Abstract
In this study, we describe a practical and facile synthesis of deuterium-labeled indoles via acid-catalyzed hydrogen–deuterium exchange. 3-Substituted indoles were efficiently deuterated through treatment with 20 wt % D2SO4 in CD3OD at 60–90 °C. A deuterium incorporation reaction of 3-unsubstituted indoles was accomplished through treatment with CD3CO2D at 150 °C. The in situ preparation of a 20 wt % D2SO4/CH3OD/D2O solution enabled a large-scale and low-cost synthesis of auxins, indole-3-acetic acid-d5 and indole-3-butyric acid-d5.
Introduction
Deuterium compounds are widely used as an efficient tool to understand the biosynthesis of natural products, reaction mechanism, pharmacokinetics, and metabolism of bioactive compounds, by taking advantage of their features in NMR and mass spectroscopies. Besides their use in chemical kinetics, deuterated drugs have recently attracted much attention because of the deuterium pharmacokinetic isotope effect, which lowers the rate of metabolism and the distribution of metabolites.1 Indeed, deutetrabenazine2 has recently been approved by the United States Food and Drug Administration, and several deuterated compounds are currently being tested in clinical trials. An indole skeleton is one of the most common structures observed in biologically active molecules. Thus, deuterated indole compounds are in demand not only by organic chemists but also by bioorganic chemists. Particularly, polydeuterium-labeled indoles are useful for tracing the biotransformation of bioactive indoles. To access a deuterium-labeled indole, a hydrogen–deuterium (H–D) exchange reaction is considered more effective than the multistep synthesis from originally deuterium-labeled small synthons.3 Although several examples of selective deuterium incorporation at the reactive positions of indoles have been reported,4 there are limited examples of the H–D exchange reaction of all hydrogen attached to indoles.5,6 Oba’s group4 and Kańska’s group6 have reported the H–D exchange reaction of l-tryptophan to prepare pentadeuterium-labeled l-tryptophan with high isotopic purity under acidic conditions. These methods require expensive deuterium reagents, such as CF3CO2D, or repeated operations, resulting in reduced optical purity. However, 3-unsubstituted indoles cannot be exposed to acidic conditions because of their high reactivity at the 3-position.7 The polydeuteration of indoles, including 3-unsubstituted indoles, has been achieved using a transition metal catalyst.8 Although such catalytic conditions are effective for small-scale preparations, the required pyrophoric and flammable reagents and/or high-pressure conditions might not be suitable for large-scale synthesis.
In the course of our study on the biological role and biosynthesis of auxins such as indole acetic acid (IAA, 1) and indole butyric acid (IBA, 2) in plants,9 we required deuterium-labeled IAA (3) and IBA (4). Thus, we attempted to prepare a labeled compound via Fischer indole synthesis as a key step from commercially available nitrobenzene-d5 (Scheme 1).10 Although we successfully prepared the desired deuterium-labeled IBA (4), the deuteration degree was dramatically reduced under the Fischer indole synthesis condition, 1% H2SO4 in the CH3OH solution.11 To our surprise, all deuteriums attached to the indole at C4–C7 positions were partially replaced by protons. The H–D replacement at the C2 position, usually reactive under acidic conditions, also occurred and demonstrated a slight D incorporation (C2:13%, C4:95%, C5:49%, C6:91%, and C7:76% D incorporation). These results indicate that the deuteriums of IBA methyl ester 5 and CH3OH proton were exchanged under acidic conditions. This inspired a new idea for preparing deuterium-labeled indoles: indole compounds would incorporate deuterium from CD3OD in the presence of an appropriate acid catalyst. This method could be applicable to biologically important indole compounds, such as IAA (1), tryptophan (6), and other substituted indoles. In this manuscript, we describe a practical and facile method to prepare deuterium-labeled indoles via acid-catalyzed H–D exchange reactions.
Scheme 1. Synthesis of IBA-d5 via Fischer Indole Synthesis.
Results and Discussion
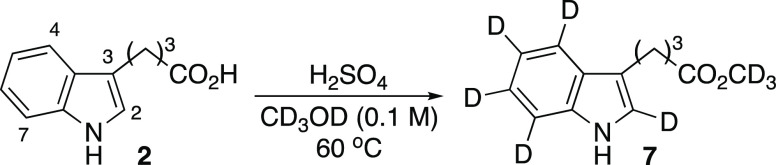
On the basis of the aforementioned idea, we first attempted the deuteration of IBA (2). Thus, 2 was dissolved in a variety of H2SO4/CD3OD solutions, and the reaction was monitored by 1H NMR spectra. The treatment of 2 with 2.4 wt % H2SO4 in CD3OD promoted the incorporation of deuteriums at the C2 and C4–C7 positions, even at room temperature, albeit with low D content. Upon continuous stirring at 60 °C for 65 h, IBA CD3 ester 7 with good D incorporation (77% average D incorporation at C2 and C4–C7) was obtained (Table 1, entry 1).12 The use of 9.7 wt % H2SO4 accelerated D incorporation. After reaction at 60 °C for 110 h, the average D incorporation reached a high of 91% (entry 2). This D content of the resulting IBA indicates that the H–D exchange reaction was completed because the deuterium content in the whole reaction system was calculated to be 91%. Further increasing the H2SO4 proportion to 20 wt % accelerated the reaction rate, resulting in a complete reaction at 45 h in 98% yield, without generating any other side products (82% average D incorporation, entry 3). Since the D content is reflected in the H+/D+ ratio of the reaction mixture, increasing the proportion of H2SO4 accelerated the reaction rate but decreased D incorporation. The reaction progress under a light shielding condition indicates that the reaction proceeded via a cationic pathway rather than a radical pathway (entries 3 and 4). Using 20 wt % D2SO4 instead of H2SO4 as the acid accelerated the reaction rate and improved D incorporation (97% average D incorporation, entry 5). Increasing the IBA concentration from 0.1 to 0.3 M slightly decreased the yield and D incorporation (93% average D incorporation, entry 6).
Table 1. Optimization of Reaction Conditions.
| entry | H2SO4 (%) | time (h) | D content (%)a | yield (%)b |
|---|---|---|---|---|
| 1 | 2.4 | 65 | 77c | n.d.d |
| 2 | 9.7 | 110 | 91c | n.d.d |
| 3 | 20 | 45 | 82e | 98 |
| 4f | 20 | 45 | 81c | n.d.d |
| 5 | 20g | 18 | 97e | 99 |
| 6h | 20g | 18 | 93e | 93 |
Indicated as the average D incorporation at C2 and C4–C7.
Isolated yield.
D content was calculated based on the 1H NMR spectra of the reaction mixture.
Not determined.
D content was calculated based on the 1H NMR spectra of the isolated product.
With light shielding.
D2SO4 was used instead of H2SO4.
The reaction was performed in a 0.3 M solution.
Once we achieved optimized reaction conditions, the substrate scope in the H–D exchange reaction was investigated, and the results are summarized in Table 2. 3-Substituted indole compounds, such as IAA (1), l-tryptophan (6), N,O-dimethyl-IBA (12), indomethacin (14), and yohimbine hydrochloride (16), were effectively deuterated in good yields with high D incorporation (>91% yield, >95% average D incorporation at C2, C4–7, entries 1–3 and 5–7). The H–D exchange reaction of l-tryptophan (6, >99% ee) was relatively slower than those of other indoles, presumably due to the presence of the amino group (entry 2). The optical purity was determined by chiral high-performance liquid chromatography (HPLC) analysis and was almost maintained (98% ee). When 6 was treated at 90 °C under the same acidic conditions, the reaction time was shortened to 20 h, and the corresponding deuterated tryptophan CD3 ester (9) was obtained in 99% yield without reducing the optical purity (>99% ee, entry 3). As predicted, introduction of an electron-withdrawing group reduced the reactivity; however, the reaction proceeded well and provided deuterated indole 11 with good D incorporation except for the C7 position (entry 4). 3-Substituted N-methylindole (12) was smoothly deuterated with high D incorporation (entry 5). An acyl group on indole nitrogen was removed under this reaction condition and then the H–D exchange reaction proceeded smoothly because of increasing the electron density (entry 6). In this reaction, C2 methyl protons were replaced with deuterium.13 As a result, it was found that various 3-substituted indoles can be effectively deuterated with a high degree of deuterium. Meanwhile, the H–D exchange reaction of carbazole (18) was remarkably slow and required more than 120 h at 90 °C to complete the reaction. Repeating the H–D exchange reaction resulted in increased D incorporation, but the yield decreased (entries 8, 9). Treatment of harmine hydrochloride (20), a naturally occurring β-carboline alkaloid, under this H–D exchange reaction provided deuterated harmine 21 in good yield (entry 10). However, only C6 and C8 protons were selectively exchanged to deuterium due to low electron density at other positions. Contrary to the results of 3-substituted indoles, C3-unsubstituted indoles, such as 2-methylindole (22) and 5-methylindole (23), resulted in complex mixtures under these conditions (entries 10 and 11). High nucleophilicity at the C3 position could cause side reactions.
Table 2. H–D Exchange Reaction of Indoles and Carbazole.
D content was calculated based on the 1H NMR spectra of the isolated product.
Isolated yield.
Isolated yield of CD3 ester.
98% ee.
The reaction was conducted in a sealed tube.
>99% ee.
Reactions were performed in a 0.05 M solution.
D content was calculated based on 1H NMR spectroscopy after N-methylation.
After isolating deuterated carbazole (70% average D incorporation), the H–D exchange reaction of this product was performed again.
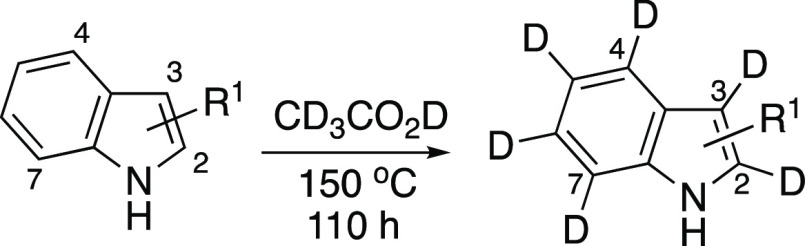
Therefore, for the H–D exchange reaction of 3-unsubstituted indoles, we considered using weaker Brønsted acid, such as acetic acid and substituted acetic acid, which could act as both an acid catalyst and a source of deuterium (Table 3). After screening several reaction conditions, we found that treating 3-unsubstituted indoles with CD3CO2D at 150 °C effectively promotes the incorporation of deuteriums at C2–C7 positions. Using more-acidic α-halogenated acetic acids, such as ClCH2CO2D, CCl3CO2D, and CF3CO2D, resulted in the production of indole dimers and oligomers.7 The H–D exchange reactions of 3-unsubstituted indoles with CD3CO2D are summarized in Table 3. The condition was found to be applicable to the indole itself and the substituted indoles bearing an alkyl group at each position. After reacting for 110 h, the corresponding deuterated indoles were obtained with moderate to good D incorporation and yield. In all cases, the C3 position of the indole was highly reactive, and the H–D exchange occurred even when dissolved in CD3CO2D and CDCl3 and also during purification by silica gel column chromatography. Therefore, the C3 deuterium was partially protonated during the isolation process. Introducing an electron-donating group increased D incorporation, but the yield decreased because of undesired oligomerisation.7 However, the substituent at around the C2 and C3 positions suppressed that side reaction due to their steric hindrance. In the reaction of 2-methylindole, methyl protons were replaced with deuterium (entry 3),13 whereas the alkyls on the benzene ring were intact (entries 4–6).
Table 3. H–D Exchange Reaction of 3-Unsubstituted Indolesa.
Reactions were performed in CD3CO2D (0.1 M) in a sealed tube at 150 °C for 110 h.
D content was calculated based on the 1H NMR spectra of the isolated product.
Isolated yield.
D content was calculated based on 1H NMR spectroscopy after N-methylation.
Indicated as average D incorporation.
As described above, we developed a facile protocol to introduce deuteriums into the indole moiety. However, the method required a somewhat large amount of expensive deuterium reagents and is not necessarily efficient for large-scale synthesis. Thus, we proceeded to study the use of an inexpensive deuterium oxide as the deuterium source. After examining several reaction conditions, we found that the treatment of IBA (2) with 20 wt % D2SO4 in CD3OD/D2O (7/3, 0.2 M) at 95 °C for 5 h followed by saponification with aqueous LiOH effectively provided deuterated IBA (4, 97% average D incorporation at C2, C4–C7) in 99% yield (Table 4, entry 1). Next, we turned our attention to the use of HC(OCH3)3 as a source of CH3OD and the in situ preparation of this solvent system (entry 2). Indeed, a D2SO4/CH3OD/D2O mixture was prepared by treating HC(OCH3)3 with D2O in the presence of D2SO4, followed by removing HCO2CH3 via common distillation in an Ar atmosphere. In this media, the reaction of 2 proceeded as smoothly as under original conditions and successfully provided deuterated IBA (4) in a high yield with a high D content (95% yield, 97% average D incorporation).14 The effective deuteration of IAA (1) with this procedure was also attained (entry 3). The reaction conditions provide economical ways to access deuterium-labeled 3-substituted indoles in quantities greater than 1 g.15
Table 4. Synthesis of Polydeuterated IAA and IBA in an Economical Waya.
| entry | substrate | solvent | time (h) | D content (%)b | yield (%)c |
|---|---|---|---|---|---|
| 1 | 2 | 70% CD3OD/D2O | 5 | 97 | 99 |
| 2d | 2 | CH(OCH3)3/D2Oe | 5 | 97 | 95 |
| 3d | 3 | CH(OCH3)3/D2Oe | 14 | 97 | 94 |
Reactions were performed in 20 wt % D2SO4 in CD3OD/D2O (7/3, 0.2 M). After the reaction was completed, the mixture was poured into an aqueous LiOH solution to hydrolyze.
Indicated as the average D content at C2 and C4–C7, which were calculated based on the 1H NMR spectra of the isolated product.
Isolated yield.
Reactions were performed on a 1 gram scale of the substrate.
In situ preparation of 20 wt % D2SO4 in CD3OD/D2O (7/3).
Conclusions
In summary, we developed a practical and facile protocol for preparing polydeuterium-labeled indoles through the H–D exchange reaction. These procedures are operationally and economically feasible and can be performed not only by organic chemists but also by researchers in other fields. We believe that the results are valuable to encourage researchers to further investigate chemical biology and drug development. In our laboratories, we are currently investigating the metabolism of indole plant hormones using deuterium-labeled indoles.
Experimental Section
General Information
All reagents and solvents were purchased from Tokyo Chemical Industry Co., Ltd.; FUJIFILM Wako Pure Chemical Corporation; Kanto Chemical Co., Ltd.; Sigma-Aldrich Co., LLC; and ISOTEC and used without further purification. Unless otherwise noted, all reactions were conducted without any inert gas. Chromatography was carried out with Wakogel C-200 silica gel (FUJIFILM Wako Pure Chemical Corporation, granule, 0.075–0.150 mm). NMR spectra were recorded at 600, 500, and 400 MHz for 1H and 150, 125, and 100 MHz for 13C on JEOL JNM-ECA600, -ECA500, and -ECZ400R spectrometers, respectively. Chemical shifts were reported in parts per million (ppm, δ) relative to the residual solvent peaks of CD3OD (3.31 ppm for 1H NMR, 49.0 ppm for 13C NMR) and (CD3)2CO (2.05 ppm for 1H NMR, 206.26 ppm for 13C NMR), and coupling constants (J values) were given in Hertz. High-performance liquid chromatography was carried out with a PU–2089 plus HPLC pump (JASCO), an LC–NetII/ADC (JASCO), and a UV–2075 plus UV/vis detector (JASCO). High-resolution mass spectra (HRMS) were measured on a JEOL Accu TOF T-100 equipped with an electrospray ionization (ESI) unit.
Preparation of Deuterated 3-Substituted Indole CD3 Ester (Tables 1 and 2)
A solution of 3-substituted indoles with 20 wt % D2SO4 in CD3OD (0.1 M) was heated at 60 °C in an NMR tube or 90 °C in a sealed tube. The reaction was monitored by 1H NMR spectroscopy. After the reaction was completed, the reaction mixture was slowly poured into a saturated aqueous NaHCO3 solution (10 mL). The resulting mixture was extracted with Et2O (3 × 10 mL). The combined organic extracts were washed with brine, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel to give deuterated 3-substituted indole CD3 ester.
Deuterated IBA CD3 Ester (7)
Treatment of IBA (2) (10.7 mg, 0.053 mmol) with 20 wt % D2SO4 in CD3OD at 60 °C afforded deuterated IBA CD3 ester 7 (11.8 mg, 99% yield, 97% average D incorporation at C2 and C4∼C7). 1H NMR (500 MHz, CD3OD) δ 7.51 (s, 0.04H), 7.31 (s, 0.03H), 7.07 (s, 0.02H), 7.00–6.97 (0.05H), 2.77 (t, J = 7.5 Hz, 2H), 2.36 (t, J = 7.5 Hz, 2H), 1.99 (tt, J = 7.5, 7.5 Hz, 2H), 13C NMR (125 MHz, CD3OD) δ 176.1, 138.1, 128.7, 122.8 (t, J = 27.4 Hz,), 121.7 (t, J = 23.8 Hz), 118.9 (t, J = 23.3 Hz, 2C), 115.3, 111.8 (t, J = 23.3 Hz), 51.1 (m), 34.4, 26.8, 25.5. HRMS (ESI-TOF) m/z [M + H]+ calcd for [C13H8D8NO2]+ 226.1683, found 226.1684.
Deuterated IAA CD3 Ester (8)
Treatment of IAA (1) (8.8 mg, 0.050 mmol) with 20 wt % D2SO4 in CD3OD at 60 °C afforded deuterated IAA CD3 ester (9.9 mg, 99% yield). 1H NMR (500 MHz, CD3OD) δ 7.51 (s, 0.07H), 7.34 (s, 0.04H), 7.15 (s, 0.03H), 7.10 (s, 0.03H), 7.01 (s, 0.03H), 3.76 (s, 2H). 13C NMR (125 MHz, CD3OD) δ 174.9, 137.9, 128.5, 124.4 (t, J = 26.8 Hz), 122.0 (t, J = 23.8 Hz), 119.4 (t, J = 23.3 Hz), 118.9 (t, J = 23.8 Hz), 111.9 (t, J = 24.4 Hz), 108.3, 51.7 (m), 31.9. HRMS (ESI-TOF) m/z [M + H]+ calcd for [C11H4D8NO2]+ 198.1370, found 198.1369.
Deuterated l-Trp-CD3 Ester (9)
Treatment of l-tryptophan (6) (11.2 mg, 0.055 mmol) with 20 wt % D2SO4 in CD3OD at 90 °C in a sealed tube afforded deuterated l-Trp CD3 ester (9) (12.5 mg, 99% yield, >99% ee). 1H NMR (500 MHz, CD3OD) δ 7.52 (s, 0.10H), 7.35 (s, 0.04H), 7.11–7.09 (0.07H), 7.02 (m, 0.03H), 3.88 (t, J = 6.3 Hz, 1H), 3.24 (dd, J = 14.3, 5.3 Hz, 1H), 3.16 (dd, J = 14.3, 7.0 Hz, 1H). 13C NMR (125 MHz, CD3OD): δ 176.2, 138.1, 128.6, 124.5 (t, J = 27.4 Hz), 122.0 (t, J = 23.9 Hz), 119.4 (t, J = 24.4 Hz), 118.7 (t, J = 23.9 Hz), 112.0 (t, J = 23.8 Hz), 110.1, 55.8, 51.8 (m), 31.0. HRMS (ESI-TOF) m/z [M + H]+ calcd for [C12H7D8N2O2]+ 227.1636, found 227.1637. Chiral HPLC analysis: CHIRAL-PAK IB N-5, i-PrOH (0.1% diethylamine): hexane (0.1% diethylamine) = 3: 7, UV 282 nm, flow rate 1.0 mL/min, 25 °C, l-isomer tR = 10.4 min, D isomer tR = 9.5 min.
Preparation of 3-(5-Chloroindol-3-yl)-propanoic Acid Methyl Ester (10)
According to Hong and Wang’s report, 3-(5-chloroindol-3-yl)-propanoic acid was prepared.16 To a solution of crude 3-(5-chloroindol-3-yl)-propanoic acid (710 mg, 3.2 mmol) in methanol (9.6 mL) was added H2SO4 (0.5 mL) at 0 °C under an Ar atmosphere. The reaction mixture was stirred at room temperature for 6 h and then 1N NaOH was added to neutralize. The mixture was extracted with Et2O (3 × 25 mL). The combined organic extracts were washed with brine, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel (hexane/AcOEt = 5:1) to give 3-(5-chloroindol-3-yl)-propanoic acid methyl ester (10) (731 mg, 96%) as a red brown solid. 1H NMR (400 MHz, CDCl3) δ 8.05 (brs, 1H), 7.56 (d, J = 2.3 HZ, 1H), 7.26 (d, J = 8.5 Hz, 1H), 7.14 (dd, J = 8.5, 2.3 Hz, 1H), 7.03 (m, 1H), 3.68 (s, 3H), 3.06 (m, 2H), 2.70 (t, J = 7.6 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ 173.6, 134.6, 128.3, 125.1, 122.9, 122.3, 118.2, 114.7, 112.1, 51.6, 34.6, 20.4. IR (neat, ATR) 3370, 1716, 1459, 1441, 1205, 1158, 1096, 1053, 895, 799 cm–1. HRMS (ESI-TOF) m/z [M – H]− calcd for [C12H11ClNO2]− 236.0478, found 236.0481.
Deuterated 3-(5-Chloroindol-3-yl)-propanoic Acid CD3 Ester (11)
Treatment of 3-(5-chloroindol-3-yl)-propanoic acid methyl ester (10) (23.8 mg, 0.10 mmol) with 20 wt % D2SO4 in CD3OD at 90 °C in a sealed tube afforded deuterated 3-(5-chloroindol-3-yl)-propanoic acid CD3 ester (11) (22.2 mg, 92% yield, 88% average D incorporation at C2, C4 and C6∼C7). 1H NMR (400 MHz, CD3OD) δ 7.48 (s, 0.02H), 7.28 (s, 0.42H), 7.07 (s, 0.02H), 7.04 (m, 0.02H), 3.01 (t, J = 7.5 Hz, 2H), 2.67 (t, J = 7.5 Hz, 2H). 13C NMR (100 MHz, CD3OD) δ 175.6, 136.4, 129.5, 125.2, 124.5 (t, J = 27.3 Hz), 122.1 (td, J = 25.0, 10.2 Hz), 118.3 (t, J = 25.0 Hz), 114.6, 113.0 (t, J = 24.9 Hz), 51.3 (sept, J = 22.5 Hz), 35.9, 21.5. HRMS (ESI-TOF) m/z [M – H]− calcd for [C12H4D7ClNO2]− 243.0918, found 243.0915.
Deuterated N-methyl-IBA CD3 Ester (13)
Treatment of N-methyl-IBA methyl ester (12) (13.9 mg, 0.06 mmol) with 20 wt % D2SO4 in CD3OD at 60 °C afforded deuterated N-methyl-IBA CD3 ester (13) (14.3 mg, 99% yield). 1H NMR (600 MHz, CD3OD) δ 7.51 (s, 0.05H), 7.28 (s, 0.08H), 7.13 (s, 0.08H), 7.01 (s, 0.08H), 6.90 (d, J = 2.1 Hz, 0.07H), 3.72 (d, J = 2.1 Hz, 3H), 2.75 (t, J = 7.5 Hz, 2H), 2.35 (t, J = 7.5 Hz, 2H), 1.97 (tt, J = 7.5, 7.5 Hz, 2H). 13C NMR (150 MHz, CD3OD) δ 176.1, 138.6, 129.2, 127.3 (t, J = 27.2 Hz), 121.8 (t, J = 23.0 Hz), 119.2 (t, J = 23.0 Hz), 119.0 (t, J = 24.4 Hz), 114.8, 109.7 (t, J = 23.0 Hz), 51.2 (m), 34.4, 32.5, 26.9, 25.3. HRMS (ESI-TOF) m/z [M + Na]+ calcd for [C14H9D8NNaO2]+ 262.1666, found 262.1665.
Deuterated 2-(5-Methoxy-2-methylindole-3-acetic Acid) CD3 Ester (15)
Treatment of indomethacin (14) (17.9 mg, 0.05 mmol) with 20 wt % D2SO4 in CD3OD at room temperature afforded deuterated 2-(5-methoxy-2-methyl-indole-3-acetic acid) CD3 ester (15) (10.8 mg, 91% yield). 1H NMR (600 MHz, CD3OD) δ 7.11 (s, 0.03H), 6.92 (s, 0.03H), 6.67 (s, 0.03H), 3.79 (s, 3H), 3.65 (s, 2H), 2.31 (m, 0.1H). 13C NMR (100 MHz, CD3OD) δ 174.9, 155.0, 135.0, 132.1, 130.1, 111.6 (t, J = 24.5 Hz), 110.9 (t, J = 24.5 Hz), 104.4, 100.9 (t, J = 24.0 Hz), 56.3, 51.6 (quint, J = 22.3 Hz), 30.9. HRMS (ESI-TOF) m/z [M + H]+ calcd for [C13H7D9NO3]+ 243.1700, found 243.1697.
Deuterated Yohimbine (17)
Treatment of yohimbine hydrochloride (16) (39.1 mg, 0.1 mmol) with 20 wt % D2SO4 in CD3OD at room temperature afforded deuterated yohimbine (17) (32.8 mg, 91% yield). 1H NMR (600 MHz, CD3OD) δ 7.37 (s, 0.03H), 7.29 (s, 0.03H), 7.03 (s, 0.03H), 6.96 (s, 0.03H), 4.23 (m, 1H), 3.39 (dd, J = 12.0, 3.0 Hz, 1H), 3.10 (dd, J = 11.6, 5.7 Hz, 1H), 2.98 (m, 1H), 2.90 (dd, J = 11.1, 2.4 Hz, 1H), 2.72 (dd, J = 15.3, 4.2 Hz, 1H), 2.62 (ddd, J = 11.6, 4.8, 4.2 Hz, 1H), 2.46 (ddd, J = 12.4, 3.0, 3.0 Hz, 1H), 2.34 (dd, J = 11.4, 3.0 Hz, 1H), 2.23 (dd, J = 11.1, 10.8 Hz, 1H), 1.99 (m, 1H), 1.91 (m, 1H), 1.64 (m, 1H), 1.56–1.46 (2H), 1.38 (m, 1H), 1.17 (ddd, J = 12.4, 12.0, 12.0 Hz, 1H). 13C NMR (100 MHz, CD3OD) δ 175.3, 138.0, 135.5, 128.2, 121.4 (t, J = 24.9 Hz), 119.2 (t, J = 23.1 Hz), 118.2 (t, J = 25.0 Hz), 111.7 (t, J =24.5 Hz), 107.7, 68.6, 62.2, 61.9, 54.2, 53.8, 51.4 (quint, J = 22.3 Hz), 41.1, 37.4, 34.6, 33.4, 24.4, 22.4. HRMS (ESI-TOF) m/z [M + H]+ calcd for [C21H20D7N2O3]+ 362.2461, found 362.2475.
Deuterated Carbazole (19)
A solution of carbazole (18) (15.7 mg, 0.094 mmol) with 20 wt % D2SO4 in CD3OD (1.9 mL, 0.05 M) was heated at 90 °C for 120 h in a sealed tube. The reaction mixture was slowly poured into a saturated aqueous NaHCO3 solution (30 mL). The resulting suspension was filtered and washed with H2O to collect the precipitate, which was subsequently dried under reduced pressure. The obtained deuterated carbazole (14.4 mg, 90% yield, 70% average D incorporation at C1–C8) was treated with 20 wt % D2SO4 in CD3OD (1.9 mL, 0.05 M) at 90 °C for 48 h in a sealed tube. Deuterated carbazole (19, 10.0 mg, 61% yield, 98% average D incorporation at C1–C8) was isolated through the same procedure as described above. 1H NMR (400 MHz, acetone-d6) δ 10.3 (brs), 8.11 (s), 7.51(s), 7.38 (s), 7.17 (s). 13C NMR (150 MHz, acetone-d6) δ 141.1, 126.0 (t, J = 24.5 Hz), 124.0, 120.6 (t, J = 24.5 Hz), 119.2 (t, J = 24.4 Hz), 111.4 (t, J = 25.9 Hz). HRMS (ESI-TOF) m/z [M – H]− calcd for [C12D8N]− 174.1159, found 174.1152.
N-Methylation of Deuterated Carbazole
To a solution of deuterated carbazole (19) (5.1 mg, 0.03 mmol) in THF (300 μL) was added NaH (60% oil dispersion, 1.5 mg, 0.06 mmol) at 0 °C under an Ar atmosphere. After being stirred at 0 °C for 20 min, methyl iodide (4 μL, 0.06 mmol) was added and stirred at rt for 1 h. The reaction was quenched with a saturated aqueous NH4Cl solution (5 mL) and extracted with AcOEt (5 mL × 3). The combined organic extracts were washed with brine, dried over anhydrous Na2SO4, and concentrated under reduced pressure to give crude N-methylcarbazole (30). Deuterium content was calculated based on the 1H NMR spectra of this crude mixture. 1H NMR (400 MHz, acetone-d6) δ 8.14 (s, 0.04H) 7.54 (s, 0.04H), 7.46 (s, 0.03H), 7.20 (s, 0.06H), 3.91 (s, 3H). HRMS (ESI-TOF) m/z [M + H]+ calcd for [C13H4D8N]+ 190.1472, found 190.1473.
Deuterated Harmine (21)
Treatment of harmine hydrochloride (20) (24.9 mg, 0.10 mmol) with 20 wt % D2SO4 in CD3OD at 90 °C afforded deuterated harmine (21) (20.3 mg, 94% yield). 1H NMR (600 MHz, CD3OD) δ 8.14 (d, J = 5.7 Hz, 1H), 7.97 (s, 1H), 7.78 (d, J = 5.7 Hz, 1H), 7.03 (s, 0.02H), 6.85 (d, J = 8.4 Hz, 0.02H), 3.90 (s, 3H), 2.76 (s, 3H). 13C NMR (100 MHz, CD3OD) δ 162.5, 144.1, 142.0, 137.9, 136.3, 130.2, 123.4, 116.3, 113.3, 110.7 (t, J = 25.0 Hz), 95.2 (t, J = 24.5 Hz), 56.0, 19.5. HRMS (ESI-TOF) m/z [M + H]+ calcd for [C13H11D2N2O]+ 215.1153, found 215.1146.
General Procedure of the H–D Exchange Reaction of 3-Unsubstituted Indole (Table 3)
A solution of 3-unsubstituted indoles in CD3CO2D (0.1 M) was heated at 150 °C for 110 h in a sealed tube. The mixture was concentrated under reduced pressure. The residue was purified by column chromatography on silica gel (hexane/AcOEt = 7/1) to give deuterated indoles.
Deuterated Indole (24)
A solution of indole (29.3 mg, 0.25 mmol) in CD3CO2D (2.5 mL, 0.1 M) was heated at 150 °C to afford deuterated indole (24) (22.8 mg, 77% yield). 1H NMR (600 MHz, CD3OD) δ 7.53 (m, 0.86H), 7.36 (m, 0.68H), 7.20 (s, 0.02H), 7.08 (m, 0.72H), 6.99 (m, 0.57H), 6.42 (s, 0.39H). 13C NMR (100 MHz, CD3OD) δ 137.6, 129.7–129.2, 125.4–125.0, 122.2–121.8, 121.3–120.8, 120.0–119.4, 112.3–111.7, 102.2–101.7. HRMS (ESI-TOF) m/z [M + H]+ calcd for [C8H1D5N]+ 121.0814, found 121.0813.
N-Methylation of Deuterated Indole
To a solution of deuterated indole (24) (15.1 mg, 0.13 mmol) in THF (1.3 mL) was added NaH (60% oil dispersion, 8 mg, 0.20 mmol) at 0 °C under an Ar atmosphere. After being stirred at 0 °C for 20 min, methyl iodide (17 μL, 0.27 mmol) was added and stirred at rt for 1 h. The reaction was quenched with a saturated aqueous NH4Cl solution (5 mL) and extracted with AcOEt (5 mL × 3). The combined organic extracts were washed with brine, dried over anhydrous Na2SO4, and concentrated under reduced pressure to give crude N-methylindole (31). Deuterium content was calculated based on the 1H NMR spectra of this crude mixture. 1H NMR (600 MHz, acetone-d6) δ 7.57–7.53 (0.86H), 7.39–7.35 (0.68H), 7.21–7.19 (0.02H), 7.18–7.12 (0.58H), 7.06–7.00 (0.72H), 6.41 (s, 0.57H), 3.82 (s, 3H).
Deuterated 1-Ethylindole (25)
A solution of 1-ethylindole (20.5 mg, 0.14 mmol) in CD3CO2D (1.4 mL, 0.1 M) was heated at 150 °C to afford deuterated 1-ethylindole (25) (16.6 mg, 81% yield). 1H NMR (400 MHz, acetone-d6) δ 7.56–7.53 (0.74H), 7.44–7.40 (0.53H), 7.28 (d, J = 3.2 Hz, 0.12H), 7.16–7.11 (0.49H), 7.03–6.98 (0.26 H), 6.43–6.41 (0.81 H), 4.24 (q, J = 7.4 Hz, 2H), 1.41 (t, J = 7.4 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 135.6, 128.5, 126.7 (t, J = 27.8 Hz), 121.2 (t, J = 11.1 Hz), 120.9, 119.1, 109.1, 100.8, 40.9, 15.4. HRMS (ESI-TOF) m/z [M – H]− calcd for [C10H6D4N]− 148.1064, found 148.1069.
Deuterated 2-Methylindole (26)
A solution of 2-methylindole (22) (14.7 mg, 0.11 mmol) in CD3CO2D (1.1 mL, 0.1 M) was heated at 150 °C to afford deuterated 2-methylindole (26) (13.8 mg, 92% yield). 1H NMR (500 MHz, CD3OD) δ 7.39–7.35 (0.34H), 7.23 (s, 0.30H), 6.99–6.96 (0.04H), 6.93–6.89 (0.10H), 6.09 (s, 1H), 2.38–2.35 (m, 0.14H). 13C NMR (125 MHz, CD3OD) δ 137.9, 136.4, 130.5, 121.1–118.8 (3C), 110.9 (t, J = 24.4 Hz), 100.1 (m), 12.7 (m). HRMS (ESI-TOF) m/z [M + H]+ calcd for [C9H1D7N1]+ 137.1096, found 137.1094.
Deuterated 4-Methylindole (27)
A solution of 4-methylindole (12.4 mg, 0.10 mmol) in CD3CO2D (1.0 mL, 0.1 M) was heated at 150 °C to afford deuterated 4-methylindole (27) (13.0 mg, 96% yield). 1H NMR (600 MHz, CD3OD) δ 7.21–7.16 (0.11H), 6.97 (s, 0.67H), 6.80–6.76 (0.04H), 6.45 (s, 0.53H), 2.50 (s, 3H).13C NMR (125 MHz, CD3OD) δ 137.2, 130.3, 129.1, 124.8–124.1, 122.4–121.5, 119.7 (t, J = 20.3 Hz), 109.5 (t, J = 19.2 Hz), 100.6–100.1, 18.9. HRMS (ESI-TOF) m/z [M + H]+ calcd for [C9H3D5N1]+ 135.0971, found 135.0970.
Deuterated 5-Methylindole (28)
A solution of 5-methylindole (23) (13.1 mg, 0.10 mmol) in CD3CO2D (1.0 mL, 0.1 M) was heated at 150 °C to afford deuterated 5-methylindole (28) (7.7 mg, 57% yield). 1H NMR (600 MHz, CD3OD) δ 7.31 (s, 0.07H), 7.24 (s, 0.33H), 7.16–7.14 (0.02H), 6.93–6.89 (0.03H), 6.32 (s, 0.28H), 2.39 (m, 3H). 13C NMR (125 MHz, CD3OD) δ 135.9, 129.6, 128.7, 125.6–124.8, 123.8–123.1, 120.4 (t, J = 22.7 Hz), 111.4 (t, J = 23.8 Hz), 101.3 (t, J = 26.3 Hz), 21.5. HRMS (ESI-TOF) m/z [M + H]+ calcd for [C9H3D5N]+ 135.0971, found 135.0965.
Deuterated 7-Ethylindole (29)
A solution of 7-ethylindole (13.7 mg, 0.10 mmol) in CD3CO2D (1.0 mL, 0.1 M) was heated at 150 °C to afford deuterated 7-ethylindole (29) (11.5 mg, 78% yield). 1H NMR (600 MHz, CD3OD) δ7.38–7.33 (0.16H), 7.19 (s, 0.03H), 6.94–6.88 (0.27H), 6.41 (s, 0.44H), 2.89 (q, J = 7.6 Hz, 2H), 1.33 (t, J = 7.6 Hz, 3H). 13C NMR (125 MHz, CD3OD): δ 136.2, 129.3 (t, J = 9.6 Hz), 127.9, 125.0 (t, J = 9.6 Hz), 120.5 (t, J = 19.1 Hz), 120.0, 118.8 (t, J = 15.6 Hz), 102.5–102.3, 25.2, 14.7. HRMS (ESI-TOF) m/z [M + H]+ calcd for [C10H5D5N]+ 149.1127, found 149.1123.
General Procedure of the Economically Efficient H–D Exchange Reaction of 3-Substituted Indole (Table 4)
To a mixture of HC(OCH3)3 (51 mL, 0.47 mol) and D2O (24.5 mL, 1.35 mol) was added D2SO4 (6.5 mL, 0.12 mol) dropwise at 0 °C under an Ar atmosphere. After being stirred at rt for 20 min, generated HCO2CH3 (28.6 mL, 0.47 mol) was removed by general distillation under an Ar atmosphere to prepare 20 wt % D2SO4 in CH3OD/D2O (7/3) solution. A solution of IAA (1) or IBA (2) (1.0 g) in 20 wt % D2SO4 in a CH3OD/D2O (7/3) solution (0.2 M) was heated at 95 °C for 5–14 h. The mixture was cool to ambient temperature and poured into a 6 wt % LiOH aqueous solution (100 mL). After being stirred at rt for 1 h, 6 N HCl was added to the mixture to be an acidic solution (pH = 2). The resulting mixture was extracted with Et2O (100 mL × 3). The combined organic extracts were washed with brine (100 mL), dried over anhydrous Na2SO4, and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel (hexane/AcOEt = 1/1) to provide deuterated IAA (3) or IBA (4) (97% average D incorporation at C2 and C4–C7).
Deuterated IBA (4)
A solution of IBA (2) (1.0 g, 4.92 mmol) in 20 wt % D2SO4 in a CH3OD/D2O (7/3) solution (24.6 mL, 0.2 M) was heated at 95 °C for 5 h to provide deuterated IBA (4) (967 mg, 95% yield, 97% average D incorporation at C2 and C4–C7) as colorless crystals. 1H NMR (600 MHz, CD3OD) δ 7.53 (s, 0.05H), 7.32 (s, 0.03H), 7.07 (s, 0.03H), 7.01 (s, 0.03H), 6.98 (s, 0.03H), 2.79 (t, J = 7.8 Hz, 2H), 2.34 (t, J = 7.5 Hz, 2H), 1.99 (tt, J = 7.8, 7.5 Hz, 2H). 13C NMR (150 MHz, CD3OD) δ 177.8, 138.1, 128.7, 122.7 (t, J = 27.3 Hz), 121.7 (t, J = 23.0 Hz), 119.0 (t, J = 23.0 Hz), 118.9 (t, J = 23.0 Hz), 115.4, 111.8 (t, J = 24.5 Hz), 34.5, 26.9, 25.5. HRMS (ESI-TOF) m/z [M+H]+ calcd for [C12H9D5NO2]+ 209.1338, found 209.1332.
Deuterated IAA (3)
A solution of IAA (1) (1.0 g, 5.7 mmol) in 20 wt % D2SO4 in a CH3OD/D2O (7/3) solution (28.6 mL, 0.2 M) was heated at 95 °C for 14 h to provide deuterated IAA (3) (959 mg, 94% yield, 95% average D incorporation at C2 and C4–C7) as brown solid. 1H NMR (600 MHz, CD3OD) δ 7.54 (s, 0.03H), 7.33 (s, 0.03H), 7.15 (s, 0.03H), 7.09 (s, 0.02H), 7.01 (0.03H), 3.73 (s, 2H). 13C NMR (150 MHz, CD3OD) δ 176.5, 137.9, 128.6, 124.4 (t, J = 27.3 Hz), 121.9 (t, J = 23.0 Hz), 119.3 (t, J = 24.5 Hz), 119.0 (t, J = 24.5 Hz), 111.8 (t, J = 23.0 Hz), 108.7, 31.9. HRMS (ESI-TOF) m/z [M + H]+ calcd for [C10H5D5NO2]+ 181.1025, found 181.1030.
Acknowledgments
This work was supported by a Kanagawa University Grant for Joint Research. The authors thank Haruka Yamamoto and Misaki Shimada for their contributions at the early stage of this study.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c02940.
Details of the results and 1H and 13C NMR spectra and MS spectra for all deuterated compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Gant T. G. Using deuterium in drug discovery: leaving the label in the drug. J. Med. Chem. 2014, 57, 3595–3611. 10.1021/jm4007998. [DOI] [PubMed] [Google Scholar]; b Cargnin S.; Serafini M.; Pirali T. A primer of deuterium in drug design. Future Med. Chem. 2019, 11, 2039–2042. 10.4155/fmc-2019-0183. [DOI] [PubMed] [Google Scholar]; c Pirali T.; Serafini M.; Cargnin S.; Genazzani A. A. Applications of deuterium in medicinal chemistry. J. Med. Chem. 2019, 62, 5276–5297. 10.1021/acs.jmedchem.8b01808. [DOI] [PubMed] [Google Scholar]
- Dean M.; Sung V. W. Review of deutetrabenazine: a novel treatment for chorea associated with Huntington’s disease. Drug Des., Dev. Ther. 2018, 12, 313–319. 10.2147/DDDT.S138828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Atzrodt J.; Derdau V.; Kerr W. J.; Reid M. C-H Functionalisation for Hydrogen isotope exchange. Angew. Chem., Int. Ed. 2018, 57, 3022–3047. 10.1002/anie.201708903. [DOI] [PubMed] [Google Scholar]; b Atzrodt J.; Derdau V.; Fey T.; Zimmermann J. The renaissance of H/D exchange. Angew. Chem., Int. Ed. 2007, 46, 7744–7765. 10.1002/anie.200700039. [DOI] [PubMed] [Google Scholar]; c Lockley W. J. S.; Heys J. R. Metal-catalysed hydrogen isotope exchange labelling: a brief overview. J. Labelled Compd. Radiopharm. 2010, 53, 635–644. 10.1002/jlcr.1851. [DOI] [Google Scholar]; d Sawama Y.; Monguchi Y.; Sajiki H. Efficient H–D exchange reaction using heterogeneous platinum-group metal on carbon-H2-D2O system. Synlett 2012, 23, 959–972. 10.1055/s-0031-1289696. [DOI] [Google Scholar]; and references cited therein.
- a Zhang J.; Zhang S.; Gogula T.; Zou H. Versatile regioselective deuteration of indoles via transition-metal-catalyzed H/D exchange. ACS Catal. 2020, 10, 7486–7494. 10.1021/acscatal.0c01674. [DOI] [Google Scholar]; b Kerr W. J.; Lindsay D. M.; Owens P. K.; Reid M.; Tuttle T.; Campos S. Site-selective deuteration of N-heterocycles via iridium-catalyzed hydrogen isotope exchange. ACS Catal. 2017, 7, 7182–7186. 10.1021/acscatal.7b02682. [DOI] [Google Scholar]; c Murray A. T.; Challinor J. D.; Gulácsy C. E.; Lujan C.; Hatcher L. E.; Pudney C. R.; Raithby P. R.; John M. P.; Carbery D. R. Modelling flavoenzymatic charge transfer events: development of catalytic indole deuteration strategies. Org. Biomol. Chem. 2016, 14, 3787. 10.1039/C6OB00361C. [DOI] [PubMed] [Google Scholar]; d Lioe H.; O’Hair R. A. J.; Reid G. E. Gas-phase reactions of protonated tryptophan. J. Am. Soc. Mass Spectrom. 2004, 15, 65–76. 10.1016/j.jasms.2003.09.011. [DOI] [PubMed] [Google Scholar]; and references cited therein.
- Oba Y.; Kato S.; Ojika M.; Inouye S. Biosynthesis of luciferin in the sea firefly, Cypridina hilgendorfii: l-tryptophan is a component in Cypridina luciferin. Tetrahedron Lett. 2002, 43, 2389–2392. 10.1016/S0040-4039(02)00257-5. [DOI] [Google Scholar]
- Winnicka E.; Kańska M. Synthesis of l-tryptophan labeled with hydrogen isotopes in the indole ring. J. Radioanal. Nucl. Chem. 2009, 279, 675–678. 10.1007/s10967-007-7310-8. [DOI] [Google Scholar]
- a Guo T.; Han S.-L.; Liu Y.-C.; Liu Y.; Liu H.-M. Convenient synthesis of antiproliferative 2,3-dihydro-2,3′-bisindoles via dimerization of N-H indole derivatives. Tetrahedron Lett. 2016, 57, 1097–1099. 10.1016/j.tetlet.2016.01.093. [DOI] [Google Scholar]; b Dupeyre G.; Lemoine P.; Ainseba N.; Michel S.; Cachet X. A one-pot synthesis of 7-phenylindolo[3,2-a]carbazoles from indoles and β-nitrostyrenes, via an unprecedented reaction sequence. Org. Biomol. Chem. 2011, 9, 7780–7790. 10.1039/c1ob06108a. [DOI] [PubMed] [Google Scholar]; c Li Y.-X.; Ji K.-G.; Wang H.-X.; Ali S.; Liang Y.-M. Iodine-induced regioselective C-C and C-N Bonds formation of N-protected indoles. J. Org. Chem. 2011, 76, 744–747. 10.1021/jo1023014. [DOI] [PubMed] [Google Scholar]; d Pal B.; Giri V. S.; Jaisankar P. First indium trichloride catalyzed self-addition of indoles: One pot synthesis of indolylindolines. Catal. Commun. 2005, 6, 711–715. 10.1016/j.catcom.2005.07.003. [DOI] [Google Scholar]; e Ishii H.; Murakami K.; Sakurada E.; Hosoya K.; Murakami Y. Polymerisation of indole. Part 2. A new indole trimer. J. Chem. Soc., Perkin Trans. 1 1988, 8, 2377–2385. 10.1039/P19880002377. [DOI] [Google Scholar]
- a Matsubara S.; Asano K.; Kajita Y.; Yamamoto M. C-H Bond activation by water on a palladium or platinum metal surface. Synthesis 2007, 2007, 2055–2059. 10.1055/s-2007-983738. [DOI] [Google Scholar]; b Yamamoto M.; Oshima K.; Matsubara S. Platinum catalyzed H–D exchange reaction of various aromatic compounds under hydrothermal condition. Heterocycles 2006, 67, 353–359. 10.3987/COM-05-S(T)36. [DOI] [Google Scholar]; c Esaki H.; Ito N.; Sakai S.; Maegawa T.; Monguchi Y.; Sajiki H. General method of obtaining deuterium-labeled heterocyclic compounds using neutral D2O with heterogeneous Pd/C. Tetrahedron 2006, 62, 10954–10961. 10.1016/j.tet.2006.08.088. [DOI] [Google Scholar]; d Yau W.-M.; Gawrisch K. Deuteration of indole and N-methylindole by Raney nickel catalyst. J. Labelled Compd. Radiopharm. 1999, 42, 709–713. . [DOI] [Google Scholar]
- a Watanabe M.; Shigihara M.; Hirota Y.; Takato S.; Sato A.; Kakei Y.; Kikuchi R.; Ishii T.; Soeno K.; Nakamura A.; Shimada Y. Effect of an auxin biosynthesis inhibitor, p-phenoxyphenyl boronic acid, on auxin biosynthesis and development in rice. Biosci., Biotechnol., Biochem. 2021, 85, 510–519. 10.1093/bbb/zbaa033. [DOI] [PubMed] [Google Scholar]; b Kakei Y.; Yamazaki C.; Suzuki M.; Nakamura A.; Sato A.; Ishida Y.; Kikuchi R.; Higashi S.; Kokubo Y.; Ishii T.; Soeno K.; Shimada Y. Small-molecule auxin inhibitors that target YUCCA are powerful tools for studying auxin function. Plant J. 2015, 84, 827–837. 10.1111/tpj.13032. [DOI] [PubMed] [Google Scholar]
- a Muralirajan K.; Cheng C.-H. Regioselective synthesis of indoles via rhodium-catalyzed C-H activation directed by an in-situ generated redox-neutral group. Adv. Synth. Catal. 2014, 356, 1571–1576. 10.1002/adsc.201400224. [DOI] [Google Scholar]; b Rosillo M.; Arnáiz E.; Abdi D.; Blanco-Urgoiti J.; Domínguez G.; Pérez-Castells J. Combination of RCM and the Pauson-Khand reaction: One-step synthesis of tricyclic structures. Eur. J. Org. Chem. 2008, 2008, 3917–3927. 10.1002/ejoc.200800332. [DOI] [Google Scholar]
- Schmidt A. M.; Eilbracht P. Tandem hydroformylation-hydrazone formation-Fischer indole synthesis: a novel approach to tryptamides. Org. Biomol. Chem. 2005, 3, 2333–2343. 10.1039/b503396a. [DOI] [PubMed] [Google Scholar]
- Details of D-content, see Supporting Information.
- Liu M.; Chen T.; Chen T.; Yin S.-F. A facile and general acid-catalyzed deuteration at methyl groups of N-heteroarylmethanes. Org. Biomol. Chem. 2017, 15, 2507–2511. 10.1039/C7OB00062F. [DOI] [PubMed] [Google Scholar]
- Treatment of IBA with 20 wt % D2SO4, HC(OEt)3 and D2O at 60 °C provided complex mixtures. This result indicated the formic acid and/or ethyl formate caused side reactions.
- Preparation of 3-substituted indoles costs approximately $12 per mmol, when purchase the reagents from either Sigma-Aldrich Co., LLC., Tokyo Chemical Industry Co., Ltd. or Taiyo Nippon Sanso Co.
- Li G.; Huang L.; Xu J.; Sun W.; X J.; Hong L.; Wang R. Sodium Iodide/Hydrogen Peroxide-Mediated Oxidation/Lactonization for the Construction of Spirocyclic Oxindole-Lactones. Adv. Synth. Catal. 2016, 358, 2873–2877. 10.1002/adsc.201600441. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.