec H, Topkaya C. The effects of carbohydrate-rich drink on perioperative discomfort, insulin response and arterial pressure in spinal aesthesia. J Res Med Sci. 2011;16:1483–1489. [PMC free article] [PubMed] [Google Scholar]
- 26.Aguilar-Nascimento JE, Dock-Nascimento DB. Reducing preoperative fasting time: a trend based on evidence. World J Gastrointest Surg. 2010;2:57–60. doi: 10.4240/wjgs.v2.i3.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lauwick SM, Kaba A, Maweja S, Hamoir EE, Joris JL. Effects of oral preoperative car bohydrate on early postoperative outcome after thyroidectomy. Acta Anaesthesiol Belg. 2009;60:67–73. [PubMed] [Google Scholar]
- 28.Yilmaz N, Cekmen N, Bilgin F, Erten E, Ozhan MO, Coşar A. Preoperative carbohydrate nutrition reduces postoperative nausea and vomiting compared to preoperative fasting. J Res Med Sci. 2013;18:827–832. [PMC free article] [PubMed] [Google Scholar]


