Abstract
Introduction: Some patients with idiopathic membranous nephropathy (iMN) do not respond to cyclophosphamide plus steroids treatment, and we define them as non-responsive iMN. The combined regimen of rituximab (RTX) and tacrolimus (TAC) has an excellent effect on this kind of non-responsive iMN patients; however, the optimal dose is still unclear. In this retrospective study, we comapred the efficacy and safety of ultra-low dose RTX plus low-dose TAC therapy versus standard TAC monotherapy in patients with non-responsive iMN. Materials and methods: Sixty-seven Chinese non-responsive iMN patients were included. There were 41 patients received standard tacrolimus monotherapy (TAC) and 26 patients received ultra-low dose rituximab plus low dose tacrolimus (RTX/TAC) combination therapy. All patients were observed for 12 months. Results: 18 patients (18/26, 69.2%) in the RTX/TAC group and 17 patients (17/41, 41.5%) in the TAC group achieved clinical response after 12-month follow-up (P=0.044). The median time for achieving response in the two groups was 3.0 months. As indicated by Kaplan-Meier curve, the response rate in the RTX/TAC group was higher than that in the TAC group (P=0.015). 24-hour proteinuria, serum albumin, estimated glomerular filtration rate (eGFR) and serum creatinine in the two groups were comparable at baseline; howerver, after 12-month follow up, they were significantly improved in the RTX/TAC group compared with the TAC group (P<0.05). B-cell depletion was achieved in all patients in the RTX/TAC group during the whole follow-up period. Pneumonia, urinary tract infections and glucose intolerance were the major side effects observed in this study. All adverse events were mild, and the cumulative incidence was lower in the RTX/TAC group compared with that in the TAC group (9 (34.6%) vs 27 (65.9%), P=0.023). Conclusion: The combination of ultra-low dose rituximab and low dose tacrolimus is more effective in inducing proteinuria response, improving eGFR and serum albumin in non-responsive iMN patients than standard tacrolimus monotherapy. The combined treatment also has higher safty.
Keywords: Rituximab, tacrolimus, non-responsive, idiopathic membranous nephropathy, low-dose
Introduction
Membranous nephropathy (MN) is the most common cause of the nephrotic syndrome in nondiabetic adults. Due to air pollution and increasing cases of renal biopsy, the prevalence of idiopathic membranous nephropathy (iMN) has dramatically increased in China in recent years [1]. For patients who are at high risk of progression (namely the patients who have the proteinuria above 4000 mg/d and fail to respond to a 6-month treatment with antihypertensive and antiproteinuric agents), combination therapy of a cyclophosphamide and glucocorticoids is the preferred initial therapy [2,3]. Studies over ten years in iMN patients treated with corticosteroids combined with cyclophosphamide have reported that about 10% patients would be non-responsive to the classic regimens and were very likely to progress to ESRD [4,5]. For these non-responsive patients, it is urgent to figure out the iMN pathogenesis and look for more effective therapies.
iMN is an antibody-mediated disease induced by deposits of immunoglobulins and complement components on the subepithelial layer of the glomerular capillary wall [6]. Antibodies from lymphocytes (mainly B lymphocytes) in situ bind to antigen in target cells, which leads to abnormal immunoreaction, resulting in glomerular filtration barrier damage. Researchers measured specific antigens expressed in glomerular podocytes in iMN patients including the M-type phospholipase A2 receptor (PLA2R), thrombospondin type-1 domain-containing 7A (THSD7A), neural epidermal growth factor-like 1 (NELL-1), providing a better understanding of the pathogenesis of iMN and possible means of intervention.
The calcineurin inhibitor tacrolimus (TAC) reduces proliferation of intra-renal T lymphocytes and stabilizes the podocyte cytoskeleton, thus alleviates immunoreaction of iMN [7]. However, the high tendency to relapse after TAC withdrawal or tapering [8,9] and the concern about nephrotoxicity after long-term use limited its application. As B cell plays an important role in the pathogenesis of membranous nephropathy, anti-B lymphocytes therapy using CD20 monoclonal antibody has recently been used as a more selective and promising agent for the treatment of both naïve or refractory iMN [10,11]. However, the study from Ming-Hui Zhao’s group in 2017 showed that only 41.7% of patients with non-responsive iMN acheived response in a median of 12 months of RTX treatment [8].
Recent reports revealed an increased response rate when different doses of rituximab were added to calcineurin inhibitors in refractory iMN, lupus nephritis and other autoimmune diseases. The combination therapy presented better toleration, less relapse and almost no additional side effect due to the quick calcineurin inhibitor tapering [12,13]. Thus, we launched a research of comparison between TAC therapy and combination therapy of ultra-low dose RTX and low dose TAC to observe the response and relapse rate in patients with non-responsive iMN.
Materials and methods
Patients
For this retrospective and single-center study, we collected data of adult non-responsive iMN patients at Tongji Hospital of Tongji Medical College of HuaZhong University of Science and Technology in Wuhan from November 2015 to January 2021, and divided them into two groups: the RTX/TAC group (patients received low dose tacrolimus in combination with ultra-low dose rituximab) and the TAC group (standard tacrolimus therapy). Inclusion criteria: (i) biopsy-proved MN, (ii) proteinuria above 4000 mg/d and non-responsive to previous therapy, defined as not meeting response level after at least 6-month treatmen, and (iii) estimated glomerular filtration rate (eGFR) >30 mL/min/1.73 m2. Exclusive criteria: (i) membranous nephropathy secondary to other causes: positivity for Hepatitis B, C or HIV; malignancy; autoimmune diseases; untreated infection, (ii) with hepatitis B or diabetes, (iii) pregnant or breast feeding females and those planning a pregnancy or using unreliable contraception, (iiii) malignancy or had a life expectancy of less than 1 year. All patients provided written informed consent. The research was approved by the Ethics Committee of Tongji Hospital of Tongji Medical Colleage of HuaZhong University of Science and Technology (No. TJ-IRB20210632). Informed consent was obtained for sampling tissue and blood.
Treatment and follow-up
Rituximab
The ultra-low dose RTX was defined as infusion dosage of 100 mg. The RTX was reconstituted in saline to a final concentration of about 0.4 mg/mL (general infusion volume was 250 ml) and infused at a controlled speed, the whole time-span for fulfilling the infusion was above 2 hours according to each patient’s tolerability. The circulative B cells were monitored in the next morning after administration, once a month for the first 3 months and then once every three months. B-cell depletion was defined as <5 B cells/ul and <1% of total lymphocytes in the circulation. If circulating B cells didn’t achieve the standard, addintional ultra-low dose RTX was applied in that month to maintain circulating B cell depletion until 12 months. The dosing interval was adjusted at the discretion of the attending physician.
Tacrolimus
TAC group
Tacrolimus monotherapy was given at an initial dose of 0.05 mg/kg/day p.o. divided into 2 equal doses given at 12 h intervals and adjusted to a serum concentration level at 4 to 10 ng/mL for 6 months. Then the dose was tapered gradually to 0.5 mg per day at the end of 12 months. TAC dosage was reduced by 30% when 30% increase in serum creatinine was detected as compared with the baseline value, and TAC was withdrawn if the renal function was not improved after 2 weeks. Two patients in the TAC group once reached complete response by TAC monotherapy but relapsed within half a year.
RTX/TAC group
Low dose tacrolimus was given at 0.025 mg/kg/d initially, once the patients have achieved partial or complete response, the dose was tapered to 0.5 mg per day. When proteinuria level was maintained or the relapse occured, the tacrolimus was kept at the initial dose or even increased. Therefore, the duration of the taper varied individually.
Baseline data was established with a serial of laboratory measurerments before rituximab administration. The patients visited doctors every month for the first three months and then once every three months. The follow-up lasted for at least 12 months. Follow-up was terminated when the patients switched to other immunosuppressant or started regular renal replacement therapy. The final follow-up for this study ended in January, 2021.
Outcomes
The primary clinical outcome was complete or partial response in 12 months after initial therapy. Secondary clinical outcomes included time to treatment response up to 12 months, time for anti-PLA2R antibody to turn negative and adverse events. The data of proteinuria, creatinine clearance and serum albumin were systematically assessed. Complete response was defined as urinary protein <0.3 g/day and serum albumin >35 g/L. Partial response was defined as proteinuria <3.5 g/day and >50% reduction from baseline. The remaining ones were defined as no response. Relapse was charactered as recurrence of proteinuria >3.5 g/day after a period of partial or complete response. We consider end-stage renal disease (ESRD) as the end points.
Statistical analysis
Statistical analysis was performed using statistical software SPSS 23.0 (SPSS Inc., Chicago, IL, USA). Parametric data were expressed as means ± standard deviation (SD). Nonparametric data were expressed as median [interquartile range (IQR)]. Response rates were expressed as frequencies or percentages and their 95% CIs. T-test was used for analysis of normally distributed data in different groups. Kruskal-Wallis test or the Mann-Whitney U-test was used for analysis of semi-quantitative data. ‘Chi-squared’ test, Fisher’s exact test or one-way analysis of variation (ANOVA) was used for analysis of qualitative data. All probabilities were two-tailed and the level of significance was set at 0.05.
Results
Patients
Since November 2015, in Tongji Hospital of Tongji Medical Colleage of HuaZhong University of Science and Technology, there were 621 patients with biopsy-proved idiopathic membranous nephropathy. Among them, 156 had proteinuria <4000 mg/24 h, 223 showed a complete or partial response after the initial immunosuppressive treatment, 66 lost to follow-up (paritially due to COVID-19 pandemic in 2020), and 75 did not use immunosuppressors for sufficient time. There were 101 eligible patients, 33 of them switched to other immunosuppressors such as MMF, Leflunomide, etc. The remaining 68 were assigned to the RTX/TAC combination therapy group (26 patients) and the TAC monotherapy group (41 patients). One patient in RTX/TAC group withdrew the consent before administration (shown in Figure 1).
Figure 1.
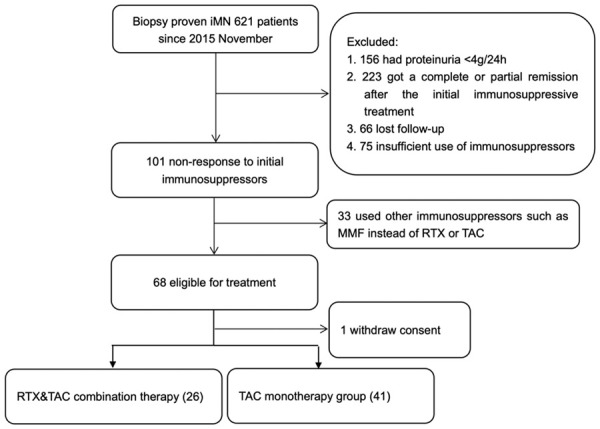
Flow chart of the trial. The flowchart shows the process of patient selection in this retrospective cohort study. The cohort included 621 patients who were biopsy-proved iMN in Tongji Hospital from November 2015 to March 2020. After screening under the conditions shown in this figure, 67 eligible patients (26 in the RTX&TAC group and 41 in the TAC group) were included in the final analysis.
Basic information
Mean age of the participants was 41.7 (RTX/TAC) vs 44.3 (TAC) years in the two groups. There were 7 and 10 females in the two groups, respectively (RTX/TAC vs TAC). They had experienced a median of 22.43 (IQR 12.4-24.9 months) vs 19.95 (IQR 12.7-21.9 months) months of iMN history since their percutaneous renal biopsy operation. The positive rates of anti-PLA2R antibody on pathological specimens were 88.9% vs 85.2% in RTX/TAC group and TAC group.
All the patients had been administered at least one course (mean 2.23 vs 1.85 courses, range 1-4) of immunosuppressant therapy, including glucocorticoids + cyclophosphamide, cyclosporine and/or TAC + small dose glucocorticoids, mycophenolate mofetil and leflunomide. Among all the participants, 14 of 26 in the RTX/TAC group and 26 of 41 patients in the TAC group achieved complete or partial response during previous therapies but relapsed within one year. Other baseline characteristics in the two groups were comparable with no significant differences (Table 1).
Table 1.
Baseline characteristics of patients with non-responsive iMN in RTX/TAC and TAC groups
| Patients | RTX/TAC | TAC | P value |
|---|---|---|---|
| Female/Total (n) | 7/26 | 10/41 | 0.744 |
| Age (years) (mean ± SD) | 41.73±14.89 | 44.34±10.57 | 0.404 |
| History (months) [median (IQR)] | 22.43 (12.4-24.9) | 19.95 (12.7-21.9) | 0.128 |
| Diabetes mellitus/Total (n) | 3/26 | 6/41 | 0.999 |
| Prior immunosuppressive therapy (n) (%) | |||
| 1 | 7 (26.9%) | 20 (48.8%) | 0.770 |
| 2 | 9 (34.6%) | 12 (29.3%) | 0.788 |
| ≥3 | 10 (38.5%) | 9 (22.0%) | 0.172 |
| Previous therapies | |||
| Prednisone + cyclophosphamide, n | 26 | 41 | |
| Prednisone + cyclosporine, n | 2 | 3 | |
| Prednisone + tacrolimus, n | 6 | 2 | |
| Mycophenolate mofetil, n | 7 | 13 | |
| Prednisone + leflunomide, n | 8 | 6 | |
| Immunoglobulin G infusion, n | 9 | 11 | |
| Systolic blood pressure (mean ± SD) | 132.94±14.23 | 128.70±14.41 | 0.349 |
| Diastolic blood pressure (mean ± SD) | 86.77±9.35 | 82.98±9.83 | 0.122 |
| Proteinuria (g/24 h) (mean ± SD) | 5875.31±1548.62 | 5580.43±1510.02 | 0.441 |
| Circulation B cells (mean ± SD) | 219.2±112.91 | 208.7±49.39 | 0.607 |
| Albumin (g/L) (mean ± SD) | 25.07±5.56 | 25.85±5.92 | 0.592 |
| eGFR (mL/min/1.73 m2) (mean ± SD) | 94.23±24.67 | 88.48±19.75 | 0.324 |
| Creatinine (umol/L) (mean ± SD) | 84.50±21.62 | 84.39±22.74 | 0.984 |
| Cholesterol (mmol/l) (mean ± SD) | 6.13±1.67 | 7.05±2.06 | 0.424 |
| PLA2R positive/Total (pathology) (%) | 24/26 (92.3) | 36/41 (87.8) | 0.697 |
| PLA2R positive/Total (plasma) (%) | 18/26 (69.2) | 31/41 (75.6) | 0.584 |
| Tacrolimus initial dose (mg) | 1.41±0.36 | 2.48±0.47 | 0.01 |
| Diuretics, n (%) | 7/26 (26.9) | 12/41 (29.3) | 0.997 |
| Previous remission, n (%) | 14/26 (53.8) | 26/41 (63.4) | 0.456 |
eGFR: Estimated glomerular filtration rate; P value <0.05 statistically significant; RTX: Rituximab; TAC: Tacrolimus.
Rituximab regimen
All of the 26 patients in RTX/TAC group used RTX under a B-cell driven protocol. They were followed up every 1-3 month, an additional dose was decided based on the number of circulating B cells measured at each follow-up. At the 12th month of follow-up, the cumulative dose per person was 219.2±112.9 mg (Table 2), with an average of 3 injections per patient in 12th month. All 26 patients in the RTX/TAC group achieved B-cell depletion during follow-up (Figure 2).
Table 2.
Comparison of laboratory indices in the two groups after 12 months follow-up
| Patients | RTX + TAC | TAC | P value |
|---|---|---|---|
| Rituximab dose (mg) (mean ± SD) | 219.2±112.9 | 0.00 | 0.00 |
| Proteinuria (g/24 h) (mean ± SD) | 2158.1±2412.9 | 3732.1±3190.3 | 0.037 |
| Albumin (g/L) (mean ± SD) | 36.45±8.04 | 32.08±8.65 | 0.046 |
| Creatinine (umol/L) (mean ± SD) | 77.81±19.01 | 92.49±23.01 | 0.011 |
| eGFR (mL/min/1.73 m2) (mean ± SD) | 98.25±25.06 | 81.74±21.50 | 0.006 |
| PLA2R positive rates (positive/total) (%) | 2/26 (7.69) | 7/41 (17.1) | 0.465 |
| Median time for achieving partial response (month) (IQR) | 3 (1.0-6.0) | 3 (1.5-8.25) | 0.272 |
| Time for antibody to turn negative (month) (IQR) | 3.13 (1.97-7.25) | 4.89 (2.65-7.89) | 0.021 |
| 24 h-proteinuria reduction of non-responsive iMN | -1585±2842 | 588.7±2316.3 | 0.033 |
eGFR: Estimated glomerular filtration rate; P value <0.05 indicates statistically significant difference; RTX: Rituximab; TAC: Tacrolimus.
Figure 2.
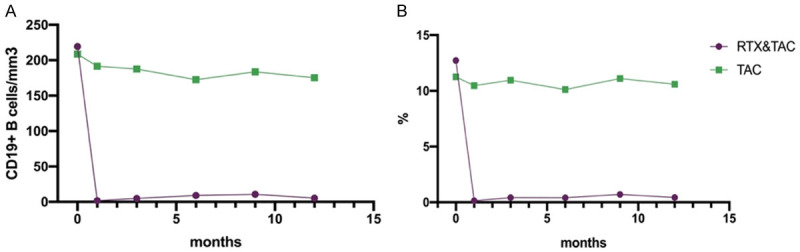
Changes in peripheral B lymphocytes (A) and proportion of peripheral B lymphocytes (B) after administration of RTX/TAC or TAC in the two groups.
Tacrolimus regimen
In this study, patients of the two groups were both treated with TAC, but the dose of TAC used in the RTX/TAC group was less compared with that in the TAC group. The initial dose of the two groups was 1.33±0.41 mg/d and 2.62±0.72 mg/d respectively, P<0.01 (Table 1). The TAC concentration of all patients in the TAC group reached the target range.
Kidney outcomes
The level of urinary protein in the RTX/TAC group decreased from 5875.31±1548.62 mg/24 h to 2158.1±2412.9 mg/24 h (P<0.001) and from 5580.43±1510.02 to 3732.1±3190.3 mg/24 h (P=0.015) in the TAC group (Table 2). The 24 h-proteinuria in both groups decreased significantly and the improvement of proteinuria in the RTX/TAC group was better than that in the TAC group at the end of follow-up (Figure 3). The serum albumin, eGFR and serum creatinine of the two groups were similar to 24 h-proteinuria, and they were improved significantly from the baseline (P<0.05), but the improvement was better in the RTX/TAC group at the last one or two follow-ups (P<0.05) (Figure 3).
Figure 3.
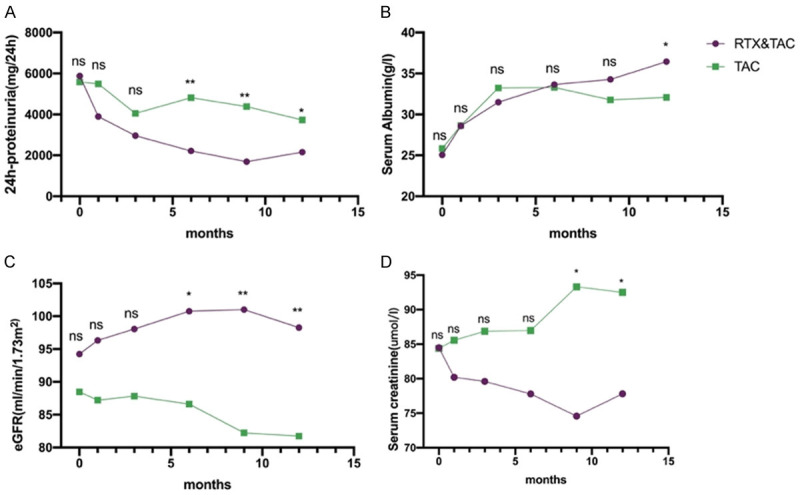
Changes in 24 h-proteinuria (A), serum albumin (B), eGFR (C) and serum creatinine (D) in the two groups at baseline, during the periods of therapy and follow-up. Compare the two groups at different time points (ns, no significant difference, *P<0.05, **P<0.01, P<0.05 indicates significant difference).
To obtain similar observation periods in the two groups, we truncated the follow-up at 12 months. 18 of 26 (69.2%) in the RTX/TAC group vs 17 of 41 patients (41.5%) in the TAC group achieved partial or complete response at 12th month, P=0.044 (Table 3). There were significant differences in the current and cumulative response rates after 6th month of follow-up between the two groups, the data are shown in Table 3. We presented the cumulative response of the two groups using Kaplan-Meier curve, and the RTX/TAC group showed a better response (P=0.015) (Figure 4).
Table 3.
Remission in patients of the two groups
| Months | Current remission rate | Cumulative remission rate | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| RTX/TAC | TAC | p value | RTX/TAC | TAC | p value | |
| 1-month (RR/T) (%) | 7/26 (26.9) | 5/41 (12.2) | 0.191 | 7/26 (26.9) | 5/41 (12.2) | 0.191 |
| 1-month (CR/T) (%) | 0 | 0 | 1 | 0 | 0 | 1 |
| 3-month (RR/T) (%) | 13/26 (50.0) | 13/41 (31.7) | 0.198 | 13/26 (50.0) | 13/41 (31.7) | 0.198 |
| 3-month (CR/T) (%) | 1/26 (3.85) | 0 | 0.388 | 2/26 (7.69) | 0 | 0.147 |
| 6-month (RR/T) (%) | 19/26 (73.1) | 14/41 (34.2) | 0.003 | 19/26 (73.1) | 15/41 (36.6) | 0.006 |
| 6-month (CR/T) (%) | 6/26 (23.1) | 2/41 (4.9) | 0.048 | 6/26 (23.1) | 2/41 (2.44) | 0.048 |
| 9-month (RR/T) (%) | 18/26 (69.2) | 17/41 (41.5) | 0.044 | 20/26 (76.9) | 18/41 (43.9) | 0.011 |
| 9-month (CR/T) (%) | 7/26 (26.9) | 4/41 (9.76) | 0.092 | 8/26 (30.8) | 4/41 (9.76) | 0.048 |
| 12-month (RR/T) (%) | 18/26 (69.2) | 17/41 (41.5) | 0.044 | 20/26 (76.9) | 20/41 (48.8) | 0.040 |
| 12-month (CR/T) (%) | 9/26 (34.6) | 3/41 (7.32) | 0.028 | 9/26 (34.6) | 4/41 (9.76) | 0.024 |
CR: Complete remission; PR: Partial remission; P value <0.05 indicates statistically significant differnce; RR=CR + PR, T: Total number of patients in each group.
Figure 4.
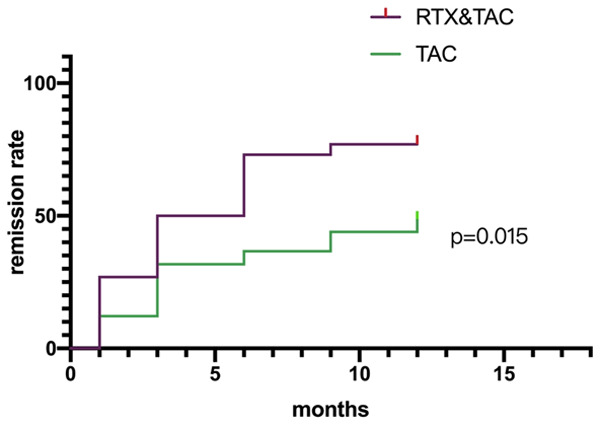
Cumulative response of the two groups. Shown are Kaplan-Meier estimates of the time to response of different treatment groups. Using the Log-rank (Mantel-Cox) test, there was a significant difference in response rate between the two groups (P=0.015).
The average time for anti-PLA2R antibody to turn negative in the RTX/TAC group was 3.13 (1.97-7.25) months, which was shorter than 4.89 (2.65-7.89) months in the TAC group (P=0.021). 2 patients maintained positive of anti-PLA2R antibody in the RTX/TAC group while there were 7 patients in the TAC group (P=0.465) at the end of follow-up.
Relapse
In this study, 1 patient in the RTXTAC group and 3 patients in the TAC group relapsed after partial response. No relapse happened after complete response in both groups.
Adverse effects
Rituximab was well tolerated in most patients. No severe adverse events were observed during infusions in all the patients. Only 2 patients presented chills and rash during the infusion which diminished quickly after administration of anti-allergic drugs, and the discomforts did not reappear in the same patients in the next injection (Table 4). Long-term use of TAC may cause pneumonia, urinary tract infections and glucose intolerance (Table 4). The cumulative incidence of the adverse events were lower in the RTX/TAC group compared with that in the TAC group (9 (34.6) vs 27 (65.9), P=0.023), as shown in Table 4.
Table 4.
Adverse events
| Adverse events, n | RTX/TAC | TAC | P value |
|---|---|---|---|
| Allergic reaction, n (%) | 4 (15.4) | 3 (7.3) | 0.417 |
| Hypertension, n (%) | 0 | 5 (12.2) | 0.148 |
| Infection, n (%) | 3 (11.5) | 11 (26.8) | 0.217 |
| Urinary tract infection, n (%) | 2 (7.7) | 5 (12.2) | 0.697 |
| Pneumonia, n (%) | 1 (3.8) | 6 (14.6) | 0.234 |
| IGT/Diabetes, n (%) | 2 (7.7) | 8 (19.5) | 0.295 |
| Total, n (%) | 9 (34.6) | 27 (65.8) | 0.023 |
IGT: IGT: Impaired glucose tolerance.
Discussion
In this retrospective study, we exhibited the therapeutic and side effects in the RTX/TAC group and the TAC group. Results showed that the combination therapy of ultra-low dose RTX and low dose TAC had better response rate than monotherapy of standard TAC. The response rate in the RTX/TAC group was consistent with the data (60-80%) reported in a previous paper adopting different doses of RTX [14]. Otherwise, the patients in the RTX/TAC group presented fewer adverse effects. Data above demonstrated that the RTX/TAC combination therapy seemed to be a better choice for non-responsive iMN patients.
A cytotoxic agent (commonly cyclophosphamide) combined with glucocorticoids is the preferred initial therapy for patients who are at high risk of progression. Cyclophosphamide is an alkylating agent that prevents cell mitosis by cross-linking DNA strands and decreasing DNA synthesis [15]. Nonspecific and selective inhibitor for cell cycle phase mainly plays inhibitory and subtractive effects on B lymphocytes, with many side effects, especially infection and reproductive toxicity [16,17]. When Cyclophosphamide treatment fails to achieve response, it is generally recommended to switch to calcineurin inhibitors (TAC or cyclosporine, now we prefer TAC). TAC selectively inhibits calcineurin and impairs the transcription of interleukin-2 (IL-2) and several other cytokines in T lymphocytes, resutling in reducing T cell proliferation and improving iMN [7]. Calcineurin inhibitors can generally induce a quicker relief of clinical symptoms, especially in nephrotic syndrome patients and are widely used in clinical therapy in recent years. However, its related nephrotoxicity and relapse after tapering are important issues of concern, which have limited its clinical applications in long-term stability of the patient’s remission [11].
Selective CD20 monoclonal antibody-RTX was first invented in 1997 and used for non-Hodgkin lymphoma, chronic lymphocytic leukemia, and it is now recommended for iMN as well. RTX seems to be safer and shows a better patient compliance. However, the optimal dose for rituximab is still uncertain. There are two mainstreams of RTX in the treatment of iMN, one was given in the MENTOR trial [11], namely 1 g initially and another 1 g 14 days later. The other regimen was 375 mg/m2 every week and lasted for four weeks [18,19]. Some studies suggested that even low-dose treatment with RTX can lead to expectant treatment response in patients with iMN [19]. In the RTX-treated autoimmune diseases, it is reported that ultra-low dose RTX maintenance could also deplete circulating B lymphocytes and exert therapeutic effects [20]. Although it is easy to achieve the depletion of circulating B lymphocytes with low-dose rituximab, the therapeutic effect was not satisfying [14]. Considering the different action mechanisms of the two drugs and the side effects brought by high-dose drugs, we tried RTX combined with TAC to treat non-responsive iMN.
This study showed that the RTX/TAC treatment was effective in 76.9% of non-responsive iMN patients during the 12-month follow-up period, which was better than the 41.7% of Chinese patients with non-responsive iMN achieving response in a median of 12 months of RTX monotherapy [10]. Compared with other regimes, the combination of ultra-low dose RTX and TAC has consistent response rate which varied from 60% to 76% in first- or second-line therapy [11,14,21]. This may have contributed to the dual inhibitory effects on both B and T lymphocytes with the combination therapy.
RTX/TAC therapy in this cohort maintained the number of circulative B lymphocytes below 5 cells/ul for 12 months, which is a full time low level of B lymphocytes state in peripheral circulation. As for the other method of administration, the number of B lymphocytes generally increased from about 6 months [22]. CD20 is expressed on pre-B to fully differentiated B lymphocytes, however, ultimately differentiated plasma cells do not express CD20 marker. The histologic lesion of iMN under light microscope is diffuse thickening of the glomerular basement membrane (GBM) throughout all glomeruli with a diffuse granular pattern of immunoglobulin G (IgG) and C3 deposits along the GBM. These deposits are mainly produced by plasma cells. However, after the process of affinity maturation in germinal centers, plasma cells have an indeterminate lifespan, ranging from days to months [23]. Thus, prolonging the low level of circulative B lymphocytes may bring more benefits to patients in response period. Although there were 8 patients in RTX/TAC group who did not achieve response (including those who relapsed after response), the 24 h-proteinuria of these non-responsive patients were still significantly reduced (Table 2). Thus, ultra-low dose rituximab plus low dose tacrolimus showed better effect on non-responsive iMN patients.
RTX eliminated B lymphocytes, which may weaken the immunity. However, as RTX was at ultra-low doses, there was no significant difference between the two groups in the concern of infection. In the first 12 months of the study, 83 patients received intravenous RTX, with an average injection interval of 3.8 months. Multiple times of RTX injection may lead to the production of circulative rituximab antibodies and affect its efficacy. In this study, we counted the intervals between the first three doses. The mean interval was 3.17, 2.92, and 3.05 months (Figure 5), and there was no statistical difference among each other. For at least 12 months, we did not observe decreased effect of RTX on circulating B lymphocytes due to antibody production.
Figure 5.
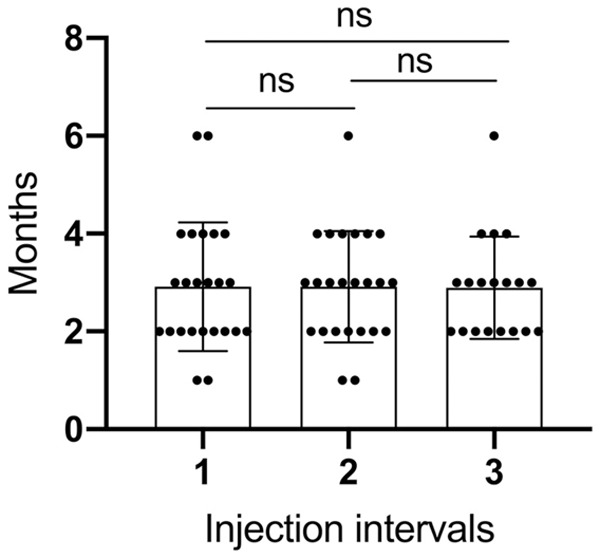
The first three dosing intervals for patients receiving rituximab. There is no significant statistical difference between the three dosing intervals.
In this study, relapse was observed in one patient in the RTX/TAC group while 3 in the TAC group after achieving partial response. Some studies pointed out that RTX could maintain iMN remission up to 24 months [11]. TAC usually induces more rapid remission [11], and the combination therapy may have advantages of both.
Limitations: This study also had two limitations. One is the small sample size. The other is that most of the patients used qualitative measurement to monitor serum level of anti-PLA2R antibody, which did not reflect the trend of antibody titers. The patient’s anti-PLA2R antibody titers will be measured in later follow up.
In conclusion, ultra-low dose rituximab plus low dose tacrolimus therapy is more effective and safer than standard tacrolimus therapy, implying that it might be a potential option for patients with non-responsive iMN.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grants 81974087, 81770684, 81974087, 81770681, 81974086, 81700597).
Disclosure of conflict of interest
None.
References
- 1.Xu X, Wang G, Chen N, Lu T, Nie S, Xu G, Zhang P, Luo Y, Wang Y, Wang X, Schwartz J, Geng J, Hou FF. Long-term exposure to air pollution and increased risk of membranous nephropathy in China. J Am Soc Nephrol. 2016;27:3739–3746. doi: 10.1681/ASN.2016010093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ponticelli C, Zucchelli P, Passerini P, Cesana B, Locatelli F, et al. A 10-year follow-up of a randomized study with methylprednisolone and chlorambucil in membranous nephropathy. Kidney Int. 1995;48:1600–4. doi: 10.1038/ki.1995.453. [DOI] [PubMed] [Google Scholar]
- 3.Radhakrishnan J, Cattran DC. The KDIGO practice guideline on glomerulonephritis: reading between the (guide)lines--application to the individual patient. Kidney Int. 2012;82:840–856. doi: 10.1038/ki.2012.280. [DOI] [PubMed] [Google Scholar]
- 4.Ponticelli C, Altieri P, Scolari F, Passerini P, Roccatello D, Cesana B, Melis P, Valzorio B, Sasdelli M, Pasquali S, Pozzi C, Piccoli G, Lupo A, Segagni S, Antonucci F, Dugo M, Minari M, Scalia A, Pedrini L, Pisano G, Grassi C, Farina M, Bellazzi R. A randomized study comparing methylprednisolone plus chlorambucil versus methylprednisolone plus cyclophosphamide in idiopathic membranous nephropathy. J Am Soc Nephrol. 1998;9:444–450. doi: 10.1681/ASN.V93444. [DOI] [PubMed] [Google Scholar]
- 5.Jha V, Ganguli A, Saha TK, Kohli HS, Sud K, Gupta KL, Joshi K, Sakhuja V. A randomized, controlled trial of steroids and cyclophosphamide in adults with nephrotic syndrome caused by idiopathic membranous nephropathy. J Am Soc Nephrol. 2007;18:1899–1904. doi: 10.1681/ASN.2007020166. [DOI] [PubMed] [Google Scholar]
- 6.Larsen CP, Messias NC, Silva FG, Messias E, Walker PD. Determination of primary versus secondary membranous glomerulopathy utilizing phospholipase A2 receptor staining in renal biopsies. Mod Pathol. 2013;26:709–715. doi: 10.1038/modpathol.2012.207. [DOI] [PubMed] [Google Scholar]
- 7.Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992;13:136–142. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- 8.Chen M, Li H, Li XY, Lu FM, Ni ZH, Xu FF, Li XW, Chen JH, Wang HY Chinese Nephropathy Membranous Study Group. Tacrolimus combined with corticosteroids in treatment of nephrotic idiopathic membranous nephropathy: a multicenter randomized controlled trial. Am J Med Sci. 2010;339:233–238. doi: 10.1097/MAJ.0b013e3181ca3a7d. [DOI] [PubMed] [Google Scholar]
- 9.Ramachandran R, Hn HK, Kumar V, Nada R, Yadav AK, Goyal A, Kumar V, Rathi M, Jha V, Gupta KL, Sakhuja V, Kohli HS. Tacrolimus combined with corticosteroids versus Modified Ponticelli regimen in treatment of idiopathic membranous nephropathy: randomized control trial. Nephrology (Carlton) 2016;21:139–146. doi: 10.1111/nep.12569. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Cui Z, Zhang YM, Qu Z, Wang F, Meng LQ, Cheng XY, Liu G, Zhou FD, Zhao MH. Rituximab for non-responsive idiopathic membranous nephropathy in a Chinese cohort. Nephrol Dial Transplant. 2018;33:1558–1563. doi: 10.1093/ndt/gfx295. [DOI] [PubMed] [Google Scholar]
- 11.Fervenza FC, Appel GB, Barbour SJ, Rovin BH, Lafayette RA, Aslam N, Jefferson JA, Gipson PE, Rizk DV, Sedor JR, Simon JF, McCarthy ET, Brenchley P, Sethi S, Avila-Casado C, Beanlands H, Lieske JC, Philibert D, Li T, Thomas LF, Green DF, Juncos LA, Beara-Lasic L, Blumenthal SS, Sussman AN, Erickson SB, Hladunewich M, Canetta PA, Hebert LA, Leung N, Radhakrishnan J, Reich HN, Parikh SV, Gipson DS, Lee DK, da Costa BR, Jüni P, Cattran DC MENTOR Investigators. Rituximab or cyclosporine in the treatment of membranous nephropathy. N Engl J Med. 2019;381:36–46. doi: 10.1056/NEJMoa1814427. [DOI] [PubMed] [Google Scholar]
- 12.Waldman M, Beck LH Jr, Braun M, Wilkins K, Balow JE, Austin HA 3rd. Membranous nephropathy: pilot study of a novel regimen combining cyclosporine and rituximab. Kidney Int Rep. 2016;1:73–84. doi: 10.1016/j.ekir.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kronbichler A, Brezina B, Gauckler P, Quintana LF, Jayne DRW. Refractory lupus nephritis: when, why and how to treat. Autoimmun Rev. 2019;18:510–518. doi: 10.1016/j.autrev.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Seitz-Polski B, Dahan K, Debiec H, Rousseau A, Andreani M, Zaghrini C, Ticchioni M, Rosenthal A, Benzaken S, Bernard G, Lambeau G, Ronco P, Esnault VLM. High-dose rituximab and early remission in PLA2R1-related membranous nephropathy. Clin J Am Soc Nephrol. 2019;14:1173–1182. doi: 10.2215/CJN.11791018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall AG, Tilby MJ. Mechanisms of action of, and modes of resistance to, alkylating agents used in the treatment of haematological malignancies. Blood Rev. 1992;6:163–173. doi: 10.1016/0268-960x(92)90028-o. [DOI] [PubMed] [Google Scholar]
- 16.Clements PJ, Yu DT, Levy J, Paulus HE, Barnett EV. Effects of cyclophosphamide on B- and T-lymphocytes in rheumatoid arthritis. Arthritis Rheum. 1974;17:347–353. doi: 10.1002/art.1780170403. [DOI] [PubMed] [Google Scholar]
- 17.Pryor BD, Bologna SG, Kahl LE. Risk factors for serious infection during treatment with cyclophosphamide and high-dose corticosteroids for systemic lupus erythematosus. Arthritis Rheum. 1996;39:1475–1482. doi: 10.1002/art.1780390906. [DOI] [PubMed] [Google Scholar]
- 18.Ruggenenti P, Cravedi P, Chianca A, Perna A, Ruggiero B, Gaspari F, Rambaldi A, Marasa M, Remuzzi G. Rituximab in idiopathic membranous nephropathy. J Am Soc Nephrol. 2012;23:1416–1425. doi: 10.1681/ASN.2012020181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cravedi P, Ruggenenti P, Sghirlanzoni MC, Remuzzi G. Titrating rituximab to circulating B cells to optimize lymphocytolytic therapy in idiopathic membranous nephropathy. Clin J Am Soc Nephrol. 2007;2:932–937. doi: 10.2215/CJN.01180307. [DOI] [PubMed] [Google Scholar]
- 20.den Broeder AA, Verhoef LM, Fransen J, Thurlings R, van den Bemt BJF, Teerenstra S, Boers N, den Broeder N, van den Hoogen FHJ. Ultra-low dose of rituximab in rheumatoid arthritis: study protocol for a randomised controlled trial. Trials. 2017;18:403. doi: 10.1186/s13063-017-2134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cravedi P, Sghirlanzoni MC, Marasa M, Salerno A, Remuzzi G, Ruggenenti P. Efficacy and safety of rituximab second-line therapy for membranous nephropathy: a prospective, matched-cohort study. Am J Nephrol. 2011;33:461–468. doi: 10.1159/000327611. [DOI] [PubMed] [Google Scholar]
- 22.Dahan K, Debiec H, Plaisier E, Cachanado M, Rousseau A, Wakselman L, Michel PA, Mihout F, Dussol B, Matignon M, Mousson C, Simon T, Ronco P GEMRITUX Study Group. Rituximab for severe membranous nephropathy: a 6-month trial with extended follow-up. J Am Soc Nephrol. 2017;28:348–358. doi: 10.1681/ASN.2016040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amanna IJ, Slifka MK. Mechanisms that determine plasma cell lifespan and the duration of humoral immunity. Immunol Rev. 2010;236:125–138. doi: 10.1111/j.1600-065X.2010.00912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


