Abstract
Lifestyle is one of the most powerful instruments shaping mankind; the lifestyle includes many aspects of interactions with the environment, from nourishment and education to physical activity and quality of sleep. All these factors taken in complex affect neuroplasticity and define brain performance and cognitive longevity. In particular, physical exercise, exposure to enriched environment and dieting act through complex modifications of microglial cells, which change their phenotype and modulate their functional activity thus translating lifestyle events into remodelling of brain homoeostasis and reshaping neural networks ultimately enhancing neuroprotection and cognitive longevity.
Keywords: Microglia, Neuroplasticity, Enriched environment, Physical exercise, Lifestyle modifications, Diet
Introduction: lifestyle, neural plasticity and cognitive performance
Adaptive behaviours are paramount for survival: in complex multicellular organisms, environmental challenges instigate life-long morpho-functional restructuring of the nervous system, known as neural plasticity. Lifestyle is one of the most powerful instruments shaping mankind; the lifestyle includes many aspects of interactions with the environment, from nourishment and education to physical activity and quality of sleep. There is compelling evidence demonstrating that exposure of animals to environmental stimulation including enriched environment, social engagement and physical activity affects neural plasticity and impacts synaptic connectivity and neuronal morphology; similarly, dieting not only affects the organism as a whole but also reshapes structure and modifies functions of the nervous system.
In rodents engaged in physical activity (usually in a form of free access to the running wheel), an increase in the neuronal arborisation, length and complexity of dendrites, spine morphology and synaptic densities has been documented; these morphological changes develop in paralleled with an increased expression of glutamate receptors and amplification of long-term potentiation (LTP) in several brain regions [1–4]. This plastic remodelling seems to be associated with an increase in production of brain-derived neurotrophic factor (BDNF) [5]. The morpho-functional changes translate into improved cognitive performance including learning and memory [6, 7], prolong cognitive longevity [8–10] and reduce the risk of dementia [8, 11–13].
Intellectual engagement represents another lifestyle factor that, by instigating neural plasticity, positively impacts on cognitive longevity by increasing cognitive reserve. There is convincing evidence demonstrating the role of education, occupational activities, creativity challenges and social engagement in prolonging physiological cognitive ageing and delaying dementia [14–18]. Similarly, dieting has been shown to affect brain metabolism, neuronal plasticity, and synaptic connectivity [19–22] thus impacting of cognitive performance and cognitive longevity [23, 24].
Cellular mechanisms of lifestyle action on the brain remain to be fully elucidated; there is mounting evidence highlighting the role of neuroglia. Neuroglia are the principal homeostatic and defensive arm of the nervous system, which is critical for neural plasticity and cognitive performance. In particular, neuroglia are responsible for the ability of brain to compensate life-long pathological challenges thus preserving cognitive reserve [25]. Physical activity and enriched environment has been shown to significantly increase the complexity, volume and surface area of astrocytes, enhance astrocytic coverage of synapses and blood vessels and positively modulate astrocyte-dependent neurogenesis in adult neurogenic niches [25–29]. Dieting also affects astrocytes: for example, caloric restriction induces substantial increase in astrocytic complexity and increase in astrocytic synaptic coverage, which enhances control over extracellular glutamate and K+ thus augmenting long-term potentiation in the hippocampus of mice [30]. Astrocytes have been proposed to be a critical element in translating lifestyle factors into brain plasticity and cognitive capabilities [31]. Finally, diet, physical exercise, and environmental enrichment act on oligodendrocytes thus promoting myelination in physiology and pathology [32–35].
In this paper we shall overview the effects of lifestyle factors on the plasticity of the third major type of neuroglia— microglial cells, which contribute to brain physiology and represent the principal arm of the defence system of the central nervous system (CNS).
Plasticity of microglia
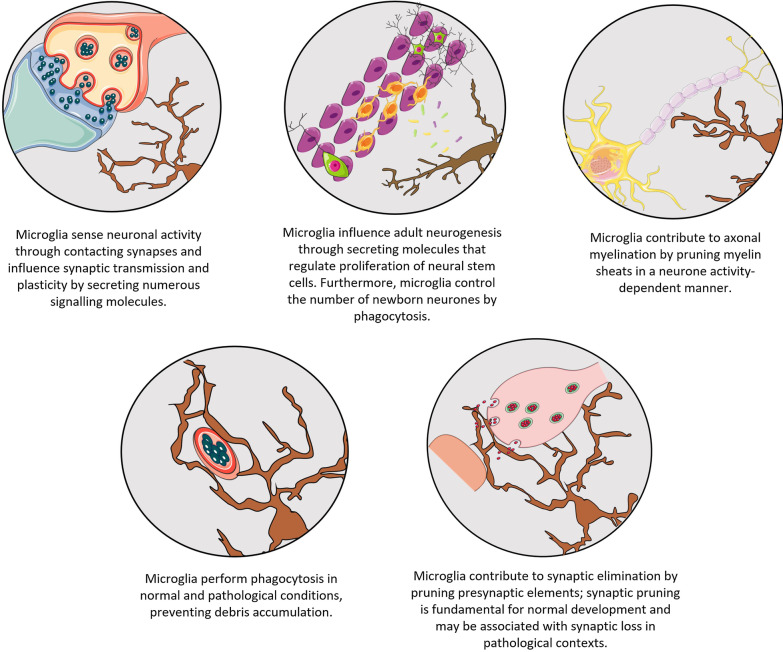
Microglia are the neural cells of the non-neural origin [36]: microglial precursors in the form of foetal macrophages invade the neural tube early in development [37, 38]. These precursors disseminate throughout the brain and the spinal cord and undergo the most remarkable metamorphosis. Mature microglial cells are as different from macrophages as they could be: the latter are spherical or amoeboid, while the former possess highly elaborated processes resembling, in their fundamental design, neural cells. The profound morphological transfiguration is accompanied by similarly profound physiological change: microglial cells acquire a multitude of receptors to neurotransmitters and neurohormones, while retaining "immune" receptors as a legacy from their myeloid ancestry, making microglia are, arguably, the most "sensitive" cells of the CNS. In the normal brain, microglia appear in the form of 'never resting' cells which constantly survey the nervous tissue by their highly ramified and moving processes; hence these cells are defined as 'surveilling microglia'. In addition, microglia perform numerous physiological functions related to regulation of synaptic behaviour, shaping synaptic contacts and regulating adult neurogenesis thus ultimately modulating cognitive processes (Fig. 1) [39–44]. Microglia are highly heterogeneous and plastic cells, which present numerous distinct morphological shapes and functional states depending on the brain region, age and context [45, 46].
Fig. 1.
Microglial functions in physiology and pathophysiology
Lifestyle effects on microglia
Physical exercise
Physical exercise modifies density, morphological appearance, and molecular profile of microglia (Table 1). Ten days of physical exercise in the running wheel stimulates microglia proliferation in the superficial cortical layers [47], and favours ramified surveilling microglial state in the mouse hippocampus [48]. Microglia seem to translate numerous lifestyle modifications into changes in adult neurogenesis within neurogenic niches [49]. Physical exercises are known to stimulate neurogenesis, potentate survival of newborn neurones and improve memory [50–53]. Physical exercise induced rather profound changes in microglial phenotype, as these changes persisted even after cell isolation and maintenance in culture. Addition of purified (by FACS sorting of microglia isolated from transgenic Csf1r-GFP mice expressing GFP under control of Csf1r gene) microglia isolated from the brains of animals subjected to 3 weeks of voluntary running to the culture of hippocampal neurones obtained from sedentary mice activated neural cells and increased neurogenesis. These effects of microglia were mediated through colony-stimulating factor 1 (CSF-1) and its receptor signalling axis. Conversely, microglia from aged animals or young sedentary animals were not effective in recruiting and stimulating neuronal precursors [54]. The same CSF-1 signalling cascade underlies emergence of stress resilience following physical exercise [55]. Positive regulation of neurogenesis may also be mediated by an increase in microglial production of BDNF, which is well known enhancer of neurogenesis [56]. Voluntary physical activity increases the proportion of BDNF-expressing microglia in aged (but not in adult) mice microglia [57], while microglial levels of BDNF were found to correlate with the density of newly generated neurones. Physical activity also increases microglial production of pro-neurogenic insulin-like growth factor (IGF1), which may mediate local microglia-neural precursor cells communications [58]
Table 1.
Effects of physical exercise on microglia
| Species | CNS region | Experimental paradigm | Microglial changes | References |
|---|---|---|---|---|
| Mice | Whole brain | Treadmill for 6 weeks | In EAE model (transfer of encephalitogenic T cells), exercise protected the CNS against autoimmune inflammation by reducing microglial-derived ROS production, neurotoxicity and pro-inflammatory responses | [71] |
| Mice | Hippocampus or remaining brain | Running wheel for 10 weeks | Aged mice showed a greater proportion of CD86 and MHC II positive microglia. In aged females, access to a running wheel decreased proportion of CD86 and MHC II positive microglia in the hippocampus whereas aged males in the running group showed a decrease in the proportion of CD86 positive microglia in the brain and an increase in the proportion of MHC II positive microglia in hippocampus and brain | [63] |
| Mice | Hippocampus | Treadmill for 8 weeks | Treadmill running inhibited the excessive reactivity of microglia in hippocampus of the fluoride-toxic mice, accompanied with the alteration of neuroactive ligand-receptor interaction pathway | [140] |
| Mice | Spinal cord | Running wheel for 8 weeks | Exercise reduced microglial reactivity thus preventing age-related loss of motor neurones | [141] |
| Mice | Hippocampus | Treadmill for 9 days | Exercise protected against LPS-induced memory impairment with concomitant attenuation of IL-1β, TNF-α and IL-10 mRNA expression. Exercise abolished LPS-induced response of astrocytes and microglia | [59] |
| Rat | Hippocampus and striatum | Running wheel for 4 weeks | Exercise reduced microglial reactivity and partially prevented damage to dopaminergic neurones in a rat model of PD | [69] |
| Mice | Hippocampus | Running wheel for 2 weeks | Microglia mediate exercise-induced increase in neural precursor cell activity through fractalkine signalling | [54] |
| Mice | Hippocampus | Running wheel for 10 days | Exercise increases microglial proliferation without morphological, antigenic or transcriptional changes | [48] |
| Mice | Cortices | Running wheel for 10 days | Exercise led to regional increase in microglia proliferation | [47] |
| Mice | Striatum and Substantia nigra | Treadmill for 4 weeks | Exercise prevented dopaminergic neuronal loss by suppressing microglial reactivity in a PD model | [68] |
| Mice | Hippocampus | Treadmill for 12 weeks | Exercise preserved hippocampal cognitive function, suppressed β-amyloid accumulation in the hippocampus in APP/PS1 mice, and attenuates oxidative stress possibly through modulating microglia | [65] |
| Rat | Hippocampus | Treadmill for 4 weeks | Exercise inhibited reactive gliosis following STZ insult, reduced expression of pro-inflammatory mediators and enhanced expression of anti-inflammatory cytokine in the hippocampus | [142] |
EAE Experimental autoimmune encephalomyelitis, LPS lipopolysaccharide, APP/PS1 amyloid precursor protein/presenilin1 mouse Alzheimer's disease model mice, PD Parkinson disease, ROS reactive oxygen species
Although precise description of effects of physical exercise on microglia and mechanisms involved in physiological conditions needs more investigations, there is a large body of evidence indicating that physical exercise promotes microglia-dependent neuroprotection in numerous pathological contexts. For instance, physical exercise protects against lipopolysaccharide (LPS)-induced neuroinflammation and associated cognitive impairment. This protection is associated with suppressed expression of IL-1β, TNFα and IL-10 mRNA in the hippocampus, indicating reduced microglial pro-inflammatory response as an underlying mechanism by which physical exercise might protect CNS [59]. Similar mechanisms may be operational in ageing. Physical exercise, for example, reduces the ratio between pro- (IL-1β, IL-6 and TNFα) and anti-inflammatory (IL-10) cytokines in the hippocampus of aged rats [60]. Microglia are the main source of cytokines in the ageing brain [61], hence are likely to be responsible. Voluntary exposure to the running wheel for 8 weeks attenuated microglial proliferation in the hippocampus of aged mice [62], whereas running for 10 weeks reduced microglial reactivity in the hippocampus and other brain regions of aged rats [63]. Aged mice show greater expression of reactivity markers CD68 and MHCII; exposure of aged females to physical activity decreased densities of CD68 + and MHCII + microglia in the hippocampus, whereas in males CD68 + microglia decreased and MHCII + microglia increased in hippocampus [63]. These data suggest that the effects of physical exercise on microglial immunological profiles vary with age, sex and brain region, probably reflecting microglial heterogeneity. Treadmill exercise for 10 days attenuated cognitive decline and reduced glycolysis, glycolytic capacity, and PFKB3 enzyme in aged mice; similarly, senescent markers such as β-galactosidase and P16INK4A, were also reduced suggesting the exercise-related improved cognition is orchestrated by a normalisation of the metabolic profile and functionality of microglia [64].
Physical exercise-induced microglial plasticity contributes to neuroprotection in neurodegenerative diseases. In the APP/PS1 mice Alzheimer`s disease (AD) model, twelve weeks of treadmill exercise decreased β-amyloid deposits and improved cognitive processes possibly through hippocampal microglia modulation [65]. Treadmill exercise improved cognitive performance including spatial learning and exploratory activity, and reduced β-amyloid deposits and microglial reactivity [66]. In the Tg2576 mice AD model, three weeks of voluntary wheel running significantly reduced hippocampal levels of IL-1β and TNF-α and decreased soluble β-amyloid40 as well as soluble fibrillar β-amyloid [67]. These results indicate that physical exercise can shift the immune response in the brain of an AD mouse model by transforming microglia to an antigen presenting phenotype, thus reducing β-amyloid burden, alleviating AD pathology and improving cognition [67].
In a Parkinson`s disease (PD) MPTP mouse model, treadmill exercise for 4 weeks ameliorated dopaminergic neuronal loss by suppressing microglial reactivity, preventing loss of nigrostriatal neurones and improving motor balance and coordination dysfunction [68]. In 6-hydroxydopamine PD rat model running wheel exercise for four weeks suppressed microglia reactivity and partially prevented neuronal damage and cognitive decline [69]. This potent modulation of microglia, which have a significant neuroprotective role in the PD brain [70], highlights microglia as a key cellular element translating beneficial effects of physical exercise in PD.
Physical exercise and related microglial changes seem to protect the CNS in the experimental autoimmune encephalomyelitis model induced by transfer of encephalitogenic T-cells. High-intensity six-week continuous treadmill training reduced microglia reactive oxygen species formation, neurotoxicity and pro-inflammatory response, which are all involved in the propagation of autoimmune neuroinflammation [71].
Environmental enrichment
Environmental enrichment is defined as a brain stimulating environment composed by physical (such as puzzle boxes, toys, numerous feeders, ropes and running wheels) and social (relationships with peers) elements [72]. In humans, environmental enrichment corresponds to intellectual, social and physical engagement, which contributes to cognitive longevity [73]. Exposure to enriched environment results in well characterised beneficial effects on the CNS including boosting adult neurogenesis, synaptic plasticity, cellular physiology, and remodelling of neuroglia resulting in cognitive improvements and acceleration of neurological recovery following insults of various aetiology (Table 2) [31, 72, 74–77].
Table 2.
Effects of enriched environment on microglia
| Species | Brain region | Experimental paradigm | Microglial changes | References |
|---|---|---|---|---|
| Mice | Hippocampus, amygdala and hypothalamus | EE for 32 and 48 weeks | EE reduced expression of pro-inflammatory cytokines, increased Iba1 expression, and induced microglial hypertrophy and increased ramification | [79] |
| Mice | Hippocampus | EE for 7–8 weeks | EE prevents microgliosis induced by human β-amyloid oligomers, as evidenced by morphology, mRNA changes, and brain interstitial fluid cytokine levels | [81] |
| Mice | Hippocampus and hypothalamus | EE for 6 weeks | EE housing blocks pro-inflammatory cytokine gene induction and promotes arginase 1 mRNA expression in brain-sorted microglia, indicating that EE favours an anti-inflammatory activation state | [143] |
| Mice | Hippocampus and neocortex | EE for 6 weeks | EE in APP/PS1 mice amyloidosis model led to improved short-term memory, reduced microgliosis and increased microglial phagocytic activity | [83] |
| Mice | EE for 4–6 weeks | EE acting through enhanced β-adrenergic signalling reduces microgliosis in response to direct exposure to β-amyloid | [82] | |
| Mice | Hippocampus | EE, PE, and EE + PE for 7 weeks | EE led to an increased microglial number at 5 and 10 months while PE and EE + PE increased microglial numbers only at 10 months | [78] |
| Mice | Amygdala | EE or PE for 40 days | EE Increased microglial proliferation | [144] |
| Rat | Hippocampus | EE for 12 weeks | EE ameliorates cognitive comorbidities associated with type I diabetes mellitus, possibly by reducing hyperactivity in the hypothalamic–pituitary–adrenal axis and microglial reactivity in diabetic animals | [91] |
| Mice | Hippocampus | EE for 87 weeks | Long-term EE reduces microglia morphological diversity of the molecular layer of dentate gyrus | [88] |
| Mice | Lateral septum | EE for 32 weeks | Following dengue infection, EE led to a reduction of microglial morphological diversity | [145] |
| Mice | Hippocampus, septum, olfactory bulb and brainstem | EE for 16 weeks | EE alleviated microgliosis, promoted faster viral clearance, decreased viral dissemination, reduced disease progression, and decreased CNS damage in a model of limbic encephalitis | [90] |
| Mice | Hippocampus | EE for 12 weeks | EE attenuated microgliosis, damage to the extracellular matrix and promoted virus clearance in a model of viral encephalitis | [89] |
| Mice | Striatum | EE for 7 weeks | Glioma-bearing mice housed in EE have increased branching and patrolling activity microglia, besides increased phagocytic activity | [92] |
| Pig | Frontal cortex | EE for 3 weeks | EE piglets displayed a signature consistent with a relative decrease in microglial activity compared to those in the standard condition | [146] |
EE enriched environment, PE physical exercise, APP/PS1 amyloid precursor protein/presenilin1 mouse Alzheimer's disease model mice
Environmental enrichment affects microglial densities. When 3-, 8- and 13-month-old C57BL/6 wild-type mice have been subjected to seven weeks of enriched environment, running wheel or combination of both, the density of Iba1 positive microglia in hippocampus increased. Enriched environment alone appeared to be more effective in increasing Iba1-labelled microglia; physical exercise on its own or in combination with environmental enrichment affected microglial density only in older animals. [78]. A longer environmental enrichment for 32–48 weeks promotes healthy ageing by reducing microglial expression of pro-inflammatory cytokines and MHCII. At the morphological level, environmental enrichment leads to microglial hypertrophy and increased ramification in hippocampus, hypothalamus and amygdala without changing microglial density [79]. In LPS-injection model of neuroinflammation, subjecting rats to enriched environment for 12 h/day for 7 weeks caused significant reduction of microglial reactivity with decreased expression of chemokines including Ccl2, Ccl3, Cxcl2, and cytokines including TNF-α and IL-1β [80].
Similarly, in the context of AD model, animals` exposure to the enriched environment boosts neuroprotection, which is, at least in part, associated with changes in microglia. In particular, exposure to enriched environment protected against direct β-amyloid toxicity through alleviating microglial reactivity, increasing microglial morphological complexity and decreasing expression of inflammatory cytokines such as IL-1β and TNF-α [81]. The underlying mechanism connecting environmental stimulation to the status of microglia is represented by an increased noradrenergic stimulation of the brain. This effect is mediated through activation of β-adrenoceptors: feeding mice with β-adrenergic agonist isoproterenol mimicked effects of environmental enrichment, whereas treating mice undergoing enriched environment with the β-adrenergic antagonist propranolol inhibited positive effect of environmental stimulation. This effect was also absent in transgenic animals lacking β1,2 adrenoceptors [82].
In the APP/PS1 mice AD model, environment enrichment for six weeks starting from 12 months of age, improved short-term memory, reduced microglial reactivity while increasing microglia phagocytic activity; the β-amyloid burden however remained unaffected [83]. Similarly, increased microglial phagocytic activity has been observed in 5xFAD AD mouse model subjected to six weeks of enriched environment as compared with animals in standard environment. In addition, exposure to environmental stimulation rescued adult neurogenesis and memory deficits simultaneously preventing β-amyloid dissemination [84]. An increase in microglial phagocytosis activity following exposure to enriched environment may also improve physiological ageing, known to suppress microglial phagocytic machinery [85].
Exposure to enriched environment does not always enhance microglial profiles. Systemic non-neurotropic dengue virus infection, for example, results in an increased size and complexity of microglia; exposure to the enriched environment reduced microglial diversity in lateral septum, with significant correlation between morphological complexity and the levels of TNF-α in the circulation [86]. At the same time, sedentary lifestyle negatively impacted on microglial reactivity, thus diminishing microglial neuroprotection [87]. Similar loss of morphological diversity occurs in the molecular layer of dentate gyrus in mice housed in long-term enriched environment, suggesting different microglial morphotypes may have different physiological roles in various environments, and that long-term enriched environment may be associated with adaptive microglial response to cognitive stimuli [88]. In Piry rhabdovirus model of encephalitis, mice exposed to enriched environment presented less CNS infection and substantially faster virus clearance, less microgliosis and less damage to the extracellular matrix than animals housed in standard environment [89]. In cocal virus infection, mice dwelling in standard environment demonstrated significant weight loss and higher mortality as compared with animals exposed to environmental stimulation. Additionally, enriched environment led to better locomotor and exploratory activity associated with less neuroinvasion and reduced microglial reactivity, revealing that enriched environment drives a more effective immune response in a mouse model of virus encephalitis [90].
In type 1 diabetic rats, enriched environment reduced microglial reactivity, improved memory and ameliorated cognitive comorbidities associated with diabetes [91]. Environmental stimulation also shapes microglial plasticity in glioma: glioma-bearing mice exposed to environmental stimulation have increased branching and patrolling activity of microglia, besides increased phagocytic activity [92].
Diet
Healthy diet, especially being applied in combination with other modifiable lifestyle factors discussed above, emerges as a promising strategy for preventing cognitive decline and promoting brain health [93, 94]. The adoption of a friendly diet or caloric restriction is positively associated with cognitive performance throughout lifespan, leading to cognitive improvements during infancy, adolescence and adulthood [95–97] and preserving cognitive functions in elderly [98]. The mechanisms by which diet affects the brain include modulation of synaptic plasticity, neuroglial support and adult neurogenesis [99]. On the other hand, high-fat diet is associated with obesity and cognitive impairments [100], which induces poor lifestyle choices leading to weight gain in a self-accelerating cycle [101].
Obesity is a world-wide concern which affects millions of people and represents an important risk factor for neurological disorders [102]. It is often caused by high-fat intake [103] and it is associated with impaired cognitive functions, neurodegenerative pathologies and atrophy in many brain areas including frontal lobe, anterior cingulate gyrus, hippocampus and hypothalamus [104]. Detrimental effects of high-fat diet on the brain include synaptic loss and microglial reactivity [105], the latter playing multiple roles in damaging the brain and affecting cognition [106–108]. On the other hand, the adoption of friendly low-fat diet rich in plant foods, moderate intake of fish, poultry and wine, and low intake of fat and red and processed meat promotes microglia-dependent neuroprotection through mitigating microglia-mediated brain disturbance [109].
Microglial appearance and functional activities are strongly affected by diet (Table 3); microglial changes contribute to brain response to both high-fat detrimental and low-fat friendly dieting. In Yucatan minipigs, for example, maternal high-fat diet during gestation and lactation modifies offspring's microglial density and morphology. These alterations occurred differently in hippocampus and prefrontal cortex; in the prefrontal cortex microglial density increased whereas in the hippocampus it remained unchanged compared to standard diet group; at a morphological level, anterior prefrontal cortex, dorsolateral prefrontal cortex and hippocampus presented higher number of unipolar microglia whereas orbitofrontal cortex presented higher number of multipolar microglia, both compared to standard diet group in hippocampus [110]. This brain region-dependent microglial response induced by high-fat diet is also observed in humans. Post-mortem analysis of the brain tissue obtained from obese individuals (body mass index, BMI, > 30) revealed increased microglial proliferation and morphological changes indicative of microglial reactivity (enlarged cell bodies and shortened processes) in the hypothalamus as compared with normal individuals (BMI < 25). At the same time in the cortex microglia kept physiological morphology (small cell bodies and ramified processes) in both obese and non-obese individuals [111].
Table 3.
Effects of diet on microglia
| Species | Brain region | Experimental paradigm | Microglial changes | References |
|---|---|---|---|---|
| Mice | Hypothalamus and total brain | LFD (6.5% fat), HFD (42% fat) and caloric restriction (40% less) for 6 and 24 months | HFD increased the number of microglia in the hypothalamus and both number and soma size of microglia were increased in the cerebellum during aging in HFD mice. Under basal- or LPS-induced inflammatory conditions, gene expression analysis of the total brain microglia population or hypothalamus tissue showed similar findings in HFD and LFD mice. Caloric restriction in LFD mice prevented the increased expression of phagocytic markers in white matter microglia with aging, and this protective effect of caloric restriction was not observed in HFD mice. Because running wheel access did not affect white matter microglia activation in either diet, dietary fat as well as caloric content may play an important role in the inflammatory process in brain aging | [123] |
| Mice | Hypothalamus | Standard diet (13,2% fat) or HFD (42% fat) for 28 days | HFD led to microglial reactivity and neuronal stress in the mediobasal hypothalamus. Microglial depletion abrogated HFD-induced hypothalamic inflammation besides to enhance leptin signalling and reduce food intake | [147] |
| Mice | Hippocampus and amygdala | HFD (60.3% fat) for 3 days | In the hippocampus, HFD induced enlarged synaptophysin boutons, indicative of neurodegeneration. In the amygdala, HFD exacerbated the effects of ageing on microglial priming (morphology) and significantly suppressed microglial phagocytosis | [115] |
| Mice | White matter | Western diet (42% fat) | WD diet induced an ageing-related metabolic dysfunction associated with impaired myelin-debris clearance in microglia, which is mediated by TGF-β signalling and disrupts lesion recovery after demyelination. Blocking TGF-β restores microglia responsiveness and myelin-debris clearance following demyelinating injury | [148] |
| Mice | Nucleus accumbens | high-caloric chocolate cafeteria diet for 43 days | This high-caloric diet led to microglial reactivity with increased expression of pro-inflammatory factors and abnormal responses after amphetamine-induced hyperlocomotion. Chronic inhibition of microglial reactivity normalised these behavioural alterations | [149] |
| Mice and Human | Hypothalamus |
Mice: HFD (60% fat) for 8 weeks Human: post-mortem samples from obese individuals (BMI > 30) |
HFD induced microglia number in the hypothalamus of mice. Gene expression analysis of isolated microglia found downregulation of genes important for sensing signals in microenvironment. In obese humans, it was found signs of hypothalamic gliosis and exacerbated microglial dystrophy | [111] |
| Mice | Hippocampus | HFD (60% fat) for 8 weeks | HFD partially disrupted the rhythmicity of circadian clock genes in microglia, besides disruption on microglial immune gene expression. HFD induced a shift of substrate utilisation on microglia, with decreased glutamate and glucose metabolism and an overall increase of lipid metabolism during active period of the animals | [150] |
| Mice | Hypothalamus | Caloric restriction (40% of the ad libitum food intake) in HFD and LFD animals for 23 months | Caloric restriction in combination with LFD affected microglial morphology and decreased expression of phagocytic markers (Mac2/Lgals3, Dectin-1/Clec7a and CD16/CD32 in microglia | [123] |
| Mice | Hippocampus | Luteolin intake (20 mg/d) for 4 weeks | In aged animals, luteolin food supplement improved spatial memory and restored expression of inflammatory markers compared with that of young animals | [131] |
| Rat | - | EO and EP intake (2%) for 8 weeks | In aged animals, this diet improved working memory. Then, blood serum was used to assess microglial response in vitro. BV-2 microglia treated with blood serum from EO- and EP-fed rat showed reduced expression of NO and TNF-α respectively | [133] |
| Mice | Hippocampus | HFD and LFD with or without blueberry (4%) for 5 months | HFD supplemented with blueberry had fewer microglia compared to LFD and HFD ones. BV-2 microglia treated with serum collected from mice fed the diets with blueberry produced less NO compared to HFD mice. HFD + blueberry mice presented higher levels of hippocampal BDNF and DCX-positive cells compared to mice fed HFD | [139] |
| Mice | Frontal cortex | Caloric restriction (70% of the ad libitum food intake) for 6 weeks and 6 and 12 months | Caloric restriction for 6- and 12 months counteracted ageing-induced microglial changes such as Ca2+ signalling and processes motility toward a younger phenotype. Even shot-term caloric restriction (6 weeks) beginning in old age significantly improved microglial motility and Ca2+ signalling | [124] |
LFD low-fat diet, HFD high fat diet, EO Euterpe oleracea, EP Euterpe precatoria, WD Western diet
Distinct microglial response depends on the length of dieting. Hypothalamic microglia from mice fed with high-fat diet for 3 days upregulated expression of pro-inflammatory mediators including IL-1β, Cd74, Irf8 and IL-6. However, keeping the same mice on high-fat diet for eight weeks reduced expression of pro-inflammatory mediators and increased expression of anti-inflammatory molecules such as IL-10 and Pparg [111]. Early exposure (at postnatal days 21–60) of mice to a high-fat diet, triggered reactive microgliosis with increased expression of IL-1β and TNF-α, reduced neurogenesis and promoted immature morphology of dendritic spines along with reduced levels of scaffold protein Shank2 suggesting defective connectivity. In addition, these animals demonstrated cognitive impairment with spatial memory alterations [112]. Incubation of primary cultured microglia with palmitate, a saturated fatty acid present in high fat diet led to a secretion of exosomes which induced immature dendritic spine phenotype [112].
Similar changes were observed in mice fed with high-fat diet for eight weeks: animals showed reduced presence of synaptic markers, altered microglial morphology and cognitive disruption [105]. Recently, the mechanisms underlying obesity-associated cognitive decline were found to be influenced by reactive microglia. High-fat diet for 18 weeks made mice obese, which was associated with decreased dendritic spine density, increased microglial reactivity, increased microglial phagocytosis of synapses, which ultimately provoked cognitive impairment [106]. Reducing microglial reactivity with: (i) partial knockdown of fractalkine receptor CX3CR1; (ii) minocycline treatment, or (iii) annexin-V treatment prevented obesity-associated cognitive impairment. These data highlight microglial contribution to the synaptic loss and cognitive impairment associated with obesity [106].
In ageing, exposure to high-fat diet triggers reactive microgliosis in mice and in an APP/PS1 mouse model of amyloidosis [113]. Certain evidence indicates that even short-term consumption of high-fat diet may trigger cognitive deficits [114], although it remains doubtful whether this may be translated to humans. It has been suggested that the detrimental diet disrupts the ageing process by worsening the impact of ageing on microglial function and morphology, priming microglia in brain areas important for cognitive functions including hippocampus and amygdala [115, 116].
Appropriate diet therefore is critical for the brain performance and cognitive capabilities. In this context, diet-dependent modulation of microglia emerges as a non-pharmacological and non-invasive strategy to improve cognition and prolong cognitive longevity (Table 3) [117, 118]. Caloric restriction, in particular, protects the brain from age-dependent diseases and prolongs cognitive longevity [119–122]. Caloric restriction in combination with low-fat diet abolished age-dependent increase in Iba1 immunoreactivity, microglial density and expression of phagocytic marker Mac-2 in the white matter tract of hippocampus, the fimbria [123]. Caloric restriction for 6–12 months at 70% of ad libitum food intake has been shown to alleviate age-dependent dystrophic changes in microglia, manifested by decreased Ca2+ signalling and disorganised motility of microglial processes [124]. Combining the low-fat diet with caloric restriction reduced white matter microglial reactivity during ageing, modulating their morphology and reducing phagocytic markers [123]. Since microglial dystrophy is critical for ageing-induced brain dysfunction and cognitive decline, caloric restriction emerges as a non-pharmacological, cost-effective, and clinically relevant microglial modulator for rejuvenation of microglia.
Healthy diet with high content of flavonoids and phenolic compounds, present in plants, vegetables, and wine, protects cognition in subjects aged 65 or older. After 10 year`s follow up, individuals with highest flavonoids intake presented better cognitive performance compared with individuals with lowest intake: on average Mini-Mental State Examination score loss was 2.1 points in the latter, whereas in the former the score loss was only 1.2 points [125]. Polyphenol-rich diet is similarly associated with cognitive improvements in elderly [126]. Flavonoids and phenolic compounds are well known for their ability to affect microglial status, in particular by reducing microglial reactivity and restoring microglial homeostatic functions with beneficial influence on cognitive functions with consequent reduction in microglia-derived neuroinflammation and cognitive improvements [127–130]. Luteolin, a plant derived flavonoid, suppressed expression of pro-inflammatory genes in BV-2 microglia; whereas luteolin consumption significantly improved spatial working memory and reduced expression of inflammatory markers in hippocampi of aged (22–24 months old) mice [131]. Luteolin interacts with several signalling cascades modulating microglial transcriptomic profile and promoting anti-inflammatory and anti-oxidative phenotype, thus strengthening neuroprotection [132]. Similar outcomes were observed in ageing (19–21) months old rats fed with polyphenol rich açaí palm tree pulps; dietary supplementation with pulps of Euterpe oleracea (EO) and Euterpe precatoria (EP) for eight weeks improved working memory as tested by Morris water maze; the EO-supplemented diet, but not an EP one also improved reference memory. Treatment of BV-2 microglial cell line with serum obtained from rats receiving EO or EP rich diet reduced production of nitric oxide (NO) and expression of TNF-α [133].
Blueberries represent another source of polyphenols and anthocyanins well known to improve cognition especially in ageing [134–136], reduce microglial reactivity [137, 138], and counterbalance brain dysfunctions induced by high-fat diet [139]. In particular, mice exposed to the diet supplemented with blueberry showed significantly less Iba1 immunoreactivity and lower microglial density, together with higher levels of BDNF and larger number of newborn neurones. The BV-2 microglial cell lines treated with serum collected from mice fed with blueberry produced less NO [139].
Conclusion: mens sana in corpore sana—microglia translate friendly lifestyle into brain health and cognitive longevity
The brain is endowed with remarkable plastic capacity both in structure and cellular functioning, which allows life-long adaptation to the exposome. The adoption of healthy lifestyle including regular exercise, intellectual engagement and friendly diet significantly impacts the brain, affecting different areas at different levels of nervous tissue organisation modulating brain-wide homoeostatic systems such as blood–brain barrier and glymphatic clearance, remodelling cellular networks and modifying molecular cascades in neural and non-neural cells. As a holistic therapy, these lifestyle factors have been associated with improvements in cognitive performance, speedy recovery in the contexts of neurotrauma, stroke and neuroinfections, promoting healthy ageing and arresting or retarding neurodegenerative alterations, which as yet, cannot be managed pharmacologically. Lifestyle challenges, at least in part, are translated through changes in microglial phenotypes, that underlie multiple beneficial adjustments of the nervous tissue (Fig. 2).
Fig. 2.
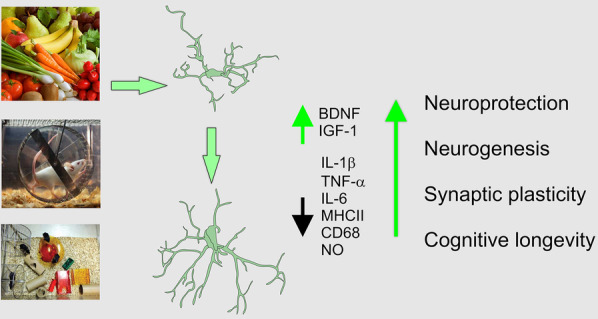
Microglia translate lifestyle into the neuroprotection and cognitive longevity. The adoption of friendly lifestyle induces morphological and functional plasticity of microglia, these plastic changes translate, at least in part, intellectual engagement, physical exercise and healthy diets into the brain health through enhanced neuroprotection, neurogenesis, and synaptic plasticity. Images of microglia has been re-drawn from ref [86] with permission
Acknowledgements
No.
Authors' contributions
Authors contributed equally. Both authors read and approved the final manuscript.
Funding
No funding.
Availability of data and materials
Not applicable.
Declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
No completing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Marcus Augusto-Oliveira, Email: marcusadeoliveira@outlook.com.
Alexei Verkhratsky, Email: Alexej.Verkhratsky@manchester.ac.uk.
References
- 1.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu Q, Li X, Wang J, Li Y. Effect of exercise training on long-term potentiation and NMDA receptor channels in rats with cerebral infarction. Exp Ther Med. 2013;6:1431–1436. doi: 10.3892/etm.2013.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eadie BD, Redila VA, Christie BR. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J Comp Neurol. 2005;486:39–47. doi: 10.1002/cne.20493. [DOI] [PubMed] [Google Scholar]
- 4.Stranahan AM, Khalil D, Gould E. Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus. 2007;17:1017–1022. doi: 10.1002/hipo.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillips C, Baktir MA, Srivatsan M, Salehi A. Neuroprotective effects of physical activity on the brain: a closer look at trophic factor signaling. Front Cell Neurosci. 2014;8:170. doi: 10.3389/fncel.2014.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/S0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- 8.Chang M, Jonsson PV, Snaedal J, Bjornsson S, Saczynski JS, Aspelund T, Eiriksdottir G, Jonsdottir MK, Lopez OL, Harris TB, et al. The effect of midlife physical activity on cognitive function among older adults: AGES–Reykjavik Study. J Gerontol A Biol Sci Med Sci. 2010;65:1369–1374. doi: 10.1093/gerona/glq152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dik M, Deeg DJ, Visser M, Jonker C. Early life physical activity and cognition at old age. J Clin Exp Neuropsychol. 2003;25:643–653. doi: 10.1076/jcen.25.5.643.14583. [DOI] [PubMed] [Google Scholar]
- 10.Larson EB, Wang L, Bowen JD, McCormick WC, Teri L, Crane P, Kukull W. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- 11.Gronek P, Balko S, Gronek J, Zajac A, Maszczyk A, Celka R, Doberska A, Czarny W, Podstawski R, Clark CCT, et al. Physical activity and Alzheimer's disease: a narrative review. Aging Dis. 2019;10:1282–1292. doi: 10.14336/AD.2019.0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meng Q, Lin MS, Tzeng IS. Relationship between exercise and Alzheimer's disease: a narrative literature review. Front Neurosci. 2020;14:131. doi: 10.3389/fnins.2020.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reas ET, Laughlin GA, Bergstrom J, Kritz-Silverstein D, Richard EL, Barrett-Connor E, McEvoy LK. Lifetime physical activity and late-life cognitive function: the Rancho Bernardo study. Age Ageing. 2019;48:241–246. doi: 10.1093/ageing/afy188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phillips C. Lifestyle modulators of neuroplasticity: how physical activity, mental engagement, and diet promote cognitive health during aging. Neural Plast. 2017;2017:3589271. doi: 10.1155/2017/3589271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ball K, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M, Morris JN, Rebok GW, Smith DM, Tennstedt SL, et al. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA. 2002;288:2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Curlik DM, 2nd, Shors TJ. Training your brain: Do mental and physical (MAP) training enhance cognition through the process of neurogenesis in the hippocampus? Neuropharmacology. 2013;64:506–514. doi: 10.1016/j.neuropharm.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Staff RT, Hogan MJ, Williams DS, Whalley LJ. Intellectual engagement and cognitive ability in later life (the "use it or lose it" conjecture): longitudinal, prospective study. BMJ. 2018;363:k4925. doi: 10.1136/bmj.k4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krell-Roesch J, Vemuri P, Pink A, Roberts RO, Stokin GB, Mielke MM, Christianson TJ, Knopman DS, Petersen RC, Kremers WK, et al. Association between mentally stimulating activities in late life and the outcome of incident mild cognitive impairment, with an analysis of the APOE epsilon4 genotype. JAMA Neurol. 2017;74:332–338. doi: 10.1001/jamaneurol.2016.3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agrawal R, Gomez-Pinilla F. 'Metabolic syndrome' in the brain: deficiency in omega-3 fatty acid exacerbates dysfunctions in insulin receptor signalling and cognition. J Physiol. 2012;590:2485–2499. doi: 10.1113/jphysiol.2012.230078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomez-Pinilla F, Tyagi E. Diet and cognition: interplay between cell metabolism and neuronal plasticity. Curr Opin Clin Nutr Metab Care. 2013;16:726–733. doi: 10.1097/MCO.0b013e328365aae3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy T, Dias GP, Thuret S. Effects of diet on brain plasticity in animal and human studies: mind the gap. Neural Plast. 2014;2014:563160. doi: 10.1155/2014/563160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamada D, Wada K, Sekiguchi M. Modulation of long-term potentiation of cortico-amygdala synaptic responses and auditory fear memory by dietary polyunsaturated fatty acid. Front Behav Neurosci. 2016;10:164. doi: 10.3389/fnbeh.2016.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nurk E, Refsum H, Drevon CA, Tell GS, Nygaard HA, Engedal K, Smith AD. Intake of flavonoid-rich wine, tea, and chocolate by elderly men and women is associated with better cognitive test performance. J Nutr. 2009;139:120–127. doi: 10.3945/jn.108.095182. [DOI] [PubMed] [Google Scholar]
- 24.Devore EE, Kang JH, Breteler MM, Grodstein F. Dietary intakes of berries and flavonoids in relation to cognitive decline. Ann Neurol. 2012;72:135–143. doi: 10.1002/ana.23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verkhratsky A, Augusto-Oliveira M, Pivoriunas A, Popov A, Brazhe A, Semyanov A. Astroglial asthenia and loss of function, rather than reactivity, contribute to the ageing of the brain. Pflugers Arch. 2021;473:753–774. doi: 10.1007/s00424-020-02465-3. [DOI] [PubMed] [Google Scholar]
- 26.Leardini-Tristao M, Andrade G, Garcia C, Reis PA, Lourenco M, Moreira ETS, Lima FRS, Castro-Faria-Neto HC, Tibirica E, Estato V. Physical exercise promotes astrocyte coverage of microvessels in a model of chronic cerebral hypoperfusion. J Neuroinflammation. 2020;17:117. doi: 10.1186/s12974-020-01771-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uda M, Ishido M, Kami K, Masuhara M. Effects of chronic treadmill running on neurogenesis in the dentate gyrus of the hippocampus of adult rat. Brain Res. 2006;1104:64–72. doi: 10.1016/j.brainres.2006.05.066. [DOI] [PubMed] [Google Scholar]
- 28.de Senna PN, Bagatini PB, Galland F, Bobermin L, do Nascimento PS, Nardin P, Tramontina AC, Goncalves CA, Achaval M, Xavier LL. Physical exercise reverses spatial memory deficit and induces hippocampal astrocyte plasticity in diabetic rats. Brain Res. 2017;1655:242–251. [DOI] [PubMed]
- 29.Sirevaag AM, Greenough WT. Plasticity of GFAP-immunoreactive astrocyte size and number in visual cortex of rats reared in complex environments. Brain Res. 1991;540:273–278. doi: 10.1016/0006-8993(91)90517-Y. [DOI] [PubMed] [Google Scholar]
- 30.Popov A, Denisov P, Bychkov M, Brazhe A, Lyukmanova E, Shenkarev Z, Lazareva N, Verkhratsky A, Semyanov A. Caloric restriction triggers morphofunctional remodeling of astrocytes and enhances synaptic plasticity in the mouse hippocampus. Cell Death Dis. 2020;11:208. doi: 10.1038/s41419-020-2406-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Augusto-Oliveira M, Verkhratsky A. Mens sana in corpore sano: lifestyle changes modify astrocytes to contain Alzheimer's disease. Neural Regen Res. 2021;16:1548–1549. doi: 10.4103/1673-5374.303023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng J, Sun X, Ma C, Li BM, Luo F. Voluntary wheel running promotes myelination in the motor cortex through Wnt signaling in mice. Mol Brain. 2019;12:85. doi: 10.1186/s13041-019-0506-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoon H, Kleven A, Paulsen A, Kleppe L, Wu J, Ying Z, Gomez-Pinilla F, Scarisbrick IA. Interplay between exercise and dietary fat modulates myelinogenesis in the central nervous system. Biochim Biophys Acta. 2016;1862:545–555. doi: 10.1016/j.bbadis.2016.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaddock-Heyman L, Erickson KI, Kienzler C, Drollette ES, Raine LB, Kao SC, Bensken J, Weisshappel R, Castelli DM, Hillman CH, et al. Physical activity increases white matter microstructure in children. Front Neurosci. 2018;12:950. doi: 10.3389/fnins.2018.00950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Svatkova A, Mandl RC, Scheewe TW, Cahn W, Kahn RS, Hulshoff Pol HE. Physical exercise keeps the brain connected: biking increases white matter integrity in patients with schizophrenia and healthy controls. Schizophr Bull. 2015;41:869–878. doi: 10.1093/schbul/sbv033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garaschuk O, Verkhratsky A. Microglia: the neural cells of nonneural origin. Methods Mol Biol. 2019;2034:3–11. doi: 10.1007/978-1-4939-9658-2_1. [DOI] [PubMed] [Google Scholar]
- 37.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ginhoux F, Lim S, Hoeffel G, Low D, Huber T. Origin and differentiation of microglia. Front Cell Neurosci. 2013;7:45. doi: 10.3389/fncel.2013.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Augusto-Oliveira M, Arrifano GP, Lopes-Araujo A, Santos-Sacramento L, Takeda PY, Anthony DC, Malva JO, Crespo-Lopez ME. What do microglia really do in healthy adult brain? Cells. 2019;8. [DOI] [PMC free article] [PubMed]
- 40.Kettenmann H, Kirchhoff F, Verkhratsky A. Microglia: new roles for the synaptic stripper. Neuron. 2013;77:10–18. doi: 10.1016/j.neuron.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 41.Tay TL, Carrier M, Tremblay ME. Physiology of microglia. Adv Exp Med Biol. 2019;1175:129–148. doi: 10.1007/978-981-13-9913-8_6. [DOI] [PubMed] [Google Scholar]
- 42.Tremblay ME, Stevens B, Sierra A, Wake H, Bessis A, Nimmerjahn A. The role of microglia in the healthy brain. J Neurosci. 2011;31:16064–16069. doi: 10.1523/JNEUROSCI.4158-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cserep C, Posfai B, Denes A. Shaping neuronal fate: functional heterogeneity of direct microglia-neuron interactions. Neuron.2020. [DOI] [PubMed]
- 44.Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 45.Tan YL, Yuan Y, Tian L. Microglial regional heterogeneity and its role in the brain. Mol Psychiatry.2019. [DOI] [PMC free article] [PubMed]
- 46.Stratoulias V, Venero JL, Tremblay ME, Joseph B. Microglial subtypes: diversity within the microglial community. EMBO J. 2019;38:e101997. doi: 10.15252/embj.2019101997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ehninger D, Kempermann G. Regional effects of wheel running and environmental enrichment on cell genesis and microglia proliferation in the adult murine neocortex. Cereb Cortex. 2003;13:845–851. doi: 10.1093/cercor/13.8.845. [DOI] [PubMed] [Google Scholar]
- 48.Olah M, Ping G, De Haas AH, Brouwer N, Meerlo P, Van Der Zee EA, Biber K, Boddeke HW. Enhanced hippocampal neurogenesis in the absence of microglia T cell interaction and microglia activation in the murine running wheel model. Glia. 2009;57:1046–1061. doi: 10.1002/glia.20828. [DOI] [PubMed] [Google Scholar]
- 49.Valero J, Paris I, Sierra A. Lifestyle shapes the dialogue between environment, microglia, and adult neurogenesis. ACS Chem Neurosci. 2016;7:442–453. doi: 10.1021/acschemneuro.6b00009. [DOI] [PubMed] [Google Scholar]
- 50.Opendak M, Gould E. Adult neurogenesis: a substrate for experience-dependent change. Trends Cogn Sci. 2015;19:151–161. doi: 10.1016/j.tics.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 51.Farioli-Vecchioli S, Mattera A, Micheli L, Ceccarelli M, Leonardi L, Saraulli D, Costanzi M, Cestari V, Rouault JP, Tirone F. Running rescues defective adult neurogenesis by shortening the length of the cell cycle of neural stem and progenitor cells. Stem Cells. 2014;32:1968–1982. doi: 10.1002/stem.1679. [DOI] [PubMed] [Google Scholar]
- 52.Kempermann G, Fabel K, Ehninger D, Babu H, Leal-Galicia P, Garthe A, Wolf SA. Why and how physical activity promotes experience-induced brain plasticity. Front Neurosci. 2010;4:189. doi: 10.3389/fnins.2010.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Speisman RB, Kumar A, Rani A, Foster TC, Ormerod BK. Daily exercise improves memory, stimulates hippocampal neurogenesis and modulates immune and neuroimmune cytokines in aging rats. Brain Behav Immun. 2013;28:25–43. doi: 10.1016/j.bbi.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vukovic J, Colditz MJ, Blackmore DG, Ruitenberg MJ, Bartlett PF. Microglia modulate hippocampal neural precursor activity in response to exercise and aging. J Neurosci. 2012;32:6435–6443. doi: 10.1523/JNEUROSCI.5925-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fleshner M, Greenwood BN, Yirmiya R. Neuronal-glial mechanisms of exercise-evoked stress robustness. In: Pariante CM, Lapiz-Bluhm MD, editors Behavioral neurobiology of stress-related disorders. Berlin: Springer; 2014. pp. 1–12. [DOI] [PubMed]
- 56.Aimone JB, Li Y, Lee SW, Clemenson GD, Deng W, Gage FH. Regulation and function of adult neurogenesis: from genes to cognition. Physiol Rev. 2014;94:991–1026. doi: 10.1152/physrev.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Littlefield AM, Setti SE, Priester C, Kohman RA. Voluntary exercise attenuates LPS-induced reductions in neurogenesis and increases microglia expression of a proneurogenic phenotype in aged mice. J Neuroinflammation. 2015;12:138. doi: 10.1186/s12974-015-0362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Llorens-Martin M, Torres-Aleman I, Trejo JL. Mechanisms mediating brain plasticity: IGF1 and adult hippocampal neurogenesis. Neuroscientist. 2009;15:134–148. doi: 10.1177/1073858408331371. [DOI] [PubMed] [Google Scholar]
- 59.Mota BC, Kelly AM. Exercise alters LPS-induced glial activation in the mouse brain. Neuronal Signal. 2020;4:NS20200003. [DOI] [PMC free article] [PubMed]
- 60.Gomes da Silva S, Simoes PS, Mortara RA, Scorza FA, Cavalheiro EA, da Graca Naffah-Mazzacoratti M, Arida RM. Exercise-induced hippocampal anti-inflammatory response in aged rats. J Neuroinflammation. 2013;0:61. [DOI] [PMC free article] [PubMed]
- 61.Barrientos RM, Kitt MM, Watkins LR, Maier SF. Neuroinflammation in the normal aging hippocampus. Neuroscience. 2015;309:84–99. doi: 10.1016/j.neuroscience.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kohman RA, DeYoung EK, Bhattacharya TK, Peterson LN, Rhodes JS. Wheel running attenuates microglia proliferation and increases expression of a proneurogenic phenotype in the hippocampus of aged mice. Brain Behav Immun. 2012;26:803–810. doi: 10.1016/j.bbi.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kohman RA, Bhattacharya TK, Wojcik E, Rhodes JS. Exercise reduces activation of microglia isolated from hippocampus and brain of aged mice. J Neuroinflammation. 2013;10:885. doi: 10.1186/1742-2094-10-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mela V, Mota BC, Milner M, McGinley A, Mills KHG, Kelly ÁM, Lynch MA. Exercise-induced re-programming of age-related metabolic changes in microglia is accompanied by a reduction in senescent cells. Brain Behav Immun. 2020;87:413–428. doi: 10.1016/j.bbi.2020.01.012. [DOI] [PubMed] [Google Scholar]
- 65.Zhang X, He Q, Huang T, Zhao N, Liang F, Xu B, Chen X, Li T, Bi J. Treadmill exercise decreases Aβ deposition and counteracts cognitive decline in APP/PS1 mice, possibly via hippocampal microglia modifications. Front Aging Neurosci. 2019;11:78. doi: 10.3389/fnagi.2019.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ke HC, Huang HJ, Liang KC, Hsieh-Li HM. Selective improvement of cognitive function in adult and aged APP/PS1 transgenic mice by continuous non-shock treadmill exercise. Brain Res. 2011;1403:1–11. doi: 10.1016/j.brainres.2011.05.056. [DOI] [PubMed] [Google Scholar]
- 67.Nichol KE, Poon WW, Parachikova AI, Cribbs DH, Glabe CG, Cotman CW. Exercise alters the immune profile in Tg2576 Alzheimer mice toward a response coincident with improved cognitive performance and decreased amyloid. J Neuroinflammation. 2008;5:13–13. doi: 10.1186/1742-2094-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sung YH, Kim SC, Hong HP, Park CY, Shin MS, Kim CJ, Seo JH, Kim DY, Kim DJ, Cho HJ. Treadmill exercise ameliorates dopaminergic neuronal loss through suppressing microglial activation in Parkinson's disease mice. Life Sci. 2012;91:1309–1316. doi: 10.1016/j.lfs.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 69.Real CC, Doorduin J, Kopschina F, García PV, de Paula FD, Britto D, Luiz R, de Vries EFJ. Evaluation of exercise-induced modulation of glial activation and dopaminergic damage in a rat model of Parkinson’s disease using [11C]PBR28 and [18F]FDOPA PET. J Cereb Blood Flow Metab. 2017;39:989–1004. doi: 10.1177/0271678X17750351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Badanjak K, Fixemer S, Smajić S, Skupin A, Grünewald A. The Contribution of Microglia to Neuroinflammation in Parkinson's Disease. Int J Mol Sci. 2021; 22. [DOI] [PMC free article] [PubMed]
- 71.Zaychik Y, Fainstein N, Touloumi O, Goldberg Y, Hamdi L, Segal S, Nabat H, Zoidou S, Grigoriadis N, Katz A et al. High-intensity exercise training protects the brain against autoimmune neuroinflammation: regulation of microglial redox and pro-inflammatory functions. Front Cell Neurosci. 2021;15. [DOI] [PMC free article] [PubMed]
- 72.McDonald MW, Hayward KS, Rosbergen ICM, Jeffers MS, Corbett D. Is environmental enrichment ready for clinical application in human post-stroke rehabilitation? Front Behav Neurosci. 2018;12:135. [DOI] [PMC free article] [PubMed]
- 73.Neupert SD, Growney CM, Zhu X, Sorensen JK, Smith EL, Hannig J. BFF: bayesian, fiducial, and frequentist analysis of cognitive engagement among cognitively impaired older adults. Entropy. 2021;23:428. doi: 10.3390/e23040428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bekinschtein P, Oomen CA, Saksida LM, Bussey TJ. Effects of environmental enrichment and voluntary exercise on neurogenesis, learning and memory, and pattern separation: BDNF as a critical variable? Semin Cell Dev Biol. 2011;22:536–542. doi: 10.1016/j.semcdb.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 75.Ohline SM, Abraham WC. Environmental enrichment effects on synaptic and cellular physiology of hippocampal neurons. Neuropharmacology. 2019;145:3–12. doi: 10.1016/j.neuropharm.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 76.Rodriguez JJ, Terzieva S, Olabarria M, Lanza RG, Verkhratsky A. Enriched environment and physical activity reverse astrogliodegeneration in the hippocampus of AD transgenic mice. Cell Death Dis. 2013;4:e678. doi: 10.1038/cddis.2013.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rodriguez JJ, Noristani HN, Olabarria M, Fletcher J, Somerville TD, Yeh CY, Verkhratsky A. Voluntary running and environmental enrichment restores impaired hippocampal neurogenesis in a triple transgenic mouse model of Alzheimer's disease. Curr Alzheimer Res. 2011;8:707–717. doi: 10.2174/156720511797633214. [DOI] [PubMed] [Google Scholar]
- 78.Singhal G, Morgan J, Corrigan F, Toben C, Jawahar MC, Jaehne EJ, Manavis J, Hannan AJ, Baune BT. Short-Term Environmental enrichment is a stronger modulator of brain glial cells and cervical lymph node T cell subtypes than exercise or combined exercise and enrichment. Cell Mol Neurobiol. 2021;41:469–486. doi: 10.1007/s10571-020-00862-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ali S, Liu X, Queen NJ, Patel RS, Wilkins RK, Mo X, Cao L. Long-term environmental enrichment affects microglial morphology in middle age mice. Aging (Albany NY) 2019;11:2388–2402. doi: 10.18632/aging.101923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Williamson LL, Chao A, Bilbo SD. Environmental enrichment alters glial antigen expression and neuroimmune function in the adult rat hippocampus. Brain Behav Immun. 2012;26:500–510. doi: 10.1016/j.bbi.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu H, Gelyana E, Rajsombath M, Yang T, Li S, Selkoe D. Environmental enrichment potently prevents microglia-mediated neuroinflammation by human amyloid beta-protein oligomers. J Neurosci. 2016;36:9041–9056. doi: 10.1523/JNEUROSCI.1023-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xu H, Rajsombath MM, Weikop P, Selkoe DJ. Enriched environment enhances β-adrenergic signaling to prevent microglia inflammation by amyloid-β. EMBO Mol Med. 2018;10:e8931. doi: 10.15252/emmm.201808931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stuart KE, King AE, King NE, Collins JM, Vickers JC, Ziebell JM. Late-life environmental enrichment preserves short-term memory and may attenuate microglia in male APP/PS1 mice. Neuroscience. 2019;408:282–292. doi: 10.1016/j.neuroscience.2019.04.015. [DOI] [PubMed] [Google Scholar]
- 84.Ziegler-Waldkirch S, d'Errico P, Sauer J-F, Erny D, Savanthrapadian S, Loreth D, Katzmarski N, Blank T, Bartos M, Prinz M, et al. Seed-induced Aβ deposition is modulated by microglia under environmental enrichment in a mouse model of Alzheimer's disease. EMBO J. 2018;37:167–182. doi: 10.15252/embj.201797021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Koellhoffer EC, McCullough LD, Ritzel RM. Old maids: aging and its impact on microglia function. Int J Mol Sci. 2017;18:769. doi: 10.3390/ijms18040769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gomes GF, Peixoto R, Maciel BG, Santos KFD, Bayma LR, Feitoza Neto PA, Fernandes TN, de Abreu CC, Casseb SMM, de Lima CM, et al. Differential microglial morphological response, TNFalpha, and viral load in sedentary-like and active murine models after systemic non-neurotropic dengue virus infection. J Histochem Cytochem. 2019;67:419–439. doi: 10.1369/0022155419835218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carvalho-Paulo D, Bento Torres Neto J, Filho CS, de Oliveira TCG, de Sousa AA, dos Reis RR, dos Santos ZA, de Lima CM, de Oliveira MA, Said NM et al. Microglial morphology across distantly related species: phylogenetic, environmental and age influences on microglia reactivity and surveillance states. Front Immunol. 2021;12. [DOI] [PMC free article] [PubMed]
- 88.de Oliveira TCG, Carvalho-Paulo D, de Lima CM, de Oliveira RB, Santos Filho C, Diniz DG, Bento Torres Neto J, Picanço-Diniz CW. Long-term environmental enrichment reduces microglia morphological diversity of the molecular layer of dentate gyrus. Eur J Neurosc. 2020. [DOI] [PubMed]
- 89.de Sousa AA, Reis R, Bento-Torres J, Trévia N, Lins NAdA, Passos A, Santos Z, Diniz JAP, Vasconcelos PFdC, Cunningham C et al. Influence of enriched environment on viral encephalitis outcomes: behavioral and neuropathological changes in albino swiss mice. PLOS ONE. 2011;6:e15597. [DOI] [PMC free article] [PubMed]
- 90.Freitas P, Lima AVL, Carvalho KGB, Cabral TDS, Farias AM, Rodrigues APD, Diniz DG, Picanco Diniz CW, Diniz Junior JAP. Limbic encephalitis brain damage induced by cocal virus in adult mice is reduced by environmental enrichment: neuropathological and behavioral studies. Viruses. 2020;13. [DOI] [PMC free article] [PubMed]
- 91.Piazza FV, Segabinazi E, Centenaro LA, do Nascimento PS, Achaval M, Marcuzzo S. Enriched environment induces beneficial effects on memory deficits and microglial activation in the hippocampus of type 1 diabetic rats. Metabolic Brain Dis. 2014;29:93–104. [DOI] [PubMed]
- 92.Garofalo S, Porzia A, Mainiero F, Di Angelantonio S, Cortese B, Basilico B, Pagani F, Cignitti G, Chece G, Maggio R et al. Environmental stimuli shape microglial plasticity in glioma. Elife. 2017: 6 [DOI] [PMC free article] [PubMed]
- 93.Rakesh G, Szabo ST, Alexopoulos GS, Zannas AS. Strategies for dementia prevention: latest evidence and implications. Ther Adv Chronic Dis. 2017;8:121–136. doi: 10.1177/2040622317712442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Baranowski BJ, Marko DM, Fenech RK, Yang AJT, MacPherson REK. Healthy brain, healthy life: a review of diet and exercise interventions to promote brain health and reduce Alzheimer's disease risk. Appl Physiol Nutr Metab. 2020;45:1055–1065. doi: 10.1139/apnm-2019-0910. [DOI] [PubMed] [Google Scholar]
- 95.Nyaradi A, Oddy WH, Hickling S, Li J, Foster JK. The relationship between nutrition in infancy and cognitive performance during adolescence. Front Nutr. 2015;2:2. doi: 10.3389/fnut.2015.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Isaacs EB, Morley R, Lucas A. Early diet and general cognitive outcome at adolescence in children born at or below 30 weeks gestation. J Pediatr. 2009;155:229–234. doi: 10.1016/j.jpeds.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 97.McEvoy CT, Hoang T, Sidney S, Steffen LM, Jacobs DR, Jr, Shikany JM, Wilkins JT, Yaffe K. Dietary patterns during adulthood and cognitive performance in midlife: the CARDIA study. Neurology. 2019;92:e1589–e1599. doi: 10.1212/WNL.0000000000007243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Morris MC, Tangney CC, Wang Y, Sacks FM, Barnes LL, Bennett DA, Aggarwal NT. MIND diet slows cognitive decline with aging. Alzheimers Dement. 2015;11:1015–1022. doi: 10.1016/j.jalz.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pani G. Neuroprotective effects of dietary restriction: Evidence and mechanisms. Semin Cell Dev Biol. 2015;40:106–114. doi: 10.1016/j.semcdb.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 100.Martin AA, Davidson TL. Human cognitive function and the obesogenic environment. Physiol Behav. 2014;136:185–193. doi: 10.1016/j.physbeh.2014.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Spyridaki EC, Avgoustinaki PD, Margioris AN. Obesity, inflammation and cognition. Cur Opin Behav Sci. 2016;9:169–175. doi: 10.1016/j.cobeha.2016.05.004. [DOI] [Google Scholar]
- 102.Bhat ZF, Morton JD, Mason S, Bekhit AEA, Bhat HF. Obesity and neurological disorders: dietary perspective of a global menace. Crit Rev Food Sci Nutr. 2019;59:1294–1310. doi: 10.1080/10408398.2017.1404442. [DOI] [PubMed] [Google Scholar]
- 103.Hu S, Wang L, Yang D, Li L, Togo J, Wu Y, Liu Q, Li B, Li M, Wang G, et al. Dietary fat, but not protein or carbohydrate, regulates energy intake and causes adiposity in mice. Cell Metab. 2018;28:415–431e414. doi: 10.1016/j.cmet.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 104.Raji CA, Ho AJ, Parikshak NN, Becker JT, Lopez OL, Kuller LH, Hua X, Leow AD, Toga AW, Thompson PM. Brain structure and obesity. Hum Brain Mapp. 2010;31:353–364. doi: 10.1002/hbm.20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bocarsly ME, Fasolino M, Kane GA, LaMarca EA, Kirschen GW, Karatsoreos IN, McEwen BS, Gould E. Obesity diminishes synaptic markers, alters microglial morphology, and impairs cognitive function. Proc Natl Acad Sci USA. 2015;112:15731–15736. doi: 10.1073/pnas.1511593112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cope EC, LaMarca EA, Monari PK, Olson LB, Martinez S, Zych AD, Katchur NJ, Gould E. Microglia play an active role in obesity-associated cognitive decline. J Neurosci. 2018;38:8889–8904. doi: 10.1523/JNEUROSCI.0789-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Miller AA, Spencer SJ. Obesity and neuroinflammation: a pathway to cognitive impairment. Brain Behav Immun. 2014;42:10–21. doi: 10.1016/j.bbi.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 108.Garcia-Caceres C, Balland E, Prevot V, Luquet S, Woods SC, Koch M, Horvath TL, Yi CX, Chowen JA, Verkhratsky A, et al. Role of astrocytes, microglia, and tanycytes in brain control of systemic metabolism. Nat Neurosci. 2019;22:7–14. doi: 10.1038/s41593-018-0286-y. [DOI] [PubMed] [Google Scholar]
- 109.Hornedo-Ortega R, de Pablos RM, Cerezo AB, Richard T, Garcia-Parrilla MC, Troncoso AM, Microglia-mediated neuroinflammation and mediterranean diet. In: Preedy VR, Watson RR, editors The mediterranean diet. Elsevier: Amsterdam. 2020 [DOI] [PMC free article] [PubMed]
- 110.Val-Laillet D, Kanzari A, Guerin S, Randuineau G, Coquery N. A maternal Western diet during gestation and lactation modifies offspring's microglial cell density and morphology in the hippocampus and prefrontal cortex in Yucatan minipigs. Neurosci Lett. 2020;739:135395. doi: 10.1016/j.neulet.2020.135395. [DOI] [PubMed] [Google Scholar]
- 111.Baufeld C, Osterloh A, Prokop S, Miller KR, Heppner FL. High-fat diet-induced brain region-specific phenotypic spectrum of CNS resident microglia. Acta Neuropathol. 2016;132:361–375. doi: 10.1007/s00401-016-1595-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vinuesa A, Bentivegna M, Calfa G, Filipello F, Pomilio C, Bonaventura MM, Lux-Lantos V, Matzkin ME, Gregosa A, Presa J, et al. Early exposure to a high-fat diet impacts on hippocampal plasticity: implication of microglia-derived exosome-like extracellular vesicles. Mol Neurobiol. 2019;56:5075–5094. doi: 10.1007/s12035-018-1435-8. [DOI] [PubMed] [Google Scholar]
- 113.Graham LC, Harder JM, Soto I, de Vries WN, John SW, Howell GR. Chronic consumption of a western diet induces robust glial activation in aging mice and in a mouse model of Alzheimer's disease. Sci Rep. 2016;6:21568. doi: 10.1038/srep21568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Spencer SJ, D'Angelo H, Soch A, Watkins LR, Maier SF, Barrientos RM. High-fat diet and aging interact to produce neuroinflammation and impair hippocampal- and amygdalar-dependent memory. Neurobiol Aging. 2017;58:88–101. doi: 10.1016/j.neurobiolaging.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Spencer SJ, Basri B, Sominsky L, Soch A, Ayala MT, Reineck P, Gibson BC, Barrientos RM. High-fat diet worsens the impact of aging on microglial function and morphology in a region-specific manner. Neurobiol Aging. 2019;74:121–134. doi: 10.1016/j.neurobiolaging.2018.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Butler MJ, Cole RM, Deems NP, Belury MA, Barrientos RM. Fatty food, fatty acids, and microglial priming in the adult and aged hippocampus and amygdala. Brain Behav Immun. 2020;89:145–158. doi: 10.1016/j.bbi.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Johnson RW. Feeding the beast: can microglia in the senescent brain be regulated by diet? Brain Behav Immun. 2015;43:1–8. doi: 10.1016/j.bbi.2014.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pena-Altamira E, Petralla S, Massenzio F, Virgili M, Bolognesi ML, Monti B. Nutritional and pharmacological strategies to regulate microglial polarization in cognitive aging and alzheimer's disease. Front Aging Neurosci. 2017;9:175. doi: 10.3389/fnagi.2017.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Witte AV, Fobker M, Gellner R, Knecht S, Floel A. Caloric restriction improves memory in elderly humans. Proc Natl Acad Sci USA. 2009;106:1255–1260. doi: 10.1073/pnas.0808587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Leclerc E, Trevizol AP, Grigolon RB, Subramaniapillai M, McIntyre RS, Brietzke E, Mansur RB. The effect of caloric restriction on working memory in healthy non-obese adults. CNS Spectr. 2020;25:2–8. doi: 10.1017/S1092852918001566. [DOI] [PubMed] [Google Scholar]
- 121.Fontana L, Ghezzi L, Cross AH, Piccio L. Effects of dietary restriction on neuroinflammation in neurodegenerative diseases. J Exp Med.2021;218. [DOI] [PMC free article] [PubMed]
- 122.Bok E, Jo M, Lee S, Lee BR, Kim J, Kim HJ. Dietary Restriction and Neuroinflammation: A Potential Mechanistic Link. Int J Mol Sci. 2019;20. [DOI] [PMC free article] [PubMed]
- 123.Yin Z, Raj DD, Schaafsma W, van der Heijden RA, Kooistra SM, Reijne AC, Zhang X, Moser J, Brouwer N, Heeringa P, et al. Low-fat diet with caloric restriction reduces white matter microglia activation during aging. Front Mol Neurosci. 2018;11:65. doi: 10.3389/fnmol.2018.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Olmedillas Del Moral M, Frohlich N, Figarella K, Mojtahedi N, Garaschuk O. Effect of caloric restriction on the in vivo functional properties of aging microglia. Front Immunol. 2020;11:750. doi: 10.3389/fimmu.2020.00750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Letenneur L, Proust-Lima C, Le Gouge A, Dartigues JF, Barberger-Gateau P. Flavonoid intake and cognitive decline over a 10-year period. Am J Epidemiol. 2007;165:1364–1371. doi: 10.1093/aje/kwm036. [DOI] [PubMed] [Google Scholar]
- 126.Valls-Pedret C, Lamuela-Raventos RM, Medina-Remon A, Quintana M, Corella D, Pinto X, Martinez-Gonzalez MA, Estruch R, Ros E. Polyphenol-rich foods in the Mediterranean diet are associated with better cognitive function in elderly subjects at high cardiovascular risk. J Alzheimers Dis. 2012;29:773–782. doi: 10.3233/JAD-2012-111799. [DOI] [PubMed] [Google Scholar]
- 127.Rangarajan P, Karthikeyan A, Dheen ST. Role of dietary phenols in mitigating microglia-mediated neuroinflammation. Neuromolecular Med. 2016;18:453–464. doi: 10.1007/s12017-016-8430-x. [DOI] [PubMed] [Google Scholar]
- 128.Hornedo-Ortega R, Cerezo AB, de Pablos RM, Krisa S, Richard T, Garcia-Parrilla MC, Troncoso AM. Phenolic compounds characteristic of the mediterranean diet in mitigating microglia-mediated neuroinflammation. Front Cell Neurosci. 2018;12:373. doi: 10.3389/fncel.2018.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jang S, Johnson RW. Can consuming flavonoids restore old microglia to their youthful state? Nutr Rev. 2010;68:719–728. doi: 10.1111/j.1753-4887.2010.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Flanagan E, Muller M, Hornberger M, Vauzour D. Impact of flavonoids on cellular and molecular mechanisms underlying age-related cognitive decline and neurodegeneration. Curr Nutr Rep. 2018;7:49–57. doi: 10.1007/s13668-018-0226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jang S, Dilger RN, Johnson RW. Luteolin inhibits microglia and alters hippocampal-dependent spatial working memory in aged mice. J Nutr. 2010;140:1892–1898. doi: 10.3945/jn.110.123273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dirscherl K, Karlstetter M, Ebert S, Kraus D, Hlawatsch J, Walczak Y, Moehle C, Fuchshofer R, Langmann T. Luteolin triggers global changes in the microglial transcriptome leading to a unique anti-inflammatory and neuroprotective phenotype. J Neuroinflammation. 2010;7:3. doi: 10.1186/1742-2094-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Carey AN, Miller MG, Fisher DR, Bielinski DF, Gilman CK, Poulose SM, Shukitt-Hale B. Dietary supplementation with the polyphenol-rich acai pulps (Euterpe oleracea Mart and Euterpe precatoria Mart) improves cognition in aged rats and attenuates inflammatory signaling in BV-2 microglial cells. Nutr Neurosci. 2017;20:238–245. [DOI] [PubMed]
- 134.Youdim KA, Shukitt-Hale B, Martin A, Wang H, Denisova NA, Bickford PC, Joseph JA. Short-term dietary supplementation of blueberry polyphenolics: beneficial effects on aging brain performance and peripheral tissue function. Nutr Neurosci. 2000;3:383–397. doi: 10.1080/1028415X.2000.11747338. [DOI] [Google Scholar]
- 135.Goyarzu P, Malin DH, Lau FC, Taglialatela G, Moon WD, Jennings R, Moy E, Moy D, Lippold S, Shukitt-Hale B, et al. Blueberry supplemented diet: effects on object recognition memory and nuclear factor-kappa B levels in aged rats. Nutr Neurosci. 2004;7:75–83. doi: 10.1080/10284150410001710410. [DOI] [PubMed] [Google Scholar]
- 136.Joseph JA, Shukitt-Hale B, Denisova NA, Bielinski D, Martin A, McEwen JJ, Bickford PC. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J Neurosci. 1999;19:8114–8121. doi: 10.1523/JNEUROSCI.19-18-08114.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Willis LM, Freeman L, Bickford PC, Quintero EM, Umphlet CD, Moore AB, Goetzl L, Granholm AC. Blueberry supplementation attenuates microglial activation in hippocampal intraocular grafts to aged hosts. Glia. 2010;58:679–690. doi: 10.1002/glia.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lau FC, Bielinski DF, Joseph JA. Inhibitory effects of blueberry extract on the production of inflammatory mediators in lipopolysaccharide-activated BV2 microglia. J Neurosci Res. 2007;85:1010–1017. doi: 10.1002/jnr.21205. [DOI] [PubMed] [Google Scholar]
- 139.Carey AN, Gildawie KR, Rovnak A, Thangthaeng N, Fisher DR, Shukitt-Hale B. Blueberry supplementation attenuates microglia activation and increases neuroplasticity in mice consuming a high-fat diet. Nutr Neurosci. 2019;22:253–263. doi: 10.1080/1028415X.2017.1376472. [DOI] [PubMed] [Google Scholar]
- 140.Wang J, Yue B, Zhang X, Guo X, Sun Z, Niu R. Effect of exercise on microglial activation and transcriptome of hippocampus in fluorosis mice. Sci Total Environ. 2021;760:143376. doi: 10.1016/j.scitotenv.2020.143376. [DOI] [PubMed] [Google Scholar]
- 141.Giorgetti E, Panesar M, Zhang Y, Joller S, Ronco M, Obrecht M, Lambert C, Accart N, Beckmann N, Doelemeyer A, et al. Modulation of microglia by voluntary exercise or CSF1R inhibition prevents age-related loss of functional motor units. Cell Rep. 2019;29:1539–1554 e1537. doi: 10.1016/j.celrep.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 142.Lu Y, Dong Y, Tucker D, Wang R, Ahmed ME, Brann D, Zhang Q. Treadmill exercise exerts neuroprotection and regulates microglial polarization and oxidative stress in a streptozotocin-induced rat model of sporadic Alzheimer's disease. J Alzheimers Dis. 2017;56:1469–1484. doi: 10.3233/JAD-160869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chabry J, Nicolas S, Cazareth J, Murris E, Guyon A, Glaichenhaus N, Heurteaux C, Petit-Paitel A. Enriched environment decreases microglia and brain macrophages inflammatory phenotypes through adiponectin-dependent mechanisms: Relevance to depressive-like behavior. Brain Behav Immun. 2015;50:275–287. doi: 10.1016/j.bbi.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 144.Ehninger D, Wang LP, Klempin F, Romer B, Kettenmann H, Kempermann G. Enriched environment and physical activity reduce microglia and influence the fate of NG2 cells in the amygdala of adult mice. Cell Tissue Res. 2011;345:69–86. doi: 10.1007/s00441-011-1200-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gomes GF, Peixoto R, Maciel BG, Santos KFD, Bayma LR, Feitoza Neto PA, Fernandes TN, de Abreu CC, Casseb SMM, de Lima CM, et al. Differential microglial morphological response, TNFα, and viral load in sedentary-like and active murine models after systemic non-neurotropic dengue virus infection. J Histochem Cytochem. 2019;67:419–439. doi: 10.1369/0022155419835218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Brown SM, Bush SJ, Summers KM, Hume DA, Lawrence AB. Environmentally enriched pigs have transcriptional profiles consistent with neuroprotective effects and reduced microglial activity. Behav Brain Res. 2018;350:6–15. doi: 10.1016/j.bbr.2018.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Valdearcos M, Robblee MM, Benjamin DI, Nomura DK, Xu AW, Koliwad SK. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep. 2014;9:2124–2138. doi: 10.1016/j.celrep.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bosch-Queralt M, Cantuti-Castelvetri L, Damkou A, Schifferer M, Schlepckow K, Alexopoulos I, Lutjohann D, Klose C, Vaculciakova L, Masuda T, et al. Diet-dependent regulation of TGF. Nat Metab. 2021;3:211–27. [DOI] [PMC free article] [PubMed]
- 149.Gutierrez-Martos M, Girard B, Mendonca-Netto S, Perroy J, Valjent E, Maldonado R, Martin M. Cafeteria diet induces neuroplastic modifications in the nucleus accumbens mediated by microglia activation. Addict Biol. 2018;23:735–749. doi: 10.1111/adb.12541. [DOI] [PubMed] [Google Scholar]
- 150.Milanova IV, Kalsbeek MJT, Wang XL, Korpel NL, Stenvers DJ, Wolff SEC, de Goede P, Heijboer AC, Fliers E, la Fleur SE, et al. Diet-induced obesity disturbs microglial immunometabolism in a time-of-day manner. Front Endocrinol (Lausanne) 2019;10:424. doi: 10.3389/fendo.2019.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.