Abstract
Objective: To explore the effect and complications of esketamine combined with ultrasound-guided nerve block in children with lower extremity fractures. Methods: 120 children with fractures of lower extremities were included into the observation group (OG) and control group (CG) according to the specific anesthesia method. The OG was given esketamine combined with ultrasound-guided nerve block, and the CG was given ultrasound-guided nerve block combined with general anesthesia. Serum norepinephrine (NE), epinephrine (E), renin (R), mean arterial pressure (MAP), heart rate (HR), oxyhemoglobin saturation (SpO2) and respiration rate (RR) were measured before, at 10, 20, and 30 min after anesthesia. The incidence of clinical complications and the anesthetic effects were compared between the two groups. The mini-mental state examination (MMES) scale was performed to evaluate the cognitive function of children in the two groups before and after surgery. Results: At 10 min and 20 min after anesthesia, the CG showed higher MAP and HR than the OG (P<0.05); however, RR and SpO2 showed no difference between the two groups (P>0.05). At 30 min after anesthesia, HR and MAP were not significantly different between the two groups (P>0.05); NE, E, and R showed no significant difference before surgery (P>0.05), which were higher in CG than those in the OG after surgery (P<0.05). The success rate of nerve block and anesthesia were 91.67% and 85.00%, respectively in the OG, which were higher than 88.33% and 83.33% in the CG (P>0.05). The OG had a complication rate of 8.33%, significantly lower than that of 20.00% in the CG (P<0.05). Conclusion: Esketamine combined with ultrasound-guided nerve block anesthesia was superior to ultrasound-guided nerve block combined with general anesthesia in children with lower extremity fractures, with fewer compilations.
Keywords: Esketamine, ultrasound-guided nerve block, lower extremity fractures in children, complications
Introduction
Fractures of lower extremities are common in children and are mainly treated by open reduction and manual reduction followed by external fixation [1]. The motion of active children may cause displacement of the fracture following open reduction and external fixation, which seriously prolongs bone healing process [2]. Therefore, open reduction therapy is recommended in clinical practice, but for pediatric anesthesia, the increased risk is associated with younger age, lower body weight, and incompletely developed respiratory tract [3].
A study has found that spinal anesthesia requires appropriate positioning, which is more likely to cause pain and adverse reactions such as increased heart beat and blood pressure during surgery [4]. Esketamine has a significant inhibitory effect on the human circulatory system and can enhance the excitement of central sympathetic nerves. Ultrasound-guided nerve block anesthesia is simple to operate and has a low incidence of complications. It has been widely used in pediatric anesthesia [5]. At present, there have been clinical reports on esketamine combined with ultrasound-guided nerve block anesthesia. A controlled study of 100 children with fractures showed that ultrasound-guided esketamine anesthesia could exert the anesthetic effect faster, and patients were not awake during the surgery, with shorter postoperative extubation time and lower incidence of agitation [6]. This study aimed to explore the effect and complications of esketamine combined with ultrasound-guided nerve block in children with lower extremity fractures.
Materials and methods
General data
A total of 120 children with open reduction and internal fixation in our hospital from September 2018 to December 2019 were selected and were divided into an observation group (OG) and a control group (CG) according to the anesthesia methods. The OG included 32 males and 28 females, aged 9-14 years, with the average age of (11.15 ± 1.11) years, body weight 35-57 kg, and operation time: (55.8 ± 11.5) min. The CG included 33 males and 27 females, aged 8-15 years, with the average age of (11.12 ± 1.13) years, body weight 34-56 kg; and operation time: (54.4 ± 10.3) min. According to the American Society of Anesthesiologists (ASA) Physical Status Classification [7], there were 78 cases of grade I and 42 cases of grade II. Inclusion criteria: patients with ASA grade I-II, lower extremity fractures, without contraindication of nerve block, and without upper respiratory tract infection during past two weeks. Exclusion criteria: patients with liver and kidney disfunctions, coagulation dysfunction, pathological fractures and cognitive impairment. The family members of this study signed the informed consent form and this study was obtained the approval of the ethics committee of the First Affiliated Hospital of Hainan Medical College.
Anesthesia method
Both groups underwent open reduction and internal fixation and fasted for 8 hours before surgery. Atropine (0.02 mg/kg) was delivered by intramuscular injection. An anesthetic machine with bag for delivery of oxygen or oxygen mask was used to aid the respiratory system. Ultrasound-guided nerve block combined with general anesthesia was performed in the CG, and after general anesthesia, ultrasound-guided sciatic nerve block and femoral nerve block were performed. Sciatic nerve block: In the lateral decubitus position, a vertical line was drawn at the midpoint of the line between the greater trochanter of femur and the posterior superior iliac spine. The curved low-frequency ultrasound probe was parallel to the line and scanned along the vertical line. When the continuity plane of the iliac bone was interrupted and the sciatic nerve behind the iliac bone was scanned, the scanning plane was moved slightly along the vertical line to the proximal end, and this plane was the puncture plane. The buttock area was disinfected and placed with surgical drape, and the needle was inserted under ultrasound guidance to avoid the superior gluteal artery and vein. After no blood return was produced, 0.3 mL/kg of 0.375% ropivacaine was injected on the ilium surface near the greater sciatic foramen. Femoral nerve block: The patient was placed in the supine position to disinfect the inguinal area, and the ultrasound probe was placed on the lateral side of the inguinal femoral artery to determine the iliopsoas femoral nerve, femoral artery and femoral vein. Under the guidance of high-frequency ultrasound probe, the needle was punctured in the plane. Water separation technology was used to confirm that the puncture needle was located near the nerve. After no blood return was produced, 0.3 mL/kg of 0.375% ropivacaine was injected. When the femoral nerve was soaked in the hypoechoic area of local anesthetic, the needle was withdrawn. The ultrasound-guided puncture method in the OG was the same as that in the CG, and 0.5 mg/kg esketamine was added on the basis of the same dose of ropivacaine.
Observation indicators and evaluation methods
Mean arterial pressure (MAP), heart rate (HR), oxyhemoglobin saturation (SpO2), respiration rate (RR), epinephrine (E), norepinephrine (NE), renin (R) before and at 10 min, 20 min, and 30 min after anesthesia were observed. The complications were recorded after anesthesia and during the surgery. The effectiveness of nerve block was evaluated by puncture. No pain indicated complete nerve block; The child who did not hold back indicated excellent nerve block; Slow response and small amplitude of the limb movement indicated good nerve block; Otherwise, the nerve block was poorly performed. Evaluation of anesthesia effect: excellent = quiet during the entire surgery, no need to increase anesthesia dose; good = slight pain during the surgery, a small amount of anesthetic needs to be added; poor = strong pain with obvious limb reaction.
Cognitive function score: The mini-mental state examination (MMES) was used to evaluate cognitive function. The total score is 30 points, the cut-off point of non-education is 17 points, the cut-off point of every 1 year of education is 1 point, and the cut-off point of >6 years of education is 24 points. Those whose preoperative cognitive function score was lower than the cut-off value was considered as cognitive impairment, and would be excluded in this study.
Statistical analysis
SPSS18.0 statistical software was used for data analysis. The measurement data (x ± s) were compared by t test. The count data were examined using X2 test. P<0.05 was considered statistically significant.
Results
Comparison of general data between the two groups
There was no significant difference in age, gender, operation time and body mass between the two groups (P>0.05, Table 1).
Table 1.
Comparison of the clinical general materials between the two groups (x ± s)/[n (%)]
| General materials | Observation group (n = 60) | Control group (n = 60) | t/X2 | P | |
|---|---|---|---|---|---|
| Gender | Male | 32 | 33 | 0.034 | 0.855 |
| Female | 28 | 27 | |||
| Average age (years) | 11.15±1.11 | 11.12±1.13 | 0.147 | 0.883 | |
| Average weight (kg) | 45.98±5.44 | 46.19±5.12 | 0.218 | 0.828 | |
| ASA | Grade I | 40 | 38 | 0.147 | 0.702 |
| Grade II | 20 | 22 | |||
The hemodynamic changes of the two groups at multiple time points
There was no significant difference in HR, MAP, RR and SpO2 between the two groups before anesthesia (P>0.05). The HR and MAP of the CG at 10 min were significantly higher than those at 20 min and 30 min after anesthesia (P<0.05) and they were not significant different among multiple time points in the OG (P>0.05). The two groups showed no significant difference in RR and SpO2 at multiple time-point (P>0.05) (Table 2).
Table 2.
Changes of hemodynamic indices between the two groups (x ± s)
| Signs | Group | n | Time-points | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Before anesthesia | At 10 min after anesthesia | At 20 min after anesthesia | At 30 min after anesthesia | |||
| HR (beats/min) | Observation group | 60 | 112.3±8.1 | 126.3±7.8 | 109.8±7.6 | 101.4±7.5 |
| Control group | 60 | 111.2±7.5 | 132.5±8.9 | 122.6±8.7 | 119.8±6.2 | |
| t | 0.772 | 4.058 | 8.583 | 14.647 | ||
| P | 0.442 | <0.001 | <0.001 | <0.001 | ||
| MAP | Observation group | 60 | 100.25±13.58 | 91.29±11.25 | 90.26±11.74 | 93.28±8.62 |
| Control group | 60 | 99.13±12.64 | 104.26±9.13 | 100.38±10.23 | 96.32±13.24 | |
| t | 0.468 | 6.934 | 5.034 | 2.471 | ||
| P | 0.641 | <0.001 | <0.001 | 0.015 | ||
| RR | Observation group | 60 | 13.2±2.1 | 16.5±3.2 | 16.4±2.8 | 17.5±3.2 |
| Control group | 60 | 14. ± 2.8 | 17.1±2.2 | 16.9±3.1 | 17.2±2.6 | |
| t | 1.771 | 1.197 | 0.927 | 0.564 | ||
| P | 0.079 | 0.234 | 0.356 | 0.574 | ||
| SpO2 | Observation group | 60 | 98.5±1.9 | 99.2±0.9 | 98.5±3.1 | 98.3±3.2 |
| Control group | 60 | 97.9±3.2 | 98.6±1.8 | 98.8±2.7 | 98.6±2.5 | |
| t | 1.249 | 1.93 | 0.565 | 0.572 | ||
| P | 0.214 | 0.056 | 0.573 | 0.568 | ||
Success rate of ultrasound-guided nerve block and anesthesia in the two groups
The excellent and good rate of ultrasoundguided nerve block was 91.67% in the OG and 88.33% in the CG. The difference between the two groups was not statistically significant (P>0.05). The excellent and good rate of anesthesia was 85.00% in the OG and 83.33% in the CG. The difference between the two groups was not statistically significant (P>0.05, Table 3).
Table 3.
Condition of anesthesia between the two groups [n (%)]
| Group | n | Ultrasound-guided nerve block | Condition of anesthesia | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Excellent | Good | Poor | Excellent rate | Excellent | Good | Poor | Excellent rate | ||
| Observation group | 60 | 44 | 11 | 5 | 55 (91.67) | 48 | 3 | 9 | 51 (85.00) |
| Control group | 60 | 40 | 13 | 7 | 53 (88.33) | 45 | 5 | 10 | 50 (83.33) |
| X2 | 0.37 | 0.063 | |||||||
| P | 0.543 | 0.803 | |||||||
Changes of MMSE scores before and after surgery in the two groups
The OG showed increased MMSE score compared with the CG (P<0.05), and MMSE score was significantly improved in both groups compared with that before surgery (P<0.05, Figure 1).
Figure 1.
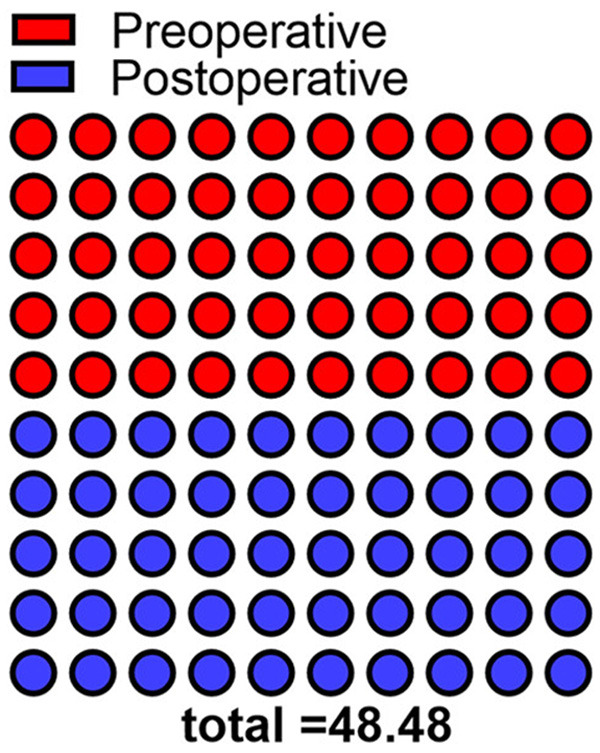
Comparison of postoperative cognitive function between the two groups. Postoperative MMSE score in the observation group was 23.11, significantly higher than that of 21.13 in the control group (P<0.05).
The postoperative complications of the two groups
The incidences of nausea and vomiting, postoperative irritability, and intravascular injury in the OG were significantly lower than those in the CG (P<0.05, Table 4).
Table 4.
Incidence of complications between the two groups [n (%)]
| Group | n | Horner syndrome | Recurrent laryngeal nerve block | Intravascular injury | Sick and vomit | Postoperative irritability | Total incidence |
|---|---|---|---|---|---|---|---|
| Observation group | 60 | 1 | 1 | 0 | 2 | 1 | 5 (8.33) |
| Control group | 60 | 3 | 2 | 0 | 4 | 3 | 12 (20.00) |
| X2 | 11.298 | ||||||
| P | <0.05 |
Changes of serum stress indices and condition of anesthesia before and after surgery in both groups
There was no significant difference in serum NE, R and E between the two groups before surgery (P>0.05). The above-mentioned serum stress indices of the CG after surgery were significantly lower than those of the OG (P<0.05, Figure 2). The postoperative anesthesia time, pain disappearance time and anesthesia recovery time in the OG were significantly shorter than those in the CG (P<0.05, Figure 3).
Figure 2.
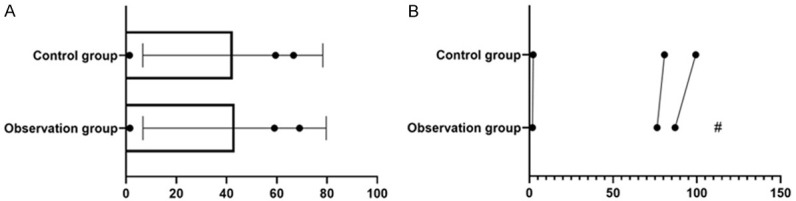
Changes of serum stress indices between the two groups. There was no significant difference on preoperative NE, E and R between the two groups (t = -1.005, 0.950, 0.727, P>0.05). E and R of the observation group were significantly lower than those of the control group (t = -2.047, -4.142, -2.842, P<0.05). # indicates that the difference of the same index is statistically significant compared with the control group.
Figure 3.
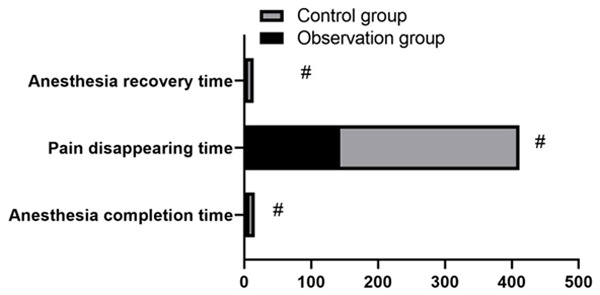
Anesthesia conditions of the two groups. The difference in anesthesia time, anesthesia recovery time and pain disappearance time was statistically significant (t = -1.478, -23.308, -6.855, P<0.05). # means that the difference of the same indicator between groups is statistically significant.
Discussion
Lower extremity fracture is a common fracture disease, and some children often experience anxiety and fear after fracture [8]. Therefore, a reasonable surgical plan can accelerate fracture healing, and the effectiveness of analgesia is the premise to ensure the smooth operation [9]. Currently, the common option of surgical anesthesia is intraspinal anesthesia, which requires appropriate positioning, aggravating the pain and fear of patients and easily causing pain when transported to the operating room, which is not conducive to the smooth operation. Anesthesia can also cause complications such as increased heart rate and blood pressure [10]. The choice of anesthesia and drugs before surgery is crucial. On the one hand, good anesthetic method should be selected to achieve desirable analgesic effects; On the other hand, damage should be minimized [11,12]. General anesthesia is a widely used anesthesia method and can be easily managed. However, general anesthesia also has the drawback of affecting the stability of blood flow and the effect of the surgery [13,14].
Studies have shown that ultrasound-guided nerve block can make up for the shortcomings of general anesthesia and improve the effect of anesthesia [15,16]. In this study, ultrasound-guided nerve block was combined with esketamine to observe the hemodynamic changes and postoperative complication rate. Esmolol ketamine was used in small surgical and diagnostic procedures. Ketamine is a lipophilic compound. Ketamine can quickly enter the central nervous system, and the onset of anesthesia after IV ketamine is approximately 25-30 s. The study found that the HR, MAP and RR of the two groups at 10 min after anesthesia were higher than before anesthesia (P<0.05); However, these effects were not observed at 20 and 30 min after anesthesia (P>0.05); There was no significant difference in RR and SpO2 (P>0.05), confirming that these indices gradually returned to normal with time following esketamine combined with ultrasound-guided nerve block. A study of 151 children with forearm fractures showed that there were no adverse events in the application of esketamine, which confirmed that the application of esketamine in emergency department fractures had a good application prospect and could exert a better sedative effect [17]. A systematic review and meta-analysis of 16 ketamine trials with a total of 1485 patients showed that ketamine reduced the incidence of shivering after anesthesia compared with placebo, which confirmed that ketamine was effective in preventing the occurrence of shivering events after anesthesia [18]. The author of this study believed that esketamine is a chiral cyclohexanone derivative that can block N-methyl-D-aspartic acid receptors to exert analgesic effects. This drug is often used in combination with sleeping pills to induce and implement general anesthesia, and is also used as a supplement of local anesthetic drugs in children anesthesia, with good effect. This study found that the success rate of nerve block and anesthesia of the OG was superior to that in the CG, with no significant difference (P>0.05), indicating that both groups achieved better nerve block and anesthesia effect under the same ultrasound-guided nerve block method, but the OG had better nerve block and anesthesia effect, which may be related to the difference in the application of drugs [19,20]. The reason may be related to the advantages of rapid onset, rapid fading and few side effects of esketamine. When performing surgery on lower limb fracture, vital signs, agitation, and physical activity should be closely monitored. The dose of anesthetic drug should be increased if necessary [21-26]. During the surgery, the monitoring of the respiratory cycle should be strengthened to ensure the smoothness of the patient’s breathing during the surgery. This study showed that the incidence of complications in the OG was significantly lower than that in the CG, suggesting that esketamine combined with ultrasound-guided nerve block can also reduce the incidence of complications.
In summary, the use of esketamine combined with ultrasound-guided nerve block for anesthesia in children with lower extremity fractures can effectively reduce the incidence of complications, and the combined effect of the two is better than one alone. The limitation of this study is that the controlled study of the underlying diseases is not carried out on the included subjects, which may lead to the presence of underlying diseases affecting the accuracy of the investigation results. Therefore, it should be planned to be corrected in the next investigation.
Disclosure of conflict of interest
None.
References
- 1.Matsumura T, Takahashi T, Miyamoto O, Saito T, Kimura A, Takeshita K. Clinical outcome of conversion from external fixation to definitive internal fixation for open fracture of the lower limb. J Orthop Sci. 2019;24:888–893. doi: 10.1016/j.jos.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Ömeroğlu H. Basic principles of fracture treatment in children. Eklem Hastalik Cerrahisi. 2018;29:52–57. doi: 10.5606/ehc.2018.58165. [DOI] [PubMed] [Google Scholar]
- 3.Korobeinikov A, Popkov D. Use of external fixation for juxta-articular fractures in children. Injury. 2019;50(Suppl 1):S87–S94. doi: 10.1016/j.injury.2019.03.043. [DOI] [PubMed] [Google Scholar]
- 4.LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet] Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012. Esketamine. 2019 Oct 18. PMID: 31869190. [Google Scholar]
- 5.Wang J, Huang J, Yang S, Cui C, Ye L, Wang SY, Yang GP, Pei Q. Pharmacokinetics and safety of esketamine in Chinese patients undergoing painless gastroscopy in comparison with ketamine: a randomized, open-label clinical study. Drug Des Devel Ther. 2019;13:4135–4144. doi: 10.2147/DDDT.S224553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jonkman K, Duma A, Olofsen E, Henthorn T, van Velzen M, Mooren R, Siebers L, van den Beukel J, Aarts L, Niesters M, Dahan A. Pharmacokinetics and bioavailability of inhaled esketamine in healthy volunteers. Anesthesiology. 2017;127:675–683. doi: 10.1097/ALN.0000000000001798. [DOI] [PubMed] [Google Scholar]
- 7.Kannan T. ASA grading: a step forward. J Perioper Pract. 2017;27:54–58. doi: 10.1177/175045891702700304. [DOI] [PubMed] [Google Scholar]
- 8.Gastaldon C, Papola D, Ostuzzi G, Barbui C. Esketamine clinical trials: reply to Maju et al. Epidemiol Psychiatr Sci. 2020;29:e122. doi: 10.1017/S204579602000027X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao XG, Zhang M, Yue XA, Zhang H, Xue H. Application of nerve block in total knee arthroplasty under general anesthesia. Zhongguo Gu Shang. 2020;33:363–7. doi: 10.12200/j.issn.1003-0034.2020.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Helen L, O’Donnell BD, Moore E. Nerve localization techniques for peripheral nerve block and possible future directions. Acta Anaesthesiol Scand. 2015;59:962–74. doi: 10.1111/aas.12544. [DOI] [PubMed] [Google Scholar]
- 11.Moucha CS, Weiser MC, Levin EJ. Current strategies in anesthesia and analgesia for total knee arthroplasty. J Am Acad Orthop Surg. 2016;24:60–73. doi: 10.5435/JAAOS-D-14-00259. [DOI] [PubMed] [Google Scholar]
- 12.Turbitt LR, Mariano ER, El-Boghdadly K. Future directions in regional anaesthesia: not just for the cognoscenti. Anaesthesia. 2020;75:293–297. doi: 10.1111/anae.14768. [DOI] [PubMed] [Google Scholar]
- 13.Kohno Y, Koishi K, Fujii T, Nishiyama T. A case report of ultrasound guided peripheral nerve block for lower extremity amputation of a patient with anti-phospholipid syndrome. Masui. 2013;62:718–720. [PubMed] [Google Scholar]
- 14.Shamim F, Hameed M, Siddiqui N, Abbasi S. Ultrasound-guided peripheral nerve blocks in high-risk patients, requiring lower limb (Above and below knee) amputation. Int J Crit Illn Inj Sci. 2018;8:100–103. doi: 10.4103/IJCIIS.IJCIIS_60_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park YU, Cho JH, Lee DH, Choi WS, Lee HD, Kim KS. Complications after multiple-site peripheral nerve blocks for foot and ankle surgery compared with popliteal sciatic nerve block alone. Foot Ankle Int. 2018;39:731–735. doi: 10.1177/1071100717753954. [DOI] [PubMed] [Google Scholar]
- 16.Ghodki PS, Shalu PS, Sardesai SP. Ultrasound-guided adductor canal block versus femoral nerve block for arthroscopic anterior cruciate ligament repair under general anesthesia. J Anaesthesiol Clin Pharmacol. 2018;34:242–246. doi: 10.4103/joacp.JOACP_172_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel D, Talbot C, Luo W, Mulvaney S, Byrne E. The use of esketamine sedation in the emergency department for manipulation of paediatric forearm fractures: a 5 year study. Injury. 2021;52:1321–1330. doi: 10.1016/j.injury.2020.12.033. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Y, Mannan A, Han Y, Liu H, Guan HL, Gao X, Dai MS, Cao JL. Efficacy and safety of prophylactic use of ketamine for prevention of postanesthetic shivering: a systematic review and meta analysis. BMC Anesthesiol. 2019;19:245. doi: 10.1186/s12871-019-0910-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desai N, El-Boghdadly K, Albrecht E. Peripheral nerve blockade and novel analgesic modalities for ambulatory anesthesia. Curr Opin Anaesthesiol. 2020;33:760–767. doi: 10.1097/ACO.0000000000000928. [DOI] [PubMed] [Google Scholar]
- 20.Ling C, Liu XQ, Li YQ, Wen XJ, Hu XD, Yang K. Ultrasound-guided fascia iliaca compartment block combined with general anesthesia for amputation in an acute myocardial infarction patient after percutaneous coronary intervention: a case report. World J Clin Cases. 2019;7:2567–2572. doi: 10.12998/wjcc.v7.i17.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al Rawahi K, Khan R, Kaul N, Azharuddin M. Peripheral nerve stimulator-induced electrostimulation at the P6 point reduces the incidence of post-spinal hypotension in patients undergoing post-trauma orthopaedic surgery. Southern African Journal of Anaesthesia & Analgesia. 2013;19:216–218. [Google Scholar]
- 22.Alfred VM, Srinivasan G, Zachariah M. Comparison of ultrasound with peripheral nerve stimulator-guided technique for supraclavicular block in upper limb surgeries: a randomized controlled trial. Anesth Essays Res. 2018;12:50–54. doi: 10.4103/aer.AER_211_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bansal L, Attri JP, Verma P. Lower limb surgeries under combined femoral and sciatic nerve block. Anesth Essays Res. 2016;10:432–436. doi: 10.4103/0259-1162.177186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cicekci F, Yildirim A, Önal Ö, Celik JB, Kara I. Ultrasound-guided adductor canal block using levobupivacaine versus periarticular levobupivacaine infiltration after totalknee arthroplasty: a randomized clinical trial. Sao Paulo Med J. 2019;137:45–53. doi: 10.1590/1516-3180.2018.0269101218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, Lam D, King H, Credaroli E, Harmon E, Vadivelu N. Novel regional anesthesia for outpatient surgery. Curr Pain Headache Rep. 2019;23:69. doi: 10.1007/s11916-019-0809-6. [DOI] [PubMed] [Google Scholar]
- 26.Sahin SH, Colak A, Arar C, Tutunculer E, Sut N, Yılmaz B, Birtane M. A retrospective trial comparing the effects of different anesthetic techniques on phantom pain after lower limb amputation. Curr Ther Res Clin Exp. 2011;72:127–137. doi: 10.1016/j.curtheres.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


