Abstract
Objective: To explore the effect of miR-1307-5p which specifically inhibits transforming growth factor beta-induced gene (TGFBI) on the biologic behavior of osteoarthritis (OA) chondrocytes. Methods: We detected miR-1307-5p and TGFBI expression in the cartilage tissue specimens of OA patients and mice, respectively. RNA22 was applied to predict the target gene of miR-1307-5p, and we further verified the relationship by performing a dual luciferase reporter experiment. Enzyme-linked immunosorbent assay was used to measure the expression of matrix metalloproteinase inhibitor-1 (TIMP-1), interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) in the culture medium of mouse chondrocytes. Quantitative reverse transcription-polymerase chain reaction and western blot were used to measure the expression of Bax and Bcl-2. MTT method was applied to detect the proliferation activity of chondrocytes, while flow cytometry was implemented to detect the apoptosis of chondrocytes. Results: The expression of miR-1307-5p in cartilage tissue specimens of OA patients was up-regulated, while TGFBI expression was down-regulated. Compared with normal mice cartilage tissue specimens, the expression of miR-1307-5p in cartilage tissue specimens of OA mouse was increased, while TGFBI expression was decreased (both P<0.05). The results of the dual luciferase reporter experiment showed that TGFBI was a target gene of miR-1307-5p. In cell experiments, compared with the normal group, TIMP-1 and Bcl-2 expression, and cell proliferation activities in all model groups were decreased. IL-1β, IL-6, TNF-α, Bax expression, and cell apoptosis rates were increased (all P<0.05). Compared with the blank group, TIMP-1 and Bcl-2 expression, and cell proliferation activities in the miR-1307-5p inhibitor group and the TGFBI group were increased, while IL-1β, IL-6, TNF-α, and Bax expression, and cell apoptosis rates were decreased (all P<0.05). The changes in all indicators in the miR-1307-5p mimic group were opposite to those of the miR-1307-5p inhibitor group (all P<0.05). There were no significant differences concerning all indicators between the blank group and the NC group, and between the blank group and the miR-1307-5p mimic + TGFBI group (all P>0.05). Conclusion: The suppression of miR-1307-5p expression can increase TGFBI expression, promoting the proliferation of chondrocytes in OA mice, while inhibiting their apoptosis.
Keywords: miR-1307-5p, TGFBI, osteoarthritis, chondrocytes, proliferation, apoptosis
Introduction
Osteoarthritis (OA) is also known as degenerative osteoarthrosis. The death of chondrocytes and destruction of joint integrity, which are induced by degenerated articular cartilage, are characteristics of OA [1]. More than 60% of people aged over 60 years old suffer from various degrees of OA. Its prevalence rises with age and life and health are seriously affected [2,3]. With the development of OA, joint pain and even loss of joint function will develop. The degeneration of joints is almost irreversible, and there is still no effective treatment. As a result, it is particularly important to explore the pathogenesis and early diagnostic methods of OA [4].
MicroRNA, which has a length of 18-24 nucleotides, is encoded by an endogenous gene. It mainly combines with the 3’-untranslated region of mRNA to promote the degradation of mRNA target genes or induce translational silencing. Accordingly, intracellular activities such as cell apoptosis, proliferation, and differentiation are influenced [5,6]. The proliferation, apoptosis, and inflammatory reactions of chondrocytes, the unique cells in articular cartilage, are important for the development of OA. In the skeletal system, microRNA regulates the proliferation and apoptosis of chondrocytes, and affects the growth and development of cartilage. Wang et al. reported that overexpressed miR-142-3p could inhibit the apoptosis of chondrocytes and the production of inflammatory factors like interleukin-1 (IL-1) and interleukin-6 (IL-6) [7]; Li et al. found that the expression of miR-16-5p in OA chondrocytes was significantly increased when compared to healthy cartilage. In other words, miR-16-5p was involved in the occurrence and development of OA [8]. Previous studies have confirmed that the expression of miR-1307-5p in rheumatoid arthritis synovial cells, which is stimulated by TNF-α, is up-regulated. This is related to the expression of osteoclast-related genes [9]. In addition, it has been found that miR-1307-3p can inhibit the differentiation of adipose stem cells into chondrocytes [10]. However, the role of miR-1307-5p in OA and its effect on the biologic behavior of OA chondrocytes are not clear. Therefore, we aimed to explore the mechanism with miR-1307-5p.
Transforming growth factor beta-induced gene (TGFBI) is also called BIGH3 gene. It is first obtained in lung adenocarcinoma cell lines stimulated by transforming growth factor-β (TGF-β). It has been verified that TGFBI participates in biologic processes like cell growth, differentiation, and damage repair [11]. Ruiz et al. found that the expression of TGFBI was down-regulated in OA. When TGFBI was silenced, bone marrow mesenchymal stem cells partially lost their induction of chondrocytes’ anabolic markers. Additionally, they found that the protection of bone marrow mesenchymal stem cells provided to chondrocytes was mainly attributed to the existence of TGFBI mRNA and protein in cell vesicles. Therefore, they suppose that TGFBI is a chondroprotective factor released by mesenchymal stem/stromal cells and an anabolic regulator of cartilage homeostasis [12]. On the basis of previous studies, we studied the effect of miR-1307-5p specifically inhibiting TGFBI on the proliferation and apoptosis of OA chondrocytes.
Materials and methods
Screening of gene expression omnibus (GEO) chip and analysis of differential gene expression
OA was the key word applied to search for OA-related gene expression chips in the GEO database, with GSE79258 chips for subsequent analysis. Based on robust multiarray average algorithm, background correction and standardized preprocessing of the expression data was conducted using Affy package (http://www.bioconductor.org/packages/release/bioc/html/affy.html). With limma package in R language (http://master.bioconductor.org/packages/release/bioc/html/limma.html). miRNAs that were significantly differentially expressed in OA patients were identified and a heat map of differential gene expression was drawn. Differential genes were those with adj. P-value less than 0.05 and |log fold change| over 1.
Collection of human tissue specimens
In total, 23 cartilage tissue specimens were acquired from OA patients who received total knee arthroplasty between March 2019 and March 2020 in our hospital. Additionally, normal articular cartilage tissue specimens were collected from 18 patients with femoral neck fracture. The expressions of miR-1307-5p and TGFBI in human cartilage tissue specimens were measured using quantitative reverse transcription-polymerase chain reaction (qRT-PCR). This study was approved by the Ethics Committee of our hospital. Informed consent was signed by the patients or their family members.
Construction of OA mouse model
Totally, 45 specific pathogen free mice (male or female) aged 10 to 11 weeks old were selected. They were provided with free drinking water and food. After 7 days of feeding, these mice were randomly allocated to the OA group (30 mice) and the normal group (5 mice). In the OA group, the mice were anesthetized by the intraperitoneally injection of 1% sodium pentobarbital. Thereafter, they were fixed. With the aid of a dissecting microscope, surgery was carried out: (1) cut the inside of patellar ligament using a microblade; (2) find the joint cavity; (3) separate the fat pad between femoral condyles; (4) find the medial meniscus tibial ligament of knee joint; (5) cut the tibial ligament with a microblade to create damage on the joint surface; (6) disinfectthe surface; (7) suture the skin. Eight mice sutured immediately after incising the skin on the inside of one knee joint, were assigned to the sham group. After operation, the mice were returned to the cage. In order to prevent infection, penicillin was injected daily. The 7 remaining mice were allocated to the normal group, and received no treatment. After 25 days, we closely observed the mice in the OA group. The modeling was considered to be successful when there was increased synovial fluid, obvious synovial hyperplasia in the joint cavity, and severe cartilage defects in the mice [13]. The success rate of modeling in our study was 82.5%.
Cultivation, grouping, and transfection of chondrocytes
The mouse cartilage tissue specimens were cut into small cubes about 1 mm3. Thereafter, 1 mg/mL type II collagenase (article number: 17101-015, Shanghai Limin Industrial Co., Ltd., China) was added, and the mixture was incubated in an incubator. Two hours later, 10% fetal bovine serum (article number: 26140079, Shanghai Yingxin Laboratory Instrument Co., Ltd., China) was supplied to terminate the digestion. Undigested tissue pieces were removed by filtering through a 100 mesh nylon screen. After centrifuging, the supernatant was discarded. Meanwhile, the cells were suspended in the growth medium (article number: 1965-084, Thermo Fisher Scientific Inc., USA) provided with 100 U/mL penicillin and streptomycin, and cultured at 37°C and 5% CO2. The medium was replaced every 2 to 3 days.
Mouse chondrocytes were divided into 7 groups, including: normal group (normal mouse chondrocytes), blank group (mouse chondrocytes in the modeling group and was transfected with blank plasmid), NC group (mouse chondrocytes in the modeling group and was transfected with miR-1307-5p negative control plasmid), miR-1307-5p mimic group (mouse chondrocytes in the modeling group were transfected with miR-1307-5p mimic plasmid), miR-1307-5p inhibitor group (mouse chondrocytes in the modeling group were transfected with miR-1307-5p inhibitor plasmid), TGFBI group (mouse chondrocytes in the modeling group were transfected with TGFBI overexpressed plasmid), and miR-1307-5p mimic combined with TGFBI group (mouse chondrocytes in the modeling group were transfected with both miR-1307-5p mimic and TGFBI overexpressed plasmid). In our study, plasmids used for transfection were bought from Shanghai Jima Pharmaceutical Technology Co., Ltd., China. The cells were transfected in accordance with the instruction of Lipofectamin 3000 transfection kit (Thermo Fisher Scientific Inc., USA). After transfecting for 48 h, the efficiency was measured by qRT-PCR.
Verification of target relationship between miR-1307-5p and TGFBI
With the assistance of biologic information website RNA22, we predicted whether TGFBI was the direct target gene of miR-1307-5p. Thereafter, we conducted a dual luciferase report experiment to verify whether there was a target relationship between miR-1307-5p and TGFBI. Based on the binding site between the 3’ untranslated region (3’UTR) of TGFBI and miR-1307-5p, the wild sequence (TGFBI-3’UTR-WT) and mutant sequence (TGFBI-3’UTR-MUT) were designed. The 3’UTR fragment of TGFBI was double-digested and cloned to the upstream of pmirGLO to construct the wild sequence. The mutant sequence was established by mutating the binding site between TGFBI and miR-1307-5p. Both TGFBI-3’UTR-WT and TGFBI-3’UTR-MUT were inserted in a miR-1307-5p mimic and miR-1307-5p negative control plasmid, and the reorganized plasmids were transfected into 293T cells, respectively. The transfection was performed based on the instruction of Lipofectamin 3000 transfection kit (article number: 11668500, Thermo Fisher Scientific Inc., USA). In addition, a dual luciferase gene kit was implemented to detect luciferase activity.
Enzyme-linked immunosorbent assay (ELISA)
After transfecting for 48 h, the mice cells in each group were seeded into 96-well plates. The concentration of matrix metalloproteinase inhibitor-1 (TIMP-1), interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) in the supernatant were separately measured according to the instructions of TIMP-1 kit (article number: TX20653, Shanghai Yingxin Laboratory Instrument Co., Ltd., China), IL-1β kit (article number: 69-21178, Wuhan Moshake Biological Technology Co., Ltd., China), IL-6 kit (article number: 69-23048, Wuhan Moshake Biological Technology Co., Ltd., China), and TNF-α kit (article number: 69-99985, Wuhan Moshake Biological Technology Co., Ltd., China).
qRT-PCR
Following the Trizol method, the total RNA of OA patients’ cartilage tissue specimens, which were preserved during joint replacement surgery, was extracted. Its concentration and purity were determined with an ultraviolet spectrophotometer (device number: SP-3803AA, Shanghai Spectrum Instruments Co., Ltd., China). cDNA was synthesized using a reverse transcription kit (article number: 18091050, Thermo Fisher Scientific Inc., USA). Thereafter, the reverse transcriptase was inactivated in a warm bath at 85°C. The reaction system was prepared based on the kit instructions. The reaction conditions are: pre-denaturation (90°C, 30 s), denaturation (90°C, 10 s), annealing (55°C, 30 s), and extension (70°C, 45 s), with a total of 40 cycles. U6 was applied as the internal control of miR-1307-5p, while GAPDH was used as the internal control for the rest of the genes. The primer sequences are displayed in Table 1. The relative expression of each target gene was quantified using 2-ΔΔCT method. This method was applicable to both animal and cell specimens.
Table 1.
Primer sequences
| Target gene | Primer sequence (5’-3’) |
|---|---|
| miR-1307-5p | F: TGCGGTTTTGGCAATGGTAGAAC |
| R: CCAGTGCAGGGTCCGAGGT | |
| TGFBI | F: CTAAAGCCCACGAAACCTGA |
| R: CACGGAAGAGTCCAAGCCAC | |
| Bax | F: TCCACCAAGAAGCTGAGCGAG |
| R: GTCCAGCCCATGATGGTTCT | |
| Bcl-2 | F: CCGCTCGAGGATCAGACCTTTGAATGATTC |
| R: ATAAGAATGCGGCCGCCTCTGTGAATCCCGTTTGAA | |
| GAPDH | F: ATGGTGAAGGTCGGAGTGAAC |
| R: CTCGCTCCTGGAAGATGGT | |
| U6 | F: GCTTCGGCAGCAATATACTAAAAT |
| R: CGCTTCACGAATTTGCGTGTCAT |
Note: TGFBI: transforming growth factor beta-induced gene.
Western blot
Liquid nitrogen was added to the mouse cartilage tissue specimens, and the specimens were grinded into powder. Thereafter, appropriate protein lysis solution (article number: 88216, Thermo Fisher Scientific Inc., USA) was supplied to make them lysised. Thirty minutes later, the mixture was centrifuge at 1,500 r/min for 20 minutes. The supernatant was reserved, and a bicinchoninic acid kit was applied to detect the concentration of protein. The protein was separated in a polyacrylamide gel, and transferred to a nitrocellulose membrane with the wet transfer method. The membrane was then immersed in tris-buffered saline with tween (TBST) containing 5% skimmed milk powder for 1 h. The primary antibody of TGFBI (1:1500, article number: ab170874, Abcam, UK), Bax (1:1000, article number: ab182734, Abcam, UK), and Bcl-2 (1:1,500, article number: ab196495, Abcam, UK) were added drop by drop. After incubating overnight at 4°C, the membrane was rinsed with TBST 3 times. After that, goat anti-rabbit (1:2,500, article number: ab205718, Abcam, UK), the secondary antibody, was put in and the mixture was incubated at room temperature for 1 h. Similarly, the membrane was rinsed with TBST 3 times. Finally, the electrogenerated chemiluminescence reaction solution was applied to image in a dark room. Image J software was implemented to detect the relative expression level of protein. The procedure for cell specimens was the same as above.
MTT
After transfecting for 48 h, the transfected cells, which were digested using 0.25% trypsin, were collected. Single cell suspensions were prepared at a concentration of 3*106 cells/mL and seeded into 96-well plates, with 150 μL in each well. The plate was left at 37°C and 5% CO2. After culturing for 24 h, 48 h, and 72 h, the plates were taken out, and 15 μL of MTT solution (5 mg/mL, article number: M1025, Beijing Solarbio Science & Technology Co., Ltd., China) was added dropwise. The optic density value of every well at 570 nm was obtained using a microplate reader (device number: HBS-1096A, Nanjing Detie Experimental Equipment Co., Ltd., China).
Flow cytometry
After 48 hours of transfection, the transfected cells were digested using 0.25% trypsin. Thereafter, they were centrifuged to remove the supernatant. After washing with phosphate buffer saline, the cell concentration was regulated to 5*105 cells/mL, and a volume of 1.5 mL cell suspensions was added into 6-well plates. Annexin V/propidium iodide staining solution was used to stain the cells in each well for 20 minutes. The apoptosis of the cells was measured by flow cytometry (device number: Attune Nxt, Thermo Fisher Scientific Inc., USA).
Statistical methods
All data were analyzed using SPSS statistical software version 21.0. The measurement data were calculated as mean ± standard deviation (x̅ ± sd); independent sample t test was applied for inter-group comparison, while one-way variance combined with post-hoc Bonferroni or LSD test was used for multiple groups comparison. The difference was significant when P value was <0.05.
Results
Screening of differential genes
We conducted the analysis with GSE79258 chip. Compared with the normal articular cartilage tissue specimens, a total of 3 miRNA expressions in OA patients’ cartilage tissue specimens were up-regulated, while 3 miRNA expressions were down-regulated (Figure 1A). Referring to related literature, we included miR-1307-5p in the follow-up study. Previous studies showed that miR-1307-5p is dysregulated in rheumatoid arthritis [9]. In addition, it was found that miR-1307-3p can inhibit the differentiation of adipose stem cells into chondrocytes [10]. However, its role in OA, and whether or not it can influence the biologic behavior of OA chondrocytes, are not clear. Here, we aimed to explore the mechanism concerned with miR-1307-5p in OA. Furthermore, we analyzed the downstream genes applying the RNA22 database, and found that there was a specific binding site between miR-1307-5p and TGFBI (Figure 1B). TGFBI, which is secreted by mesenchymal stromal cells, can maintain cartilage homeostasis and inhibit the expression of inflammatory factors, displaying a protective effect in OA [12]. Results of dual luciferase reporter experiment showed that miR-1307-5p mimic significantly inhibited the luciferase activity of TGFBI-3’UTR-WT (P<0.05), but exhibited no suppression on the luciferase activity of TGFBI-3’UTR-MUT (Figure 1C).
Figure 1.
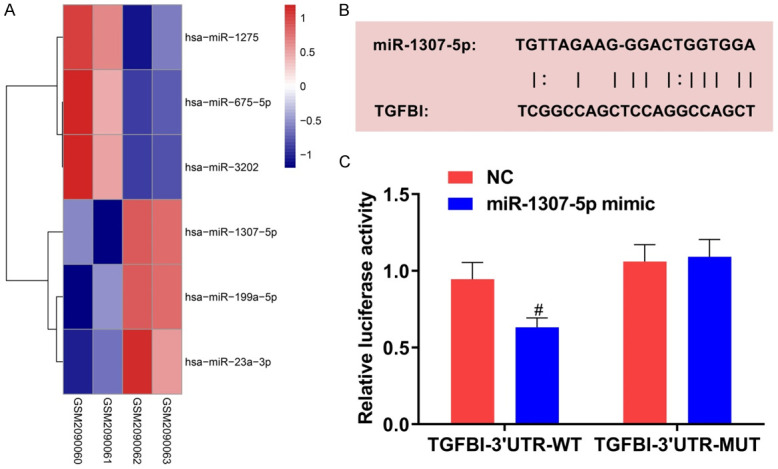
Screening of differential genes and verification of the target relationship between miR-1307-5p and TGFBI. A: Screening of differential miRNA based on GEO database; B: There is a binding site between TGFBI and miR-1307-5p; C: The dual luciferase report experiment revealed the target relationship between miR-1307-5p and TGFBI. Compared with chondrocytes transfected with NC and TGFBI-3’UTR-WT, #P<0.05. TGFBI: transforming growth factor beta-induced gene; 3’UTR: 3’ untranslated region; GEO: Gene Expression Omnibus.
The expression of miR-1307-5p and TGFBI mRNA in OA patients’ cartilage tissue specimens
To clarify whether miR-1307-5p participated in the pathogenesis of OA, we firstly measured miR-1307-5p expression in the cartilage tissue specimens of OA patients. As displayed in Figure 2A, miR-1307-5p expression in the cartilage tissue specimens of OA patients was significantly higher than that in the normal articular cartilage tissue specimens (P<0.001). Thereafter, we detected the expression of TGFBI mRNA and found that TGFBI mRNA expression in cartilage tissue specimens of OA patients was less than that in normal articular cartilage tissue specimens (P<0.001, Figure 2B). Additionally, there was a negative correlation between miR-1307-5p and TGFBI mRNA expression in cartilage tissue specimens (P<0.05, Figure 2C).
Figure 2.
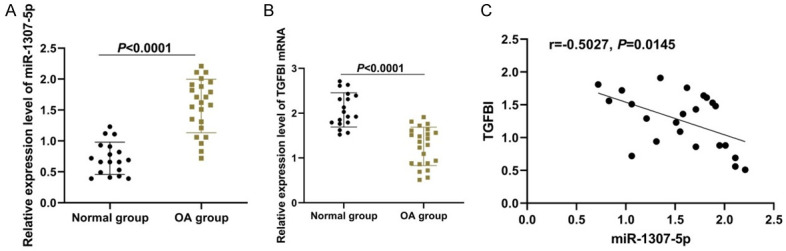
Comparison of miR-1307-5p and TGFBI expression in patients between the two groups. A: Relative miR-1307-5p expression in both groups; B: Relative TGFBI mRNA expression in both groups; C: Correlation between miR-1307-5p and TGFBI mRNA expression. OA: Osteoarthritis; TGFBI: transforming growth factor beta-induced gene.
Expression level of related mRNA and protein in mouse cartilage tissue specimens
As shown in Figure 3, compared with the normal mouse cartilage tissue specimens, miR-1307-5p expression in cartilage tissue specimens of OA mouse was significantly increased, while TGFBI expression was decreased (both P<0.05); there were no significant differences in miR-1307-5p and TGFBI expression between the normal group and the sham group (both P>0.05).
Figure 3.
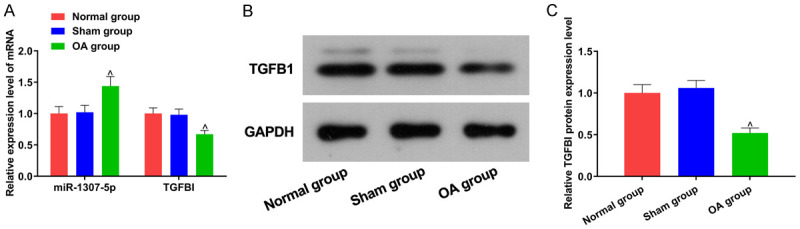
Comparison of miR-1307-5p and TGFBI expression in mice among the three groups. A: Relative miR-1307-5p and TGFBI mRNA expression; B: TGFBI protein expression; C: Relative expression level of TGFBI protein. Compared with the normal mice cartilage tissue specimens, ^P<0.05. OA: Osteoarthritis; TGFBI: transforming growth factor beta-induced gene.
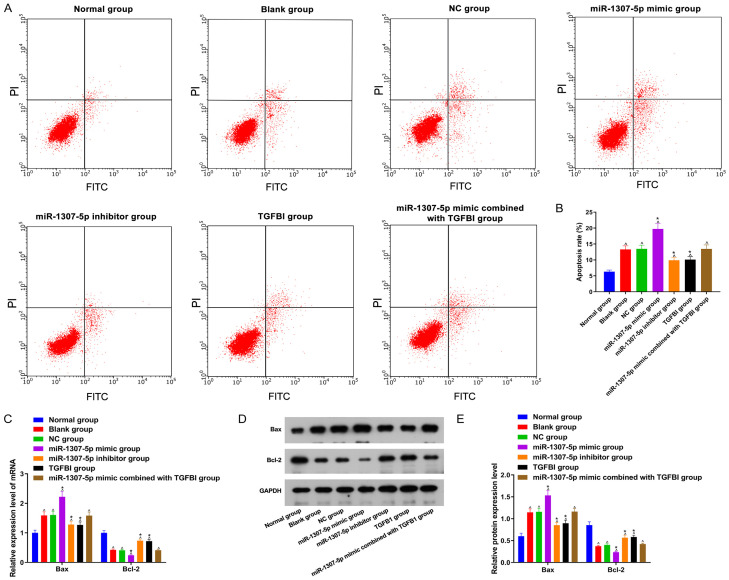
TIMP-1, IL-1β, IL-6, and TNF-α expression
As displayed in Figure 4, TIMP-1 expression in the blank group, NC group, miR-1307-5p mimic group, miR-1307-5p inhibitor group, TGFBI group, and miR-1307-5p mimic combined with TGFBI group were decreased when compared with the normal group, while IL-1β, IL-6, and TNF-α expression were increased (all P<0.05). Compared with the blank group, TIMP-1 expression in the miR-1307-5p inhibitor group and the TGFBI group were increased, while IL-1β, IL-6, and TNF-α expression were reduced (all P<0.05). There were no significant differences concerning TIMP-1, IL-1β, IL-6, and TNF-α expression between the NC group and the 1307-5p mimic combined with the TGFBI group (all P>0.05). The changes of TIMP-1, IL-1β, IL-6, and TNF-α expression in the miR-1307-5p mimic group were opposite to those in the miR-1307-5p inhibitor group (all P<0.05).
Figure 4.
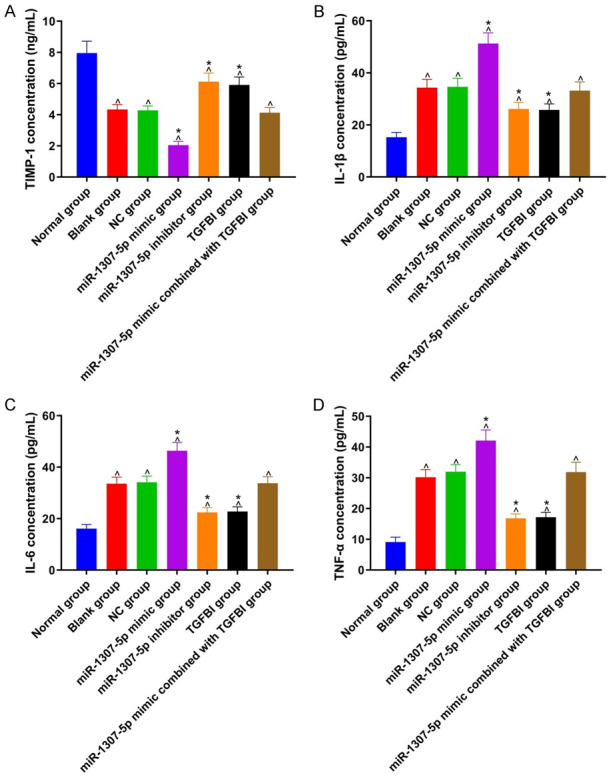
Comparison of TIMP-1, IL-1β, IL-6, and TNF-α expression among 7 groups. A: TIMP-1 expression in each group; B: IL-1β expression in each group; C: IL-6 expression in each group; D: TNF-α expression in each group. Compared with the normal group, ^P<0.05; compared with the blank group, *P<0.05. TIMP-1: matrix metalloproteinase inhibitor-1; IL-1β: interleukin-1β; IL-6: interleukin-6; TNF-α: tumor necrosis factor-α; TGFBI: transforming growth factor beta-induced gene.
Overexpressed TGFBI counteracted the proliferation inhibition induced by miR-1307-5p
As shown in Figure 5, cell proliferation activity in the blank group, NC group, miR-1307-5p mimic group, miR-1307-5p inhibitor group, TGFBI group, and miR-1307-5p mimic combined with TGFBI group were lower than those in the normal group (all P<0.05). Compared with the blank group, cell proliferation activity in the miR-1307-5p inhibitor group and TGFBI group were increased, but significantly decreased in the miR-1307-5p mimic group (both P<0.05). There were no significant differences on cell proliferation activity between the blank group and the NC group, and between the blank group and the miR-1307-5p mimic combined with TGFBI group (both P>0.05).
Figure 5.
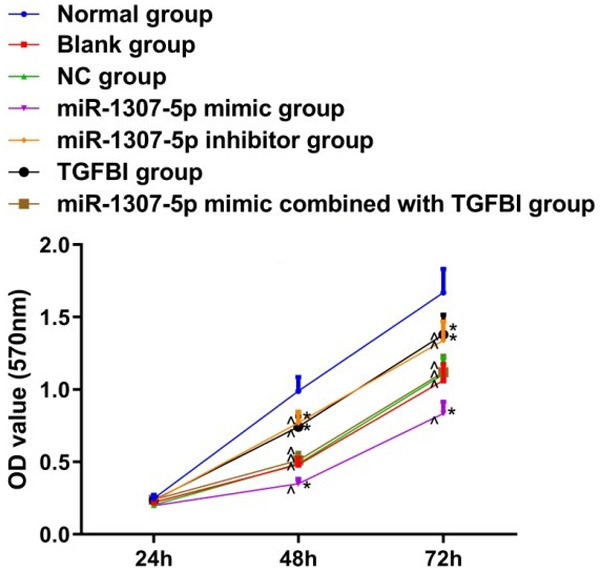
Comparison of cell proliferation activity among 7 groups. Compared with the normal group, ^P<0.05; compared with the blank group, *P<0.05. OD: optical density; TGFBI: transforming growth factor beta-induced gene.
Inhibited miR-1307-5p or overexpressed TGFBI suppressed chondrocyte apoptosis
As displayed in Figure 6, compared with the normal group, Bcl-2 expression in the blank group, NC group, miR-1307-5p mimic group, miR-1307-5p inhibitor group, TGFBI group, and miR-1307-5p mimic combined with TGFBI group were decreased, while Bax expression and chondrocyte apoptosis were increased (all P<0.05). Compared with the blank group, Bcl-2 expression in the miR-1307-5p inhibitor group and the TGFBI group were increased, while Bax expression and chondrocytes apoptosis were decreased. Compared with the blank group, Bcl-2 expression in the miR-1307-5p mimic group and TGFBI group were decreased, while Bax expression and chondrocyte apoptosis were increased (all P<0.05). There were no significant differences on Bcl-2 expression, Bax expression, and chondrocytes apoptosis between the blank group and the NC group, and between the blank group and the miR-1307-5p mimic combined TGFBI group (all P>0.05).
Figure 6.
Comparison of chondrocyte apoptosis among 7 groups. A: Chondrocyte apoptosis in each group; B: The rate of chondrocyte apoptosis in each group; C: Bcl-2 and Bax mRNA expression in each group; D: Bcl-2 and Bax protein expression in each group; E: Expression of Bcl-2 and Bax protein in each group. Compared with the normal group, ^P<0.05; compared with the blank group, *P<0.05. TGFBI: transforming growth factor beta-induced gene.
Discussion
OA occurs in large and small joints throughout the whole body, and is most commonly observed in knee and hip joints. It is a disease accompanied by pathological features such as tissue lesions, cartilage degeneration, and cartilage matrix disintegration, which in turn cause inflammation and hyperplasia of bone. As a result, joint pain is developed [14,15]. It was reported that OA was one of the top four causes of disability. However, there is no effective treatment for OA as a result of its complicated pathology and the adverse effects of drugs [16].
Recently, the role of microRNA in OA has attracted more and more attention. He et al. reported that miR-582-3p could reduce the progression of OA by targeting Yes-associated protein 1 [17]. Cao et al. found that miR-204-5p could inhibit the proliferation of chondrocytes in OA rat model established by surgery, improving the OA-like phenotype of chondrocytes [18]. Here, our results obtained from the tissue and cell specimens revealed that expression of miR-1307-5p in OA was up-regulated, while TGFBI was down-regulated. In addition, the proliferation and apoptosis of chondrocytes were influenced. The specific role of miR-1307-5p and TGFBI in OA were thus revealed. The change in inflammatory factors expressed in OA is one of the characteristics of OA. In order to explore these changes in inflammatory factors, we selected four representative ones, including TIMP-1, IL-1β, IL-6, and TNF-α, by consulting relevant literature. In addition, it was reported that the TIMP-1 expressed in OA was decreased, while IL-1β, IL-6, and TNF-α were increased [19,20]. In our study, we found the abnormal expression of inflammatory mediators (like TIMP-1, IL-1β, IL-6, and TNF-α) in OA chondrocytes through ELISA. To be specific, the expression of TIMP-1 was decreased, while IL-1β, IL-6, and TNF-α were increased. The role of miR-1307, in diseases has been continuously clarified. Yang et al. reported that the rs7911488-T allele could promote the expression of miR-1307, while suppress the expression of Prrx1, accelerating the proliferation and migration of colorectal cancer cells [21]. Yang et al. also found that the overexpression of miR-1307-3 could inhibit the deposition of cartilage matrix proteoglycans and reduce the expression of cartilage-related markers. Also, the differentiation of human adipose stem cells into chondrocytes was inhibited by specifically targeting BMPR2 and its downstream signaling pathways [10]. Using qRT-PCR, we confirmed that the expression of miR-1307-5p in OA cartilage tissue specimens and cells was increased. Subsequently, we further studied TGFBI, the possible target gene of miR-1307-5p.
Previous studies on TGFBI are mostly focused on tumors [22,23]. Iwafuchi et al. confirmed that TGFBI participated in the cell-collagen interaction [24]. Jung et al. found that the absence of TGFBI could cause the disintegration of articular cartilage matrix, disclosing a protective role of TGFBI in bone tissue [25]. In our study, we found that the expression of TGFBI in OA patients’ cartilage tissue specimens was lower than that in normal cartilage tissue specimens. By detecting luciferase activity, we verified that TGFBI was the target gene of miR-1307-5p. We also verified through experiments that the silencing of miR-1307-5p or overexpression of TGFBI could promote the expression of Bcl-2 and TIMP-1 and the proliferation of chondrocytes, while inhibit the expression of IL-1β, IL-6, TNF-α, and Bax, and the apoptosis of chondrocytes. The influence of overexpressed miR-1307-5p was opposite. Additionally, the effect of overexpressed TGFBI could be reversed by the excessive expression of miR-1307-5p. Studies on TGFBI and OA have found that TGF-β-induced bone marrow mesenchymal stem cells can suppress chondrocytes’ degradation of metabolites and reduce the expression of inflammatory factors. It has also been confirmed that GFBI is synthesized in response to TGF-β and can inhibit cell adhesion. These results suggest that GFBI can be used as a chondroprotective factor in OA to maintain the homeostasis of cartilage [12]. Accordingly, we suppose that the regulation of TGFBI in inflammatory factors and the biologic behavior of chondrocytes might be related to TGF-β and bone marrow mesenchymal stem cells, which is also the focus of our subsequent study.
However, our results are acquired from in vitro experiments. Subsequently, we will perform animal experiments to verify our conclusion.
In summary, the silencing of miR-1307-5p can inhibit the development of OA inflammation, facilitate the proliferation of chondrocytes, and suppress the apoptosis of chondrocytes. Further studies on miR-1307-5p might contribute to finding of a novel treatment for OA.
Disclosure of conflict of interest
None.
References
- 1.Paolillo FR, Paolillo AR, João JP, Frascá D, Duchêne M, João HA, Bagnato VS. Ultrasound plus low-level laser therapy for knee osteoarthritis rehabilitation: a randomized, placebo-controlled trial. Rheumatol Int. 2018;38:785–793. doi: 10.1007/s00296-018-4000-x. [DOI] [PubMed] [Google Scholar]
- 2.Felson DT, Naimark A, Anderson J, Kazis L, Castelli W, Meenan RF. The prevalence of knee osteoarthritis in the elderly. The framingham osteoarthritis study. Arthritis Rheum. 1987;30:914–918. doi: 10.1002/art.1780300811. [DOI] [PubMed] [Google Scholar]
- 3.Collins JA, Diekman BO, Loeser RF. Targeting aging for disease modification in osteoarthritis. Curr Opin Rheumatol. 2018;30:101–107. doi: 10.1097/BOR.0000000000000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allas L, Brochard S, Rochoux Q, Ribet J, Dujarrier C, Veyssiere A, Aury-Landas J, Grard O, Leclercq S, Vivien D, Ea HK, Maubert E, Cohen-Solal M, Boumediene K, Agin V, Baugé C. EZH2 inhibition reduces cartilage loss and functional impairment related to osteoarthritis. Sci Rep. 2020;10:19577. doi: 10.1038/s41598-020-76724-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qu Q, Wang L, Bing W, Bi Y, Zhang C, Jing X, Liu L. miRNA-126-3p carried by human umbilical cord mesenchymal stem cell enhances endothelial function through exosome-mediated mechanisms in vitro and attenuates vein graft neointimal formation in vivo. Stem Cell Res Ther. 2020;11:464. doi: 10.1186/s13287-020-01978-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang R, Qin L, Shi J. MicroRNA-199a-3p suppresses high glucose-induced apoptosis and inflammation by regulating the IKKβ/NF-κB signaling pathway in renal tubular epithelial cells. Int J Mol Med. 2020;46:2161–2171. doi: 10.3892/ijmm.2020.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X, Guo Y, Wang C, Yu H, Yu X, Yu H. MicroRNA-142-3p inhibits chondrocyte apoptosis and inflammation in osteoarthritis by targeting HMGB1. Inflammation. 2016;39:1718–1728. doi: 10.1007/s10753-016-0406-3. [DOI] [PubMed] [Google Scholar]
- 8.Li L, Jia J, Liu X, Yang S, Ye S, Yang W, Zhang Y. MicroRNA-16-5p controls development of osteoarthritis by targeting SMAD3 in chondrocytes. Curr Pharm Des. 2015;21:5160–5167. doi: 10.2174/1381612821666150909094712. [DOI] [PubMed] [Google Scholar]
- 9.Takamura Y, Aoki W, Satomura A, Shibasaki S, Ueda M. Small RNAs detected in exosomes derived from the MH7A synovial fibroblast cell line with TNF-α stimulation. PLoS One. 2018;13:e0201851. doi: 10.1371/journal.pone.0201851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Z, Li R, Ao J, Wa QD, Zhang Y, Chen L, Wen J, Chen B, Pan W, Li B, Tian XB. miR-1307-3p suppresses the chondrogenic differentiation of human adipose-derived stem cells by targeting BMPR2. Int J Mol Med. 2018;42:3115–3124. doi: 10.3892/ijmm.2018.3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song X, Cai L, Li Y, Zhu J, Jin P, Chen L, Ma F. Identification and characterization of transforming growth factor β induced gene (TGFBIG) from Branchiostoma belcheri: insights into evolution of TGFBI family. Genomics. 2014;103:147–153. doi: 10.1016/j.ygeno.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Ruiz M, Toupet K, Maumus M, Rozier P, Jorgensen C, Noël D. TGFBI secreted by mesenchymal stromal cells ameliorates osteoarthritis and is detected in extracellular vesicles. Biomaterials. 2020;226:119544. doi: 10.1016/j.biomaterials.2019.119544. [DOI] [PubMed] [Google Scholar]
- 13.Yao H, Xu J, Wang J, Zhang Y, Zheng N, Yue J, Mi J, Zheng L, Dai B, Huang W, Yung S, Hu P, Ruan Y, Xue Q, Ho K, Qin L. Combination of magnesium ions and vitamin C alleviates synovitis and osteophyte formation in osteoarthritis of mice. Bioact Mater. 2021;6:1341–1352. doi: 10.1016/j.bioactmat.2020.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jahanban-Esfahlan R, Mehrzadi S, Reiter RJ, Seidi K, Majidinia M, Baghi HB, Khatami N, Yousefi B, Sadeghpour A. Melatonin in regulation of inflammatory pathways in rheumatoid arthritis and osteoarthritis: involvement of circadian clock genes. Br J Pharmacol. 2018;175:3230–3238. doi: 10.1111/bph.13898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brzeszczyńska J, Brzeszczyński F, Hamilton DF, McGregor R, Simpson A. Role of microRNA in muscle regeneration and diseases related to muscle dysfunction in atrophy, cachexia, osteoporosis, and osteoarthritis. Bone Joint Res. 2020;9:798–807. doi: 10.1302/2046-3758.911.BJR-2020-0178.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng G, Zhan Y, Tang Q, Chen T, Zheng F, Wang H, Wang J, Wu D, Li X, Zhou Y, Wang X, Wu Y, Zhou Y, Xu H, Tian N, Zhang X. Monascin inhibits IL-1β induced catabolism in mouse chondrocytes and ameliorates murine osteoarthritis. Food Funct. 2018;9:1454–1464. doi: 10.1039/c7fo01892d. [DOI] [PubMed] [Google Scholar]
- 17.He J, Su X, Xie W. MiR-582-3p alleviates osteoarthritis progression by targeting YAP1. Mol Immunol. 2020;128:258–267. doi: 10.1016/j.molimm.2020.10.022. [DOI] [PubMed] [Google Scholar]
- 18.Cao J, Han X, Qi X, Jin X, Li X. miR-204-5p inhibits the occurrence and development of osteoarthritis by targeting Runx2. Int J Mol Med. 2018;42:2560–2568. doi: 10.3892/ijmm.2018.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alonso B, Bravo B, Mediavilla L, Gortazar AR, Forriol F, Vaquero J, Guisasola MC. Osteoarthritis-related biomarkers profile in chronic anterior cruciate ligament injured knee. Knee. 2020;27:51–60. doi: 10.1016/j.knee.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Liu W, Chen Y, Zeng G, Yang T, Song W. INSR mediated by transcription factor KLF4 and DNA methylation ameliorates osteoarthritis progression via inactivation of JAK2/STAT3 signaling pathway. Am J Transl Res. 2020;12:7953–7967. [PMC free article] [PubMed] [Google Scholar]
- 21.Yang M, Liu X, Meng F, Zhang Y, Wang M, Chen Y, Guo X, Chen W, Wang W. The rs7911488-T allele promotes the growth and metastasis of colorectal cancer through modulating miR-1307/PRRX1. Cell Death Dis. 2020;11:651. doi: 10.1038/s41419-020-02834-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shang D, Song B, Liu Y. Epirubicin suppresses proliferative and metastatic potential by downregulating transforming growth factor-β-induced expression in urothelial carcinoma. Cancer Sci. 2018;109:980–987. doi: 10.1111/cas.13403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hung MS, Chen IC, You L, Jablons DM, Li YC, Mao JH, Xu Z, Hsieh MJ, Lin YC, Yang CT, Liu ST, Tsai YH. Knockdown of Cul4A increases chemosensitivity to gemcitabine through upregulation of TGFBI in lung cancer cells. Oncol Rep. 2015;34:3187–3195. doi: 10.3892/or.2015.4324. [DOI] [PubMed] [Google Scholar]
- 24.Iwafuchi Y, Morioka T, Oyama Y, Nozu K, Iijima K, Narita I. A case of transforming growth factor-β-induced gene-related oculorenal syndrome: granular corneal dystrophy type II with a unique nephropathy. Case Rep Nephrol Dial. 2016;6:106–113. doi: 10.1159/000449129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JM, Lee EH, Kim IS, Kim JE. Tgfbi deficiency leads to a reduction in skeletal size and degradation of the bone matrix. Calcif Tissue Int. 2015;96:56–64. doi: 10.1007/s00223-014-9938-4. [DOI] [PubMed] [Google Scholar]