Abstract
Objective: This study aimed to explore the relationship between peripheral blood miR-29a and sepsis-related encephalopathy (SAE). Methods: A total of 120 patients with sepsis admitted to our hospital from March 2018 to October 2019 were selected as research subjects. They were divided into a SAE group (30 cases) and an unrelated encephalopathy group (90 cases) according to whether the patients were complicated with SAE. The levels of miR-29a in the peripheral blood, neuron-specific enolase (NSE), S100β calcium binding protein (S100β) and interleukin-6 (IL-6) in serum were determined, and the relationship between miR-29a in the peripheral blood and the diagnosis and prognosis prediction in SAE patients was analyzed. Results: Compared with the unrelated encephalopathy group, the levels of miR-29a in peripheral blood, NSE, S100β and IL-6 in serum of patients in the SAE group were elevated notably. miR-29a in the peripheral blood, and NSE, S100β, IL-6 in the serum of patients who died and survived within 28 days were detected, and the levels of these four indexes in the death group were significantly higher than those in the survival group. Correlation analysis revealed that miR-29a in the peripheral blood was positively correlated with the levels of NSE, S100β and IL-6 in serum. According to Receiver Operating Characteristic (ROC) curve analysis, miR-29a in the peripheral blood can be used as a potential biomarker to predict whether sepsis is complicated with SAE and the relative prognosis. Conclusion: miR-29a is closely associated with the development of SAE, and miR-29a in the peripheral blood can be used as a potential biological index to predict whether sepsis is complicated with SAE and indications of a poor prognosis.
Keywords: miR-29a, sepsis, diagnosis, prognosis
Introduction
Sepsis is the leading cause of death in both medical and surgical intensive care units (ICU). In most cases, impaired consciousness of varying severity is an early warning sign of the development of sepsis. Sepsis-associated encephalopathy (SAE) is the most common type of encephalopathy in the ICU and it is defined as a state of diffuse brain dysfunction caused by inflammatory responses to various infections, in which the inflammatory process does not directly affect the symptoms of the central nervous system, and the main symptom is impaired consciousness [1]. Most patients develop SAE symptoms before meeting the criteria of sepsis, which ranges from illness behaviors and acute phase symptoms in delirium coma, and can lead to long-term cognitive impairment [2]. In addition, SAE has been identified as associated with increased mortality, high hospitalization costs, and prolonged length of stay, followed by persistent cognitive deficits and limitations in physical effects [3]. Therefore, early identification and timely intervention of brain injury are crucial for the survival and prognosis of sepsis patients.
miRNA is a small non-coding RNA molecule that is considered to be related to the occurrence and progression of various tumors, which can regulate post-transcriptional levels by binding to the 3’-UTR of target mRNA [4]. miRNA biogenesis is dynamic and has great diversity, and maladjusted miRNA plays a key part in the progress of various diseases [5]. Circulating miRNA can also be used as a potential biomarker for the diagnosis and prognosis of various diseases, as well as other known diseases and symptoms [6]. miR-29a, for example, exhibits its specificity in pancreatic tumor cells because of its up-regulation during proliferation and metastasis [7]. It is also suggested that miR-29 expression is down-regulated in cervical cancer tissues compared with tissues from the control group [8]. As a tumor suppressor, miR-29a can inhibit the progress of thyroid tumors, hepatocellular carcinoma and glioma [9]. Hence, it can be seen that miR-29a may play an important part in the regulation of various diseases. In this article, we focused on the expression and application value of miR-29a in SAE.
Materials and methods
General data
A total of 120 patients with sepsis admitted to The First People’s Hospital of Chenzhou from March 2018 to October 2019 were recruited as research subjects. They were divided into a SAE group (30 cases) and an unrelated encephalopathy group (90 cases) according to whether they were complicated with SAE.
Inclusion and exclusion criteria
Inclusion criteria
Patients met the diagnostic criteria of sepsis or SAE [10,11]; patients agreed and signed the informed consent. This experiment was approved by the Ethics Committee of our hospital.
Exclusion criteria
Patients who underwent cardiopulmonary cerebral resuscitation; patients who were complicated with cerebrovascular accidents; patients who were complicated with intracranial lesions; and patients who were complicated with other metabolic encephalopathy.
Main instruments
The main instruments used in this study included: centrifuge (Wuhan Khayal Bio-Technology Co., Ltd.), mirVanaTM miRNA Isolation Kit (Shanghai Huzhen Industrial Co., Ltd.), ultraviolet and visible spectrophotometer (Wuhan Chundu Biotechnology Co., Ltd.), TaqMan MicroRNA reverse transcription kit (Beijing Biolab Technology Co., Ltd.), real-time fluorescence quantification PCR system (Shenzhen Enco Science and Technology Co., Ltd.), and miRNA RT-qPCR research kit (Shanghai Genetimes Biotechnology Co., Ltd.). miR-29a and internal reference primer synthesis were provided by Shanghai Beyotime Biotechnology Co., Ltd., as shown in Table 1.
Table 1.
Primer sequence
| Upstream primer (5’-3’) | Downstream primer (5’-3’) | |
|---|---|---|
| miR-29a | CTGCTGATCATGGGCCTCCT | CTCCACAGGCTCGGGTTGTT |
| U6 | CTCGCTTCGGCAGCACA | AACGCTTCACGAATTTGCGT |
Methods
Five ml of fasting venous blood were taken from both groups of patients early in the morning. The serum was separated by centrifugation (10×g at 4°C for 15 min) after standing for 20 min and stored at -80°C under liquid nitrogen freezing. Serum neuron-specific enolase (NSE), S100 calcium binding protein β (S100β) and Interleukin-6 (IL-6) levels were determined by enzyme-linked immunosorbent assay (ELISA). The total RNA was extracted according to the instructions of mirVanaTM miRNA Isolation Kit. The concentration of RNA was measured by ultraviolet and visible spectrophotometer. According to TaqMan MicroRNA reverse transcription kit, RNA was reverse transcribed into cDNA, and PCR amplification experiment was conducted with cDNA as template and U6 as internal reference gene. Based on the miRNA RT-qPCR research kit, miR-29a was quantitatively determined by real-time fluorescence quantitative PCR system. PCR amplification cycle conditions were as follows: 95°C for 30 s, 95°C for 5 s, and 60°C for 30 s, with a total of 40 cycles. Three duplicate wells were set in each group, and the experiment was repeated three times. The results were expressed by 2-ΔΔCT.
Statistical analysis
SPSS 24.0 (IBM Corp, Armonk, NY, USA) was applied for statistical analysis, and GraphPad Prism 8 (GraphPad Software, San Diego, USA) was used for data plotting. The t-test was used to represent the measurement data, expressed as mean ± standard deviation (x̅±sd). The count data were expressed as number of cases/percentage [n (%)], and the chi-square test was applied for comparison between groups. The diagnostic value of serum miR-29a in patients with SAE was analyzed by the Receiver Operating Characteristic (ROC) curve. The correlation was analyzed by Pearson Correlation Coefficient. P<0.05 was considered statistically significant.
Results
General data of patients in the two groups
There was no statistically significant difference in age, gender, living habits and other aspects between the two groups (P>0.05), as shown in Table 2.
Table 2.
Comparison of general data between the two groups [n (%)] (x̅±sd)
| Classification | SAE group (n=30) | Unrelated encephalopathy group (n=90) | t/χ2 value | p value |
|---|---|---|---|---|
| Gender | 0.342 | 0.900 | ||
| Male | 17 (56.67) | 42 (46.67) | ||
| Female | 13 (43.33) | 48 (53.33) | ||
| Age (years) | 57.61±4.16 | 56.91±4.85 | 0.708 | 0.480 |
| Height (cm) | 171.61±6.91 | 172.14±6.17 | 0.596 | 0.551 |
| Weight (kg) | 54.62±4.62 | 55.19±4.18 | 0.629 | 0.530 |
| Hypertension history | 0.878 | 0.348 | ||
| Present | 4 (13.33) | 19 (21.11) | ||
| Absent | 26 (86.67) | 71 (78.89) | ||
| Diabetes history | 0.658 | 0.417 | ||
| Present | 7 (23.33) | 28 (31.11) | ||
| Absent | 23 (76.67) | 62 (68.89) | ||
| Smoking | 0.117 | 0.732 | ||
| Present | 20 (66.67) | 63 (70.00) | ||
| Absent | 10 (33.33) | 27 (30.00) | ||
| Drinking | 0.378 | 0.538 | ||
| Present | 24 (80.00) | 67 (74.44) | ||
| Absent | 6 (20.00) | 23 (25.56) | ||
| Days in ICU | 4.51±1.15 | 3.97±146 | 1.842 | 0.067 |
| Death within 28 days | 7 (23.33) | 10 (11.11) | 2.764 | 0.096 |
miR-29a expression in the two groups
miR-29a in SAE and unrelated encephalopathy groups were (1.46±0.12) and (1.17±0.15), respectively. Compared with the unrelated encephalopathy group, miR-29a in the peripheral blood of patients in the SAE group was significantly elevated (P<0.05), as shown in Figure 1.
Figure 1.
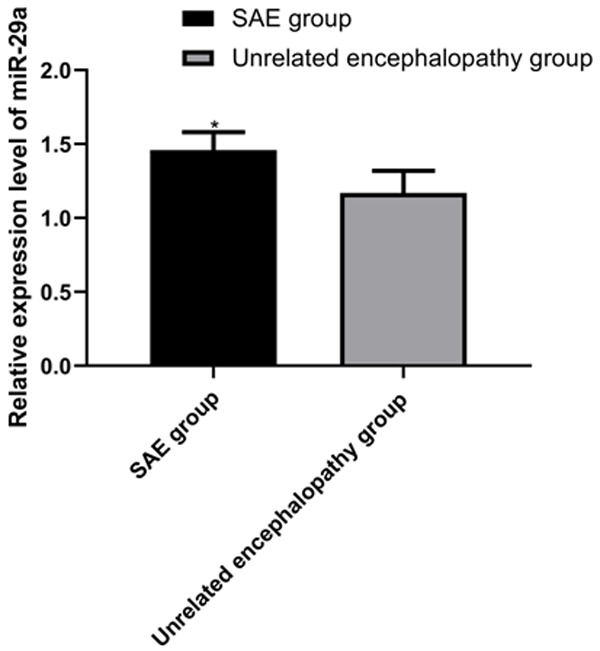
miR-29a expression in the two groups. According to the results, compared with the unrelated encephalopathy group, miR-29a in peripheral blood of patients in the SAE group elevates markedly (P<0.05), Note: * vs. the unrelated encephalopathy group (P<0.05).
NSE, S100β, and IL-6 expression in the two groups
In the SAE group, the expression of NSE, S100β, and IL-6 were (10.16±2.11), (0.27±0.06), and (229.15±29.61), respectively. In the unrelated encephalopathy group, the expression of NSE, S100β, and IL-6 were (8.62±1.62), (0.18±0.04), and (196.71±18.62), respectively. The above results revealed that NSE, S100β and IL-6 levels were significantly higher in the SAE group than in the unrelated encephalopathy group (P<0.05). More details are shown in Figure 2.
Figure 2.
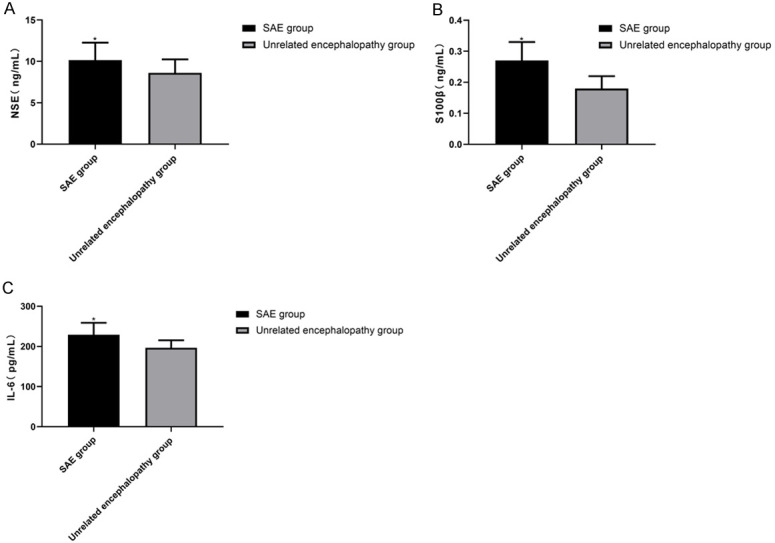
NSE, S100β, and IL-6 expression in the two groups. According to the results, NSE, S100β and IL-6 levels are notably higher in the SAE group than in the unrelated encephalopathy group (P<0.05). A. NSE expression in the two groups. B. S100β expression in the two groups. C. IL-6 expression in the two groups. Note: * vs. the unrelated encephalopathy group (P<0.05).
miR-29a expression in the dead and surviving patients within 28 days
miR-29a expression in patients who died and survived for 28 days were (1.54±0.19) and (1.28±0.15), respectively. Compared with the surviving patients, miR-29a in dead patients was considerably higher (P<0.05). More details are shown in Figure 3.
Figure 3.
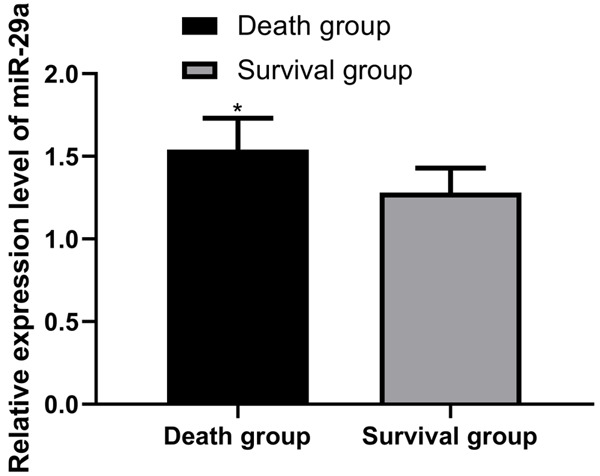
miR-29a expression in the dead and surviving patients within 28 days. Compared with the surviving patients, the index level of the dead patients was considerably higher (P<0.05). Note: * vs. the survival group (P<0.05).
NSE, S100β and IL-6 expression in the dead and surviving patients within 28 days
In dead patients, the expression of NSE, S100β and IL-6 were (13.41±2.18), (0.30±0.08), and (238.16±27.62), respectively. In the surviving patients, however, the expression of NSE, S100β and IL-6 were (10.17±1.61), (0.21±0.06), and (216.81±24.16), respectively. Compared with the survival group, these levels in the death group were significantly higher (P<0.05). More details are shown in Figure 4.
Figure 4.
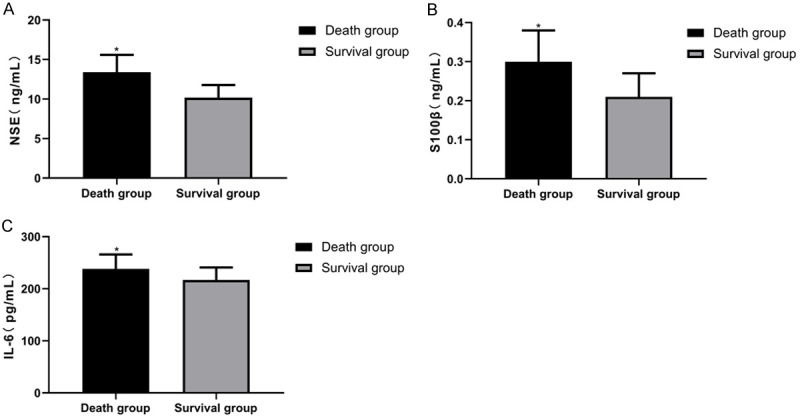
NSE, S100β and IL-6 expression in the dead and surviving patients within 28 days. Compared with the survival group, the levels of NSE, S100β and IL-6 in the death group are considerably higher (P<0.05). A. NSE expression in dead and surviving patients within 28 days. B. S100β expression in the dead and surviving patients within 28 days. C. IL-6 expression in the dead and surviving patients within 28 days. Note: * vs. the survival group (P<0.05).
Correlation analysis of miR-29a levels with NSE, S100β and IL-6 levels
We applied Pearson Correlation Coefficient for analysis, and found that miR-29a in the peripheral blood was markedly positively associated with the levels of NSE, S100β and IL-6 in serum (r=0.599, 0.659, 0.520; P<0.05). More details are shown in Figure 5.
Figure 5.
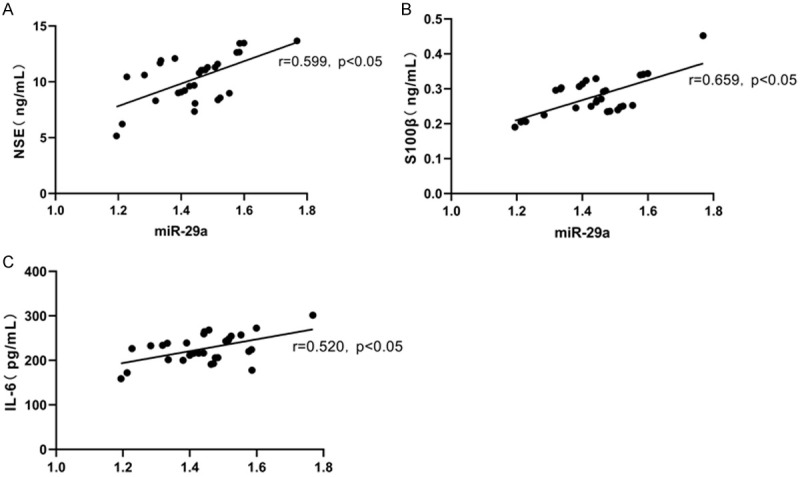
Correlation analysis of miR-29a levels with NSE, S100β and IL-6 levels. miR-29a in the peripheral blood is significantly positively associated with of NSE, S100β and IL-6 in serum (r=0.599, P=0.0005; r=0.659, P<0.0001; r=0.520, P=0.0032). A. Correlation analysis of peripheral blood miR-29a levels with serum NSE levels. B. Correlation analysis of peripheral blood miR-29a levels with serum S100β levels. C. Correlation analysis of peripheral blood miR-29a levels with serum IL-6 levels.
Value of miR-29a for diagnosing SAE
By plotting the ROC curves, it was found that the area under the curve (AUC) of miR-29a for the diagnosis of SAE was 0.872, as shown in Figure 6 and Table 3.
Figure 6.
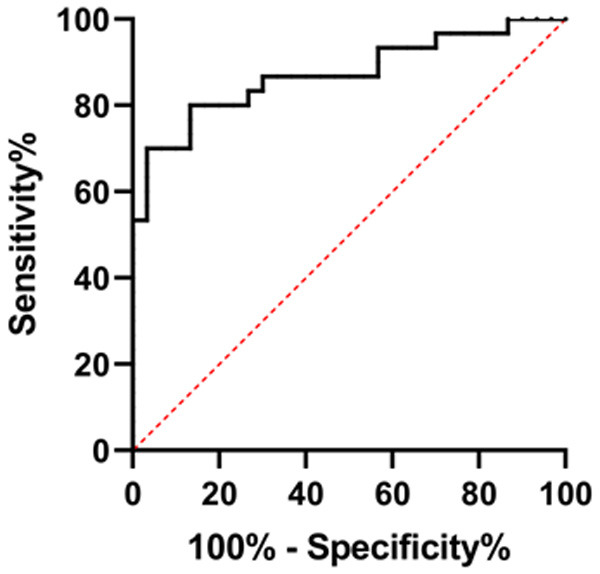
ROC curve of miR-29a in diagnosing SAE patients.
Table 3.
ROC parameters of miR-29a in diagnosing SAE patients
| Index | AUC | 95% CI | S.E | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| miR-29a | 0.872 | 0.7796 to 0.9649 | 0.047 | 73.33 | 86.67 |
Prognostic value of miR-29a for indicating a poor prognosis in SAE patients
By plotting the ROC curve, it was found that the AUC of miR-29a predicting poor prognosis was 0.876, as detailed in Figure 7 and Table 4.
Figure 7.
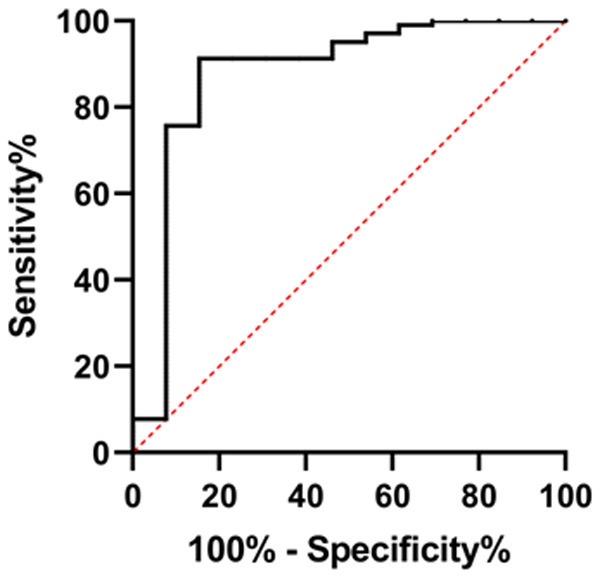
ROC curve of miR-29a in predicting poor patient prognosis.
Table 4.
ROC parameters of miR-29a in predicting poor patient prognosis
| Index | AUC | 95% CI | S.E | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| miR-29a | 0.876 | 0.7436 to 1.000 | 0.067 | 75.73 | 84.62 |
Discussion
Approximately 70% of patients with SAE develop severe polyneuropathy and myopathy, manifesting as generalized weakness, within days to weeks after the onset of sepsis [12]. SAE affects about one third of sepsis patients and is a risk factor for long-term disability and death [13]. miR-29a is a conserved miRNA involved in the regulation of several coordinated post-transcriptional programs that affect different biological processes [14]. It has been found to effectively suppress the development of many human diseases [15]. For example, it has been reported that miR-29b can act as a tumor suppressor, and its down-regulation enhances the metastasis of endometrial cancer cells [16]. There are also studies showing that exosomes derived from mesenchymal stem cells regulate angiogenesis and osteogenesis, while exosomes derived from mesenchymal stem cells loaded with miR-29a may become potential therapeutic targets for osteoporosis [17]. In this study, we observed that miR-29a level was significantly elevated in the peripheral blood of patients in the SAE group, suggesting that miR-29a may participate in the pathogenesis of SAE, and its differential expression between SAE patients and those without SAE may hold promise as a specific and sensitive marker for the indication of SAE in patients. In this case, we drew the ROC curve of serum miR-29a in diagnosing SAE patients. The AUC of miR-29a in diagnosing SAE patients was 0.872, suggesting that miR-29a may have great potential as a molecular marker for the diagnosis of SAE patients.
The development of illness behavior may be associated with proinflammatory factors [18]. NSE is an intracellular glycolytic enzyme which is mainly expressed by neurons, and can be detected in serum after neuronal injury [19]. Elevated levels of NSE in the first few days after cardiac arrest may indicate a sustained release of NSE, which may reflect an association between persistent secondary neurological injury and poor prognosis [20]. S100β is a protein of the S-100 protein family. It is glial-specific and mainly expressed by mature astrocytes [21]. Elevated S100β levels can accurately reflect the existence of neuropathological conditions, including craniocerebral trauma and neurodegenerative diseases [22]. IL-6 is a typical cytokine to maintain balance in vivo. When the steady state is disrupted by infection or tissue damage, IL-6 will be produced immediately, which helps the host resist this emergency pressure by activating an acute phase of the immune response [23]. However, the maladjusted excessive or continuously synthesized IL-6 can have pathological effects on the acute systemic inflammatory response syndrome and chronic immune-mediated diseases, respectively [24,25]. In this study, NSE, S100β and IL-6 increased in SAE, and Pearson correlation coefficient analysis revealed a positive relationship between miR-29a and them, suggesting that NSE, S100β, IL-6 and miR-29a may have synergistic effects on the occurrence and progression of diseases in SAE patients. However, the specific regulatory mechanisms of NSE, S100β, IL-6 and miR-29a in patients with SAE have not been elaborated in clinical practice. Subsequent experiments revealed that the levels of NSE, S100β, IL-6 and miR-29a in dead patients were remarkably higher than those in the surviving patients within 28 days. The above data suggested that the continuous increase of miR-29a levels may be related to a poor prognosis of SAE patients. We also analyzed the value of miR-29a in predicting a poor prognosis in SAE patients by ROC curves, and obtained an AUC of 0.876, suggesting that miR-29a may have a higher efficacy in predicting poor prognosis in SAE patients. Therefore, the detected expression of serum miR-29a may be a reliable biomarker for early diagnosis and prognosis prediction of SAE patients, but the specific regulation mechanism needs further discussion.
The current experiment is still deficient. We did not explore the specific mechanism of the effect between NSE, S100β, IL-6 and miR-29a, nor did we explore the effect of miR-29a on the cellular and biological functions of SAE patients. We will continue to study and improve the results.
In summary, miR-29a is highly expressed in SAE patients, and serum miR-29a may be used as a molecular marker for early diagnosis and prognosis prediction of SAE patients.
Acknowledgements
This study is financially supported by the Chenzhou Science and Technology Project Fund (ZDYF2020107).
Disclosure of conflict of interest
None.
References
- 1.Molnar L, Fulesdi B, Nemeth N, Molnar C. Sepsis-associated encephalopathy: a review of literature. Neurol India. 2018;66:352–361. doi: 10.4103/0028-3886.227299. [DOI] [PubMed] [Google Scholar]
- 2.Chung HY, Wickel J, Brunkhorst FM, Geis C. Sepsis-associated encephalopathy: from delirium to dementia? J Clin Med. 2020;9:703. doi: 10.3390/jcm9030703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ren C, Yao RQ, Zhang H, Feng YW, Yao YM. Sepsis-associated encephalopathy: a vicious cycle of immunosuppression. J Neuroinflammation. 2020;17:14. doi: 10.1186/s12974-020-1701-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang JY, Zhang Q, Wang DD, Yan W, Sha HH, Zhao JH, Yang SJ, Zhang HD, Hou JC, Xu HZ, He YJ, Hu JH, Zhong SL, Tang JH. MiR-29a: a potential therapeutic target and promising biomarker in tumors. Biosci Rep. 2018;38:BSR20171265. doi: 10.1042/BSR20171265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen L, Heikkinen L, Wang C, Yang Y, Sun H, Wong G. Trends in the development of miRNA bioinformatics tools. Brief Bioinform. 2019;20:1836–1852. doi: 10.1093/bib/bby054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Correia de Sousa M, Gjorgjieva M, Dolicka D, Sobolewski C, Foti M. Deciphering miRNAs’ action through miRNA editing. Int J Mol Sci. 2019;20:6249. doi: 10.3390/ijms20246249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dooley J, Lagou V, Garcia-Perez JE, Himmelreich U, Liston A. miR-29a-deficiency does not modify the course of murine pancreatic acinar carcinoma. Oncotarget. 2017;8:26911–26917. doi: 10.18632/oncotarget.15850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zamani S, Sohrabi A, Hosseini SM, Rahnamaye-Farzami M, Akbari A. Deregulation of miR-21 and miR-29a in cervical cancer related to HPV infection. Microrna. 2019;8:110–115. doi: 10.2174/2211536607666181017124349. [DOI] [PubMed] [Google Scholar]
- 9.An Q, Liu T, Wang MY, Yang YJ, Zhang ZD, Lin ZJ, Yang B. circKRT7-miR-29a-3p-COL1A1 axis promotes ovarian cancer cell progression. Onco Targets Ther. 2020;13:8963–8976. doi: 10.2147/OTT.S259033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 12.Helbing DL, Bohm L, Witte OW. Sepsis-associated encephalopathy. CMAJ. 2018;190:E1083. doi: 10.1503/cmaj.180454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heming N, Mazeraud A, Verdonk F, Bozza FA, Chretien F, Sharshar T. Neuroanatomy of sepsis-associated encephalopathy. Crit Care. 2017;21:65. doi: 10.1186/s13054-017-1643-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dai Y, Chen Z, Zhao W, Cai G, Wang Z, Wang X, Hu H, Zhang Y. miR-29a-5p regulates the proliferation, invasion, and migration of gliomas by targeting DHRS4. Front Oncol. 2020;10:1772. doi: 10.3389/fonc.2020.01772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang T, Sui D, You D, Yao S, Zhang L, Wang Y, Zhao J, Zhang Y. MiR-29a-5p inhibits proliferation and invasion and induces apoptosis in endometrial carcinoma via targeting TPX2. Cell Cycle. 2018;17:1268–1278. doi: 10.1080/15384101.2018.1475829. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Habata S, Iwasaki M, Sugio A, Suzuki M, Tamate M, Satohisa S, Tanaka R, Saito T. BAG3 increases the invasiveness of uterine corpus carcinoma cells by suppressing miR-29b and enhancing MMP2 expression. Oncol Rep. 2015;33:2613–2621. doi: 10.3892/or.2015.3831. [DOI] [PubMed] [Google Scholar]
- 17.Lu GD, Cheng P, Liu T, Wang Z. BMSC-derived exosomal miR-29a promotes angiogenesis and osteogenesis. Front Cell Dev Biol. 2020;8:608521. doi: 10.3389/fcell.2020.608521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shulyatnikova T, Verkhratsky A. Astroglia in sepsis associated encephalopathy. Neurochem Res. 2020;45:83–99. doi: 10.1007/s11064-019-02743-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mastroianni A, Panella R, Morelli D. Invisible hemolysis in serum samples interferes in NSE measurement. Tumori. 2020;106:79–81. doi: 10.1177/0300891619867836. [DOI] [PubMed] [Google Scholar]
- 20.Chung-Esaki HM, Mui G, Mlynash M, Eyngorn I, Catabay K, Hirsch KG. The neuron specific enolase (NSE) ratio offers benefits over absolute value thresholds in post-cardiac arrest coma prognosis. J Clin Neurosci. 2018;57:99–104. doi: 10.1016/j.jocn.2018.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michetti F, D’Ambrosi N, Toesca A, Puglisi MA, Serrano A, Marchese E, Corvino V, Geloso MC. The S100B story: from biomarker to active factor in neural injury. J Neurochem. 2019;148:168–187. doi: 10.1111/jnc.14574. [DOI] [PubMed] [Google Scholar]
- 22.Czupryna P, Grygorczuk S, Pancewicz S, Swierzbinska R, Zajkowska J, Krawczuk K, Dunaj J, Filipiuk J, Kruszewska E, Borawski K, Moniuszko-Malinowska A. Evaluation of NSE and S100B in patients with tick-borne encephalitis. Brain Behav. 2018;8:e01160. doi: 10.1002/brb3.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rose-John S. Interleukin-6 family cytokines. Cold Spring Harb Perspect Biol. 2018;10:a028415. doi: 10.1101/cshperspect.a028415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka T, Narazaki M, Kishimoto T. Interleukin (IL-6) immunotherapy. Cold Spring Harb Perspect Biol. 2018;10:a028456. doi: 10.1101/cshperspect.a028456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akbari M, Hassan-Zadeh V. IL-6 signalling pathways and the development of type 2 diabetes. Inflammopharmacology. 2018;26:685–698. doi: 10.1007/s10787-018-0458-0. [DOI] [PubMed] [Google Scholar]


