Abstract
Food ladders are clinical tools already widely used in Europe for food reintroduction in milk- and egg-allergic children. Previously developed milk and egg ladders have limited applicability to Canadian children due to dietary differences and product availability. Herein we propose a Canadian version of cow’s milk and egg food ladders and discuss the potential role that food ladders may have in the care of children with IgE-mediated allergies to cow’s milk and/or egg, as either a method of accelerating the acquisition of tolerance in those who would outgrow on their own, or as a form of modified oral immunotherapy in those with otherwise persistent allergy.
Keywords: Food ladders, Food allergy, Cow’s milk allergy, Egg allergy, Oral immunotherapy
To the editor,
Cow’s milk and egg are among the most common food allergies in young children. IgE-mediated milk and egg allergies are not only significant causes of food-induced anaphylaxis in children but are associated with numerous other adverse medical and psychosocial outcomes including nutritional and growth concerns and impaired quality of life for both children and their caregivers [1–4]. Although milk and egg allergies have historically been regarded to have a good prognosis, with many children outgrowing their food allergy in childhood, recent studies suggest that the rate of resolution may be slowing over time, with only 50% resolution by 5–6 years of age and increasing persistence of these allergies into adolescence or adulthood [5–7]. High baseline sIgE levels, such as those greater than 10kUA/L for egg and milk, may predict persistence of allergy [5–7]. Management of food allergies has historically been limited to avoidance with periodic reassessment. However, there is increasing recognition that children with egg and milk allergy may tolerate baked/processed forms of milk and egg, and that ongoing ingestion of these forms may help with resolution of their food allergy [8, 9]. Conformational changes in immune-activating epitopes that occur during the baking or heating processes alter the allergenicity of milk and egg and may allow for tolerance [10].
Milk and egg ladders (henceforth called food ladders) are tools designed to guide patients through a home-based gradual stepwise introduction of increasingly allergenic forms of milk and egg in a demedicalized setting. Originally designed in the United Kingdom for the management of non-IgE-mediated food allergies, food ladders extrapolate from previous evidence that the vast majority of egg- and milk-allergic children are able to tolerate extensively heated forms of these allergens, such as in baked goods [11–15]. Regular ingestion of tolerated forms of milk and egg may induce accelerated tolerance, allowing liberalization of the diet to more allergenic forms of the food over time. Food ladders are now widely used in Europe for this purpose and are included in the British Society for Allergy & Clinical Immunology’s guidelines for the management of egg allergy [16]. According to a 2017 survey of 114 healthcare professionals from around the globe, 68% of respondents reported that they utilized milk ladders [17]. In Canada, food ladders appear to be increasingly being adopted by allergists.
Despite increasing use, there is a paucity of published research on food ladders. Ball and Luyt studied the role of milk ladders in 86 milk-allergic children with a history of mild reactions to milk [18], and ultimately 91% of children in their study were able to tolerate the majority of dairy products within 4–6 months. While 43% experienced minor adverse reactions, there were no cases of anaphylaxis. To our knowledge, there are no studies published to date describing egg ladder use.
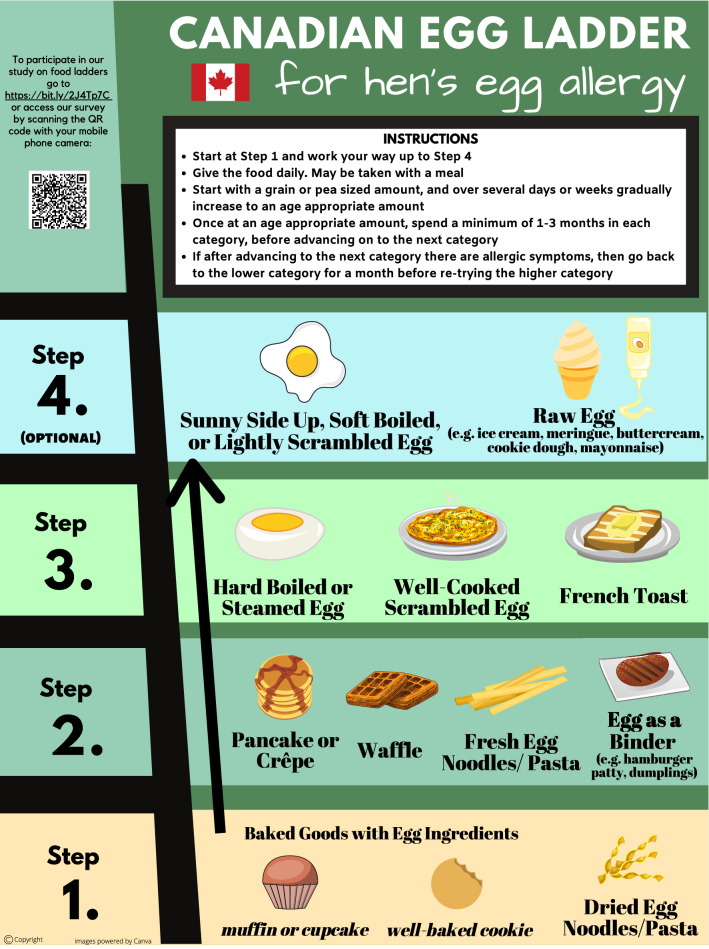
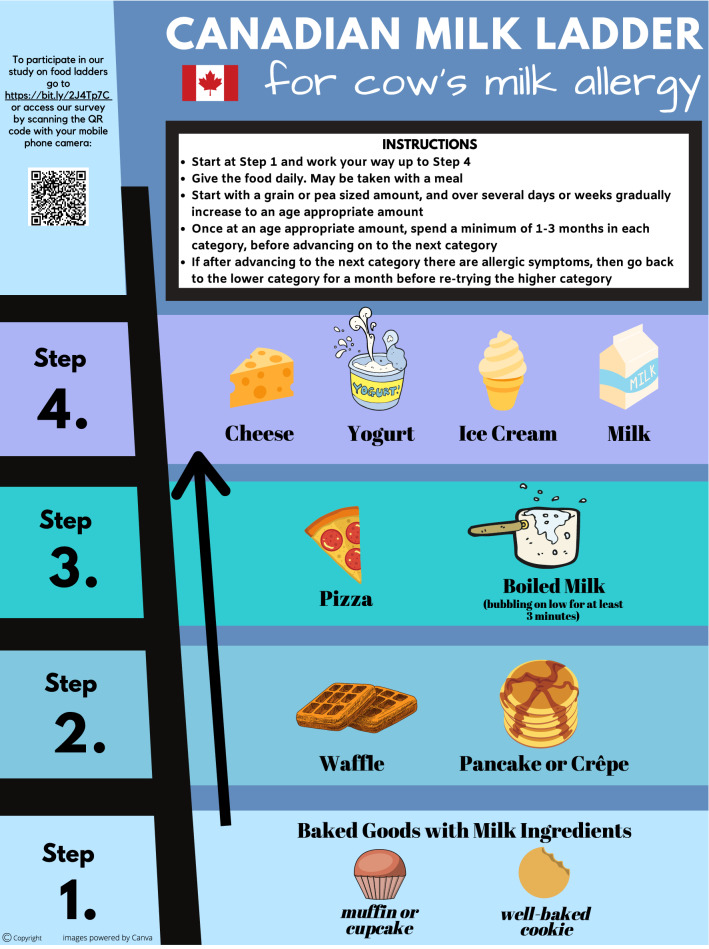
European versions of food ladders have limited applicability to the Canadian diet, as they include foods that may be seldomly consumed in many Canadian households. Hence, we developed the Canadian Food Ladders using foods more typically consumed by Canadian children [19, 20] (Figs. 1, 2).
Fig. 1.
Canadian egg ladder
Fig. 2.
Canadian milk ladder
There are four “Steps” in each ladder, with the least allergenic forms of milk or egg in Step 1, progressing to the most allergenic forms in Step 4. Children are typically introduced to their relevant allergen at Step 1, starting with a grain- or pea-sized amount of food. If tolerated, the child should consume the food on a daily basis. The serving size offered is gradually increased as tolerated over several days to weeks until an age-appropriate amount is reached. We advise that children continue to consume age-appropriate serving sizes of foods at that step on a daily basis for at least 1–3 months before advancing to the next step in the ladder. If IgE-mediated allergy symptoms occur with the introduction of a new food on the ladder, the child should return to consuming previously tolerated foods for at least 1 month before again cautiously attempting to advance on the ladder. Parents should be counselled by the allergist overseeing their child’s food ladder use on how to recognize and manage allergic reactions. If a child is confirmed to be fully tolerant to foods on a higher step of the ladder, they need not start at Step 1; rather, they may start at the step corresponding to foods currently tolerated. Caregivers are advised that children can progress as slowly through the food ladder as tolerated and desired, as even consuming baked goods regularly (Step 1) has been shown to promote tolerance [8, 9].
The Canadian Food Ladders are intended for use in preschool-aged children with a history of only mild IgE-mediated reactions to milk and/or egg. The use of food ladders is likely safest in preschool-aged children based on safety data extrapolated from studies on oral immunotherapy revealing higher rates of anaphylaxis in older children compared to preschoolers [21, 22]. Contraindications to the use of food ladders include a previous life-threatening episode of anaphylaxis or asthma that remains inadequately controlled on medium dose inhaled steroid therapy. Relative contraindications include both medical and socioeconomic factors, such as a recent severe asthma exacerbation, a language barrier or cognitive impairment. Food ladders should be initiated at the recommendation of an allergist, and patients using a food ladder should receive regular follow up (we suggest at least every 6 months).
Data in regard to efficacy of oral immunotherapy for food allergies has been promising, with excellent safety and effectiveness in the preschool aged group [21, 23]. We propose that food ladders be considered a modified form of oral immunotherapy for preschoolers with very high baseline sIgE levels or older children, representing phenotypes that would be unlikely to outgrow their allergy via strict avoidance. Similar to oral immunotherapy, food ladders consist of the regular administration of small doses of food allergen and likely lead to similar immune changes that assist in establishing tolerance. In addition, food ladders have the added benefit of allowing children to gradually expand their diet, whether by promoting tolerance or following the natural progression of resolution of their food allergy in a home setting, while potentially using fewer healthcare resources than other models of oral immunotherapy delivery (especially due to the lack of need for oral food challenges or multiple visits for conventional oral immunotherapy with the unheated food). Similar to other models of oral immunotherapy, food ladders have the potential to alleviate food-allergy related anxiety and improve quality of life for families and their children with milk and egg allergy.
However, while food ladders are a promising tool for facilitating dietary expansion for children with milk or egg allergies, further research is needed to improve confidence with their use. Further safety and efficacy data are needed, particularly for the egg ladder where this data is mainly extrapolated from baked egg ingestion and oral immunotherapy studies. Additionally, with further study, this concept may ultimately prove safe and appropriate for older children and adults. And although we propose that food ladders be considered a modified form of oral immunotherapy, long term data is needed to establish whether their use truly increases reaction thresholds and protects against potential accidental exposures. Finally, qualitative data from patients and evaluation of the impact of food ladder use on quality of life and food allergy-related anxiety is also needed. As such, our group is currently examining the experience of Canadian allergists and their patient families with the Canadian Food Ladders.
Conclusion
Food ladders offer a flexible and proactive approach to management of lower risk egg- or milk-allergic children. They have the potential to facilitate gradual dietary expansion and accelerate the resolution of allergy. For children with persistent allergy beyond the preschool age, we propose that food ladders be considered a modified form of oral immunotherapy. While food ladders are not appropriate for use in all children with egg and milk allergies, they are a promising tool with evidence supporting efficacy and safety extrapolated from studies on oral immunotherapy as well as from limited studies of milk ladder use.
Acknowledgements
We would like to acknowledge the following allergists for their contribution to the development of the Canadian Food Ladders: Drs Scott Cameron, Stuart Carr, Victoria Cook, Stephanie Erdle, Tom Gerstner, Kyla Hildebrand, Sandy Kapur, Sara Leo, Per Lidman, Mary McHenry, Gregory Rex. We would also like to acknowledge Dr Jonathan Hourihane for inspiring our group’s early discussions regarding implementing food ladders in our practices in Canada.
Authors' contributions
AC was a major contributor in writing the manuscript and contributed to conceptual development. ESC, JY, TV, LS, EA, RM and TW were main developers of the concept of the Canadian Food Ladders and determining the final content and recommended use. BW contributed intellectually with the ladder development and designed the ladders. In addition to her role above, TW played a major role in developing the manuscript. All authors read and approved the final manuscript.
Funding
No funding was received for the development of the Canadian Food Ladders or for the preparation of this manuscript.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
ESC has received research support from DBV Technologies; has been a member of advisory boards for Pfizer, Pediapharm, Leo Pharma, Kaleo, DBV, AllerGenis, Sanofi Genzyme, Bausch Health, Avir Pharma; is a member of the healthcare advisory board for Food Allergy Canada; and was co-lead of the CSACI oral immunotherapy guidelines. JY received speaking honoraria from UBC CPD, Sanofi, AstraZeneca, Pfizer, Stallergenes Greer, Novartis, Pediapharm, Medexus Pharma. She has served on advisory/consultancy committees for Sanofi, Pfizer, HealthLinkBc, Stallergenes Greer, and LEO Pharma. TV has served on advisory boards and received honoraria from Aralez, Bausch Health, and Pfizer. EA served on the Healthcare advisory board, and Food Allergy Canada. She received moderator/speaker fees from Novartis, GSK, and AstraZeneca. RM was a moderator/speaker for Astra Zeneca, Pediapharm, and Novartis. He has been on advisory boards for Sanofi, and ALK.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Alanna Chomyn, Email: alanna.chomyn@alumni.ubc.ca.
Tiffany Wong, Email: Tiffany.Wong@cw.bc.ca.
References
- 1.Grabenhenrich LB, Dölle S, Moneret-Vautrin A, Köhli A, Lange L, Spindler T, et al. Anaphylaxis in children and adolescents: the European Anaphylaxis Registry. J Allergy Clin Immunol. 2016;137(4):1128–1137.e1. doi: 10.1016/j.jaci.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 2.Sinai T, Goldberg MR, Nachshon L, Amitzur-Levy R, Yichie T, Katz Y, et al. Reduced final height and inadequate nutritional intake in cow's milk-allergic young adults. J Allergy Clin Immunol Pract. 2019;7(2):509–515. doi: 10.1016/j.jaip.2018.11.038. [DOI] [PubMed] [Google Scholar]
- 3.Protudjer JL, Jansson SA, Östblom E, Arnlind MH, Bengtsson U, Dahlén SE, et al. Health-related quality of life in children with objectively diagnosed staple food allergy assessed with a disease-specific questionnaire. Acta Paediatr. 2015;104(10):1047–54. doi: 10.1111/apa.13044. [DOI] [PubMed] [Google Scholar]
- 4.Howe L, Franxman T, Teich E, Greenhawt M. What affects quality of life among caregivers of food-allergic children? Ann Allergy Asthma Immunol. 2014;113(1):69–74.e2. doi: 10.1016/j.anai.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 5.Wood RA. The natural history of food allergy. Pediatrics. 2003;111(6 Pt 3):1631–1637. [PubMed] [Google Scholar]
- 6.Wood RA, Sicherer SH, Vickery BP, Jones SM, Liu AH, Fleischer DM, et al. The natural history of milk allergy in an observational cohort. J Allergy Clin Immunol. 2013;131(3):805–812. doi: 10.1016/j.jaci.2012.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sicherer SH, Wood RA, Vickery BP, Jones SM, Liu AH, Fleischer DM, et al. The natural history of egg allergy in an observational cohort. J Allergy Clin Immunol. 2014;133(2):492–499. doi: 10.1016/j.jaci.2013.12.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim JS, Nowak-Węgrzyn A, Sicherer SH, Noone S, Moshier EL, Sampson HA. Dietary baked bilk accelerates the resolution of cow’s milk allergy in children. J Allergy Clin Immunol. 2011;128(1):125–131.e2. doi: 10.1016/j.jaci.2011.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peters RL, Dharmage SC, Gurrin LC, Koplin JJ, Ponsonby AL, Lowe AJ, Tang ML, Tey D, Robinson M, Hill D, Czech H, Thiele L, Osborne NJ, Allen KJ. HealthNuts study. The natural history and clinical predictors of egg allergy in the first 2 years of life: a prospective, population-based cohort study. J Allergy Clin Immunol. 2014;133(2):485–91. doi: 10.1016/j.jaci.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 10.Nowak-Wegrzyn A, Fiocchi A. Rare, medium, or well done? The effect of heating and food matrix on food protein allergenicity. Curr Opin Allergy Clin Immunol. 2009;9(3):234–237. doi: 10.1097/ACI.0b013e32832b88e7. [DOI] [PubMed] [Google Scholar]
- 11.Urisu A, Ando H, Morita Y, Wada E, Yasaki T, Yamada K, Komada K, Torii S, Goto M, Wakamatsu T. Allergenic activity of heated and ovomucoid-depleted egg white. J Allergy Clin Immunol. 1997;100(2):171–176. doi: 10.1016/S0091-6749(97)70220-3. [DOI] [PubMed] [Google Scholar]
- 12.Eigenmann PA. Anaphylactic reactions to raw eggs after negative challenges with cooked eggs. J Allergy Clin Immunol. 2000;105(3):587–588. doi: 10.1067/mai.2000.104255. [DOI] [PubMed] [Google Scholar]
- 13.Nowak-Wegrzyn A, Bloom KA, Sicherer SH, Shreffler WG, Noone S, Wanich N, Sampson HA. Tolerance to extensively heated milk in children with cow’s milk allergy. J Allergy Clin Immunol. 2008;122(2):342–347. doi: 10.1016/j.jaci.2008.05.043. [DOI] [PubMed] [Google Scholar]
- 14.Kim JS, Nowak-Wegrzyn A, Sicherer SH, Noone S, Moshier EL, Sampson HA. Dietary baked milk accelerates the resolution of cow's milk allergy in children. J Allergy Clin Immunol. 2011;128:125–131. doi: 10.1016/j.jaci.2011.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lambert R, Grimshaw KEC, Ellis B, Jaitly J, Roberts G. Evidence that eating baked egg or milk influences egg or milk allergy resolution: a systematic review. Clin Exp Allergy. 2017;47:829–837. doi: 10.1111/cea.12940. [DOI] [PubMed] [Google Scholar]
- 16.Clark AT, Skypala I, Leech SC, Eqan PW, Dugué P, Brathwaite N, et al. British Society for Allergy and Clinical Immunology guidelines for the management of egg allergy. Clin Exp Allergy. 2010;40:1116–1129. doi: 10.1111/j.1365-2222.2010.03557.x. [DOI] [PubMed] [Google Scholar]
- 17.Athanasopoulou P, Deligianni E, Dean T, Dewey A, Venter C. Use of baked milk challenges and milk ladders in clinical practice: a worldwide survey of healthcare professionals. Clin Exp Allergy. 2017;47:430–434. doi: 10.1111/cea.12890. [DOI] [PubMed] [Google Scholar]
- 18.Ball HB, Luyt D. Home-based cow’s milk reintroduction using a mild ladder in children less than 3 years old with IgE-mediated cow’s milk allergy. Clin Exp Allergy. 2019;49:911–920. doi: 10.1111/cea.13366. [DOI] [PubMed] [Google Scholar]
- 19.Venter C, Brown T, Shah N, et al. Diagnosis and management of non-IgE-mediated cow’s milk allergy in infancy—a UK primary care practical guide. Clin Transl Allergy. 2013;3:23. doi: 10.1186/2045-7022-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Irish Food Allergy Network. Egg ladder. 2018. http://ifan.ie/wp-content/uploads/2018/02/Egg-ladder-2018-after-JOBH-JF-AB-RC.pdf. Accessed 11 Nov 2020.
- 21.Soller L, Abrams EM, Carr S, Kapur S, Rex GA, Leo S, et al. First real-world safety analysis of preschool peanut oral immunotherapy. J Allergy Clin Immunol Pract. 2019;7:2759–2767. doi: 10.1016/j.jaip.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 22.Chu DK, Wood RA, French S, Fiocchi A, Jordana M, Waserman S, et al. Oral immunotherapy for peanut allergy (PACE): a systematic review and meta-analysis of efficacy and safety. Lancet. 2019;393:2222–2232. doi: 10.1016/S0140-6736(19)30420-9. [DOI] [PubMed] [Google Scholar]
- 23.Soller L, Abrams EM, Carr S, Kapur S, Rex GA, Leo S, et al. First real-world effectiveness analysis of preschool peanut oral immunotherapy. J Allergy Clin Immunol Pract. 2020 doi: 10.1016/j.jaip.2020.10.045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.