Abstract
Chronic kidney disease (CKD) is a global public health problem, seemingly affecting individuals from low-income and-middle-income countries (LMICs) disproportionately, especially in sub-Saharan Africa. Despite the growing evidence pointing to an increasing prevalence of CKD across Africa, there has not been an Africa-wide concerted effort to provide reliable estimates that could adequately inform health services planning and policy development to address the consequences of CKD. Therefore, we established the CKD in Africa (CKD-Africa) Collaboration. To date, the network has curated data from 39 studies conducted in 12 African countries, totalling 35 747 participants, of which most are from sub-Saharan Africa. We are, however, continuously seeking further collaborations with other groups who have suitable data to grow the network. Although many successful research consortia exist, few papers have been published (with none from Africa) detailing the challenges faced and lessons learnt in setting up and managing a research consortium. Drawing on our experience, we describe the steps taken and the key factors required to establish a functional collaborative consortium among researchers in Africa. In addition, we present the challenges we encountered in building our network, how we managed those challenges and the benefit of such a collaboration for Africa. Although the CKD-Africa Collaboration is focused primarily on CKD research, many of the lessons learnt can be applied more widely in public health research in LMICs.
Keywords: epidemiology
Summary box.
The Chronic Kidney Disease in Africa Collaboration (CKD-Africa Collaboration) is an African network of CKD studies that pools individual participant data to: (1) determine the burden of CKD in Africa more accurately, (2) create resources that would allow the burden of CKD to be easily tracked and (3) enable CKD projections to be made in the context of Africa.
To date, the network has curated data from 39 studies conducted in 12 African countries, totalling 35 747 participants, of which most are from sub-Saharan Africa.
The estimates generated through this network will allow for the development of policy related to screening and prevention to address the consequences of CKD in Africa, inform health services planning, and aid understanding of the mechanisms driving CKD across the continent.
This network has far-reaching potential for Africa, as it is in an ideal position to validate findings across geographical and national boundaries, to test hypotheses and to generate a new understanding of CKD progression and its complications.
Introduction
Chronic kidney disease (CKD) is one of the leading causes of morbidity and mortality, affecting 10%–16% of the general adult populations of Asia, Australia, Europe and North America,1 2 with heterogeneous prevalence in African populations.3 4 The rising burden of CKD is evidenced by its climb in ranking of global causes of disability-adjusted life-years, from 29th in 1990 to 18th in 2019.5 Currently, more than 850 million people have kidney disease,6 with a disproportionate burden of this number affecting people in low-income and middle-income countries (LMICs) where access to care is significantly limited.1 7 In recent years, studies have shown that Africans are seemingly at a high risk for developing CKD,8 are affected at a younger age9 and have a more rapid progression to kidney failure.9 10 This disproportionate risk is partly attributed to the rapid epidemiological transition, culminating in a high and rising prevalence of hypertension and type 2 diabetes mellitus,11 12 combined with a high burden of infectious diseases13 and a genetic predisposition to CKD.14 Moreover, there are different methods used to detect kidney damage, which can influence the diagnosis and staging of CKD, and consequently the reported population prevalence.15 Due to the lack of data in many African countries, and the limitations in the available data,3 4 the true burden of CKD in Africa (epidemiological, including age-standardised rates, as well as cost of care and health impact on patients, their family and society) is probably underestimated and thus remains largely unknown.
Recognising the shortfall, in 2018/2019, the Non-Communicable Disease Research Unit of the South African Medical Research Council (SAMRC) established the CKD in Africa (CKD-Africa) Collaboration. The major goal of this network is to pool data at the individual participant data (IPD) level from all relevant existing African studies. This will enable the burden of CKD in Africa to be determined more accurately, create resources for the burden of CKD to be easily tracked in future and for projections of CKD to be made in Africa. This would provide reliable estimates to develop policy solutions to address the consequences of CKD in Africa, inform health services planning, and aid the understanding of the mechanisms driving CKD across the continent.
Globally, several successful research consortia focus on communicable and non-communicable diseases,16–18 and various studies across Africa successfully employ the use of IPD.19–21 One example of a successful consortium using IPD is the CKD-Prognosis Consortium (CKD-PC),17 established to compile and analyse the best available data on kidney disease and clinical outcomes. This consortium has made a significant contribution to the definition, staging and management of CKD. However, despite the large number of participating cohorts globally, the CKD-PC has no data from Africa and thus there are uncertainties around using its findings to inform CKD management and prevention strategies in Africa.
This Practice contribution will introduce the CKD-Africa Collaboration, through describing the steps taken to establish the collaborative research consortium, as well as briefly summarising the current participating studies. Since few published papers currently exist detailing the challenges faced and lessons learnt in setting up and running a research consortium, we will present the challenges we have encountered and how they were managed. Also, we will report on the novelty and effectiveness of this kidney disease network in Africa.
Conception of the network
Establishing the CKD-Africa Collaboration, by moving the idea of an African network of studies on kidney function and CKD to a functional continental resource, required several steps. These included forming the central structure responsible for initiating and managing the network, identifying research partners, inviting these partners to join the network, and establishing the database platform, by acquiring and processing the participant-level data.
Forming the consortium central structure
We found it best to balance the central structure with a mixture of highly motivated emerging researchers to drive the initiative, with more senior members to act as advisors (online supplemental addendum A highlights the roles and responsibilities of the core team). In our case, the members of the central structure have had a long-standing working relationship, and we learnt that these shared research interests and mutual trust and support proved beneficial to building a robust and sustainable network. In addition, we found it advantageous to involve a multidisciplinary group of specialists, including nephrologists, epidemiologists, statisticians and public health specialists, given that the catalyst for building this network arose from the recognised need to provide reliable estimates to guide policy. This diverse central structure, along with the other collaborators, are instrumental in shaping the consortium.
bmjgh-2021-006454supp001.pdf (57.7KB, pdf)
Identifying collaborators and setting up a functional database platform
Building a robust and sustainable collaborative research consortium requires identifying research partners. We found that a good way to identify potential partners was by first tapping into our existing research partnerships. Indeed, as the result of our cumulative existing networks as the central structure, we had access to IPD from cross-sectional studies for about 7000 individuals even before the first formal call for participation in the consortium was sent. Also, we found that forging new networks with researchers in the broader field of non-communicable diseases was beneficial. These new networks were mainly established at international conferences and events organised by specialty organisations, such as the European Society of Hypertension, the International Society of Hypertension, the European Renal Association-European Dialysis and Transplant Association and the International Diabetes Federation. We found that these in-person conversations, at conferences, were often useful when explaining the purpose of the network and discussing complicated issues (eg, security of data storage servers). These conversations also led to the development of personal relationships with our collaborators which we felt eased correspondence throughout the data sharing process. Further research partners were sought through the systematic search of published literature.
Search strategy
The reference list of the two most recently published systematic reviews of CKD prevalence in Africa3 4 was used as the basis to identify relevant studies, further supplemented by searches of Medline via PubMed, EMBASE, relevant African journals and WHO Global Health Library databases (which included the African Index Medicus, WHO Library Information System, and Scientific Electronic Library Online) to identify more recent publications. This comprehensive search strategy was developed using the African search filter22 and appropriate keywords, including “prevalence”, “incidence”, “screening’, “diagnosis”, “risk prediction”, “chronic kidney (or renal) disease’, “kidney (or renal) dysfunction”, “decreased kidney (renal) function”, “end-stage renal disease”, “glomerular filtration rate”, “albuminuria”, “proteinuria” “Cockcroft-Gault equation”, “Modification of Diet in Renal Disease equation”, “CKD Epidemiology Collaboration equation”, strung together by MeSH terms. Additional citations were also searched by scanning the reference lists of review papers and conference proceedings. Thus, to date, the searches covers the time frame from 1 January 1995 to 31 January 2021. The search results were uploaded into the citation management database EndNote (Clarivate Analytics, Philadelphia, USA), and the duplicate check function used to identify citations retrieved from multiple sources. Unique citations were uploaded into the systematic review software, Covidence (Covidence, Melbourne, Australia), used to store and track search results in the review process.
Process for selection of eligible studies
Using the Covidence software, two team members of the core working group (CG and SS) independently reviewed the articles referenced in the published systematic reviews3 4 and those obtained through the systematic search processes. In instances where either team member determined that a study may be eligible based on the title or abstract review, a full-text article review was conducted. Disagreements between reviewers, after full-text review, was resolved by discussion and consensus. There was no restriction on language since translators were available, if needed, to evaluate titles/abstracts and full-text articles for languages other than those for which the team members were fluent. In instances where multiple surveys were conducted in different countries or in different calendar years and reported within the same article, each survey was accounted for separately.
Determining the membership criteria for the CKD-Africa Collaboration required careful consideration. With the aim of combining the IPD curated across the network to enhance the ability to examine multiple health outcomes related to CKD, in addition to the ability to report more accurate estimates on the burden of CKD across Africa, a set of common attributes were defined as prerequisites for membership into the network at inception:
Studies of observational research design with a priori hypotheses and defined study objectives, participant-level information, and primary data collection.
Studies reporting, or allowing computation of, the prevalence of CKD. CKD could be defined based on estimated glomerular filtration rate (eGFR) and/or the presence of proteinuria/albuminuria, according to the Kidney Disease Improving Global Outcomes (KDIGO) guidelines.23 In the instance where eGFR is used to define CKD, investigators are required to report on the methods used to determine creatinine levels.
Studies with ethical approval from their respective ethics organisations, with deidentified data.
Studies with a minimum sample size of 300 participants for adult cohorts, aged 18 years and older. The justification for the selected sample size is that studies with small sample sizes are likely to include only few people with CKD, and consequently contribute little or nothing to the estimates in the meta-analyses, while remaining a major contributor to the heterogeneity across studies. Furthermore, we opted for participants aged above 18 years as the main aim of the consortium is to determine the burden of CKD and preventable risk factors driving the disease in Africa. The aetiology of CKD in children differs significantly from CKD in adults, with CKD in children generally caused by birth defects and hereditary diseases, whereas in adults the main drivers being diabetes and hypertension.
Studies with participants of African descent residing in Africa.
Our first formal call for participation was organised in 2018 (online supplemental addendum B), where corresponding authors of studies containing datasets that met the inclusion criteria were contacted via email, inviting them to contribute primary data for inclusion into the network. From our experience, obtaining data sets through personal contact took as little as 4 months, however, not all contacted authors responded initially. In instances of non-response, we attempted to make contact several times, with at least 6 months between calls. Since many collaborators may lack time or organisational resources to support essential data sharing tasks (eg, converting data to prespecified digital formats, drafting data sharing agreement), we found a useful technique to obtain the IPD was to minimise the additional responsibilities on the collaborator, like allowing datasets in any digital format. In addition to the non-responding investigators, we also experienced an unwillingness to participate in the network in a very limited number of cases. The main reason for the unwillingness was due to the studies not being completed at the time of our request.
bmjgh-2021-006454supp002.pdf (73.1KB, pdf)
After agreement to participate in the network, a memorandum of understanding (online supplemental addendum C), and in some cases, a material transfer agreement (online supplemental addendum D), was signed by both parties as a declaration of mutual understanding. These documents highlighted the role of the parties, the area of focus, ethical and data sharing information, intellectual property rights, commercialisation and publications. This agreement was followed by the electronic version of the data requested. In our case, we used the expanded version of the WHO STEPwise Approach to non-communicable disease risk factor Surveillance (STEPS) Instrument24 as the basis for the scope of variables required (online supplemental addendum B). In instances where investigators are unwilling or unable to transfer data, owing to legal or other logistical reasons, but are prepared to reanalyse their data according to a standard protocol, the CKD-Africa Collaboration secretariat data centre produce a computer code that can be used to generate the required summary statistics. All deidentified data are held centrally at the SAMRC. To maintain the data integrity, protective measures are employed, which include the data being kept on a virus-protected and access-restricted server at the SAMRC, which is backed-up daily. This storing procedure is as per the ‘Ethics in health research: principles, processes and structures’, second edition (2015) requirements for electronic data storage, which is the SAMRC’s Ethics Committee’s terms of reference.
bmjgh-2021-006454supp003.pdf (136.1KB, pdf)
bmjgh-2021-006454supp004.pdf (122.9KB, pdf)
The process of searching for collaborators, getting everyone on board, finalising the legal aspects of the arrangement and data acquisition is a lengthy and sometimes challenging process. However, it is laudable how generally able and willing investigators conducting CKD research in Africa have been to participate in this network. This shows the level of awareness of the devastating consequences of the disease among the African CKD research community and the desire to work collectively to address the problem. The process of data management is also a labour-intensive process. The data coding and transfer from original studies into the IPD database is done by a senior staff member, or by a student under supervision of a senior staff member of the central structure. During this process, participant characteristics and screening accuracy results for each study, using the cleaned datasets, are compared with those from the original datasets to identify any potential discrepancies. In addition to obtaining the original IPD, aggregate data are extracted from the published articles of included studies. At this point, cross-checks between the published data with the original IPD obtained from each dataset are conducted and any inconsistencies discussed with the original authors. It is crucial to record and update contact information as this will ease subsequent communication, which often occurred years after the first data request was sent.
Progress to date
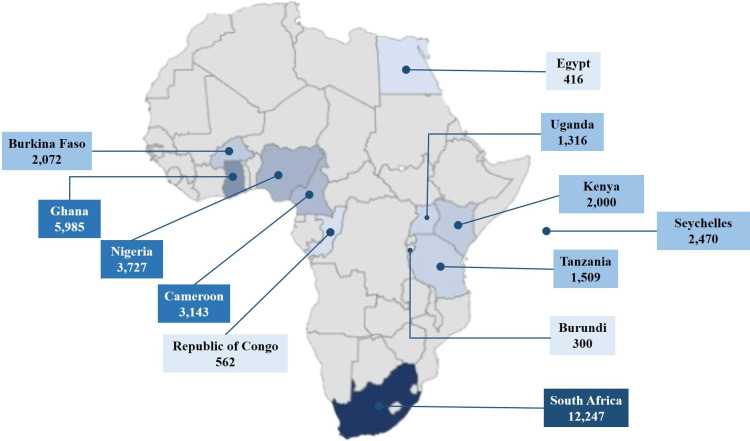
As of 1 April 2021, through our scoping efforts, we had identified 108 researchers who were the principal investigators (PIs) of 120 potential studies. Of these, 92 PIs were contacted to gauge their interest in collaborating in the consortium, as 16 PIs had either no author contact information or incorrect contact details. Of the 92 PIs contacted, 36 consented to participate in the network, with the remaining 56 PIs being either non-responsive to our call or, in two cases, unwilling to participate in the network. The consenting studies span across 12 African countries with a total of 46 276 participant-level data. To date, the network has successfully curated data from 39 studies conducted in 12 African countries, totalling 35 747 participants. Most enrolled studies are from sub-Saharan Africa, with one study representing north Africa25 (figure 1). Of the included studies, the number of participants range between 300 and 2543 per study. Some studies are still undergoing enrolment, and therefore, the number of study participants continues to grow.
Figure 1.
Distribution of African countries enrolled in the CKD-Africa Collaboration. The individual participant data (IPD) ranges from 300 participants to 12 247 participants per country. The nine shaded countries represent those for which IPD are currently available. The shading from light blue to dark blue represents the increasing number of IPD available per country, thus, the darkest shading represents the countries with the most available IPD. CKD, chronic kidney disease.
Of the participating studies (table 1), data collection of 14 studies (36%) took place before 2010,25–38 with the remaining 64% sampled between 2010 and 2017. Four of the participating studies have not been published yet. Overall, 79% of the IPD are from studies in the general population,28 30–34 36 37 39–50 7% are from studies of people with HIV-infection,27 29 51–54 6% from studies of populations with hypertension35 55 56 and 4% consists of people with diabetes mellitus57 (figure 2). The final 4% of the IPD constitutes two studies in patients with kidney failure26 58 and one study conducted in first-degree relatives of people with CKD.25 Of the 25 studies conducted in general populations, 88% (n=22) are geographically defined cohorts, with two of the remaining studies conducted among teachers recruited from primary, secondary and intermediate public schools30 39 and one study (not yet published) conducted in undergraduate students. The participants in the high-risk subpopulations were recruited from outpatient diabetes, hypertension and HIV clinics.
Table 1.
Characteristics of participating studies in CKD-Africa Collaboration
| Country | Study reference | Population | Cohort participants | Sample size (n) | Age range (years) | % Male | Creatinine measurement method | eGFR range | Proteinuria/ albuminuria |
| Burkina Faso† | Ali et al 62 | GP | Geographical cohort | 2072 | 38–69 | 50.4 | Jaffe | 11.8 to >90 | Albuminuria |
| Burundi | Cailhol et al 27 | HIV | HIV clinic cohort | 300 | 19–66 | 29.7 | Jaffe | 6.0 to >90 | Proteinuria |
| Cameroon | Choukem* | DM | Diabetes clinic cohort | 790 | 19–82 | 65.0 | Jaffe | 1.7 to >90 | NA |
| Cameroon | Feteh et al 57 | DM | Diabetes clinic cohort | 645 | 20–86 | 53.0 | Jaffe | 4.0 to >90 | NA |
| Cameroon | Kaze* | GP | Geographical cohort | 433 | 21–90 | 49.0 | Modified Jaffe | 6.9 to >90 | Albuminuria |
| Cameroon | Kaze et al 45 | GP | Geographical cohort | 500 | 19–83 | 53.4 | Modified Jaffe | 23.0 to >90 | Albuminuria |
| Cameroon | Kaze et al 46 | GP | Geographical cohort | 439 | 19–90 | 42.1 | Modified Jaffe | 12.0 to >90 | Albuminuria |
| Cameroon | Kaze et al 56 | HPT | HPT clinic cohort | 336 | 33–90 | 36.6 | Modified Jaffe | 5.0 to >90 | Albuminuria |
| Egypt | Gouda et al 25 | Other | FDR of CKD cohort | 416 | 18–75 | 43.2 | Jaffe | 32.0 to >90 | Albuminuria |
| Ghana‡ | Adjei et al 40 | GP | Geographical cohort | 2543 | 25–96 | 33.0 | Jaffe | 15.5 to >90 | Albuminuria |
| Ghana | Chadwick et al 53 | HIV | HIV clinic cohort | 677 | 20–77 | 26.1 | Jaffe | 9.8 to >90 | Proteinuria |
| Ghana | Osafo et al 55 | HPT | HPT clinic cohort | 754 | 19–90 | 21.3 | Jaffe | 1.4 to >90 | Proteinuria |
| Ghana† | Ali et al 62 | GP | Geographical cohort | 2011 | 40–61 | 45.8 | Jaffe | 12.3 to >90 | Albuminuria |
| Kenya† | Ali et al 62 | GP | Geographical cohort | 2000 | 35–67 | 46.0 | Jaffe | 13.9 to >90 | Albuminuria |
| Nigeria | Adedeji et al 51 | HIV | HIV clinic cohort | 304 | 18–80 | 54.7 | Modified Jaffe | 5.9 to >90 | Albuminuria |
| Nigeria | Alasia et al 26 | Other | Renal clinic cohort | 605 | 18–86 | 49.3 | Jaffe | 1.3 to >90 | Proteinuria |
| Nigeria | Ayodele* | GP | Cohort of students | 307 | 18–30 | 35.8 | Modified Jaffe | 39.9 to >90 | NA |
| Nigeria | Ayodele* | GP | Geographical cohort | 419 | 19–80 | 40.3 | Modified Jaffe | 37.1 to >90 | NA |
| Nigeria | Ayokunle et al 52 | HIV | HIV clinic cohort | 335 | 20–75 | 43.9 | Jaffe | 4.7 to >90 | Albuminuria |
| Nigeria | Okoye et al 32 | GP | Geographical cohort | 476 | 18–90 | 33.8 | Modified Jaffe | 22.4 to >90 | Proteinuria |
| Nigeria | Oluyombo et al 33 | GP | Geographical cohort | 972 | 18–100 | 30.4 | Modified Jaffe | 23.9 to >90 | Albuminuria |
| Nigeria | Raji et al 58 | Other | Renal clinic cohort | 309 | 18–73 | 45.3 | Modified Jaffe | 1.2 to >90 | NA |
| RoC | Ekat et al 29 | HIV | HIV clinic cohort | 562 | 18–64 | 33.8 | Jaffe | 4.0 to >90 | NA |
| SA | Adeniyi et al 39 | GP | Cohort of teachers | 455 | 22–71 | 29.7 | Modified Jaffe | 32.4 to >90 | Proteinuria |
| SA | Malan et al | GP | Cohort of teachers | 409 | 20–65 | 49.4 | Jaffe | 1.3 to >90 | Albuminuria |
| SA | Matsha et al 31 | GP | Geographical cohort | 1620 | 18–91 | 24.8 | Jaffe | 8.2 to >90 | Albuminuria |
| SA | Peer et al 34 | GP | Geographical cohort | 1086 | 22–81 | 35.9 | Jaffe | 9.0 to >90 | NA |
| SA | Rayner and Becker35 | HPT | HPT clinic cohort | 1107 | 18–94 | 48.8 | NA | NA | Albuminuria |
| SA | Schutte et al 37 | GP | Geographical cohort | 750 | 20–70 | 46.1 | Jaffe | 47.8 to >90 | NA |
| SA | Schutte et al 49 | GP | Geographical cohort | 1202 | 20–30 | 48.1 | Jaffe | 48.1 to >90 | Albuminuria |
| SA† | Ali et al 62 | GP | Geographical cohort | 2312 | 40–81 | 42.2 | Jaffe | 3.1 to >90 | Albuminuria |
| SA† | Ali et al 62 | GP | Geographical cohort | 1388 | 29–82 | 30.6 | Jaffe | 4.8 to >90 | Albuminuria |
| SA† | Ali et al 62 | GP | Geographical cohort | 1918 | 39–61 | 50.6 | Jaffe | 9.4 to >90 | Albuminuria |
| Seychelles | Pruijm et al 38 | GP | Geographical cohort | 1230 | 25–64 | 46.2 | Jaffe | 3.2 to >90 | Albuminuria |
| Seychelles | Heiniger et al 63 | GP | Geographical cohort | 1240 | 26–64 | 42.8 | Jaffe | 4.5 to >90 | Albuminuria |
| Tanzania | Peck et al 48 | GP | Geographical cohort | 1041 | 18–92 | 46.2 | Enzymatic | 25.1 to >90 | NA |
| Tanzania | Stanifer et al 50 | GP | Geographical cohort | 468 | 18–88 | 25.6 | Enzymatic | 9.2 to >90 | Albuminuria |
| Uganda | Kalyesubula et al 44 | GP | Geographical cohort | 955 | 18–87 | 33.0 | Enzymatic | 44.7 to >90 | Proteinuria |
| Uganda | Odongo et al 54 | HIV | HIV clinic cohort | 361 | 18–66 | 36.3 | Jaffe | 4.6 to >90 | Proteinuria |
*Unpublished data
†Part of the multisite study, Africa Wits-INDEPTH partnership for Genomics studies.62
‡Part of the multisite study, Research on Obesity and Diabetes among African Migrants.40 GFR is estimated by the the CKD Epidemiology Collaboration equations,64 with the ethnicity correction factor omitted.
CKD, chronic kidney disease; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; FDR, first-degree relatives; GP, general population; HPT, hypertension; NA, not applicable; RoC, Republic of Congo; SA, South Africa.
Figure 2.
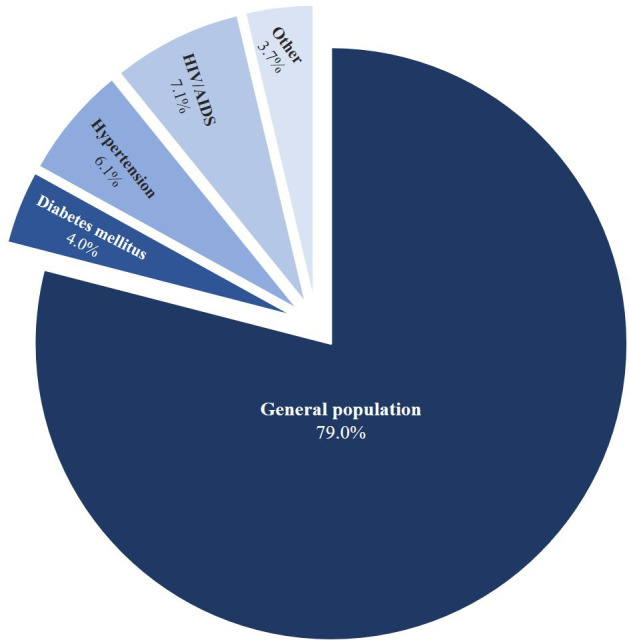
Average contribution of each subpopulation to the overall number of studies enrolled in the CKD-Africa Collaboration. CKD, chronic kidney disease.
Most studies included adults in a broad age range, with the included cohorts comprising adults between the ages of 18 –100 years. One unpublished study from Nigeria included only undergraduate students and therefore selected individuals in the age range 18–30 years. All studies recruited both male and female participants, with most having greater female participation. All, except one study used serum creatinine to estimate GFR and characterise CKD, with 76% additionally determining the presence of albuminuria or proteinuria. Only one study satisfied the 3-month chronicity criterion for diagnosing CKD.56 All the studies include participants with normal kidney function and mild-to-severe stages of CKD (CKD stages 1–4), with 32% not having participants in the most severe stage of kidney failure (stage 5 CKD). All included studies used standardised creatinine assays, with the Jaffe method59 being the most commonly used method for determining serum creatinine concentration. Three of the 38 studies44 48 50 used enzymatic methods to determine serum creatinine concentrations. All studies have data on potential confounders of the associations between kidney function and outcomes including, but not limited to, age, ethnicity, smoking, medical history and treatment, and comorbidities like diabetes mellitus, hypertension, and obesity.
Given the timeframe, we have made considerable progress in establishing the consortium, with included studies covering a fair proportion of Africa.
Expanding the consortium
As a means of strengthening the network we plan to engage with the African Renal Association and the International Society of Nephrology’s Africa Regional Board, to form a broader platform which would assist in calls for funding for this project as well as funding of needed prevalence studies in the rest of Africa not yet represented in the consortium. Also, we have scheduled a process of updating our systematic search process to identify new studies every 6 months, as a means of expanding our database. Expansion of the consortium is indeed important to obtain a representative group of study participants and sufficient statistical power for the results to be meaningful, but we are cautious to not stretch resources too thin and risk failure of the project. We also recognised early on that a balance needed to be struck between awaiting adequate IPD to generate sufficiently powered evidence and getting this evidence out to policymakers and other researchers; bearing in mind that potential collaborators and funders are more likely to join and fund a successful consortium.
Interested research groups working in the field of CKD are welcome to join the network, by contacting the CKD-Africa Collaboration manager at cindy.george@mrc.ac.za.
Funding
There are more financial constraints in LMICs than in affluent high-income countries (HICs). Foreign funding for conducting research in Africa is sparse,60 so having partners from HICs could help acquire important resources for the consortium. Besides funding from the South African National Research Foundation, our research efforts have yet to receive major funding. However, there are concerted efforts being made to attract funding from various sources, with the purpose of the funding geared at (1) capacity development, by attracting and supporting junior African researchers to undertake their postgraduate and postdoctoral projects using the consortium as basis; (2) popularising the work of the consortium, through a website, and workshops and (3) database maintenance.
Novelty and effectiveness of the CKD-Africa collaboration
There is a significant demand for research to direct and strengthen policies related to non-communicable diseases, in particular CKD research in Africa. This network is thus in the ideal position as we aim to provide evidence that could inform health services planning and shape policy and guidelines in Africa and drive the agenda for expanding CKD research. This would inevitably result in improved care for the vulnerable and most affected populations on the continent. The consortium is an ideal platform that will serve as background for future studies in which it is expected to play a major role. Indeed, this platform will allow for discussions centred on standardisation of approaches, related to study design, kidney function measurements and estimating prevalence, enhancing the interpretability of analyses, and integrating data from multiple cohort studies. Our work will create a forum for sharing analytical methods and enhance funding opportunities allowing for the development of ancillary studies using standardised methodology. Further, the potential to combine study data across the network will enhance the ability to examine multiple health outcomes related to CKD, in addition to the ability to report more accurate estimates on the burden of CKD across Africa. This will be used to inform prevention, detection and control strategies at a regional level. Through the evidence generated by the consortium we aim to actively engage policymakers to prioritise CKD reduction. Another major advantage of the large data capacity expected through this collaborative endeavour, is that this consortium could support the training of Masters students and Doctoral fellows across Africa. The consortium will also provide an opportunity for further research capacity training and networking for investigators across Africa.
IPD meta-analysis, which is the primary methodology used by the CKD-Africa Collaboration, has major advantages above those of a conventional meta-analysis. Therefore, given the controversies surrounding the use of the various equations for estimating GFR to diagnose CKD, an IPD meta-analysis will provide standardised estimates across studies. Given the larger sample size through combined studies, IPD meta-analysis will also allow the performance of subgroup analyses (eg, by region, by country, epidemiological transition and over time), which would otherwise not have been possible by any primary study. Further, while there is little opportunity to check for biases from published aggregate data, IPD can be checked for missing, invalid, out-of-range and inconsistent datapoints in the datasets, before incorporating these into the larger merged dataset. Therefore, rather than implementing conventional meta-analyses, we seek opportunities to combine primary data from different groups to implement joint analyses, given the many commonalities between participating studies that will facilitate comparative research. By providing more detailed and reliable results, IPD meta-analyses offer greater potential than aggregate-data meta-analyses to impact on study design, conduct and analysis.
Naturally, this collaborative endeavour has both strengths and limitations. The variation in populations is one of the strengths of this network. Given the distribution of studies across Africa, the populations are genetically distinct, which will provide insights on possible genetic determinants of CKD. Furthermore, these populations also differ greatly with respect to health behaviours, healthcare delivery and environments. Conversely, while commonalities in study design will facilitate joint analysis, inconsistencies in the definition and capture of variables, as well as adjudication of outcomes, can complicate analyses. For example, the Jaffe method is less expensive and more readily used in the included studies compared with enzymatic assay but is more susceptible to interference from various biomolecules, like glucose.61 However, despite the lack of standardisation of measurements of common laboratory parameters, calibration may be achieved by statistical means, given detailed descriptions of the collection processes. Also, this network can provide a unique opportunity to improve the quality of creatinine measurements by examining External Quality Assurance data of participating laboratories and encouraging those laboratories that are not participating in such programmes to do so. This allows for peer comparison and to ensure that methods being used are traceable to an internationally recognised standard. This will allow for greater precision and accuracy of eGFR measurements and permit comparisons. We do acknowledge that a single time point for serum creatinine determination for CKD diagnosis by eGFR, rather than over 3 months as recommended,23 is not ideal. However, given the resource-poor settings in most of Africa, the probability of receiving data on repeated measures is low. Another limitation is the potential risk of participant duplication, where the same individual participates in different studies. Since we receive deidentified data, the various collaborators will be required to inform the secretariat of such potential for duplication. A major limitation currently is that nearly 61% of the identified PIs have not yet responded to our call or are unwilling to participate in the network. Due to the potential large data contribution by these studies, the risk of significant bias could be introduced into our analysis. However, enrolment into the consortium continues and, to minimise the risk of bias, as far as possible, analyses will include both IPD and aggregated data from the published studies for which IPD is unavailable. In its current form, the network is only represented by 12 countries (22%) of the 54 countries in Africa, with most IPD coming from South Africa. However, with that said, this network covers all five subregions of Africa and as this is a growing network, the number of enrolled studies is expected to increase substantially over the coming years.
Conclusion
This network will aid research in the field of CKD on the African continent. With this platform to facilitate interactions among active investigators, the commitment of all teams currently involved and the broadly defined research agenda, we are confident that there will be new studies across Africa, particular in the currently under-represented countries, that will join the network. In addition, we foresee the development of new studies originating from this collaboration. In that regard, this network has far-reaching potential for Africa, as it is in an ideal position to validate findings across geographical and national boundaries, to test hypotheses and to generate a new understanding of CKD progression and its complications. Although the CKD-Africa Collaboration is focused primarily on CKD, many of our lessons learnt can be applied more widely in public health research in LMICs.
Acknowledgments
We would like to acknowledge the South African Medical Research Council (SAMRC) for infrastructure and support. Also, we are very grateful to the collaborators for showing keen interest in collaborating in this network and their continued participation, as well as all the participants from each primary study for allowing their data to be incorporated into this network.
Footnotes
Handling editor: Seye Abimbola
Collaborators: T A Adedeji; C Agyemang; A Akinsola; D D Alasia; O E Ayodele; E J Beune; P Bovet; J Cailhol; D R Chadwick; S P Choukem; S A Dada; M R Davids; M H Ekat; R T Erasmus; J Fabian; J A George; Z Gouda; H Grosskurth; R Kalyesubula; S Kapiga; F F Kaze; R Kruger; L Lammertyn; T E Matsha; C M C Mels; O C A Okoye; R Oluyombo; C Osafo; R N Peck; N Peer; R O Phillips; Y R Raji; M Ramsay; B Rayner; A A Salawu; A E Schutte; I Ssinabulya; J W Stanifer; R Wanyama.
Contributors: CG, SS, IO, MW and APK, who formed the core Writing Committee, contributed to the preparation of the manuscript. All collaborators were sent the manuscript as prepared for submission and given the opportunity to comment on the draft manuscript. The Writing Committee accepts full responsibility for the content of this manuscript.
Funding: This work was supported by the National Research Foundation (NRF) with funds from the Thuthuka Funding instrument (grant number TTK170504229669) to (CG). Several sources have supported the studies contributing to the CKD-Africa Collaboration and are described in the relevant publications.
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein), or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests: The members of the Writing Committee declare that they have no competing interests. No support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
CKD-Africa Collaboration:
T A Adedeji, C Agyemang, A Akinsola, D D Alasia, O E Ayodele, E J Beune, P Bovet, J Cailhol, D R Chadwick, S P Choukem, S A Dada, M R Davids, M H Ekat, R T Erasmus, J Fabian, J A George, Z Gouda, H Grosskurth, R Kalyesubula, S Kapiga, F F Kaze, R Kruger, L Lammertyn, T E Matsha, C M C Mels, O C A Okoye, R Oluyombo, C Osafo, R N Peck, N Peer, R O Phillips, Y R Raji, M Ramsay, B Rayner, A A Salawu, A E Schutte, I Ssinabulya, J W Stanifer, and R Wanyama
Data availability statement
The datasets depicted in this manuscript are available from the corresponding author of each primary study on reasonble request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Ethical clearance was obtained from the SAMRCs Human Research Ethics Committee for the establishment of the consortium (reference: EC016-8/2017) and further ethical clearance are sought for individual projects pertaining to the consortium.
References
- 1. GBD Chronic Kidney Disease Collaboration . Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet 2020;395:709–33. 10.1016/S0140-6736(20)30045-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ene-Iordache B, Perico N, Bikbov B, et al. Chronic kidney disease and cardiovascular risk in six regions of the world (ISN-KDDC): a cross-sectional study. Lancet Glob Health 2016;4:e307–19. 10.1016/S2214-109X(16)00071-1 [DOI] [PubMed] [Google Scholar]
- 3. Kaze AD, Ilori T, Jaar BG, et al. Burden of chronic kidney disease on the African continent: a systematic review and meta-analysis. BMC Nephrol 2018;19:125. 10.1186/s12882-018-0930-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abd ElHafeez S, Bolignano D, D'Arrigo G, et al. Prevalence and burden of chronic kidney disease among the general population and high-risk groups in Africa: a systematic review. BMJ Open 2018;8:e015069. 10.1136/bmjopen-2016-015069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. The Lancet 2020;396:1204–22. 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jager KJ, Kovesdy C, Langham R, et al. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Nephrol Dial Transplant 2019;34:1803–5. 10.1093/ndt/gfz174 [DOI] [PubMed] [Google Scholar]
- 7. Bello AK, Levin A, Lunney M, et al. Status of care for end stage kidney disease in countries and regions worldwide: international cross sectional survey. BMJ 2019;367:l5873. 10.1136/bmj.l5873 [DOI] [PubMed] [Google Scholar]
- 8. Stanifer JW, Jing B, Tolan S, et al. The epidemiology of chronic kidney disease in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Glob Health 2014;2:e174–81. 10.1016/S2214-109X(14)70002-6 [DOI] [PubMed] [Google Scholar]
- 9. Arogundade FA, Barsoum RS. Ckd prevention in sub-Saharan Africa: a call for governmental, nongovernmental, and community support. Am J Kidney Dis 2008;51:515–23. 10.1053/j.ajkd.2007.12.006 [DOI] [PubMed] [Google Scholar]
- 10. Meguid El Nahas A, Bello AK. Chronic kidney disease: the global challenge. Lancet 2005;365:331–40. 10.1016/S0140-6736(05)17789-7 [DOI] [PubMed] [Google Scholar]
- 11. George C, Mogueo A, Okpechi I, et al. Chronic kidney disease in low-income to middle-income countries: the case for increased screening. BMJ Glob Health 2017;2:e000256. 10.1136/bmjgh-2016-000256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Okunola O, Akinsola A, Ayodele O. Kidney diseases in Africa: aetiological considerations, peculiarities and burden. Afr J Med Med Sci 2012;41:119–33. [PubMed] [Google Scholar]
- 13. GBD 2015 HIV Collaborators . Estimates of global, regional, and national incidence, prevalence, and mortality of HIV, 1980-2015: the global burden of disease study 2015. Lancet HIV 2016;3:e361–87. 10.1016/S2352-3018(16)30087-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. George C, Yako YY, Okpechi IG, et al. An African perspective on the genetic risk of chronic kidney disease: a systematic review. BMC Med Genet 2018;19:187. 10.1186/s12881-018-0702-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Davids MR, Gharbi MB. Global considerations in kidney disease: Africa. 11th edn. Rector’s The Kidney, 2019. [Google Scholar]
- 16. Einterz RM, Kimaiyo S, Mengech HNK, et al. Responding to the HIV pandemic: the power of an academic medical partnership. Acad Med 2007;82:812–8. 10.1097/ACM.0b013e3180cc29f1 [DOI] [PubMed] [Google Scholar]
- 17. Matsushita K, Ballew SH, Astor BC, et al. Cohort profile: the chronic kidney disease prognosis Consortium. Int J Epidemiol 2013;42:1660–8. 10.1093/ije/dys173 [DOI] [PubMed] [Google Scholar]
- 18. Orlandi PF, Huang J, Fukagawa M, et al. A collaborative, individual-level analysis compared longitudinal outcomes across the International network of chronic kidney disease (iNETCKD) cohorts. Kidney Int 2019;96:1217–33. 10.1016/j.kint.2019.07.024 [DOI] [PubMed] [Google Scholar]
- 19. Caleyachetty R, Echouffo-Tcheugui JB, Tait CA, et al. Prevalence of behavioural risk factors for cardiovascular disease in adolescents in low-income and middle-income countries: an individual participant data meta-analysis. Lancet Diabetes Endocrinol 2015;3:535–44. 10.1016/S2213-8587(15)00076-5 [DOI] [PubMed] [Google Scholar]
- 20. Darvishian M, van den Heuvel ER, Bissielo A, et al. Effectiveness of seasonal influenza vaccination in community-dwelling elderly people: an individual participant data meta-analysis of test-negative design case-control studies. Lancet Respir Med 2017;5:200–11. 10.1016/S2213-2600(17)30043-7 [DOI] [PubMed] [Google Scholar]
- 21. Muthuri SG, Venkatesan S, Myles PR, et al. Impact of neuraminidase inhibitors on influenza A(H1N1)pdm09-related pneumonia: an individual participant data meta-analysis. Influenza Other Respir Viruses 2016;10:192–204. 10.1111/irv.12363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pienaar E, Grobler L, Busgeeth K, et al. Developing a geographic search filter to identify randomised controlled trials in Africa: finding the optimal balance between sensitivity and precision. Health Info Libr J 2011;28:210–5. 10.1111/j.1471-1842.2011.00936.x [DOI] [PubMed] [Google Scholar]
- 23. KDIGO . KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. In: Kidney Int. 3, 2013. https://kdigo.org/wp-content/uploads/2017/02/KDIGO_2012_CKD_GL.pdf [DOI] [PubMed] [Google Scholar]
- 24. Bonita R, Winkelmann R, Douglas KA. The WHO stepwise approach to surveillance (steps) of non-communicable disease risk factors. In: Global behavioral risk factor surveillance. Boston, MA: Springer, 2003. [Google Scholar]
- 25. Gouda Z, Mashaal G, Bello AK, et al. Egypt information, prevention, and treatment of chronic kidney disease (EGIPT-CKD) programme: prevalence and risk factors for microalbuminuria among the relatives of patients with CKD in Egypt. Saudi J Kidney Dis Transpl 2011;22:1055–63. [PubMed] [Google Scholar]
- 26. Alasia DD, Emem-Chioma P, Wokoma FS. A single-center 7-year experience with end-stage renal disease care in Nigeria-a surrogate for the poor state of ESRD care in Nigeria and other sub-Saharan African countries: advocacy for a global fund for ESRD care program in sub-Saharan African countries. Int J Nephrol 2012;2012:1–7. 10.1155/2012/639653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cailhol J, Nkurunziza B, Izzedine H, et al. Prevalence of chronic kidney disease among people living with HIV/AIDS in Burundi: a cross-sectional study. BMC Nephrol 2011;12:40. 10.1186/1471-2369-12-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Derra K, Rouamba E, Kazienga A, et al. Profile: Nanoro health and demographic surveillance system. Int J Epidemiol 2012;41:1293–301. 10.1093/ije/dys159 [DOI] [PubMed] [Google Scholar]
- 29. Ekat MH, Courpotin C, Diafouka M, et al. [Prevalence and factors associated with renal disease among patients with newly diagnoses of HIV in Brazzaville, Republic of Congo]. Med Sante Trop 2013;23:176–80. 10.1684/mst.2013.0170 [DOI] [PubMed] [Google Scholar]
- 30. Malan L, Hamer M, Frasure-Smith N, et al. Cohort profile: sympathetic activity and ambulatory blood pressure in Africans (SABPA) prospective cohort study. Int J Epidemiol 2015;44:1814–22. 10.1093/ije/dyu199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Matsha TE, Yako YY, Rensburg MA, et al. Chronic kidney diseases in mixed ancestry South African populations: prevalence, determinants and concordance between kidney function estimators. BMC Nephrol 2013;14:75. 10.1186/1471-2369-14-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Okoye OCA, Oviasu E, Ojogwu L. Prevalence of chronic kidney disease and its risk factors amongst adults in a rural population in Edo state, Nigeria. Journal of US-China Medical Science;8:471–81. [Google Scholar]
- 33. Oluyombo R, Ayodele OE, Akinwusi PO, et al. A community study of the prevalence, risk factors and pattern of chronic kidney disease in Osun state, South West Nigeria. West Afr J Med 2013;32:85–92. [PubMed] [Google Scholar]
- 34. Peer N, George J, Lombard C, et al. Prevalence, concordance and associations of chronic kidney disease by five estimators in South Africa. BMC Nephrol 2020;21:372. 10.1186/s12882-020-02018-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rayner B, Becker P. The prevalence of microalbuminuria and ECG left ventricular hypertrophy in hypertensive patients in private practices in South Africa. Cardiovasc J S Afr 2006;17:245–9. [PubMed] [Google Scholar]
- 36. Richter L, Norris S, Pettifor J, et al. Cohort profile: Mandela's children: the 1990 birth to twenty study in South Africa. Int J Epidemiol 2007;36:504–11. 10.1093/ije/dym016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schutte AE, Huisman HW, Schutte R, et al. Arterial stiffness profiles: investigating various sections of the arterial tree of African and Caucasian people. Clin Exp Hypertens 2011;33:511–7. 10.3109/10641963.2011.561897 [DOI] [PubMed] [Google Scholar]
- 38. Pruijm MT, Madeleine G, Riesen WF, et al. Prevalence of microalbuminuria in the general population of Seychelles and strong association with diabetes and hypertension independent of renal markers. J Hypertens 2008;26:871–7. 10.1097/HJH.0b013e3282f624d9 [DOI] [PubMed] [Google Scholar]
- 39. Adeniyi AB, Laurence CE, Volmink JA. Prevalence of chronic kidney disease and association with cardiovascular risk factors among teachers in Cape town, South Africa. Clin Kidney J;10:363–9. 10.1093/ckj/sfw138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Adjei DN, Stronks K, Adu D, et al. Cross-sectional study of association between socioeconomic indicators and chronic kidney disease in rural-urban Ghana: the RODAM study. BMJ Open 2019;9:e022610. 10.1136/bmjopen-2018-022610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Alberts M, Dikotope SA, Choma SR, et al. Health & Demographic Surveillance System Profile: The Dikgale Health and Demographic Surveillance System. Int J Epidemiol 2015;44:1565–71. 10.1093/ije/dyv157 [DOI] [PubMed] [Google Scholar]
- 42. Beguy D, Elung'ata P, Mberu B, et al. Health & demographic surveillance system profile: the nairobi urban health and demographic surveillance system (NUHDSS). Int J Epidemiol 2015;44:462–71. 10.1093/ije/dyu251 [DOI] [PubMed] [Google Scholar]
- 43. Kahn K, Collinson MA, Gómez-Olivé FX, et al. Profile: agincourt health and socio-demographic surveillance system. Int J Epidemiol 2012;41:988–1001. 10.1093/ije/dys115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kalyesubula R, Nankabirwa JI, Ssinabulya I, et al. Kidney disease in Uganda: a community based study. BMC Nephrol 2017;18:116. 10.1186/s12882-017-0521-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kaze FF, Halle M-P, Mopa HT, et al. Prevalence and risk factors of chronic kidney disease in urban adult Cameroonians according to three common estimators of the glomerular filtration rate: a cross-sectional study. BMC Nephrol 2015;16:96. 10.1186/s12882-015-0102-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kaze FF, Meto DT, Halle M-P, et al. Prevalence and determinants of chronic kidney disease in rural and urban Cameroonians: a cross-sectional study. BMC Nephrol 2015;16:117. 10.1186/s12882-015-0111-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Oduro AR, Wak G, Azongo D, et al. Profile of the navrongo health and demographic surveillance system. Int J Epidemiol 2012;41:968–76. 10.1093/ije/dys111 [DOI] [PubMed] [Google Scholar]
- 48. Peck R, Baisley K, Kavishe B, et al. Decreased renal function and associated factors in cities, towns and rural areas of Tanzania: a community-based population survey. Trop Med Int Health 2016;21:393–404. 10.1111/tmi.12651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schutte AE, Gona PN, Delles C, et al. The African prospective study on the early detection and identification of cardiovascular disease and hypertension (African-PREDICT): design, recruitment and initial examination. Eur J Prev Cardiol 2019;26:458–70. 10.1177/2047487318822354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stanifer JW, Maro V, Egger J, et al. The epidemiology of chronic kidney disease in northern Tanzania: a population-based survey. PLoS One 2015;10:e0124506. 10.1371/journal.pone.0124506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Adedeji TA, Adedeji NO, Adebisi SA, et al. Prevalence and pattern of chronic kidney disease in antiretroviral-naïve patients with HIV/AIDS. J Int Assoc Provid AIDS Care 2015;14:434–40. 10.1177/2325957415587570 [DOI] [PubMed] [Google Scholar]
- 52. Ayokunle DS, Olusegun OT, Ademola A, et al. Prevalence of chronic kidney disease in newly diagnosed patients with human immunodeficiency virus in Ilorin, Nigeria. J Bras Nefrol 2015;37:177–84. 10.5935/0101-2800.20150029 [DOI] [PubMed] [Google Scholar]
- 53. Chadwick DR, Sarfo FS, Kirk ESM, et al. Tenofovir is associated with increased tubular proteinuria and asymptomatic renal tubular dysfunction in Ghana. BMC Nephrol 2015;16:195. 10.1186/s12882-015-0192-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Odongo P, Wanyama R, Obol JH, et al. Impaired renal function and associated risk factors in newly diagnosed HIV-infected adults in Gulu Hospital, Northern Uganda. BMC Nephrol 2015;16:43. 10.1186/s12882-015-0035-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Osafo C, Mate-Kole M, Affram K, et al. Prevalence of chronic kidney disease in hypertensive patients in Ghana. Ren Fail 2011;33:388–92. 10.3109/0886022X.2011.565140 [DOI] [PubMed] [Google Scholar]
- 56. Kaze FF, Kengne A-P, Magatsing CT, et al. Prevalence and determinants of chronic kidney disease among hypertensive Cameroonians according to three common estimators of the glomerular filtration rate. J Clin Hypertens 2016;18:408–14. 10.1111/jch.12781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Feteh VF, Choukem S-P, Kengne A-P, et al. Anemia in type 2 diabetic patients and correlation with kidney function in a tertiary care sub-Saharan African hospital: a cross-sectional study. BMC Nephrol 2016;17:29. 10.1186/s12882-016-0247-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Raji YR, Ajayi SO, Akingbola TS, et al. Assessment of iron deficiency anaemia and its risk factors among adults with chronic kidney disease in a tertiary hospital in Nigeria. Niger Postgrad Med J 2018;25:197–203. 10.4103/npmj.npmj_106_18 [DOI] [PubMed] [Google Scholar]
- 59. Greenwald I. The chemistry of Jaffe's reaction for creatinine II. The effect of substitution in the creatinine molecule and a possible formula for the red tautomer. J Am Chem Soc;80:103–6. 10.1021/ja01682a033 [DOI] [Google Scholar]
- 60. van Helden P. The cost of research in developing countries. EMBO Rep 2012;13:395. 10.1038/embor.2012.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Çuhadar S, Köseoğlu M, Çinpolat Y, et al. The effect of extremely high glucose concentrations on 21 routine chemistry and thyroid Abbott assays: interference study. Biochem Med 2016;26:53–60. 10.11613/BM.2016.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ali SA, Soo C, Agongo G, et al. Genomic and environmental risk factors for cardiometabolic diseases in Africa: methods used for phase 1 of the AWI-Gen population cross-sectional study. Glob Health Action 2018;11:1507133. 10.1080/16549716.2018.1507133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Heiniger S, Viswanathan B, Gedeon J, et al. Trends in prevalence, awareness, treatment and control of high blood pressure in the Seychelles between 1989 and 2013. J Hypertens 2017;35:1465–73. 10.1097/HJH.0000000000001358 [DOI] [PubMed] [Google Scholar]
- 64. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2021-006454supp001.pdf (57.7KB, pdf)
bmjgh-2021-006454supp002.pdf (73.1KB, pdf)
bmjgh-2021-006454supp003.pdf (136.1KB, pdf)
bmjgh-2021-006454supp004.pdf (122.9KB, pdf)
Data Availability Statement
The datasets depicted in this manuscript are available from the corresponding author of each primary study on reasonble request.