Answer questions and earn CME
Abbreviations
- CI
confidence interval
- CT
computed tomography
- DAA
direct‐acting antiviral
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- IFN
interferon
- MRI
magnetic resonance imaging
- SVR
sustained virological response
Hepatitis C virus (HCV) infection is one of the most common risk factors for hepatocellular carcinoma (HCC). Direct‐acting antivirals (DAAs) are safe, well‐tolerated, cost‐effective, and offer a chance for cure for nearly all patients with HCV, with sustained virological response (SVR) rates exceeding 95%. HCV eradication with DAAs has several proven benefits, including fibrosis regression, improvement in liver function and portal hypertension, and reduced risk for all‐cause mortality 1 ; however, patients with active or treated HCC are one of the few populations in whom DAA treatment remains controversial. In this article, we discuss the impact of DAAs on HCC risk and review considerations for the use and timing of DAA therapy in patients with HCC.
DAA Therapy and Risk for Incident HCC
Patients with HCV‐related cirrhosis have a high risk for HCC, with an incidence rate of 2% to 4%. Although several studies established the association between interferon (IFN)‐induced SVR and reduced HCC risk, it was unknown whether this was related to viral eradication or a direct IFN effect. Fortunately, this association was confirmed in the DAA era with large cohort studies with similar reductions in HCC risk by up to 75% after DAA‐induced SVR (Fig. 1). 1 , 2
FIG 1.
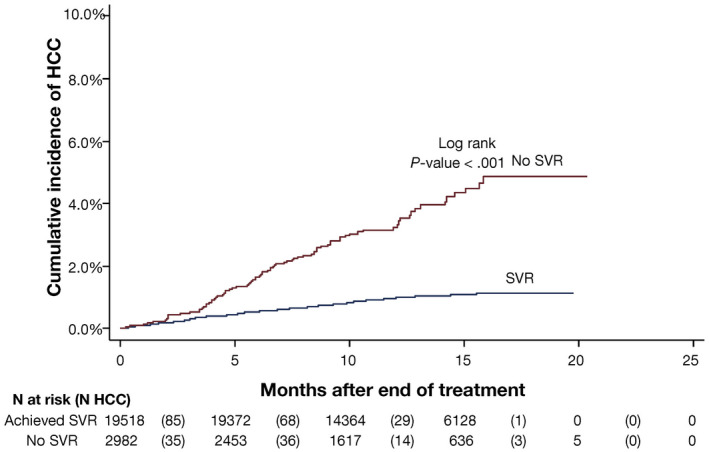
Cumulative incidence of HCC among 22,500 patients treated with DAAs who did and did not achieve SVR. Although the relative risk for HCC was reduced in patients who achieved SVR, the absolute risk for HCC persisted, with an annual incidence rate of ~0.90%. Reproduced with permission from Gastroenterology. Copyright 2017, American Gastroenterological Association. 2
Although risk for HCC post‐SVR decreases substantially, it is less clear whether the risk for HCC continues to decline in patients with cirrhosis long term. 2 In a study of more than 18,000 veterans, Kanwal et al. 3 demonstrated that HCC risk remained greater than the accepted threshold for surveillance cost‐effectiveness in patients with cirrhosis for up to 3.6 years after SVR. Using a Markov model, Zangneh et al. 4 also found that HCC surveillance was likely cost‐effective among patients with cirrhosis for up to 15 years after SVR. In this same model, surveillance in patients with F3 fibrosis post‐SVR was lower than the accepted threshold and deemed to be not cost‐effective. Notably, it may be difficult to directly translate these data to individual patients with F3 fibrosis given the potential of misclassification for cirrhosis (e.g., inaccuracy of noninvasive tests or sampling error during liver biopsy) may underestimate risk for HCC. Although future predictive models may help to identify patients with F3 fibrosis at highest risk for HCC, surveillance in this population is not currently recommended by the American Association for the Study of Liver Diseases guidelines.
DAA Therapy in Patients With Treated HCC and Risk for HCC Recurrence
In 2016, a study by Reig et al. 5 that included 58 patients with a history of HCV‐related HCC raised potential concerns about increased early HCC recurrence and more aggressive tumor biology after DAA treatment. Subsequent studies refuted these findings, and a meta‐analysis of 24 studies (n = 1820 patients) found that HCC recurrence rates varied from 0% to 59% over a 2‐year period, with a pooled risk for recurrence of 25.1% (95% confidence interval [CI]: 19.4%‐31.2%). 6 A subsequent multicenter North American cohort study found no difference in HCC recurrence after DAA treatment compared with those who did not receive DAA treatment (Fig. 2). 7 Further, among patients with HCC recurrence, there appeared to be no difference in recurrence patterns, with a similar proportion of DAA‐treated and untreated patients detected within Milan criteria (74.2% versus 78.8%; P = 0.23). The impact of DAAs on mortality in patients with HCC has been investigated, particularly in light of data showing liver dysfunction is a primary driver of mortality in patients with a history of cured HCC. 8 Singal et al. 9 found that DAAs were associated with improved survival (hazard ratio, 0.54; 95% CI: 0.33‐0.90), with fewer liver‐related deaths in the DAA‐treated group compared with those who were untreated (16.3% versus 34.0%; P = 0.03).
FIG 2.
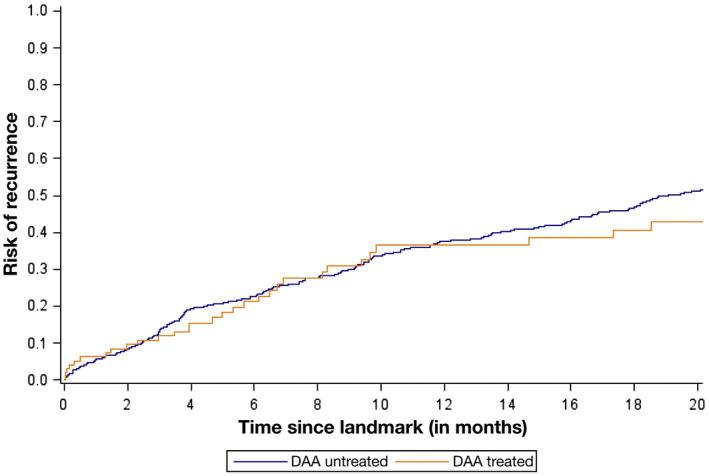
Time to HCC recurrence stratified by receipt of DAA therapy. Reproduced with permission from Gastroenterology. Copyright 2019, American Gastroenterological Association. 7
Presently, there are no convincing data to suggest that DAAs should be withheld in patients who have had complete response to HCC treatment. One of the most consistent predictors of recurrence after DAAs in the aforementioned studies was the duration between complete response to HCC treatment and initiation of DAA therapy. Risk for HCC recurrence after DAAs is also higher in patients receiving palliative treatments (e.g., transarterial chemoembolization) than those receiving traditional curative treatments (e.g., surgical resection, ablation). A national provider survey in the United States found large variation in real‐world practices of DAAs among providers. 10 At our center, we typically initiate DAAs between 4 and 6 months after complete response to HCC treatment has been identified on cross‐sectional imaging. This allows for two computed tomography (CT) or magnetic resonance imaging (MRI) scans to allow for confirmation of complete and durable response. Notably, patients with successfully treated HCC remain at risk for cancer recurrence even after SVR and should continue to receive HCC surveillance with cross‐sectional imaging (e.g., CT or MRI) every 3 to 6 months indefinitely. 11 In patients with HCC who are liver transplant candidates, decisions regarding timing of DAA treatment (e.g., before or after liver transplant) depend on organ availability and region wait times and should be individualized.
DAA Therapy in Patients With Active and/or Advanced HCC
SVR rates with DAA therapy are significantly lower in patients with active HCC. 12 The mechanism is unclear, but proposed hypotheses include HCC tumor cells serving as a reservoir for HCV and potential alterations in blood supply to HCV‐infected tumor cells impacting DAA uptake. There are few data to inform the optimal timing of DAAs in patients with active HCC. 13 Given heterogeneity in tumor biology, it is likely reasonable to initiate HCC‐directed treatment and then consider DAA therapy in the subset of patients with an objective response or stable disease. Similarly, data are limited on the benefit of DAAs in patients with advanced‐stage HCC; however, given the rapidly changing landscape of systemic therapy and recent improvements in overall survival for advanced HCC, it may be reasonable to consider DAAs in this setting. In some patients, SVR may lead to improved liver function enabling longer courses of systemic therapy. In those with tumor progression, significant liver dysfunction, and/or poor performance status, the benefit of DAAs likely remains limited.
Conclusion
DAAs have transformed the care of patients with HCV and have several established benefits, including reduction of HCC risk in patients with cirrhosis. In those with a history of treated HCC, DAAs appear safe and not associated with an increased risk for cancer recurrence. Although data are limited in patients with active HCC undergoing palliative therapies, DAAs are likely safe, potentially beneficial, and can be considered in select patients based on comorbid conditions, response to HCC treatment, and overall life expectancy.
A.G.S.’s research is supported by National Cancer Institute Grant R01 CA222900. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Potential conflict of interest: N.E.R. has no relevant conflicts of interest. A.G.S. consults for Bayer, Eisai, Genentech, Exelixis, BMS, Roche, Wako, Exact, Gycotest, and Grail and has received research support from AbbVie and Gilead.
References
- 1. Carrat F, Fontaine H, Dorival C, et al. Clinical outcomes in patients with chronic hepatitis C after direct‐acting antiviral treatment: a prospective cohort study. Lancet 2019;393:1453‐1464. [DOI] [PubMed] [Google Scholar]
- 2. Kanwal F, Kramer J, Asch SM, et al. Risk of hepatocellular cancer in HCV patients treated with direct‐acting antiviral agents. Gastroenterology 2017;153:996‐1005.e1. [DOI] [PubMed] [Google Scholar]
- 3. Kanwal F, Kramer JR, Asch S, et al. Long‐term risk of hepatocellular carcinoma in HCV patients treated with direct acting antiviral agents. Hepatology 2020;71:44‐55. [DOI] [PubMed] [Google Scholar]
- 4. Farhang Zangneh H, Wong WWL, Sander B, et al. Cost effectiveness of hepatocellular carcinoma surveillance after a sustained virologic response to therapy in patients with hepatitis C virus infection and advanced fibrosis. Clin Gastroenterol Hepatol 2019;17:1840‐1849.e16. [DOI] [PubMed] [Google Scholar]
- 5. Reig M, Mariño Z, Perelló C, et al. Unexpected high rate of early tumor recurrence in patients with HCV‐related HCC undergoing interferon‐free therapy. J Hepatol 2016;65:719‐726. [DOI] [PubMed] [Google Scholar]
- 6. Saraiya N, Yopp AC, Rich NE, et al. Systematic review with meta‐analysis: recurrence of hepatocellular carcinoma following direct‐acting antiviral therapy. Aliment Pharmacol Ther 2018;48:127‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Singal AG, Rich NE, Mehta N, et al. Direct‐acting antiviral therapy not associated with recurrence of hepatocellular carcinoma in a multicenter North American cohort study. Gastroenterology 2019;156:1683‐1692.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cabibbo G, Petta S, Barbara M, et al. Hepatic decompensation is the major driver of death in HCV‐infected cirrhotic patients with successfully treated early hepatocellular carcinoma. J Hepatol 2017;67:65‐71. [DOI] [PubMed] [Google Scholar]
- 9. Singal AG, Rich NE, Mehta N, et al. Direct‐acting antiviral therapy for hepatitis C virus infection is associated with increased survival in patients with a history of hepatocellular carcinoma. Gastroenterology 2019;157:1253‐1263.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rich NE, Yang JD, Perumalswami PV, et al. Provider attitudes and practice patterns for direct‐acting antiviral therapy for patients with hepatocellular carcinoma. Clin Gastroenterol Hepatol 2020;18:974‐983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Singal AG, Lim JK, Kanwal F. AGA clinical practice update on interaction between oral direct‐acting antivirals for chronic hepatitis C infection and hepatocellular carcinoma: expert review. Gastroenterology 2019;156:2149‐2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ji F, Yeo YH, Wei MT, et al. Sustained virologic response to direct‐acting antiviral therapy in patients with chronic hepatitis C and hepatocellular carcinoma: a systematic review and meta‐analysis. J Hepatol 2019;71:473‐485. [DOI] [PubMed] [Google Scholar]
- 13. Dang H, Yeo YH, Yasuda S, et al. Cure with interferon‐free direct‐acting antiviral is associated with increased survival in patients with hepatitis C virus‐related hepatocellular carcinoma from both east and west. Hepatology 2020;71:1910‐1922. [DOI] [PubMed] [Google Scholar]


