Abstract
Objective: To explore the application value of goal-directed fluid therapy (GDFT) in the enhanced recovery after surgery (ERAS) of patients undergoing radical lung cancer surgery (RLCS). Methods: A total of 74 patients undergoing elective RLCS based on the enhance recovery after surgery (ERAS) concept in the HanDan Central Hospital between December 2016 and December 2019 were enrolled and assigned to a group treated by regular conventional liquids (regular group, n=34) and a group treated by goal-directed fluid (GDFT group, n=40) according to the fluid infusion scheme. The two groups were compared in intraoperative fluid inflow and outflow, hemodynamic indexes at 30 min (T0) before operation, 4 h (T1) and 24 h (T2) after operation, postoperative complications, postoperative recovery, inflammatory factors at 1 day (d 0) before operation, and at 1 day (d 1) and 7 days (d 3) after operation, as well as for postoperative life quality. Results: Crystalloid fluid input, fluid infusion, and urine output of the GDFT group were all significantly less than those of the regular group (all P<0.05), and the GDFT group showed significantly lower fluctuations of MAP, cardiac index, and stroke volume (SV) than the regular group (all P<0.05). Additionally, the GDFT group showed a significantly lower overall complication rate and experienced notably earlier time to flatus and getting out-of-bed time and notably shorter hospitalization time than the regular group (all P<0.05). Moreover, the GDFT group presented with less fluctuation of IL-10, IL-6, and TNF-α levels and experienced notably higher life quality scores than the regular group. Conclusion: GDFT is beneficial to the rapid recovery of patients after RLCS, because it can exert a positive effect on maintaining the stability of hemodynamic indexes and reducing inflammation and postoperative complications.
Keywords: Goal-directed fluid, radical lung cancer surgery, ERAS, inflammation
Introduction
Lung cancer is a common form of cancer. In recent years, as the natural environment deteriorates, the diagnosis rate of lung cancer is rises annually, and cancer has become a main cause of cancer-associated death [1,2]. Currently, surgery is still the main treatment for lung cancer. As medical technology advances, conventional thoracotomy is gradually becoming an obsolete procedure because of its traumatic characteristics, and early radical lung cancer surgery (RLCS) does not satisfy the clinical needs in terms of efficacy and prognosis [3,4]. In the past few years, the concept of enhance recovery after surgery (ERAS) is gradually blooming, and its application in clinical practice has demonstrated great advantages [5].
RLCS based on the concept of ERAS can reduce postoperative complications. Postoperative fluid therapy is crucial in the prognosis of patients. Improper postoperative fluid infusion will not only give rise to a series of complications such as tissue hypoperfusion and aggravate the inflammatory reaction in patients, but it also increases the postoperative mortality of patients [6]. In recent years, the question of how to formulate an appropriate infusion treatment scheme has become a clinical focus. Conventional infusion methods are underpinned by experience, but they can no longer meet the individualized demands, so finding a suitable infusion scheme has profound clinical significance for surgical treatment of patients [7,8]. Goal-directed fluid therapy (GDFT) is an optimized individualized fluid therapy strategy, under which advanced dynamic detection methods and effective standard treatment procedures are adopted to obtain an ideal preload and oxygen delivery, thereby improving the patients’ circulation and tissue support, and finally obtaining better prognosis [9,10]. Earlier studies have determined the application of GDFT in heart and gastrointestinal surgery and have achieved good practical results [11,12]. However, there is no research on the effect of GDFT in RLCS based on the concept of ERAS.
In this study, we analyzed the application value of GDFT in ERAS of patients undergoing RLCS, with the goal of finding more suitable schemes for postoperative fluid infusion treatment of patients undergoing RLCS to better improve their prognosis and reduce complications.
Materials and methods
A total of 74 patients undergoing elective RLCS based on the concept of enhance recovery after surgery (ERAS) in the Handan Central Hospital between December 2016 and December 2019 were enrolled and assigned into groups either treated by regular conventional liquids (regular group, n=34) or a group treated by goal-directed fluid (GDFT group, n=40) according to the fluid infusion scheme. All patients were selected from those who were diagnosed with lung cancer by pathological examination and met the surgical treatment criteria. The exclusion criteria of the patients: Patients with consciousness disorders, serious trauma, or other comorbid malignant tumor diseases, and those with serious infectious diseases. All patients consented to take part in the experiment and signed the written informed consent forms, and the experiment was approved by the Ethics Committee of our hospital and in line with the Declaration of Helsinki.
Treatment methods
Patients in the two groups were all treated with RLCS under the guidance of the concept of ERAS. Those in the regular group were given the following regular fluid infusion scheme: Each patient was injected with crystalloid fluid (Ringer’s solution and normal saline) and colloidal solution (hydroxyethyl starch) at 3:1, and the systolic blood pressure and heart rate were maintained at 100-150 mm Hg (1 mm Hg=0.133 k Pa) and 60-100 beats/min, respectively, under the premise of ensuring that their fluctuation degree was not over 20%, and the hemoglobin was maintained at 80-150 g/L and SpO2 >94%.
The fluid infusion scheme for the GDFT group: Firstly, each patient was injected with fluid according to the crystal/colloid ratio to the regular group, and the stroke volume (SV) increase ≥10% was selected as the standard for further fluid infusion. Liquid (200 mL) was injected within 10 min. When the SV increase of the patient was <10%, the fluid infusion was stopped. The CVP, MAP, and ScvSpO2 of the patient were maintained at 8-12 cm H2O (1 cm H2O=0.098 k Pa), 60-110 mm Hg, and >70%, respectively. Specific intervention measures: (1) If CVP reached 8-12 cm H2O, but MAP or ScvSpO2 did not reach the standards, a vasoactive agent was used for treatment; (2) If MAP <60 mm Hg, dopamine (10-15 μg·kg-1·min-1) was used to achieve MAP of 65-70 mm Hg; (3) If MAP >100 mm Hg, nitroglycerin (0.4-0.9 μ g kg-1min-1) was adopted to achieve MAP between 90-100 mm Hg; (4) If ScvSpO2 <70%, red blood cells were injected intravenously to achieve the hematocrit to be >30%; (5) If ScvSpO2 was still lower than 70%, dobutamine (5-20 μg kg-1 min-1) was used.
Outcome measures
We recorded and compared the fluid inflow and outflow during operation of the two groups, including the crystalloid fluid consumption, colloidal solution consumption, total infusion volume, urine output, and bleeding volume. (2) We recorded and compared the hemodynamic indexes, including SV, MAP and cardiac index, at 30 min (T0) before operation and at 4 h (T1) and 24 h (T2) after operation. (3) We recorded and compared the time to flatus and first time of eating after operation, getting out-of-bed time, and hospitalization time of the two groups. (4) We adopted ELISA to quantify serum inflammatory factors, including tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and interleukin-10 (IL-10) in the two groups before operation and at 1 d and 7 d after operation, and compared them between the two groups. (5) We recorded and compared the postoperative complications of two groups, including acute lung injury, lung infection, arrhythmia, and atelectasis. (6) We adopted the MOS 36-Item Short-Form Health Survey (QLQ-C30) to evaluate the life quality of patients at discharge [13]. The survey covers body function, role function, emotion function, cognitive function, and social function, and a higher score indicates better life quality.
Statistical analyses
In our study, the collected data were analyzed statistically with SPSS 19.0 and visualized into corresponding figures with GraphPad 7. Enumeration data, expressed as percentage, were analyzed using the chi-square test, and compared between groups by the independent t test and within groups before and after operation by the Paired T test. P<0.05 suggests a significant difference.
Results
Comparison of general data
There was no significant difference between the two groups in sex, age, and body mass index (BMI), smoking history, surgical history, hemoglobin and serum creatinine indicators (all P>0.05), so the two groups were comparable (Table 1).
Table 1.
General data
| Factor | The GDFT group n=40 | The regular group n=34 | t/X2 | P-value |
|---|---|---|---|---|
| Sex | 0.218 | 0.641 | ||
| Male | 21 (52.50) | 16 (47.06) | ||
| Female | 19 (47.50) | 18 (52.94) | ||
| Age (year) | 0.110 | 0.741 | ||
| ≤61 | 18 (45.00) | 14 (41.18) | ||
| >61 | 22 (55.00) | 20 (58.82) | ||
| BMI (kg/m2) | 0.020 | 0.889 | ||
| ≤23 | 23 (57.50) | 19 (55.88) | ||
| <23 | 17 (42.50) | 15 (44.12) | ||
| Smoking history | 0.390 | 0.532 | ||
| Yes | 28 (70.00) | 26 (76.47) | ||
| No | 12 (30.00) | 8 (23.53) | ||
| Surgical history | 0.039 | 0.844 | ||
| Yes | 15 (37.50) | 12 (35.29) | ||
| No | 25 (62.50) | 22 (46.81) | ||
| Hemoglobin (g/L) | 118.05±14.05 | 117.86±13.91 | 0.058 | 0.934 |
| Platelet count (×109/L) | 285.49±22.16 | 286.38±21.93 | 0.173 | 0.863 |
| Serum creatinine (umol/L) | 64.28±10.33 | 65.37±10.42 | 0.451 | 0.654 |
Comparison of intraoperative fluid inflow and outflow
Comparison of crystalloid fluid consumption, colloidal solution consumption, total infusion volume, urine output, and blood loss between the two groups revealed that there were significant differences between the two groups in crystalloid fluid consumption, total infusion volume, urine output (all P<0.05), but no significant difference between the colloidal solution consumption and blood loss (all P>0.05) (Table 2).
Table 2.
Comparison of intraoperative fluid inflow and outflow (ml)
| Item | The GDFT group n=40 | The regular group n=34 | t/X2 | P-value |
|---|---|---|---|---|
| Crystalloid fluid consumption | 584.01±85.43 | 811.36±105.48 | 10.24 | <0.001 |
| Colloidal solution consumption | 431.76±61.92 | 423.96±60.55 | 0.545 | 0.587 |
| Total infusion volume | 1015.77±211.85 | 1235.32±259.41 | 4.008 | <0.001 |
| Urine output | 155.82±21.73 | 228.69±32.44 | 11.50 | <0.001 |
| Blood loss | 78.59±10.24 | 79.32±10.33 | 0.304 | 0.762 |
Comparison of hemodynamic indexes between the two groups at different time points
We recorded and compared the SV, MAP and cardiac index of the two groups at 30 min (T0) before operation and 4 h (T1) and 24 h (T2) after operation. At T0, there was no significant difference between the two groups in SV, MAP, and cardiac index. The MAP of the regular group decreased notably at T1 compared with that at T0, and increased greatly at T2 compared with that at T1 (both P<0.05), but no significant difference was found between T2 and T0 in the MAP of the group (P>0.05). In contrast, the MAP of the GDFT group did not fluctuate notably. Moreover, the cardiac index of the regular group decreased notably at T1 compared with that at T0 (P<0.05), but no significant difference was found between T2 and T0 in the cardiac index of the regular group (P>0.05). The cardiac index of the GDFT group also decreased notably at T1 compared with that at T0, but the fluctuation range was smaller (P<0.05), and no significant difference was found between T2 and T0 in the cardiac index of the GDFT group (P>0.05). In terms of SV, the SV of the regular group decreased at T2 compared with that at T0 (P<0.05), but no significant difference was found between T1 and T0 in the SV of the group (P>0.05). The SV of the GDFT group also decreased notably at T2 compared with that at T0, but the fluctuation range was smaller (P<0.05), and no significant difference was found between T1 and T0 in SV of the GDFT group (P>0.05) (Figure 1).
Figure 1.

Comparison of hemodynamic indexes between two groups at different time points; A. Comparison of MAP between two groups of patients. B. Comparison of Cardiac index between two groups of patients. C. Comparison of SV between two groups of patients. * indicates P<0.05.
Comparison of postoperative recovery
We recorded and compared the postoperative recovery between the two groups, finding that the GDFT group experienced notably earlier time to flatus and getting out-of-bed time and notably shorter hospitalization than the regular group (all P<0.05) (Table 3).
Table 3.
Comparison of postoperative recovery between the two groups
| Item | The GDFT group n=40 | The regular group n=34 | t/X2 | P-value |
|---|---|---|---|---|
| Time to flatus | 1.21±0.31 | 2.42±0.42 | 14.23 | <0.001 |
| Getting out-of-bed time | 1.67±0.62 | 2.51±0.64 | 5.723 | <0.001 |
| Hospitalization time | 6.59±1.02 | 8.39±1.51 | 6.084 | <0.001 |
Comparison of serum inflammatory factors
We detected and compared the serum TNF-α, IL-6, and L-10 before operation and at 1 d and 7 d after operation between the two groups. As a result, before operation, there was no significant difference between the two groups in those factors (all P>0.05), while at 1 d after operation, serum TNF-α and IL-6 in both groups increased, and IL-10 in them decreased (all P<0.05), and the regular group showed notably higher serum TNF-α and IL-6 and notably lower IL-10 than the GDFT group (all P<0.05). Moreover, at 7 d after operation, these serum inflammatory factors in both groups showed no change ((All P>0.05) (Figure 2)).
Figure 2.
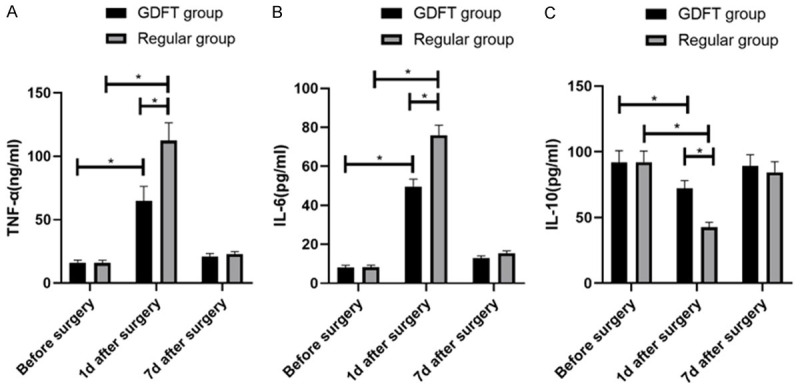
Comparison of serum inflammatory factors between the two groups. A. TNF-α expression comparison B. IL-6 expression comparison. C. IL-10 expression comparison. * indicates P<0.05.
Comparison of complications
We recorded and compared the complications of the two groups during treatment. According to the results, the GDFT group showed a complication rate of 7.50%, with 0 cases of acute lung injury, 1 case of lung infection, 1 case of arrhythmia, and 1 case of pulmonary atelectasis, while the regular group showed a complication rate of 26.47%, with 2 cases of acute lung injury, 3 cases of lung infection, 2 cases of arrhythmia, and 1 case of pulmonary atelectasis. Therefore, the GDFT group showed a notably lower complication rate than the regular group (P<0.05) (Table 4).
Table 4.
Comparison between the two groups in adverse reaction
| Complication | The GDFT group n=40 | The regular group n=34 | X2 | P-value |
|---|---|---|---|---|
| Acute lung injury | 0 | 2 (5.88) | 2.418 | 0.120 |
| Lung infection | 1 (2.50) | 3 (8.82) | 1.437 | 0.231 |
| Arrhythmia | 1 (2.50) | 3 (8.82) | 1.437 | 0.231 |
| Pulmonary atelectasis | 1 (2.50) | 1 (2.94) | 0.014 | 0.907 |
| The total incidence | 3 (7.50) | 9 (26.47) | 4.8686 | 0.027 |
Evaluation of the life quality of the two groups at 1 month after discharge
After discharge, the scores of role function, emotional function, physical function, cognitive function and social function of the GDFT group were all meaningfully higher than those of the regular group, so the GDFT group showed notably higher life quality than the regular group (P<0.05) (Table 5).
Table 5.
Life quality evaluation
| Item | The GDFT group n=40 | The regular group n=34 | t | P-value |
|---|---|---|---|---|
| Role function | 76.93±2.05 | 61.33±2.06 | 32.55 | <0.001 |
| Emotion function | 78.13±2.11 | 62.15±2.09 | 32.61 | <0.001 |
| Body function | 78.26±2.21 | 61.76±2.16 | 32.34 | <0.001 |
| Cognitive function | 80.17±3.38 | 65.42±2.37 | 21.36 | <0.001 |
| Social function | 78.93±2.25 | 63.26±2.21 | 30.10 | <0.001 |
Discussion
Since the ideal circulatory state during perioperative period was put forward for the first time in 1988, there has been a clear direction about GDFT, and studies with GDFT as the main fluid infusion concept are gradually increasing [14,15]. For instance, one study applied GDFT to head and neck surgery based on the concept of ERAS and found that compared with conventional fluid infusion methods, GDFT can reduce the liquid intake by 255 ml and obviously reduce complications [16]. Although many studies have confirmed the ability of GDFT in promoting the perioperative rehabilitation of patients, it remains to be explored whether it is equally applicable to patients undergoing RLCS.
To find a scheme more helpful to improve the postoperative rehabilitation of patients undergoing RLCS, we analyzed the application influence of GDFT based on the concept of ERAS in RLCS. First of all, we compared the liquid intake and output volume of the GDFT group and regular group during operations, and found that the crystalloid fluid consumption, total infusion volume and urine output of the GDFT group were significantly less, indicating that the GDFT scheme can effectively control the infusion volume and avoid improper fluid infusion. One earlier study has pointed out that improper clinical liquid-based therapy will not only compromise the cardiopulmonary function reserve ability of patients, especially elderly patients, but also further give rise to heart failure and various complications [17]. Afterwards, we compared the hemodynamic indexes of the two groups at different time points, and found that the hemodynamic indexes of the GDFT group fluctuated less, suggesting that the GDFT scheme could protect the patients from excessively high hemodynamic indexes that affect postoperative recovery. We also compared the postoperative recovery of two groups, and found that the GDFT group experienced notably earlier time to flatus and getting out-of-bed time and notably shorter hospitalization than the regular group. The results suggested that GDFT can not only optimize the hemodynamic indexes of patients, but also effectively promote their postoperative recovery. One previous study has explored the application of GDFT in elderly patients with bladder cancer [18], and discovered that GDFT can help stabilize the hemodynamic indexes of the patients, maintain microcirculation perfusion, and shorten patients’ hospitalization time, which are consistent with our observations.
Inflammatory reactions are a leading factor affecting the recovery of surgical patients. TNF-α and IL-6 are the main pro-inflammatory factors during inflammatory reaction, while IL-10 is an important anti-inflammatory cytokine secreted by M2 type macrophages, which can inhibit the occurrence of inflammatory reactions, and its level is inversely proportional to the intensity of inflammation [19,20]. According to our observation results, at 1 d after operation, the serum TNF-α and IL-6 in both groups increased notably, while IL-10 decreased notably, and the GDFT group showed notably lower serum TNF-α and IL-6 and higher IL-10 than the regular group. The results suggest that GDFT can strongly inhibit the occurrence of inflammatory reactions in the body during surgery, which is of great significance in reducing postoperative complications. In one earlier study [21], the application of GDFT in elderly patients with spinal stenosis was explored, and it was also found that GDFT can effectively inhibit the inflammatory reaction of patients during surgery. Moreover, we compared the postoperative complication rate and life quality between the two groups at 1 month after operation. We found that the GDFT group showed a significantly lower complication rate than the regular group after operation and experienced notably higher life quality than the regular group at 1 month after operation, which were consistent with the results we observed before.
To sum up, for patients undergoing RLCS, GDFT based on the concept of ERAS can effectively reduce patient-related complications and promote postoperative recovery, so it is worthy of popularization. However, the sample size of this study is small, so more specific application conditions and detection index selection of GDFT need further exploration.
Acknowledgements
This study is financially supported by Effect of goal-oriented liquid therapy on ERAS and early cognitive function in radical lung cancer surgery (20212173).
Disclosure of conflict of interest
None.
References
- 1.Zamarron E, Prats E, Tejero E, Pardo P, Galera R, Casitas R, Martinez-Ceron E, Romera D, Jaureguizar A, Garcia-Rio F. Static lung hyperinflation is an independent risk factor for lung cancer in patients with chronic obstructive pulmonary disease. Lung Cancer. 2019;128:40–46. doi: 10.1016/j.lungcan.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 2.Walter JE, Heuvelmans MA, de Bock GH, Yousaf-Khan U, Groen HJM, van der Aalst CM, Nackaerts K, van Ooijen PMA, de Koning HJ, Vliegenthart R, Oudkerk M. Relationship between the number of new nodules and lung cancer probability in incidence screening rounds of CT lung cancer screening: the NELSON study. Lung Cancer. 2018;125:103–108. doi: 10.1016/j.lungcan.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Zhong L, Suo J, Wang Y, Han J, Zhou H, Wei H, Zhu J. Prognosis of limited-stage small cell lung cancer with comprehensive treatment including radical resection. World J Surg Oncol. 2020;18:27. doi: 10.1186/s12957-020-1807-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aokage K, Yoshida J, Hishida T, Tsuboi M, Saji H, Okada M, Suzuki K, Watanabe S, Asamura H. Limited resection for early-stage non-small cell lung cancer as function-preserving radical surgery: a review. Jpn J Clin Oncol. 2017;47:7–11. doi: 10.1093/jjco/hyw148. [DOI] [PubMed] [Google Scholar]
- 5.Shi Q, Diao Y, Qian J. Application of single-hole thoracoscopic surgery combined with ERAS concept for respiratory function exercise in perioperative period of lung cancer. Zhongguo Fei Ai Za Zhi. 2020;23:667–672. doi: 10.3779/j.issn.1009-3419.2020.101.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamada T, Mukai N, Tsuchida K, Hayashi K. Use and safety of preoperative oral rehydration therapy using a jelly type oral rehydration solution. Masui. 2015;64:379–382. [PubMed] [Google Scholar]
- 7.Kellum JA, Mythen MG, Shaw AD. The 12th consensus conference of the acute dialysis quality initiative (ADQI XII) Br J Anaesth. 2014;113:729–731. doi: 10.1093/bja/aeu140. [DOI] [PubMed] [Google Scholar]
- 8.Lu Y, Zhang H, Teng F, Xia WJ, Sun GX, Wen AQ. Early goal-directed therapy in severe sepsis and septic shock: a meta-analysis and trial sequential analysis of randomized controlled trials. J Intensive Care Med. 2018;33:296–309. doi: 10.1177/0885066616671710. [DOI] [PubMed] [Google Scholar]
- 9.Arslan-Carlon V, Tan KS, Dalbagni G, Pedoto AC, Herr HW, Bochner BH, Cha EK, Donahue TF, Fischer M, Donat SM. Goal-directed versus standard fluid therapy to decrease ileus after open radical cystectomy: a prospective randomized controlled trial. Anesthesiology. 2020;133:293–303. doi: 10.1097/ALN.0000000000003367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Froghi F, Soggiu F, Ricciardi F, Gurusamy K, Martin DS, Singh J, Siddique S, Eastgate C, Ciaponi M, McNeil M, Filipe H, Schwalowsky-Monks O, Asis G, Varcada M, Davidson BR. Ward-based goal-directed fluid therapy (GDFT) in acute pancreatitis (GAP) trial: study protocol for a feasibility randomised controlled trial. BMJ Open. 2019;9:e028783. doi: 10.1136/bmjopen-2018-028783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wrzosek A, Jakowicka-Wordliczek J, Zajaczkowska R, Serednicki WT, Jankowski M, Bala MM, Swierz MJ, Polak M, Wordliczek J. Perioperative restrictive versus goal-directed fluid therapy for adults undergoing major non-cardiac surgery. Cochrane Database Syst Rev. 2019;12:CD012767. doi: 10.1002/14651858.CD012767.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin J, Min S, Liu D, Liu L, Lv B. Clinical and economic impact of goal-directed fluid therapy during elective gastrointestinal surgery. Perioper Med (Lond) 2018;7:22. doi: 10.1186/s13741-018-0102-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hagiwara Y, Shiroiwa T, Taira N, Kawahara T, Konomura K, Noto S, Fukuda T, Shimozuma K. Mapping EORTC QLQ-C30 and FACT-G onto EQ-5D-5L index for patients with cancer. Health Qual Life Outcomes. 2020;18:354. doi: 10.1186/s12955-020-01611-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Khafaji A, Rivers E, Shoemaker W. The prospective trial of supranormal values of survivors as therapeutic goals in high-risk surgical patients. Article of Shoemaker et al with expert commentary by Dr. Emanuel rivers. 1988. J Crit Care. 2008;23:603–606. doi: 10.1016/j.jcrc.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Bundgaard-Nielsen M, Secher NH, Kehlet H. ‘Liberal’ vs. ‘restrictive’ perioperative fluid therapy--a critical assessment of the evidence. Acta Anaesthesiol Scand. 2009;53:843–851. doi: 10.1111/j.1399-6576.2009.02029.x. [DOI] [PubMed] [Google Scholar]
- 16.Coyle MJ, Main B, Hughes C, Craven R, Alexander R, Porter G, Thomas S. Enhanced recovery after surgery (ERAS) for head and neck oncology patients. Clin Otolaryngol. 2016;41:118–126. doi: 10.1111/coa.12482. [DOI] [PubMed] [Google Scholar]
- 17.Rollins KE, Mathias NC, Lobo DN. Meta-analysis of goal-directed fluid therapy using transoesophageal Doppler monitoring in patients undergoing elective colorectal surgery. BJS Open. 2019;3:606–616. doi: 10.1002/bjs5.50188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voldby AW, Aaen AA, Moller AM, Brandstrup B. Goal-directed fluid therapy in urgent gastrointestinal surgery-study protocol for a randomised multicentre trial: the GAS-ART trial. BMJ Open. 2018;8:e022651. doi: 10.1136/bmjopen-2018-022651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schilling T, Kozian A, Senturk M, Huth C, Reinhold A, Hedenstierna G, Hachenberg T. Effects of volatile and intravenous anesthesia on the alveolar and systemic inflammatory response in thoracic surgical patients. Anesthesiology. 2011;115:65–74. doi: 10.1097/ALN.0b013e318214b9de. [DOI] [PubMed] [Google Scholar]
- 20.Gao W, Liu DD, Li D, Cui GX. Effect of therapeutic hypercapnia on inflammatory responses to one-lung ventilation in lobectomy patients. Anesthesiology. 2015;122:1235–1252. doi: 10.1097/ALN.0000000000000627. [DOI] [PubMed] [Google Scholar]
- 21.Zhang N, Liang M, Zhang DD, Xiao YR, Li YZ, Gao YG, Cai HD, Lin XZ, Lin CZ, Zeng K, Wu XD. Effect of goal-directed fluid therapy on early cognitive function in elderly patients with spinal stenosis: a case-control study. Int J Surg. 2018;54:201–205. doi: 10.1016/j.ijsu.2018.04.007. [DOI] [PubMed] [Google Scholar]


