Abbreviations
- AFP
alpha‐fetoprotein
- DCP
des‐gamma‐carboxyprothrombin
- HCC
hepatocellular carcinoma
- miRNA
microRNA
- MRI
magnetic resonance imaging
Hepatocellular carcinoma (HCC) is the fourth most common cause of cancer‐related mortality, with approximately 800,000 deaths worldwide and 27,000 deaths in the United States annually. Its 5‐year survival rate remains less than 20%, in part related to the majority of patients presenting beyond an early stage. 1 Professional societies, including the American Association for the Study of Liver Diseases, recommend semiannual surveillance using abdominal ultrasound with or without alpha‐fetoprotein (AFP) among at‐risk patients to identify HCC at an early stage, when curative options are available (Fig. 1). Herein, we review available and emerging imaging modalities and biomarkers that can be used for HCC surveillance.
FIG 1.
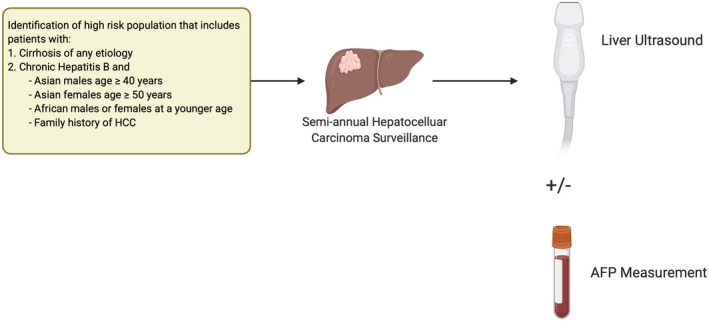
Current HCC surveillance strategy.
Imaging
Abdominal ultrasound, the current imaging cornerstone for HCC surveillance, has several advantages, including being noninvasive, inexpensive, and safe without risk for contrast or radiation exposure. Ultrasound‐based surveillance has been shown to reduce mortality by 37% in patients with chronic hepatitis B (level I data), while several cohort studies have shown a consistent association with improved curative treatment and overall survival in patients with cirrhosis, even after adjusting for lead time and length time biases (level II data). 2 , 3 However, ultrasound is operator dependent with highly variable sensitivity between providers and centers, and has a pooled sensitivity of only 45% for detecting HCC at an early stage. 4 False‐positive or indeterminate results also occur in up to 20% of patients and often lead to unnecessary diagnostic imaging. 5 Ultrasound visualization and effectiveness may be further lower in patients with obesity and nonviral cirrhosis—populations that are increasingly common in clinical practice. 6 Finally, ultrasound‐based surveillance often requires a separate appointment, with barriers such as transportation and scheduling, which can reduce utilization and negatively impact surveillance effectiveness. 7
Given growing recognition of these limitations with ultrasound‐based surveillance, there is increasing use of cross‐sectional imaging for surveillance in clinical practice. Although a computed tomography–based surveillance strategy is likely not feasible given radiation exposure and risk for contrast injury, a magnetic resonance imaging (MRI)‐based strategy would not have these same limitations. A cohort study from South Korea compared MRI‐ and ultrasound‐based surveillance in a cohort of 407 patients with cirrhosis and found MRI had significantly higher sensitivity for early HCC detection (85.7% versus 26.2%) with similar specificity (97.0% versus 94.4%). 8 Although promising, there are remaining concerns about cost‐effectiveness and lack of confirmatory data in Western non‐hepatitis B patient populations. Further, there are concerns about access, particularly in areas of the country with more limited radiological capacity. Case‐control studies have found abbreviated MRI protocols, which select specific MRI sequences to decrease in‐scanner time from 45 to 15 minutes, may address some of these concerns while preserving high sensitivity for early HCC detection; however, early promising data need to be confirmed in cohort studies.
Biomarkers
In addition to alternative imaging modalities, there is also increasing interest in blood‐based surveillance biomarkers (Fig. 2). Currently, AFP is the only biomarker that has undergone all five phases of biomarker evaluation (Table 1) and is the most commonly used biomarker in practice. Although AFP is inexpensive, objective, and widely available, its limited sensitivity to detect early‐stage HCC and false‐positive results in the setting of active viral hepatitis have historically hampered enthusiasm. However, AFP appears to be of benefit when used with ultrasound, with a meta‐analysis demonstrating increased sensitivity for early HCC detection from 45% using ultrasound alone to 63% with the two tests in combination. 4 Further, AFP false‐positive results appear to be less common in nonviral cirrhosis, and physical harms from false‐positive results appear to be mitigated in practice by providers using longitudinal changes when interpreting AFP values instead of relying on the traditional single‐threshold measurement of 20 ng/mL. 5
FIG 2.
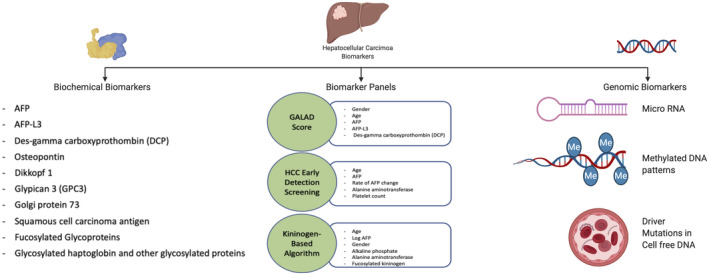
Potential biomarkers for HCC surveillance.
TABLE 1.
Phases of Biomarker Validation for Early Detection
| Phase of Development | Definition |
|---|---|
| I | Identify potential biomarkers in a preclinical setting. |
| II | Assess the ability of the assay to distinguish between cases and controls. |
| III | Analyze the ability of the assay to analyze preclinical disease. |
| IV | Assess operating characteristics of biomarker‐based screening in the target population. |
| V | Assess the impact of screening on cancer mortality. |
Several new blood‐based biomarkers, such as lectin‐bound AFP (AFP‐L3%) and des‐gamma‐carboxyprothrombin (DCP), are also under investigation (Fig. 2). Most of these biomarkers have been evaluated in only phase II (case‐control) biomarker studies and still require validation in phase III and phase IV (cohort) studies. 9 The maturation of large cohort studies, such as Early Detection Research Network HCC Early Detection Strategy Study and Texas HCC Consortium, should facilitate phase III evaluation of several biomarkers in the near future. Current data suggest that single biomarkers may not be sufficient for early HCC detection, likely related to HCC heterogeneity. Therefore, there has been increased interest in biomarker panels, combining several biomarkers with or without clinical variables. For example, GALAD is a panel that combines gender, age, AFP‐L3%, AFP, and DCP, which has been evaluated in a large multinational case‐control study with a sensitivity of 60% to 80% for detecting early‐stage HCC. 10
With advances in genomics, there also has been increased identification of dysregulated nucleic acids that can also serve as surveillance biomarkers. Circulating noncoding RNAs or microRNAs (miRNAs), such as miRNA‐21 and miRNA‐199a, have been identified in patients with early HCC. 9 Similarly, DNA abnormalities isolated from circulating tumor cells and quantitative analysis of cell‐free DNA have shown moderate sensitivity and accuracy for detecting HCC. 9 A methylated DNA marker panel was recently shown to have promising accuracy in a phase II biomarker study, with sensitivity and specificity for early HCC detection of 70% and 89%, respectively. 11 However, these biomarkers also still require validation in large cohort studies using standardized processing techniques and cutoffs.
Summary
HCC surveillance should be performed as recommended in patients with chronic hepatitis B and patients with cirrhosis to reduce HCC‐related mortality. Semiannual abdominal ultrasound and AFP is currently the best strategy to maximize early detection; however, this strategy misses more than one‐third of HCCs at an early stage, highlighting the need for better tests. MRI‐based imaging strategies may increase sensitivity for early HCC detection, although there are potential concerns about cost‐effectiveness and radiological capacity. In parallel, there are several promising blood‐based protein and genomic biomarker panels that have shown high sensitivity for early HCC detection in early case‐control studies. These biomarkers still require validation in large cohort studies but have potential to truly transform how we perform HCC surveillance among at‐risk patients.
A.G.S.’s research was conducted with support from NIH grants R01CA222900 and R01CA212008. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Potential conflict of interest: A.G.S. consults for Roche, Exact Sciences, Wako Diagnostics, Glycotest, GRAIL and Bayer.
References
- 1. Moon AM, Singal AG, Tapper EB. Contemporary epidemiology of chronic liver disease and cirrhosis. Clin Gastroenterol Hepatol 2020;18:2650‐2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 2004;130:417‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta‐analysis. PLoS Med 2014;11:e1001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tzartzeva K, Obi J, Rich NE, et al. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: a meta‐analysis. Gastroenterology 2018;154:1706‐1718.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Atiq O, Tiro J, Yopp AC, et al. An assessment of benefits and harms of hepatocellular carcinoma surveillance in patients with cirrhosis. Hepatology 2017;65:1196‐1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Simmons O, Fetzer DT, Yokoo T, et al. Predictors of adequate ultrasound quality for hepatocellular carcinoma surveillance in patients with cirrhosis. Aliment Pharmacol Ther 2017;45:169‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Singal AG, Tiro JA, Murphy CC, et al. Patient‐reported barriers are associated with receipt of hepatocellular carcinoma surveillance in a multicenter cohort of patients with cirrhosis. Clin Gastroenterol Hepatol Published online July 3, 2020. Available at: 10.1016/j.cgh.2020.06.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim SY, An J, Lim YS, et al. MRI with liver‐specific contrast for surveillance of patients with cirrhosis at high risk of hepatocellular carcinoma. JAMA Oncol 2017;3:456‐463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Parikh ND, Mehta A, Singal AG, et al. Biomarkers for the diagnosis of hepatocellular carcinoma. Cancer Epi Biomarkers Prevention 2020;29:2495‐2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Berhane S, Toyoda H, Tada T, et al. Role of the GALAD and BALAD‐2 serologic models in diagnosis of hepatocellular carcinoma and prediction of survival in patients. Clin Gastroenterol Hepatol 2016;14:875‐886.e6. [DOI] [PubMed] [Google Scholar]
- 11. Chalasani N, Bhattacharya A, Book A, et al. Improved sensitivity for early‐stage hepatocellular carcinoma detection with a novel blood‐based panel of methylated DNA and protein markers. Int Liver Cancer Assoc Ann Meet 2020. September 13, 2020 (planned for Madrid but canceled given COVID). [Google Scholar]


