Abstract
To investigate the effects of vitamin D supplementation before pregnancy on the offspring’s cognitive function in mice with advanced maternal age (AMA). Thirty-two-week-old female mice were randomly allocated into two groups: the 32 W+VD group (receiving 10 IU/g body weight vitamin D3 dissolved in 200 μl corn oil per day), and the 32 W group (receiving 200 μl corn oil per day) for one week before mating with ten-week-old male mice. Another group of eight-week-old female mice was given 200 μl corn oil for the same period of time and set as normal childbearing age controls (8 W group). The pregnancy outcomes were recorded and the offspring at the age of 6 weeks were subjected to behavioral tests. Finally, the expression level and distribution of neural cell markers in the offspring’s hippocampus were detected by immunofluorescence. Mice with AMA had higher risk of adverse pregnancy outcome, smaller litter size, and offspring development. Vitamin D supplementation in mice with AMA promoted offspring development. AMA and maternal vitamin D supplementation before pregnancy did not change the anxiety and depression of young adult offspring. AMA impaired spatial learning and memory function of offspring while vitamin D supplementation before pregnancy rescued the impairment. AMA decreased NEFH (neurofilament protein) and MAP2 (microtubule binding protein) expression in offspring hippocampus, but vitamin D supplementation before pregnancy promoted NEFH and MAP2. Vitamin D supplementation before pregnancy can rescue the impaired learning and memory in offspring born to AMA mice. Our results highlight the significant impact of maternal vitamin D supplementation on the cognitive function of offspring.
Keywords: Vitamin D supplementation, advanced maternal age, offspring’s cognitive function, pregnancy outcome
Introduction
Delaying pregnancy is becoming common in China due to the changes in the perception of fertility and gradual liberalization of the two-child policy. Adverse pregnancy outcomes for both mother and child have been reported to be correlated to advanced maternal age (AMA), such as risks for miscarriage [1], pre-eclampsia [2], gestational diabetes mellitus [3], chromosomal abnormalities [4], stillbirth [5], fetal growth restriction [6], and preterm birth [7].
Among these impairments, neurocognitive outcomes of the offspring are of great importance and debate. Some randomized controlled trials have shown that AMA aggravates anxiety behavior [8] and decreases spontaneous motor activity [9]. Epidemiologic studies show that AMA reduces the offspring’s IQ [10] and increases the risk for their offspring to develop schizophrenia and autism [11]. Nevertheless, quite a few studies obtained negative or even opposite results. For instance, a study involving 33437 singleton children found that advanced maternal age was generally associated with better cognition scores [12]. Epidemiologic studies show offspring born to mothers with AMA have higher risk of depression and anxiety, while not many randomized controlled trials and clinical trials achieved the result that offspring born to mothers with AMA have more severe depression and anxiety symptoms [13-15].
Vitamin D, as steroid hormone, has various functions in the nervous system, such as neurotrophic factor production, neurotransmitter release regulation, calcium homeostasis maintenance, oxidative stress, and inflammatory processes. It is linked to cognitive development [16], fetal brain development, and postnatal neural function [17].
Maternal vitamin D deficiency has a close association with adverse maternal and neonatal outcomes including pre-eclampsia [18], gestational diabetes mellitus [19], postpartum depression [20], emergency cesarean section delivery [21], low birth weight babies [22], small for gestational age [23] and fetal intrauterine growth restriction [24].
Vitamin D deficiency is an urgent public health issue that afflicts more than one billion children and adults globally [25], especially prevalent in pregnant women with AMA [26].
Our previous work showed that mice born to AMA mothers had worse cognitive function when compared with the offspring born to mice with normal reproductive age [27]. We speculate that vitamin D supplementation before pregnancy for mice with AMA may rescue the cognitive impairment of their offspring.
Materials and methods
Animals and vitamin D diets
Mice (C57BL/6) were approved by the Hubei Provincial Center for Disease Control and Prevention (license#: SCXK (E) 2015-0018, and animal quality certificate#: 4200006000031051, 42000600030394) and in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011). Thirty-two-week-old female virgin mice were randomly allocated into 2 groups (32 W+VD group and 32 W group) receiving normal diet. Mice in 32 W+VD group received 10 IU/g body weight vitamin D3 dissolving in 200 μl corn oil per day for consecutive 7 days by gavage, while mice in the 32 W group received 200 μl corn oil. The eight-week-old virgin female mice received the same treatment as the 32 W group did. All animals were housed at 25°C±2°C, humidity of 60%±2% and a 12-h light-dark cycle, with free access to standard laboratory chow diet and water, and acclimated to the experimental environment for one week prior to vitamin D intervention. All female mice were mated with ten-week-old male mice at 2:1 ratio after 7 days’ treatment. Successful mating was confirmed by the presence of a vaginal plug, and the day was recorded as E0.5 (Embryonic day). Adverse pregnancy outcomes were defined as miscarriage, abortion, stillbirth, overdue delivery, and fetal absorption. After behavioral tests, young adult mice were sacrificed, then the hippocampus was dissected and immediately frozen and stored at -80°C for further analysis.
Behavioral tests of offspring
Offspring at the age of 6-7 weeks old born to the mice in the above 3 groups were subjected to behavioral tests. All the offspring went through 1-day habituation in the behavioral room. All tests were finished between 8:00 a.m. and 6:00 p.m. and all apparatus were cleaned with 75% ethanol before and after each testing. Testing sessions were digitally recorded and analyzed with a computerized video tracking system (Panlab, Harvard Bioscience, Spain).
Anxiety test of offspring
Offspring anxiety behavior was measured with the open field test (OF), and the elevated plus-maze (PM) test [28,29].
In the OF test, polyvinylchloride box (50 × 50 × 40 cm) with a floor evenly divided into 25 squares was adopted to test offspring. The outer 16 cells are defined as the outer region (marked blue), the inner 8 cells defined as the inner region (marked red), and the very center cell as the center region (marked green). Mice were placed in the same corner of the test box and each was allowed to move freely for 5 min after 1 minute of adaption. A mouse was considered to be in the center region when the center of its body was in the very center region. The percentage of duration of time spent in the very center region was used to quantify the anxiety level.
In the elevated PM test, the closed arms were enclosed by a black wall (20 cm in height). Each mouse was placed in the central area of the maze facing one of the open arms. The percentage of entries to the open arms (OE%) and the percentage of time spent in the open arms (OT%) were measured to assess the anxiety level.
Depression test of offspring
The tail suspension test (TS), the forced swimming test (FW) and the sucrose preference rest (SP) were performed to evaluate the offspring depression states [30-32].
In the TS test, mice were suspended upside down 50 cm above the floor by adhesive tape while the tape was approximately 1 cm from the tip of the tail. Mice were considered immobile only when they hung completely passive. Each trial lasted for 6 min, while the last 5 min was for recording the total duration of immobility to measure the depression states.
In the FW test, mice were forced to swim in a glass cylinder (30 cm in height, 11 cm in diameter) containing water at 25°C and 20 cm deep, in which the mouse was forced to swim constantly or be immobile. Mice were considered immobile when they hung in an upright position and made only minimal movements to keep their heads above water. Each trial was tracked by a computerized video tracking system and lasted for 6 min, while the percentage of immobility duration in the last 5 min was measured to evaluate the depression state.
In the SP test, each animal was exposed to 2 bottles after a 24-h period of water deprivation, one filled with tap water and the other filled with tap water containing sucrose solution (1%). Water consumption was measured by weighing the bottle before and after the test. Sucrose preference (percentage) was calculated by the following formula: sucrose solution intake/total intake, while total intake = sucrose solution intake + tap water intake, as previously described. The positions of the 2 bottles were varied randomly and no other differences existed in the 2 bottles.
Cognitive function of offspring
The passive avoidance (PA) test, novel object recognition (NOR) test and Morris water maze (MWM) test were conducted to assess the offspring cognitive function.
The PA test was developed by Crawley [33] and widely used to assess memory. A polyvinylchloride box (29 × 14 × 30 cm) was divided into dark and the light compartments while 1 cm thick plate was in the middle to test the offspring. The dark compartment was covered with a dark lid and the light one was open illuminated with a 60 W light bulb placed 40 cm above the upper edge of the box. Stainless steel grids were placed on the floor of the dark compartment to produce foot shock. The test was divided into the following steps.
Adaptation stage: The mice were put into the box with their back to the hole and moved freely for 5 minutes. The mice with a latency greater than 180 s were removed.
Training stage: In this stage, the mice were put in the light room for 1 min.
Memory test: 24 hours after the end of training, the mice were put in the light room again. The number of mistakes made in 5 minutes and the latency (the time entering the dark room for the first time) in 2 days were recorded to measure the memory.
Memory extinction test: After 2 days of memory test, the mice were put in the light room again. The latency and the number of mistakes in 5 minutes were also recorded.
The NOR test was adopted to assess the memory [34]. The size of object A and object B were similar to mice, while the size of object C was similar to mice but the shape and color of it were completely different from object A and object B. The procedure of the test is explained:
The adaptation period: The mice were placed in the device box to move freely for 10 minutes to eliminate the fear and unfamiliarity and avoid interference with the later memory.
The familiarity period: Object A and object B were placed in a symmetrical position on both sides of the box which were 10 cm away from the wall. Then the mice were put into the device box. Each mouse was allowed for 5 minutes to be familiar with object A and object B.
The test period: Object A or object B was replaced by object C, and the mice were put into the device box. The time until contacting the new and old objects in 5 minutes was recorded by the computerized video tracking system. The effective contact was defined as directly touching or a distance of less than 2 cm between the nose of the mouse and object. Finally, the cognition index of mice was calculated as: time spent exploring the new objects/time spent for both of the new and the old objects * 100%.
The MWM test is widely used to evaluate hippocampus-dependent spatial learning and memory [35]. The maze apparatus is an open circular pool (122 cm in diameter) filled with water (22±1°C) added with white nontoxic polypropylene pellets to be opaque. The pool was located in the center of an experimental room with multiple extra-maze visual geometric cues hanging on the wall. It is virtually divided into four equal quadrants by two principal axes with the computerized video tracking system, while the end of each line demarcates four cardinal points: North (N), East (E), South (S), and West (W).
The test includes two phases, the spatial learning phase and the memory phase. For the spatial learning phase, a hidden platform (8 cm in diameter) was submerged 1.2 cm beneath the water surface and placed in a fixed location in the center of SE quadrants. Mice were released into the pool from one of the four starting positions (N, W, NE, and SW) and the releasing order changed pseudorandomly between the consecutive 4 days. The mice were trained for 4 days with 4 trials per day and tested at the fifth day. A trial was considered finished when the animal found the escape platform or the latency was beyond 60 seconds. Then the mice failed to find the hidden platform in 60 seconds were picked up again and placed on the platform for 15 more seconds at the end of each trial to be familiar with the platform. Mice were dried after each trial.
Escape latency (time reach to the platform for the first time), number of platform crossing, swim distance and swim speed were recorded to measure the spatial learning and memory.
Q-PCR analysis
We used Trizol (Life, USA) to extract total RNA of hippocampal tissue of offspring at 8 Weeks in each group. q-PCR was performed in the StepOne™ Real-Time PCR System (ABI, Singapore) using SYBR® Premix Ex Taq II (TaKaRa, Japan) and the gene-specific primers. β-actin was employed as an endogenous control. The relative expression of RNA was calculated using 2-ΔΔCt method. Primer sequences used in this experiment are shown in Table 1.
Table 1.
Primer sequences used in this experiment
| Gene | Forward primer | Reverse primer |
|---|---|---|
| β-actin | 5’GGCTGTATTCCCCTCCATCG3’ | 5’CCAGTTGGTAACAATGCCATGT3’ |
| MAP2 | 5’AGGAAGCCCAACACAAGGAC3’ | 5’TTTGTTCTGAGGCTGGCGAT3’ |
| NEFH | 5’GGCCAAGACCCCCGTC3’ | 5’GGCTTCCGGAGACTTCACAT3’ |
| NeuN | 5’CCTGGGAACCCATATGCCAAT3’ | 5’GTGGGGTAGGGGAAACTGGT3’ |
MAP2: microtubule binding protein; NEFH: neurofilament protein; NeuN: Neuron specific protein.
Immunofluorescence
Immunofluorescence was also performed on hippocampal tissues of offspring at 8 Weeks in each group and carried out by Servicebio company. The specific operations are as follows: first, the paraffin sections were deparaffinized. Subsequently, antigen retrieval was performed. Then, the primary antibody incubation and the secondary antibody incubation were performed. Next, the nuclei was counterstained with DAPI. Finally, pictures were taken after mounting.
Statistical analysis
Data are shown as the mean ± SD or n/N. Two independent sample t-tests and chi-square tests were conducted to evaluate the maternal outcomes and neonatal outcomes. In behavioral tests, escape latency of the MWM and latency of the PA were analyzed by one-way repeated-measure ANOVA while the other data were analyzed by two independent sample t-tests. Comparing 32 W group with 8 W group was to measure the effect of maternal age, while comparing 32 W+VD group with 32 W group was to explore the effects of vitamin D intervention before pregnancy for mice with AMA. Values of P<0.05 were considered significant. All analyses were conducted in SPSS 19.0.
Results
Maternal vitamin D supplementation before pregnancy improved pregnancy outcome
To explore AMA and vitamin D supplementation influence on maternal reproductive ability, we measured the duration from mating to pregnancy and litter size. Body weight at Day 2, Day 24, body length at Day 24 and gestational weight gain at E18.5 were evaluated to assess offspring development.
Mice with AMA had a higher risk of adverse pregnancy outcome and lower gestational weight gain at E18.5. Offspring born to AMA mice had smaller litter size and slower growth rate. Nevertheless, the difference in the duration from mating to pregnancy between 8 W group and 32 W group did not reach significance (Table 2).
Table 2.
Pregnancy outcomes of 8 W group and 32 W group
| 8 W group | 32 W group | t/χ2 | P | |
|---|---|---|---|---|
| Maternal outcome | ||||
| Adverse pregnancy outcomes (n/N, %) | 4/19, 21.1 | 11/19, 57.9 | 5.397 | 0.020 |
| Duration from mating to pregnancy (d) | 5.5±4.7 | 8.6±7.3 | 1.472 | 0.1507 |
| Gestational weight gain at E18.5 (g) | 17±2.5 | 12.3±2.3 | 5.705 | <0.001 |
| Neonatal outcome | ||||
| Small size (n) | 8.16±1.30 | 4.8±2.83 | 4.356 | <0.001 |
| Body weight at Day 2 (g) | 1.4±0.12 | 1.3±0.11 | 3.391 | 0.0012 |
| Body weight at Day 24 (g) | 9.7±0.23 | 7.8±0.39 | 25.49 | <0.001 |
| Body length at Day 24 (cm) | 6.9±0.21 | 6.6±0.10 | 6.594 | <0.001 |
Compared with the 32 W group, offspring born to mice with vitamin D supplementation before pregnancy had better development, while no other significant difference was found (Table 3). In summary, mice with AMA had worse pregnancy outcome, while vitamin D supplementation before pregnancy provided certain benefits.
Table 3.
Pregnancy outcomes of 32 W+VD group and 32 W group
| 32 W+VD group | 32 W group | t/χ2 | P | |
|---|---|---|---|---|
| Maternal outcome | ||||
| Adverse pregnancy outcomes (n/N, %) | 12/24, 50.0 | 11/19, 57.9 | 0.266 | 0.606 |
| Duration from mating to pregnancy (d) | 8.9±6.3 | 8.6±7.3 | 0.1260 | 0.9005 |
| Gestational weight gain at E18.5 (g) | 12.3±3.5 | 12.3±2.3 | <0.001 | >0.999 |
| Neonatal outcome | ||||
| Litter size (n) | 6.59±2.12 | 4.8±2.83 | 1.966 | 0.0583 |
| Body weight at Day 2 (g) | 1.4±0.12 | 1.3±0.11 | 3.009 | 0.0042 |
| Body weight at Day 24 (g) | 8.9±0.92 | 7.8±0.39 | 5.393 | <0.001 |
| Body length at Day 24 (cm) | 6.7±0.3 | 6.6±0.10 | 1.549 | 0.1282 |
Maternal vitamin D supplementation before pregnancy did not change the anxiety and depression in young adult offspring
We first analyzed the offspring’s anxiety status by the percentage of time spent in the very central region in the OF test, so as OT% and OE% in the elevated PM test. The slight difference in the percentage of time spent in the very central region (8 W vs 32 W: 0.556±0.375 vs 0.453±0.321; P=0.555; Figure 1A), OT% (8 W vs 32 W: 0.897±1.031 vs 1.029±1.197; P=0.818; Figure 1B) and OE% (8 W vs 32 W: 8.364±9.626 vs 10.68±10.15; P=0.644; Figure 1C) were observed between offspring born to mice in 8 W group and offspring born to mice in 32 W group, but did not reach statistical significance.
Figure 1.
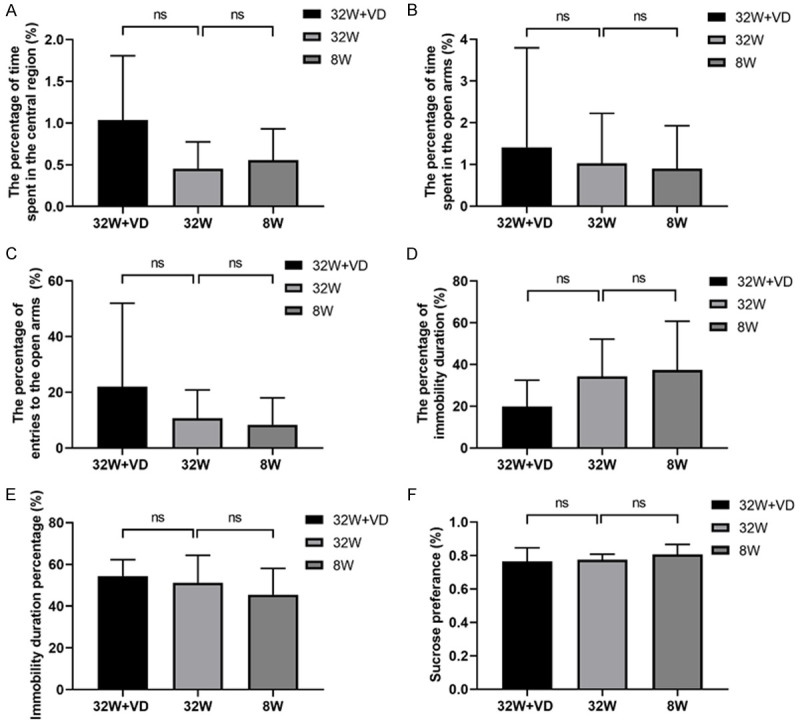
Vitamin D supplementation before pregnancy and advanced maternal age (AMA) did not change the anxiety and depression of young adult offspring. A. The percentage of time spent in the central region in the Open Field test. B. OT% in the elevated Plus Maze test. C. OE% in the elevated Plus Maze test. D. The percentage of immobility duration in the Tail Suspension test. E. Immobility duration percentage in the Forced Swimming test. F. Sucrose preference in the Sucrose Preference test. No significant difference was found in young adult offspring’s anxiety and depression states between offspring in the 32 W group and offspring in 8 W group, or in that between the 32 W+VD group and 32 W group. OT%: percentage of time spent in the open arms; OE%: percentage of entries to the open arms. All data are shown as mean ± SD, ns P>0.05.
Vitamin D supplementation before pregnancy did exert an effect on the offspring’s anxiety level in the OF test (32 W+VD vs 32 W, 1.036±0.770 vs 0.453±0.321; P=0.099; Figure 1A), though not reaching a significant level, but not in PM tests assessed by OT% (32 W+VD vs 32 W, 1.411±2.385 vs 1.029±1.197; P=0.695; Figure 1B) and OE% (32 W+VD vs 32 W, 21.96±30.01 vs 10.68±10.15; P=0.334; Figure 1C).
Offspring depression states were then evaluated by the percentage of immobility duration in both the TS and the FS tests. Similar to the anxiety tests, advanced maternal age did not exhibit a significant effect on the immobility duration percentage of the offspring (TS test, 8 W vs 32 W: 37.29±23.46 vs 34.28±17.87; P=0.772; Figure 1D; FS test, 8 W vs 32 W: 45.52±12.56 vs 51.21±13.17; P=0.378; Figure 1E). In addition, sucrose preference also confirmed that the offspring’s depression level (8 W vs 32 W: 0.8071±0.0588 vs 0.7757±0.03207; P=0.238; Figure 1F) was not affected by AMA.
Vitamin D intervention decreased the percentage of immobility duration in the TS test (32 W+VD vs 32 W, 19.89±12.59 vs 34.28±17.87; P=0.099; Figure 1D), though not reaching a significant level. However, this did not change the immobility duration percentage in the FS test (32 W+VD vs 32 W, 54.46±7.815 vs 51.21±13.17; P=0.598; Figure 1E). Vitamin D intervention did not exert any effect on the sucrose preference test either (32 W+VD vs 32 W, 0.7657±0.08018 vs 0.7757±0.03207; P=0.765; Figure 1F).
Maternal vitamin D supplementation before pregnancy rescued the spatial learning and memory of offspring
Spatial learning and memory function were measured with latency and the numbers of mistakes in the PA test, cognitive index in the NOR test, and latency and number of platforms crossing in the MWM test. Swim speed in the MWM test was evaluated to eliminate all bias due to visual and motor deficits.
Compared with offspring born to normal reproductive age females, offspring born to AMA had shorter latency (F=2383.344, P<0.001; Figure 2A) and made more mistakes (8 W vs 32 W: 1.045±0.7854 vs 2.045±0.7854; P<0.001; Figure 2B) in the PA test. In the NOR test, cognitive index (8 W vs 32 W: 0.683±0.118 vs 0.561±0.070; P=0.013; Figure 2C) in the offspring born to AMA mice was lower than that in the offspring born to mice at normal reproductive age. In the MWM test, no significant difference was found in swim speed (8 W vs 32 W: 21.75±4.675 vs 23.34±15.14; P=0.506; Figure 3A), but offspring born to AMA mice had shorter swim distance (8 W vs 32 W: 491.6±183.1 vs 407.5±167.1; P=0.017; Figure 3B), longer escape latency (F=4.682, P=0.011; Figure 3C), and lower times of platforms crossing (8 W vs 32 W: 5.164±2.478 vs 3.250±2.232; P<0.0001; Figure 3D). Besides, we observed a rise in the escape latency on the third day, indicating worse memory of mice in the 32 W group.
Figure 2.
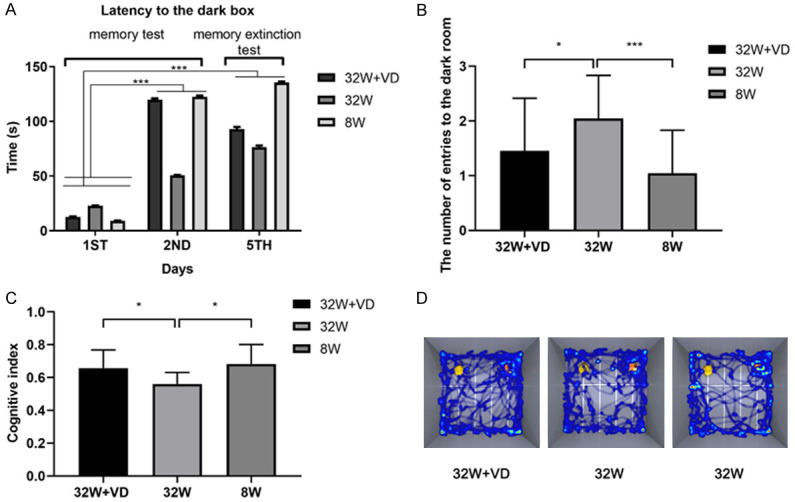
Maternal vitamin D supplementation before pregnancy rescued the spatial learning and memory impairment of offspring of AMA mice. A. Offspring in the 32 W+VD and 8 W groups had longer latency than offspring in 32 W group in the Passive Avoidance test. B. Offspring in 32 W+VD and 8 W group had fewer entries to the dark room than offspring in 32 W group on the fifth day in the Passive Avoidance test. C. Offspring in 32 W+VD and 8 W group had elevated cognitive index compared to offspring in 32 W group in the Novel Object Recognition test. D. Representative track maps in the Novel Object Recognition test showed that offspring in 32 W+VD and 8 W group had a clearer way to object A and object B than offspring in 32 W group. All data are shown as mean ± SD, *P<0.05, ***P<0.001.
Figure 3.
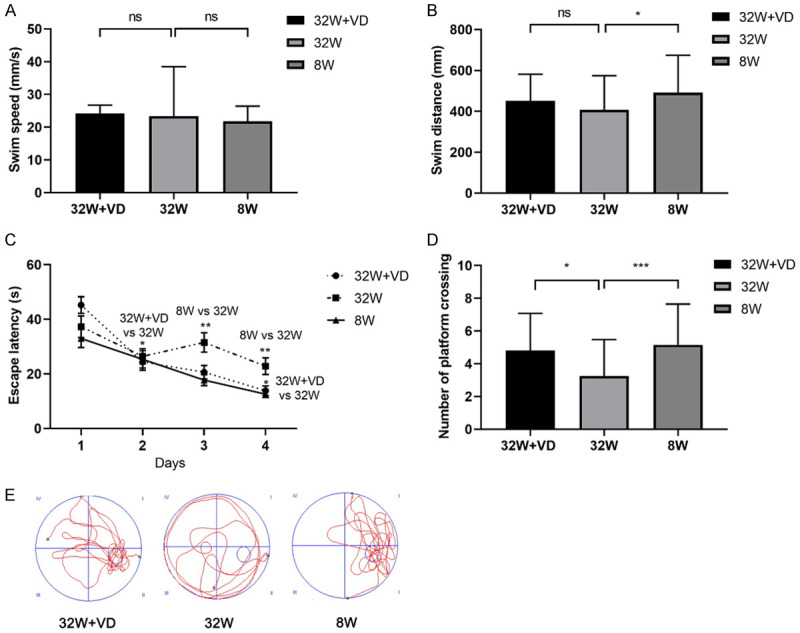
Maternal vitamin D supplementation before pregnancy rescued the spatial learning and memory impairment of offspring of AMA mice. A. No significant difference was found in swim speed. B. Offspring in the 8 W group had longer swim distance than offspring in 32 W group, but no significant difference was found in swim distance between offspring in the 32 W+VD group and offspring in 32 W group. C. Escape latency by one-way repeated-measure ANOVA in offspring in the 8 W group was longer than that of offspring in 32 W group, but we did not observe a significant difference between offspring in the 8 W group and offspring in the 32 W group. D. Offspring in the 32 W+VD group and 8 W group had a longer time of platform crossing than offspring in 32 W group. E. Representative track maps in the MWM test showed that offspring in 32 W+VD and 8 W group had a clearer way to the hidden platform than offspring in 32 W group. All data are shown as mean ± SD, ns P>0.05, *P<0.05, **P<0.01, ***P<0.001.
In comparison with offspring born to AMA mice, offspring born to AMA mice with vitamin D supplementation before pregnancy had longer latency (F=919.632, P<0.001; Figure 2A) and fewer mistakes (32 W+VD vs 32 W: 1.455±0.9625 vs 2.045±0.7854; P=0.031; Figure 2B) in the PA test. Cognitive index (32 W+VD vs 32 W: 0.658±0.110 vs 0.561±0.070; P=0.032; Figure 2C) in the offspring born to AMA mice with vitamin D supplementation before pregnancy was lower than that in the offspring born to AMA mice. In the MWM test, we observed no significant difference in swim speed (32 W+VD vs 32 W: 24.24±2.508 vs 23.34±15.14; P=0.700; Figure 3A), swim distance (32 W+VD vs 32 W: 451.7±129.8 vs 407.5±167.1; P=0.212; Figure 3B) and escape latency (F=2.007, P=0.161; Figure 3C), but offspring born to AMA mice with vitamin D supplementation before pregnancy had longer times of platforms crossing (32 W+VD vs 32 W: 4.821±2.262 vs 3.250±2.232; P=0.006; Figure 3D).
In summary, PA, NOR and MWM tests demonstrated that offspring born to AMA mice had worse spatial learning and memory, while maternal vitamin D supplementation before pregnancy rescued the impairment.
Maternal vitamin D supplementation before pregnancy promoted neural cell growth in the hippocampus of offspring
NEFH [36], MAP2 [37], and NeuN [38] are related with neural cell growth, so immunofluorescence and q-PCR analysis were conducted to evaluate expressions of neural cell markers in offspring hippocampus.
In comparison with offspring born to mice with normal reproductive age, offspring born to AMA mice had significantly lower expression in NEFH (8 W vs 32 W: 1.60±0.95 vs 1.00; P=0.0004; Figure 4A, 4B) and MAP2 (8 W vs 32 W: 2.53±0.12 vs 1.00; P<0.0001; Figure 4A, 4D), but not in NeuN (8 W vs 32 W: 1.00±0.03 vs 1.00; P=0.7953; Figure 4A, 4C) in the hippocampi of offspring.
Figure 4.
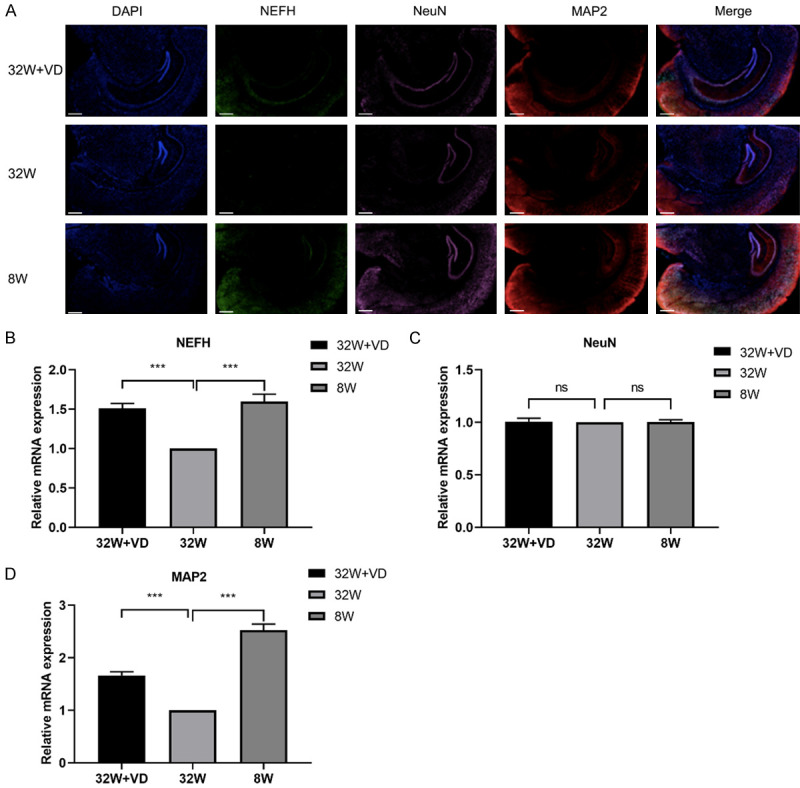
Maternal vitamin D supplementation before pregnancy promoted neural cell growth, while AMA inhibited neural cell growth in offspring’s hippocampus. A. Offspring in the 32 W+VD group and 8 W group had elevated hippocampal NEFH, NeuN, and MAP2 protein expression compared to offspring in 32 W group. Bar = 500 μm. B. Offspring in the 32 W+VD group and 8 W group had elevated hippocampal NEFH mRNA expression compared to offspring in 32 W group. C. No significant difference in NeuN mRNA expression was found between offspring hippocampi in the 32 W+VD group and 32 W group, or between the 8 W group and 32 W group. D. Offspring in the 32 W+VD group and 8 W group had elevated hippocampus MAP2 mRNA expression compared to offspring in 32 W group. All data are shown as mean ± SD, ns P>0.05, ***P<0.001.
Compared with offspring born to AMA mice, offspring born to AMA mice with vitamin D supplementation before pregnancy had elevated expression of NEFH (32 W+VD vs 32 W: 1.51±0.62 vs 1.00; P=0.0001; Figure 4A, 4B) and MAP2 (32 W+VD vs 32 W: 1.67±0.07 vs 1.00; P<0.0001; Figure 4A, 4D), while they showed no significant difference in NeuN mRNA expression but in NeuN protein expression (32 W+VD vs 32 W: 1.00±0.02 vs 1.00; P=0.7376; Figure 4A, 4D).
Discussion
The current study found that AMA not only affected maternal reproduction ability, but also had an adverse effect on perinatal outcome and neonatal development, similar to previous research [39-41]. Nevertheless, vitamin D supplementation promoted the development of offspring but had no significant effect on maternal reproduction ability and adverse pregnancy outcome. This should be verified with more detailed information and a larger sample size.
Based on data from our study and others, no significant differences in duration from mating to pregnancy and litter size were observed. We did observe a trend suggesting that great intra-group differences contributed to the negative effect after detailed analyses. As for the offspring development, AMA impaired fetal growth and vitamin D supplementation improved infant growth, as is widely recognized by experts [42-44].
Depression and anxiety are proven to impair cognitive function [45,46]. To eliminate such a possible influence, we conducted related experiments on offspring mice, including OF test, elevated PM test, TS test, FW test, and SP test. Although we concluded that AMA and maternal vitamin D supplementation before pregnancy did not change the anxiety and depression of young adult offspring, we did observe a slight difference in the indicators although this was not significant.
Our results showed that AMA impaired offspring cognitive function while vitamin D supplementation during pregnancy rescued the impairment. No such experiments have been conducted in clinical trials and randomized controlled trials. Some prospective cohort studies conclude that children of mothers with vitamin D insufficiency are at increased risk of language impairment [47] and had lower cognitive function scores [48] compared to children of mothers without insufficiency, which is similar to our results. Although some studies got the negative result such as Gale et al. in the prospective longitudinal study with 9 years of follow-up, we consider this not convincing enough as no confounders were adjusted for in this study [49].
Emotions such as anxiety and depression are proved to be correlated with the amygdala [50,51], while memory and learning are related to the hippocampus [52]. For instance, the excitation-inhibition imbalance in the amygdala is considered to be an underlying pathophysiologic mechanism of anxiety, while Erbin (Erbb2-interacting protein) in parvalbumin neurons is critical to maintain the excitation-inhibition balance in the amygdala and reveals a novel pathologic mechanism for anxiety disorders [53]. Some studies showed that stress-induced GABAergic dysfunction in the nucleus accumbens contributed to the pathophysiology of depression [54]. Besides, Negr1 (neuronal growth regulator 1) controls adult hippocampal neurogenesis and affective behaviors [55]. Microglia, brain-resident immune cells, are involved in mouse cognitive impairment [56].
The present study has several limitations. The first is that maternal emotion and cognitive were not measured to eliminate a maternal effect on offspring emotion. Second, this study is not mechanistic, and this will be investigated in future experiments. The last limitation is the small sample size. We observed slight differences in some indicators, which did not reach significant level due to small sample size and large intra-group differences. Despite these limitations, our research has its strengths: the adoption of several behavioral tests to determine the anxiety and depression states and cognitive function of offspring. To our knowledge, no random controlled trials about vitamin D supplementation and offspring cognition have been published before.
The next step of our experiments was to explore the molecular mechanism of how maternal vitamin D supplementation rescues offspring’s cognitive function. To achieve this, we not only investigated the neuronal markers, relation between vitamin D receptor level and expression of its enzymes-Cyp27a1 and Cyp27b1, in the offspring hippocampus, but also the signaling pathway that regulates vitamin D metabolism.
By virtue of this article, we would like to emphasize the significance of maternal vitamin D supplementation before pregnancy. Since maternal vitamin D supplementation was correlated to development and cognitive function of offspring, supplementation of vitamin D should be considered before pregnancy.
Acknowledgements
Supported by Natural Science Fund of Hubei Province (2019CFB823).
Disclosure of conflict of interest
None.
References
- 1.Khalil A, Syngelaki A, Maiz N, Zinevich Y, Nicolaides KH. Maternal age and adverse pregnancy outcome: a cohort study. Ultrasound Obstet Gynecol. 2013;42:634–43. doi: 10.1002/uog.12494. [DOI] [PubMed] [Google Scholar]
- 2.Zhu Y, Lu S, Bian X, Wang H, Zhu B, Wang H, Xu Z, Xu L, Yan W, Zeng Y, Chen Z, Tang S, Shen G, Miao Z. A multicenter study of fetal chromosomal abnormalities in Chinese women of advanced maternal age. Taiwan J Obstet Gynecol. 2016;55:379–84. doi: 10.1016/j.tjog.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Laopaiboon M, Lumbiganon P, Intarut N, Mori R, Ganchimeg T, Vogel JP, Souza JP, Gülmezoglu AM. Advanced maternal age and pregnancy outcomes: a multicountry assessment. BJOG. 2014;121(Suppl 1):49–56. doi: 10.1111/1471-0528.12659. [DOI] [PubMed] [Google Scholar]
- 4.Attali E, Yogev Y. The impact of advanced maternal age on pregnancy outcome. Best Pract Res Clin Obstet Gynaecol. 2021;70:2–9. doi: 10.1016/j.bpobgyn.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Waldenström U, Cnattingius S, Vixner L, Norman M. Advanced maternal age increases the risk of very preterm birth, irrespective of parity: a population-based register study. BJOG. 2017;124:1235–1244. doi: 10.1111/1471-0528.14368. [DOI] [PubMed] [Google Scholar]
- 6.Frick AP. Advanced maternal age and adverse pregnancy outcomes. Best Pract Res Clin Obstet Gynaecol. 2021;70:92–100. doi: 10.1016/j.bpobgyn.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Sohn K. The trend in the relationship of advanced maternal age to preterm birth and low birthweight. Eur J Contracept Reprod Health Care. 2017;22:363–368. doi: 10.1080/13625187.2017.1372569. [DOI] [PubMed] [Google Scholar]
- 8.Sampino S, Stankiewicz AM, Zacchini F, Goscik J, Szostak A, Swiergiel AH, Drago G, Modlinski JA, Ptak GE. Pregnancy at advanced maternal age affects behavior and hippocampal gene expression in mouse offspring. J Gerontol A Biol Sci Med Sci. 2017;72:1465–1473. doi: 10.1093/gerona/glx016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tarín JJ, Gómez-Piquer V, Manzanedo C, Miñarro J, Hermenegildo C, Cano A. Long-term effects of delayed motherhood in mice on postnatal development and behavioural traits of offspring. Hum Reprod. 2003;18:1580–7. doi: 10.1093/humrep/deg349. [DOI] [PubMed] [Google Scholar]
- 10.Myrskylä M, Silventoinen K, Tynelius P, Rasmussen F. Is later better or worse? Association of advanced parental age with offspring cognitive ability among half a million young Swedish men. Am J Epidemiol. 2013;177:649–55. doi: 10.1093/aje/kws237. [DOI] [PubMed] [Google Scholar]
- 11.Weiser M, Reichenberg A, Werbeloff N, Kleinhaus K, Lubin G, Shmushkevitch M, Caspi A, Malaspina D, Davidson M. Advanced parental age at birth is associated with poorer social functioning in adolescent males: shedding light on a core symptom of schizophrenia and autism. Schizophr Bull. 2008;34:1042–6. doi: 10.1093/schbul/sbn109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saha S, Barnett AG, Foldi C, Burne TH, Eyles DW, Buka SL, McGrath JJ. Advanced paternal age is associated with impaired neurocognitive outcomes during infancy and childhood. PLoS Med. 2009;6:e40. doi: 10.1371/journal.pmed.1000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tearne JE. Older maternal age and child behavioral and cognitive outcomes: a review of the literature. Fertil Steril. 2015;103:1381–91. doi: 10.1016/j.fertnstert.2015.04.027. [DOI] [PubMed] [Google Scholar]
- 14.Tearne JE, Robinson M, Jacoby P, Allen KL, Cunningham NK, Li J, McLean NJ. Older maternal age is associated with depression, anxiety, and stress symptoms in young adult female offspring. J Abnorm Psychol. 2016;125:1–10. doi: 10.1037/abn0000119. [DOI] [PubMed] [Google Scholar]
- 15.Lerch S, Brandwein C, Dormann C, Gass P, Chourbaji S. Mice age - does the age of the mother predict offspring behaviour? Physiol Behav. 2015;147:157–62. doi: 10.1016/j.physbeh.2015.04.041. [DOI] [PubMed] [Google Scholar]
- 16.Lisi G, Ribolsi M, Siracusano A, Niolu C. Maternal vitamin D and its role in determining fetal origins of mental health. Curr Pharm Des. 2020;26:2497–2509. doi: 10.2174/1381612826666200506093858. [DOI] [PubMed] [Google Scholar]
- 17.Landel V, Annweiler C, Millet P, Morello M, Féron F. Vitamin D, cognition and Alzheimer’s disease: the therapeutic benefit is in the D-tails. J Alzheimers Dis. 2016;53:419–44. doi: 10.3233/JAD-150943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serrano NC, Guío E, Quintero-Lesmes DC, Becerra-Bayona S, Luna-Gonzalez ML, Herrera VM, Prada CE. Vitamin D deficiency and pre-eclampsia in Colombia: PREVitD study. Pregnancy Hypertens. 2018;14:240–244. doi: 10.1016/j.preghy.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Gong Y, Xue H, Xiong J, Cheng G. Vitamin D and gestational diabetes mellitus: a systematic review based on data free of Hawthorne effect. BJOG. 2018;125:784–793. doi: 10.1111/1471-0528.15060. [DOI] [PubMed] [Google Scholar]
- 20.Aghajafari F, Letourneau N, Mahinpey N, Cosic N, Giesbrecht G. Vitamin D deficiency and antenatal and postpartum depression: a systematic review. Nutrients. 2018;10:478. doi: 10.3390/nu10040478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Savvidou MD, Makgoba M, Castro PT, Akolekar R, Nicolaides KH. First-trimester maternal serum vitamin D and mode of delivery. Br J Nutr. 2012;108:1972–5. doi: 10.1017/S0007114512000207. [DOI] [PubMed] [Google Scholar]
- 22.Fang K, He Y, Mu M, Liu K. Maternal vitamin D deficiency during pregnancy and low birth weight: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2021;34:1167–1173. doi: 10.1080/14767058.2019.1623780. [DOI] [PubMed] [Google Scholar]
- 23.Wang H, Xiao Y, Zhang L, Gao Q. Maternal early pregnancy vitamin D status in relation to low birth weight and small-for-gestational-age offspring. J Steroid Biochem Mol Biol. 2018;175:146–150. doi: 10.1016/j.jsbmb.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 24.Chen YH, Liu ZB, Ma L, Zhang ZC, Fu L, Yu Z, Chen W, Song YP, Wang P, Wang H, Xu X. Gestational vitamin D deficiency causes placental insufficiency and fetal intrauterine growth restriction partially through inducing placental inflammation. J Steroid Biochem Mol Biol. 2020;203:105733. doi: 10.1016/j.jsbmb.2020.105733. [DOI] [PubMed] [Google Scholar]
- 25.Holick MF. The vitamin D deficiency pandemic: approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord. 2017;18:153–165. doi: 10.1007/s11154-017-9424-1. [DOI] [PubMed] [Google Scholar]
- 26.Roth DE, Abrams SA, Aloia J, Bergeron G, Bourassa MW, Brown KH, Calvo MS, Cashman KD, Combs G, De-Regil LM, Jefferds ME, Jones KS, Kapner H, Martineau AR, Neufeld LM, Schleicher RL, Thacher TD, Whiting SJ. Global prevalence and disease burden of vitamin D deficiency: a roadmap for action in low- and middle-income countries. Ann N Y Acad Sci. 2018;1430:44–79. doi: 10.1111/nyas.13968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mao WJ, Wu ZY, Yang ZH, Xu YW, Wang SQ. Advanced maternal age impairs spatial learning capacity in young adult mouse offspring. Am J Transl Res. 2018;10:975–988. [PMC free article] [PubMed] [Google Scholar]
- 28.Malek FA, Möritz KU, Fanghänel J. Effects of a single inhalative exposure to formaldehyde on the open field behavior of mice. Int J Hyg Environ Health. 2004;207:151–8. doi: 10.1078/1438-4639-00268. [DOI] [PubMed] [Google Scholar]
- 29.Rodgers RJ, Dalvi A. Anxiety, defence and the elevated plus-maze. Neurosci Biobehav Rev. 1997;21:801–10. doi: 10.1016/s0149-7634(96)00058-9. [DOI] [PubMed] [Google Scholar]
- 30.Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Slattery DA, Cryan JF. Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat Protoc. 2012;7:1009–14. doi: 10.1038/nprot.2012.044. [DOI] [PubMed] [Google Scholar]
- 32.Liu MY, Yin CY, Zhu LJ, Zhu XH, Xu C, Luo CX, Chen H, Zhu DY, Zhou QG. Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat Protoc. 2018;13:1686–1698. doi: 10.1038/s41596-018-0011-z. [DOI] [PubMed] [Google Scholar]
- 33.Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 1980;13:167–70. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- 34.Zhang R, Xue G, Wang S, Zhang L, Shi C, Xie X. Novel object recognition as a facile behavior test for evaluating drug effects in AβPP/PS1 Alzheimer’s disease mouse model. J Alzheimers Dis. 2012;31:801–12. doi: 10.3233/JAD-2012-120151. [DOI] [PubMed] [Google Scholar]
- 35.Dinel AL, Lucas C, Guillemet D, Layé S, Pallet V, Joffre C. Chronic supplementation with a mix of salvia officinalis and salvia lavandulaefolia improves morris water maze learning in normal adult C57Bl/6J mice. Nutrients. 2020;12:1777. doi: 10.3390/nu12061777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calmon MF, Jeschke J, Zhang W, Dhir M, Siebenkäs C, Herrera A, Tsai HC, O’Hagan HM, Pappou EP, Hooker CM, Fu T, Schuebel KE, Gabrielson E, Rahal P, Herman JG, Baylin SB, Ahuja N. Epigenetic silencing of neurofilament genes promotes an aggressive phenotype in breast cancer. Epigenetics. 2015;10:622–32. doi: 10.1080/15592294.2015.1050173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He M, Ding Y, Chu C, Tang J, Xiao Q, Luo ZG. Autophagy induction stabilizes microtubules and promotes axon regeneration after spinal cord injury. Proc Natl Acad Sci U S A. 2016;113:11324–11329. doi: 10.1073/pnas.1611282113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weyer A, Schilling K. Developmental and cell type-specific expression of the neuronal marker NeuN in the murine cerebellum. J Neurosci Res. 2003;73:400–9. doi: 10.1002/jnr.10655. [DOI] [PubMed] [Google Scholar]
- 39.Mehari MA, Maeruf H, Robles CC, Woldemariam S, Adhena T, Mulugeta M, Haftu A, Hagose H, Kumsa H. Advanced maternal age pregnancy and its adverse obstetrical and perinatal outcomes in Ayder comprehensive specialized hospital, Northern Ethiopia, 2017: a comparative cross-sectional study. BMC Pregnancy Childbirth. 2020;20:60. doi: 10.1186/s12884-020-2740-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Care AS, Bourque SL, Morton JS, Hjartarson EP, Davidge ST. Effect of advanced maternal age on pregnancy outcomes and vascular function in the rat. Hypertension. 2015;65:1324–30. doi: 10.1161/HYPERTENSIONAHA.115.05167. [DOI] [PubMed] [Google Scholar]
- 41.Patel R, Moffatt JD, Mourmoura E, Demaison L, Seed PT, Poston L, Tribe RM. Effect of reproductive ageing on pregnant mouse uterus and cervix. J Physiol. 2017;595:2065–2084. doi: 10.1113/JP273350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lean SC, Derricott H, Jones RL, Heazell AEP. Advanced maternal age and adverse pregnancy outcomes: a systematic review and meta-analysis. PLoS One. 2017;12:e0186287. doi: 10.1371/journal.pone.0186287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fall CH, Sachdev HS, Osmond C, Restrepo-Mendez MC, Victora C, Martorell R, Stein AD, Sinha S, Tandon N, Adair L, Bas I, Norris S, Richter LM. Association between maternal age at childbirth and child and adult outcomes in the offspring: a prospective study in five low-income and middle-income countries (COHORTS collaboration) Lancet Glob Health. 2015;3:e366–77. doi: 10.1016/S2214-109X(15)00038-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bi WG, Nuyt AM, Weiler H, Leduc L, Santamaria C, Wei SQ. Association between vitamin D supplementation during pregnancy and offspring growth, morbidity, and mortality: a systematic review and meta-analysis. JAMA Pediatr. 2018;172:635–645. doi: 10.1001/jamapediatrics.2018.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gulpers BJA, Oude Voshaar RC, van Boxtel MPJ, Verhey FRJ, Köhler S. Anxiety as a risk factor for cognitive decline: a 12-year follow-up cohort study. Am J Geriatr Psychiatry. 2019;27:42–52. doi: 10.1016/j.jagp.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 46.Halahakoon DC, Lewis G, Roiser JP. Cognitive impairment and depression-cause, consequence, or coincidence? JAMA Psychiatry. 2019;76:239–240. doi: 10.1001/jamapsychiatry.2018.3631. [DOI] [PubMed] [Google Scholar]
- 47.Whitehouse AJ, Holt BJ, Serralha M, Holt PG, Kusel MM, Hart PH. Maternal serum vitamin D levels during pregnancy and offspring neurocognitive development. Pediatrics. 2012;129:485–93. doi: 10.1542/peds.2011-2644. [DOI] [PubMed] [Google Scholar]
- 48.Morales E, Guxens M, Llop S, Rodríguez-Bernal CL, Tardón A, Riaño I, Ibarluzea J, Lertxundi N, Espada M, Rodriguez A, Sunyer J. Circulating 25-hydroxyvitamin D3 in pregnancy and infant neuropsychological development. Pediatrics. 2012;130:e913–20. doi: 10.1542/peds.2011-3289. [DOI] [PubMed] [Google Scholar]
- 49.Gale CR, Robinson SM, Harvey NC, Javaid MK, Jiang B, Martyn CN, Godfrey KM, Cooper C. Maternal vitamin D status during pregnancy and child outcomes. Eur J Clin Nutr. 2008;62:68–77. doi: 10.1038/sj.ejcn.1602680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–87. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 51.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 52.Voss JL, Bridge DJ, Cohen NJ, Walker JA. A closer look at the hippocampus and memory. Trends Cogn Sci. 2017;21:577–588. doi: 10.1016/j.tics.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luo ZY, Huang L, Lin S, Yin YN, Jie W, Hu NY, Hu YY, Guan YF, Liu JH, You QL, Chen YH, Luo ZC, Zhang SR, Li XW, Yang JM, Tao YM, Mei L, Gao TM. Erbin in amygdala parvalbumin-positive neurons modulates anxiety-like behaviors. Biol Psychiatry. 2020;87:926–936. doi: 10.1016/j.biopsych.2019.10.021. [DOI] [PubMed] [Google Scholar]
- 54.Ma K, Zhang H, Wang S, Wang H, Wang Y, Liu J, Song X, Dong Z, Han X, Zhang Y, Li H, Rahaman A, Wang S, Baloch Z. The molecular mechanism underlying GABAergic dysfunction in nucleus accumbens of depression-like behaviours in mice. J Cell Mol Med. 2019;23:7021–7028. doi: 10.1111/jcmm.14596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Noh K, Lee H, Choi TY, Joo Y, Kim SJ, Kim H, Kim JY, Jahng JW, Lee S, Choi SY, Lee SJ. Negr1 controls adult hippocampal neurogenesis and affective behaviors. Mol Psychiatry. 2019;24:1189–1205. doi: 10.1038/s41380-018-0347-3. [DOI] [PubMed] [Google Scholar]
- 56.Pinto B, Morelli G, Rastogi M, Savardi A, Fumagalli A, Petretto A, Bartolucci M, Varea E, Catelani T, Contestabile A, Perlini LE, Cancedda L. Rescuing over-activated microglia restores cognitive performance in juvenile animals of the Dp(16) mouse model of down syndrome. Neuron. 2020;108:887–904. e12. doi: 10.1016/j.neuron.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


