Abstract
Background
Acute brain injuries are associated with high mortality rates and poor long-term functional outcomes. Measurement of cerebrospinal fluid (CSF) biomarkers in patients with acute brain injuries may help elucidate some of the pathophysiological pathways involved in the prognosis of these patients.
Methods
We performed a systematic search and descriptive review using the MEDLINE database and the PubMed interface from inception up to June 29, 2021, to retrieve observational studies in which the relationship between CSF concentrations of protein biomarkers and neurological outcomes was reported in patients with acute brain injury [traumatic brain injury, subarachnoid hemorrhage, acute ischemic stroke, status epilepticus or post-cardiac arrest]. We classified the studies according to whether or not biomarker concentrations were associated with neurological outcomes. The methodological quality of the studies was evaluated using the Newcastle–Ottawa quality assessment scale.
Results
Of the 39 studies that met our criteria, 30 reported that the biomarker concentration was associated with neurological outcome and 9 reported no association. In TBI, increased extracellular concentrations of biomarkers related to neuronal cytoskeletal disruption, apoptosis and inflammation were associated with the severity of acute brain injury, early mortality and worse long-term functional outcome. Reduced concentrations of protein biomarkers related to impaired redox function were associated with increased risk of neurological deficit. In non-traumatic acute brain injury, concentrations of CSF protein biomarkers related to dysregulated inflammation and apoptosis were associated with a greater risk of vasospasm and a larger volume of brain ischemia. There was a high risk of bias across the studies.
Conclusion
In patients with acute brain injury, altered CSF concentrations of protein biomarkers related to cytoskeletal damage, inflammation, apoptosis and oxidative stress may be predictive of worse neurological outcomes.
Keywords: Traumatic brain injury, Cerebrospinal fluid, Subarachnoid hemorrhage, Neurological outcomes
Introduction
Acute brain injuries are a group of neurological insults to the brain parenchyma and are associated with poor long-term functional outcomes and high mortality rates [1]. Primary brain injuries represent the initial insult to the brain and are usually considered non-reversible. Secondary brain injuries arise from insults to the brain parenchyma that occur after the initial injury (e.g., as a result of hypoxemia and/or hypotension) and increase the overall area of damaged brain tissue [2, 3]. After an acute brain injury, intrathecal expression of proteins related to brain inflammation, apoptosis and oxidative stress induces production and migration of chemotactic factors, which ultimately lead to blood–brain barrier (BBB) dysfunction, brain edema formation and intracranial hypertension [4]. This cellular response may render the brain more susceptible to secondary injuries in cases of decreased cerebral perfusion pressure and may increase the volume of non-viable tissue.
In humans, the cerebrospinal fluid (CSF) acts as a highly specific repository of cellular by-products, neurotransmitters and protein fragments as it is in close contact with the brain parenchyma and other products of neural origin [5]. Concentrations of protein biomarkers in the intrathecal space may therefore reflect the presence or severity of primary and/or secondary brain injuries. For example, in patients with traumatic brain injury (TBI), increased CSF concentrations of protein biomarkers from damaged neurons may serve as indicators of ongoing cellular damage [6], and, in patients with subarachnoid hemorrhage (SAH), higher concentrations of CSF protein biomarkers may be associated with increased risk of vasospasm and delayed cerebral ischemia [7]. CSF protein biomarkers may reflect the pathophysiological pathways involved in acute brain injuries that could be susceptible to interventions, and thus help in the development of therapies or to guide earlier intervention to improve long-term functional outcomes.
We therefore performed a systematic review to identify observational studies that have evaluated the relationship between CSF protein biomarkers in patients with acute brain injuries and neurological outcomes.
Materials and methods
Data sources
Following protocol submission to the Prospero International Prospective Register of Systematic Reviews (ID 114294), we conducted a systematic search of the literature using the MEDLINE database and the PubMed interface from inception until June 29, 2021, to identify all observational studies that evaluated CSF protein biomarkers (proteins were defined as those with at least 50 amino acids or a molecular weight greater than 4000 Da) in patients with severe acute brain injury (as a result of TBI, SAH, acute ischemic stroke, status epilepticus or post-cardiac arrest syndrome) and that reported any neurological outcome. We used the MeSH terms: (((((“Brain Injuries, Traumatic”[MeSH]) OR “Subarachnoid Hemorrhage”[MeSH]) OR “Stroke”[MeSH]) OR “Status Epilepticus”[MeSH]) OR “Post-Cardiac Arrest Syndrome”[MeSH]) AND “Biomarkers”[MeSH]. The search limits were clinical studies, human, adults 19 + (over 18 years of age) and articles written in English. We also searched the references of included articles for studies that had been missed in the initial search. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement [8].
Study selection and data abstraction
Three of the authors (AB, LARG and CDC) performed the literature search and selected the studies. We excluded studies on descriptive proteomics; those evaluating metabolites (e.g., lactate, lactate/pyruvate, glucose, glutamate, glycerol, etc.), hormones or cytokines/chemokines; those in patients with chronic degenerative or chronic traumatic injuries (e.g., multiple sclerosis, Alzheimer and Parkinson diseases, sport-related injuries, chronic traumatic encephalopathy); those in patients with autoimmune conditions (e.g., Guillain–Barré); pediatric studies; postmortem populations; studies with only physiological outcomes; and animal studies. Data abstraction regarding type of acute brain injury, source of CSF (ventricular or lumbar), number of included subjects, method used by the author to quantify the specific biomarker and neurological outcomes was performed by the same three reviewers (AB, LARG and CDC) in an independent blinded manner by completing predefined tables. Studies were classified according to whether or not the measured biomarker was associated with neurological outcome (as defined in the original study) and were grouped according to whether the brain injury was traumatic or non-traumatic. The methodological quality of the observational studies was evaluated using the Newcastle–Ottawa quality assessment scale [9]. Discrepancies in the assessment of methodologic quality and final classification of the selected studies were resolved by the involvement of a fourth author (CAS).
Results
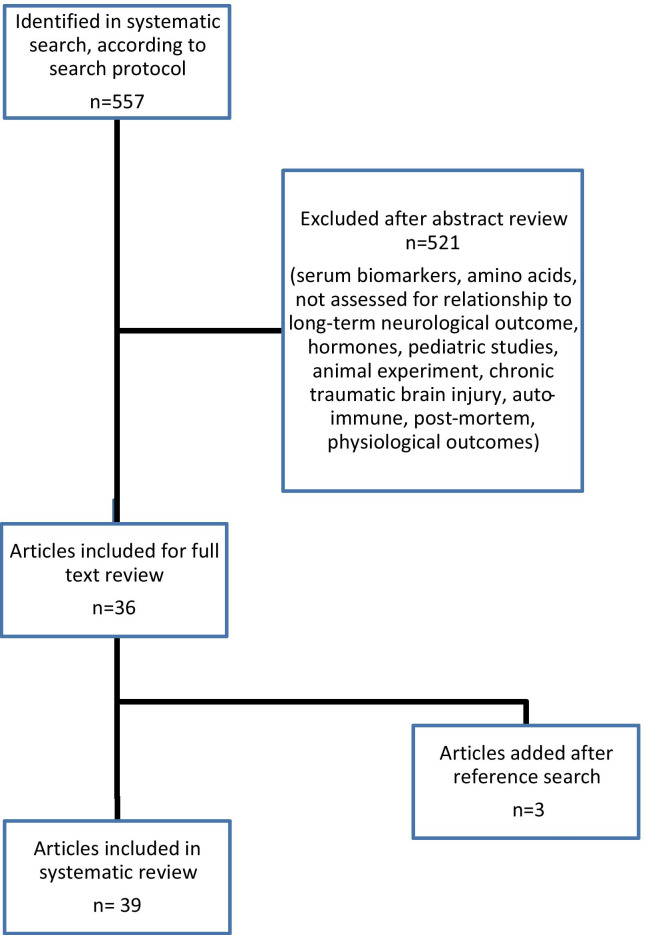
The initial search yielded 557 citations, and 39 studies met the inclusion criteria (Fig. 1). These studies had evaluated 27 CSF protein biomarkers; 26 studies had evaluated the relationship of a protein biomarker in acute brain injuries of traumatic origin [10, 12, 15, 17–22, 24–29, 32, 34, 36, 39, 41–47], 11 in acute brain injuries of non-traumatic origin [7, 11, 13, 16, 23, 30, 31, 33, 35, 37, 38] and two in acute brain injuries of mixed (traumatic and non-traumatic) origin [14, 40]. No study had reported CSF biomarkers after cardiac arrest. Thirty studies [7, 10–38] reported an association of the protein biomarker with neurological outcome (Table 1), and 9 reported no association [39–47] (Table 2).
Fig. 1.
Flowchart of included studies
Table 1.
Trials in which cerebrospinal fluid (CSF) protein biomarkers were associated with neurological outcome
| Author (ref) | Study population | CSF biomarker | Biological function of biomarker | Number of patients (ABI/control) | Source of protein (ABI/control) | Time point of first sampling* | Method of biomarker detection | Outcome measure | Relationship of biomarker with outcome | Newcastle–Ottawa risk of bias |
|---|---|---|---|---|---|---|---|---|---|---|
| Jiang et al. 2020 [10] | TBI | Caspase-3, cytochrome C, sFas and caspase-9 | Apoptosis | 45/25 | vCSF/lCSF | Day 1 after injury | ELISA | 6-month GOS | ICP and caspase-3 were significant predictors of outcome at 6 months | ★★★★★ |
| Mertens et al. 2018 [11] | Ischemic | Procarboxypeptidase U (proCPU, TAFI, proCPB2) | Inflammation, coagulation | AIS (n = 58) or TIA (n = 14)/32 | lCSF/lCSF | Day 1 after symptoms onset | ELISA | 3-month mRS | Increased proCPU levels were associated with stroke progression and worst mRS | ★★★★ |
| Kerr et al. 2018 [12] | TBI | Caspase-1, apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) | Apoptosis | 21#/30 | NS/Biobank | Day 1 after injury | ELISA | GOSE | Higher protein levels of ASC were consistent with poorer outcomes after TBI | ★★★★★ |
| Wąsik et al. 2017 [13] | SAH | Clusterin | Apoptosis | 27/25 | vCSF/lCSF | Day 1 after bleeding | ELISA | 3- month GOS | Higher levels of CSF clusterin were found 5–7 days after SAH in patients with good outcome | ★★★★ |
| Kellermann et al. 2016 [14] | Mixed | S-100β | Cytoskeleton | 45 SAH—57 TBI/no control | vCSF/no control | Day 1 after EVD placement | ELISA | GOS | In TBI and SAH patients, S-100β concentrations in CSF and serum were significantly higher in patients with unfavorable outcome (GOS 1–3) | ★★ |
| Failla et al. 2016 [15] | TBI | BDNF | Cytoskeleton | 203/10 | vCSF and serum/lCSF and serum | NR | ELISA | 1-year mortality | Higher CSF levels predicted mortality | ★★★★★★ |
| Wu et al. 2016 [16] | SAH | NLRP1, ASC and caspase 1 | Apoptosis | 24/10 | vCSF-lCSF/lCSF | Between 24 and 72 h after injury | SDS-PAGE | 3-month GOS |
Higher levels of inflammasome proteins were associated with severe SAH and poor outcome at 3 months |
★★★ |
| Papa et al. 2015 [17] | TBI | UCH-L1, MAP-2, SBDP150, SBDP145, SBDP120, MBP and S-100β | Apoptosis, cytoskeleton | 131/21 | vCSF/mixb | 6 h after injury | ELISA | 6-month mortality | MAP-2 in combination with clinical data provide enhanced prognostic capabilities for mortality at 6 months | ★★★ |
| Manevich et al. 2014 [18] | TBI | Peroxiredoxin (Prdx) VI | Redox | 21/10 | vCSF/lCSF | During EVD placement after injury | Western Blot | Scale of neurological deficits at discharge | Reduction of Prdx appeared to correlate with milder neurological deficits | ★★★ |
| Liu et al. 2014 [19] | TBI | Matrix metalloproteins (MMP-9) | Inflammation | 6/85 | vCSF/vCSF | During EVD placement | ELISA | ICP and GCS | MMP-9 was negatively correlated with the Glasgow Coma Scale | ★★★ |
| Gatson et al. 2013 [20] | TBI | NSE and Ab42 | Energy, neurodegeneration | 18/no control | vCSF/no control | Within 72 h after injury | ELISA | GOS-E and DRS | CSF oligomer levels correlated with GOS-E scores | ★★ |
| Mondello et al. 2013 [21] | TBI | Alpha-synuclein | Neurodegeneration | 12/22 | vCSF/lCSF | NR | ELISA |
6-month GOS-E Mortality |
Rising levels predicted mortality with 100% specificity and high sensitivity (83%) |
★★★ |
| Goyal et al. 2013 [22] | TBI | S100β | Cytoskeleton | 138/15 | vCSF /lCSF | First 6 days post-injury | ELISA |
GOS DRS Mortality |
Mean and peak levels were associated with mortality and GOS scores, but not with DRS | ★★★★★ |
| Zanier et al. 2013 [23] | SAH | H-FABP and tau protein | Cytoskeleton | 38/16 | vCSF/lCSF | Day 1 after injury | ELISA | GOS | Higher H-FABP and tau levels in patients with unfavorable outcome (death, vegetative state or severe disability) | ★★★★★ |
| Adamczak et al. 2012 [24] | TBI | ASC, caspase-1 and NALP-1 | Apoptosis | 23/9 | vCSF/vCSF | Within 12 h of injury and up to 72 h after injury | Western Blot | 5-month GOS |
Expression of each protein correlated significantly with the GOS at 5 months post-injury |
★★★ |
| Böhmer et al. 2011 [25] | TBI |
NSE, S-100β and glial fibrillary acidic protein |
Cytoskeleton | 20/20 | vCSF/lCSF | Between 2 and 4 h after hospitalization | ELISA | Survival | At admission, CSF NSE level predicted brain death more accurately than S-100β | ★★★★★★ |
| Stein et al. 2011 [26] | TBI | S100β, NSE | Cytoskeleton | 23/no control | vCSF/no control | Upon insertion of the EVD or as soon as possible after consent was obtained | ELISA |
ICH CH |
S-100β and NSE levels were associated with ICH and CH |
★★ |
| Darwish et al. 2010 [27] | TBI | Cytochrome c and activated caspase-9 | Apoptosis | 9/5 | vCSF/lCSF | 2 to 6 h after injury | ELISA | GOS | Activated caspase-9 showed weak correlation with poor neurologic outcome | ★★★ |
| Mondello et al. 2010 [28] | TBI | SBDP145- SBDP120 | Apoptosis | 40/24 | vCSF/vCSF | First 24 h after injury | ELISA | 3-month survival |
CSF SBDP levels predicted injury severity and mortality after severe TBI |
★★★ |
| Papa et al. 2010 [29] | TBI | UCH-L1 | Neurodegeneration | 41/25 | vCSF/vCSF | 6 h after injury | ELISA | GOS, 6-week mortality | Higher levels in patients with lower GCS score at 24 h, in those with post-injury complications, in those with 6-wk mortality and in those with a poor 6-month dichotomized GOS | ★★★★★ |
| Brouns et al. 2010 [30] | Ischemic | MBP, GFAP, S100β, NSE | Cytoskeleton, energy | 89/35 | lCSF/ lCSF | NR | ELISA |
3-month mRS Infarct volume |
MBP was a marker for infarct location. GFAP and S-100β correlated with stroke severity and outcome | ★★★ |
| Fountas et al. 2009 [31] | SAH | CRP | Inflammation | 41/no control | vCSF | Admission | Nephelometry | GOS, mRS |
Increased CRP in CSF associated with increased risk of vasospasm and bad outcome |
★★★ |
| Pineda et al. 2007 [32] | TBI | SBDP | Apoptosis | 41/11 | vCSF/vCSF | 6 h after injury | SDS-PAGE | 6-month GOS, severity of injury, computed tomography (CT) scan findings | SBDP correlated with severity of injury, computed tomography (CT) scan findings and outcome at 6 months post-injury | ★★★ |
| Lewis et al. 2007 [33] | SAH | α-2 spectrin and SBDP | Apoptosis | 20/10 | vCSF /lCSF | NR | SDS-PAGE | 6-month GOS, vasospasm | SBDP levels were significantly increased in patients with vasospasm | ★★★★ |
| Ost et al. 2006 [34] | TBI | c-tau | Cytoskeleton | 39/20 | vCSF /lCSF | First 24 h after injury | ELISA | GOSE | vCSF total tau on days 2 to 3 post-trauma correlated to morbidity and mortality at 1 year | ★★★ |
| Selakovic et al. 2005 [35] | Ischemic | NSE | Energy | 55/16 | lCSF/ lCSF | 1–2 days [21 patients], 3–4 days [14 patients], and 5–7 days [20 patients] from the onset of symptoms | ELISA |
Infarct volume, Canadian neurological scale and Barthel index |
Significant correlation between NSE concentration and infarct volume and degree of neurological and functional deficit | ★★★ |
| Kay et al. 2003 [7] | SAH | Apo-E and S-100β | Inflammation, cytoskeleton | 19/28 | vCSF/lCSF | Within 72 h after injury | ELISA | 3-month GOS | SAH patients with more severe injury and less favorable outcome had lower CSF apo-E concentration | ★★★ |
| Zemlan et al. 2002 [36] | TBI | C-tau | Cytoskeleton | 28/154 | vCSF/ lCSF | NR |
ELISA Immunoblotting |
GOS | C-tau levels-independent predictor of clinical outcome | ★★★★ |
| Aurell et al. 1991 [37] | Ischemic |
S-100β and glial fibrillary acidic protein |
Cytoskeleton | 28/18 | lCSF/lCSF | 12–48 h after onset of symptoms |
ELISA (S-100β) Radioimmunoassay (GFAP) |
Clinical state: Simplified activities of daily living test Size of infarct: computed tomography |
Increment was significantly correlated with size of infarction and clinical state of patients | ★★★★★ |
| Strand et al. 1984 [38] | Ischemic | MBP, tau-fraction, albumin, IgG and transferrin | Cytoskeleton, inflammation | 40/37 | lCSF/lCSF | 24 h after symptoms onset | Radioimmunoassay (MBP); crossed immunoelectrophoretic method (tau-fraction); electroimmunoassay (albumin, IgG and transferrin) |
Disability groups, mortality |
MBP increased with extent of brain injury; high values indicated poor short-term prognosis for the patient. No clear patterns for other markers | ★★★★★ |
ABI acute brain injury, AIS acute ischemic stroke, Apo-E apolipoprotein E, ASC apoptosis-associated speck-like protein containing a caspase recruitment domain, BDNF brain-derived neurotrophic factor, CRP C-reactive protein, lCSF lumbar CSF, C-tau cleaved tau protein, DRS Disability Rating Scale, ELISA enzyme-linked immunosorbent assay, EVD external ventricular drainage, GCS Glasgow Coma Score, GOS Glasgow Outcome Scale, GOS-E extended Glasgow Outcome Scale, H-FABP heart-type fatty acid binding protein, ICP intracranial pressure, MAP-2 microtubule-associated protein, mRS modified Rankin Scale, MBP myelin basic protein, MMP matrix metalloproteinase, NALP1 nacht leucine-rich-repeat protein-1, NR not reported, NSE neuron-specific enolase, UCH-L1 ubiquitin C-terminal hydrolase, SDS-PAGE sodium dodecyl sulfate polyacrylamide gel electrophoresis, S-100β S-100 beta, SBDP spectrin breakdown products, TAFI thrombin-activatable fibrinolysis inhibitor, TBI traumatic brain injury, TIA transient ischemic attack, vCSF ventricular CSF
#18 CSF samples; *as reported by the author
Table 2.
Trials where cerebrospinal fluid (CSF) protein biomarkers were not associated with neurological outcome
| Author (ref) | Study population | Biomarker | Biological function of biomarker | Number of patients (ABI/control) | Source of protein (ABI/control) | Time point of first sampling* | Method of biomarker detection | Outcome measure | Quality (Newcastle–Ottawa) |
|---|---|---|---|---|---|---|---|---|---|
| Jha et al. 2017 [39] | TBI | Sulfonylurea receptor-1 | Energy | 28/15 | vCSF/biobank | 24 h after injury | ELISA | 3-month GOSa | ★★★ |
| Martinez-Morillo et al. 2015 [40] | Mixed | NMP | Cytoskeleton | 30 HS, 11 IS/10 | Biobank/biobank | Median 5 (0–9) days in HS group and 1 (0–3) day in IS group | ELISA | 3-month GOS | ★★★★★ |
| Bellander et al. 2011 [41] | TBI | S-100β | Cytoskeleton | 20/no control | vCSF/no control | At admission |
Chemiluminometric immunoassays |
3–12-month GOS | ★★ |
| Grossetete et al. 2009 [42] | TBI | MMP-2 and MMP-9 | Inflammation | 6/4 | vCSF/vCSF | Following EVD insertion | Gelatin zymography and Western Blot | RLAFS and GOS | ★★★ |
| Cardali et al. 2006 [43] | TBI | α-2 spectrin and SBDP | Apoptosis | 8/2 | vCSF/vCSF | 6 h after injury | Western blot and SDS-PAGE | GOS | ★★★ |
| Farkas et al. 2005 [44] | TBI | Spectrin and SBDP | Apoptosis | 12/14 | vCSF/mix | Following EVD insertion | ELISA | GOS | ★★★ |
| Kay et al. 2003 [45] | TBI | Apo E + S100β | Inflammation, cytoskeleton | 27/28 | vCSF/lCSF | Within three days post-injury | ELISA | GOS | ★★★ |
| Franz et al. 2003 [46] | TBI | Aβ-amyloid 1–42 and tau protein | Neurodegeneration, cytoskeleton | 29/31 | 15 vCSF, 14 lCSF/lCSF | Between 1- and 284-days post-injury | ELISA | GOS | ★★★ |
| Raby et al. 1998 [47] | TBI |
β-amyloid peptide 1–42 |
Neurodegeneration | 6/24 | vCSF/vCSF | NR |
ELISA Western blot |
GOS | ★★★★ |
ABI acute brain injury, Apo-E apolipoprotein E, vCSF ventricular CSF, lCSF lumbar CSF, C-tau cleaved tau protein, DRS Disability Rating Scale, ELISA enzyme-linked immunosorbent assay, GOS Glasgow Outcome Scale, HS hemorrhagic stroke, IS ischemic stroke, NR not reported, MMP matrix metalloproteinase, NMP neurofilament medium polypeptide, SDS-PAGE sodium dodecyl sulfate polyacrylamide gel electrophoresis, RLAFS Rancho Los Amigos functional scale, S-100Β S-100 beta, SBDP spectrin breakdown products, TBI traumatic brain injury; HS hemorrhagic stroke, IS ischemic stroke
*As reported by the author
amean and peak sulfonylurea receptor-1 were elevated in patients with CT edema
Observational trials reporting biomarker associations with neurological outcome
Of the 30 trials that reported a biomarker associated with outcome, 18 included patients with TBI (n = 1345), 6 included patients with SAH (n = 258), 5 included patients with acute ischemic stroke (n = 422), and one included a mixed population (TBI and SAH) (n = 102). The main biological functions reflected by the biomarkers were related to primary brain injury (neuron cell cytoskeleton) and secondary brain injury, e.g., increased apoptosis, inflammation and energy metabolism, reduced redox response to oxidative stress and increased neurodegeneration. Specifically, concentrations of the CSF biomarkers ubiquitin carboxy-terminal hydrolase L1 (UCH-L1), microtubule-associated protein (MAP)-2, alpha-synuclein and peroxiredoxin VI were associated with a lower Glasgow Coma Scale (GCS) score on admission, worse long-term functional outcome and increased mortality. In patients with SAH, NLRP1, ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain), caspase-1 and 3, α-2 spectrin and SBDP (spectrin breakdown products), apolipoprotein-E, S-100β, H-FABP (heart-type fatty acid binding protein) and tau protein were associated with an increased risk of vasospasm, late cerebral ischemia and worse functional outcome at 3–6 months. These findings were consistent when the CSF was collected from a mixed cohort of TBI and SAH patients. In patients with acute ischemic stroke, proteins related to cytoskeleton disruption and energy metabolism were consistently associated with the size of brain infarction and clinical status (see Table 1).
Observational trials reporting no association of the biomarker with neurological outcome
Of the 9 trials that reported no association of the biomarker with neurological outcome [39–47], 8 included patients with TBI (n = 254) and 1 had a mixed population of patients with hemorrhagic or ischemic stroke (n = 51). The main biological functions assessed by the studied biomarkers included inflammation, neuronal cytoskeleton components, apoptosis, energy metabolism and neurodegeneration (Table 2).
Methodological analysis
The risk of bias among the included studies was high according to the Newcastle–Ottawa scale [9] (Tables 1 and 2). In addition, different CSF sources were used for assessment of protein biomarker concentrations (ventricular CSF, lumbar CSF, serum, biobanks) across different studies and most control group patients also had neurological conditions that may have influenced biomarker concentrations (e.g., normal pressure hydrocephalus). The studies of patients with acute ischemic stroke were the only ones in which the source of CSF was always the same in the intervention and the control group (lumbar CSF).
Discussion
Our results suggest that CSF concentrations of protein biomarkers associated with the pathophysiological pathways involved in acute brain injuries may be predictive of increased morbidity and mortality after traumatic and non-traumatic acute brain injury.
CSF proteomic expression may be altered by many factors including genetic background, the severity of the primary brain injury and secondary insults, such as hypoxemia and hypotension [48, 49]. In patients with a traumatic origin of the acute brain injury, cytoskeletal damage was associated with an increased risk of cerebral hemorrhage, intracranial hypertension and early mortality rates, suggesting severe primary brain injuries. After the initial phase of acute brain injury, the expression of proteins involved in re-establishing normal homeostasis is altered [50]. If this response is dysregulated, it may overwhelm counter-regulatory measures initiated by the body to reduce tissue injury, increasing the risk of secondary brain injuries [51]. Moreover, impairment of normal biological functions (e.g., redox function capability, dysregulated inflammation, increased apoptosis) after a primary acute brain injury may render the brain more susceptible to secondary injuries. This seems to be the case in patients with SAH in whom CSF concentrations of C-reactive protein [31], α-2 spectrin and SBDP [33], apolipoprotein E [7], H-FABP and tau protein [23] were associated with an elevated risk of vasospasm and delayed cerebral ischemia. Interestingly, in a mixed population of patients with traumatic and non-traumatic acute brain injuries, concentrations of the structural protein S-100β were higher in patients with lower Glasgow Outcome Scale (GOS) scores [14], suggesting a common pathophysiological pathway for these two types of injury.
Consequences such as acute brain edema, vasospasm or non-convulsive status epilepticus are of crucial importance in patients with acute brain injury because they may affect long- and short-term outcomes. Jha et al. [39] evaluated the ability of the protein biomarker sulfonylurea receptor-1 (Sur1) to predict the risk of cerebral edema in patients with severe TBI. Patients with evidence of edema on computed tomography (CT) had higher concentrations of Sur1 with statistically significant differences in mean (p = 0.023) and peak (p = 0.019) concentrations in patients with and without edema. Although there were no differences in functional outcome, as assessed using the 3-month GOS score, in patients with higher Sur1 concentrations, prediction of cerebral edema may indicate the need for more aggressive therapeutic measures.
It is difficult to imagine that a single biomarker could explain the complex cascades of events following acute brain injury that may be related to worse long-term outcomes. A single CSF protein biomarker may indicate derangement of a specific biological function but may not be involved in other pathophysiological pathways. Moreover, the time point at which the biomarker is measured may reflect different stages of acute brain injury (e.g., primary vs secondary injury). Thus, earlier sampling of CSF biomarkers after initial injury may provide information about the severity of the initial injury (e.g., increased risk of early mortality, extent of brain tissue involvement, risk of severe intracranial pressure), whereas more delayed measurements could provide information on risk of chronic degenerative encephalopathy or longer-term outcomes. This could be an interesting area for future study.
Our review has several limitations. First, the search strategy was based solely on the MEDLINE database, and more studies may have been identified if other databases (e.g., Embase) had been used. Second, because of insufficient data we could only provide descriptive data. We were unable to determine which protein biomarker was most associated with worse short- or long-term outcomes. Also, there was a high risk of bias among the included studies because of trials without a control group, a control group with CSF-derived from patients with other neurological conditions (e.g., with normal pressure hydrocephalus) or studies comparing lumbar and ventricular CSF without taking into account the craniocaudal gradient [52]. Finally, some studies used frozen biobank samples, which may have lower protein concentrations because of proteolysis induced by freeze–thaw and contamination. Future studies should report in a more standardized fashion to enable comparison across different studies.
Conclusions
Changes to the CSF proteome in patients with acute brain injury reflecting the pathophysiological pathways involved may be indicative of the severity of the injury and predictive of worse neurological outcomes. However, there are currently insufficient data available to recommend the routine measurement of any CSF biomarker in these patients.
Acknowledgements
Not applicable
Abbreviations
- ABI
Acute brain injury
- Apo-E
Apolipoprotein E
- CSF
Cerebrospinal fluid
- C-tau
Cleaved tau protein
- DRS
Disability Rating Scale
- ELISA
Enzyme-linked immunosorbent assay
- GOS
Glasgow Outcome Scale
- GOS-E
Extended Glasgow Outcome Scale
- H-FABP
Heart-type fatty acid binding protein
- MBP
Myelin basic protein
- MMP
Matrix metalloproteinase
- NMP
Neurofilament medium polypeptide
- NSE
Neuron-specific enolase
- RLAFS
Rancho Los Amigos functional scale
- SAH
Subarachnoid hemorrhage
- SBDP
Spectrin breakdown products
- SDS-PAGE
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- Sur1
Sulfonylurea receptor-1
- TBI
Traumatic brain injury
- UCH-L1
Ubiquitin C-terminal hydrolase
Authors’ contributions
CAS, DC and FT designed the study; AB, LARG and CDC performed the literature search and extracted the data; CAS wrote the first draft of the manuscript; FT, JLV, DC, AB, LARG and CDC reviewed the article for critical content; and all authors read and approved the final text.
Funding
Not applicable.
Availability of data and material
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
JLV is Editor-in-Chief of Critical Care. He has no other conflicts of interest. The other authors have no conflicts of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.D'Onofrio G, Jauch E, Jagoda A, Allen MH, Anglin D, Barsan WG, Berger RP, Bobrow BJ, Boudreaux ED, Bushnell C, Chan YF, Currier G, Eggly S, Ichord R, Larkin GL, Laskowitz D, Neumar RW, Newman-Toker DE, Quinn J, Shear K, Todd KH, Zatzick D. Roundtable external participants and roundtable steering committee and federal participants. NIH roundtable on opportunities to advance research on neurologic and psychiatric emergencies. Ann Emerg Med. 2010;56:551–564. doi: 10.1016/j.annemergmed.2010.06.562. [DOI] [PubMed] [Google Scholar]
- 2.Kinoshita K. Traumatic brain injury: pathophysiology for neurocritical care. J Intensive Care. 2016;4:29. doi: 10.1186/s40560-016-0138-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hernandez-Ontiveros DG, Tajiri N, Acosta S, Giunta B, Tan J, Borlongan CV. Microglia activation as a biomarker for traumatic brain injury. Front Neurol. 2013;4:30. doi: 10.3389/fneur.2013.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hinson HE, Rowell S, Schreiber M. Clinical evidence of inflammation driving secondary brain injury: a systematic review. J Trauma Acute Care Surg. 2015;78:184–191. doi: 10.1097/TA.0000000000000468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romeo MJ, Espina V, Lowenthal M, Espina BH, Petricoin EF, 3rd, Liotta LA. CSF proteome: a protein repository for potential biomarker identification. Expert Rev Proteomics. 2005;2:57–70. doi: 10.1586/14789450.2.1.57. [DOI] [PubMed] [Google Scholar]
- 6.Zemlan FP, Rosenberg WS, Luebbe PA, et al. Quantification of axonal damage in traumatic brain injury: affinity purification and characterization of cerebrospinal fluid tau proteins. J Neurochem. 1999;72:750–750. doi: 10.1046/j.1471-4159.1999.0720741.x. [DOI] [PubMed] [Google Scholar]
- 7.Kay A, Petzold A, Kerr M, Keir G, Thompson E, Nicoll J. Decreased cerebrospinal fluid apolipoprotein E after subarachnoid hemorrhage: correlation with injury severity and clinical outcome. Stroke. 2003;34:637–642. doi: 10.1161/01.STR.0000057579.25430.16. [DOI] [PubMed] [Google Scholar]
- 8.Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deeks JJ, Dinnes J, D'Amico R, Sowden AJ, Sakarovitch C, Song F, et al. Evaluating non-randomised intervention studies. Health Technol Assess. 2003;7:iii-x, 1–173. [DOI] [PubMed]
- 10.Jiang W, Jin P, Wei W, Jiang W. Apoptosis in cerebrospinal fluid as outcome predictors in severe traumatic brain injury: an observational study. Medicine (Baltimore) 2020;99:20922. doi: 10.1097/MD.0000000000020922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mertens JC, Leenaerts D, Brouns R, Engelborghs S, Ieven M, De Deyn PP, Lambeir AM, Hendriks D. Procarboxypeptidase U (proCPU, TAFI, proCPB2) in cerebrospinal fluid during ischemic stroke is associated with stroke progression, outcome and blood-brain barrier dysfunction. J Thromb Haemost. 2018;16:342–348. doi: 10.1111/jth.13914. [DOI] [PubMed] [Google Scholar]
- 12.Kerr N, Lee SW, Perez-Barcena J, Crespi C, Ibañez J, Bullock MR, Dietrich WD, Keane RW, de Rivero Vaccari JP. Inflammasome proteins as biomarkers of traumatic brain injury. PLoS ONE. 2018;13:e0210128. doi: 10.1371/journal.pone.0210128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wąsik N, Sokół B, Hołysz M, Mańko W, Juszkat R, Jagodziński PP, Jankowski R. Clusterin, a new cerebrospinal fluid biomarker in severe subarachnoid hemorrhage: a pilot study. World Neurosurg. 2017;107:424–428. doi: 10.1016/j.wneu.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Kellermann I, Kleindienst A, Hore N, Buchfelder M, Brandner S. Early CSF and Serum S-100β concentrations for outcome prediction in traumatic brain injury and subarachnoid hemorrhage. Clin Neurol Neurosurg. 2016;145:79–83. doi: 10.1016/j.clineuro.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Failla MD, Conley YP, Wagner AK. Brain-derived neurotrophic factor (BDNF) in traumatic brain injury-related mortality: interrelationships between genetics and acute systemic and central nervous system BDNF profiles. Neurorehabil Neural Repair. 2016;30:83–93. doi: 10.1177/1545968315586465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Q, Wang XL, Yu Q, Pan H, Zhang XS, Zhang QR, Wang HD, Zhang X. Inflammasome proteins in cerebrospinal fluid of patients with subarachnoid hemorrhage are biomarkers of early brain injury and functional outcome. World Neurosurg. 2016;94:472–479. doi: 10.1016/j.wneu.2016.07.039. [DOI] [PubMed] [Google Scholar]
- 17.Papa L, Robertson CS, Wang KK, Brophy GM, Hannay HJ, Heaton S, Schmalfuss I, Gabrielli A, Hayes RL, Robicsek SA. Biomarkers improve clinical outcome predictors of mortality following non-penetrating severe traumatic brain injury. Neurocrit Care. 2015;22:52–64. doi: 10.1007/s12028-014-0028-2. [DOI] [PubMed] [Google Scholar]
- 18.Manevich Y, Hutchens S, Halushka PV, Tew KD, Townsend DM, Jauch EC, Borg K. Peroxiredoxin VI oxidation in cerebrospinal fluid correlates with traumatic brain injury outcome. Free Radic Biol Med. 2014;72:210–221. doi: 10.1016/j.freeradbiomed.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu CL, Chen CC, Lee HC, Cho DY. Matrix metalloproteinase-9 in the ventricular cerebrospinal fluid correlated with the prognosis of traumatic brain injury. Turk Neurosurg. 2014;24:363–368. doi: 10.5137/1019-5149.JTN.8551-13.0. [DOI] [PubMed] [Google Scholar]
- 20.Gatson JW, Warren V, Abdelfattah K, Wolf S, Hynan LS, Moore C, Diaz-Arrastia R, Minei JP, Madden C, Wigginton JG. Detection of β-amyloid oligomers as a predictor of neurological outcome after brain injury. J Neurosurg. 2013;118:1336–1342. doi: 10.3171/2013.2.JNS121771. [DOI] [PubMed] [Google Scholar]
- 21.Mondello S, Buki A, Italiano D, Jeromin A. α-Synuclein in CSF of patients with severe traumatic brain injury. Neurology. 2013;80:1662–1668. doi: 10.1212/WNL.0b013e3182904d43. [DOI] [PubMed] [Google Scholar]
- 22.Goyal A, Failla MD, Niyonkuru C, Amin K, Fablo A, Berger RP, Wagner AK. S-100β as a prognostic biomarker in outcome prediction for patients with severe traumatic brain injury. J Neurotrauma. 2013;30:946–957. doi: 10.1089/neu.2012.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zanier ER, Zoerle T, Fiorini M, Longhi L, Cracco L, Bersano A, Branca V, Benedetti MD, De Simoni MG, Monaco S, Stocchetti N. Heart-fatty acid-binding and tau proteins relate to brain injury severity and long-term outcome in subarachnoid haemorrhage patients. Br J Anaesth. 2013;111:424–432. doi: 10.1093/bja/aet149. [DOI] [PubMed] [Google Scholar]
- 24.Adamczak S, Dale G, de Rivero Vaccari JP, Bullock MR, Dietrich WD, Keane RW. Inflammasome proteins in cerebrospinal fluid of brain-injured patients as biomarkers of functional outcome: clinical article. J Neurosurg. 2012;117:1119–1125. doi: 10.3171/2012.9.JNS12815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Böhmer AE, Oses JP, Schmidt AP, Perón CS, Krebs CL, Oppitz PP, D'Avila TT, Souza DO, Portela LV, Stefani MA. Neuron-specific enolase, S-100β, and glial fibrillary acidic protein levels as outcome predictors in patients with severe traumatic brain injury. Neurosurgery. 2011;68:1624–1630. doi: 10.1227/NEU.0b013e318214a81f. [DOI] [PubMed] [Google Scholar]
- 26.Stein DM, Kufera JA, Lindell A, Murdock KR, Menaker J, Bochicchio GV, AarABL B, Scalea TM. Association of CSF biomarkers and secondary insults following severe traumatic brain injury. Neurocrit Care. 2011;14:200–207. doi: 10.1007/s12028-010-9496-1. [DOI] [PubMed] [Google Scholar]
- 27.Darwish RS, Amiridze NS. Detectable levels of cytochrome C and activated caspase-9 in cerebrospinal fluid after human traumatic brain injury. Neurocrit Care. 2010;12:337–341. doi: 10.1007/s12028-009-9328-3. [DOI] [PubMed] [Google Scholar]
- 28.Mondello S, Robicsek SA, Gabrielli A, Brophy GM, Papa L, Tepas J, Robertson C, Buki A, Scharf D, Jixiang M, Akinyi L, Muller U, Wang KK, Hayes RL. αII-spectrin breakdown products (SBDPs): diagnosis and outcome in severe traumatic brain injury patients. J Neurotrauma. 2010;27:1203–1213. doi: 10.1089/neu.2010.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papa L, Akinyi L, Liu MC, Pineda JA, Tepas JJ, 3rd, Oli MW, Zheng W, Robinson G, Robicsek SA, Gabrielli A, Heaton SC, Hannay HJ, Demery JA, Brophy GM, Layon J, Robertson CS, Hayes RL, Wang KK. Ubiquitin C-terminal hydrolase is a novel biomarker in humans for severe traumatic brain injury. Crit Care Med. 2010;38:138–144. doi: 10.1097/CCM.0b013e3181b788ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brouns R, De Vil B, Cras P, De Surgeloose D, Mariën P, De Deyn PP. Neurobiochemical markers of brain damage in cerebrospinal fluid of acute ischemic stroke patients. Clin Chem. 2010;56:451–458. doi: 10.1373/clinchem.2009.134122. [DOI] [PubMed] [Google Scholar]
- 31.Fountas KN, Tasiou A, Kapsalaki EZ, Paterakis KN, Grigorian AA, Lee GP, Robinson JS., Jr Serum and cerebrospinal fluid C-reactive protein levels as predictors of vasospasm in aneurysmal subarachnoid hemorrhage. Clinical article Neurosurg Focus. 2009;26:E22. doi: 10.3171/2009.2.FOCUS08311. [DOI] [PubMed] [Google Scholar]
- 32.Pineda JA, Lewis SB, Valadka AB, Papa L, Hannay HJ, Heaton SC, Demery JA, Liu MC, Aikman JM, Akle V, Brophy GM, Tepas JJ, Wang KK, Robertson CS, Hayes RL. Clinical significance of alphaII-spectrin breakdown products in cerebrospinal fluid after severe traumatic brain injury. J Neurotrauma. 2007;24:354–366. doi: 10.1089/neu.2006.003789. [DOI] [PubMed] [Google Scholar]
- 33.Lewis SB, Velat GJ, Miralia L, Papa L, Aikman JM, Wolper RA, Firment CS, Liu MC, Pineda JA, Wang KK, Hayes RL. Alpha-II spectrin breakdown products in aneurysmal subarachnoid hemorrhage: a novel biomarker of proteolytic injury. J Neurosurg. 2007;107:792–796. doi: 10.3171/JNS-07/10/0792. [DOI] [PubMed] [Google Scholar]
- 34.Ost M, Nylén K, Csajbok L, Ohrfelt AO, Tullberg M, Wikkelsö C, Nellgård P, Rosengren L, Blennow K, Nellgård B. Initial CSF total tau correlates with 1-year outcome in patients with traumatic brain injury. Neurology. 2006;67:1600–1604. doi: 10.1212/01.wnl.0000242732.06714.0f. [DOI] [PubMed] [Google Scholar]
- 35.Selakovic V, Raicevic R, Radenovic L. The increase of neuron-specific enolase in cerebrospinal fluid and plasma as a marker of neuronal damage in patients with acute brain infarction. J Clin Neurosci. 2005;12(5):542–547. doi: 10.1016/j.jocn.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 36.Zemlan FP, Jauch EC, Mulchahey JJ, Gabbita SP, Rosenberg WS, Speciale SG, Zuccarello M. C-tau biomarker of neuronal damage in severe brain injured patients: association with elevated intracranial pressure and clinical outcome. Brain Res. 2002;947:131–139. doi: 10.1016/S0006-8993(02)02920-7. [DOI] [PubMed] [Google Scholar]
- 37.Aurell A, Rosengren LE, Karlsson B, Olsson JE, Zbornikova V, Haglid KG. Determination of S-100 and glial fibrillary acidic protein concentrations in cerebrospinal fluid after brain infarction. Stroke. 1991;22:1254–1258. doi: 10.1161/01.STR.22.10.1254. [DOI] [PubMed] [Google Scholar]
- 38.Strand T, Alling C, Karlsson B, Karlsson I, Winblad B. Brain and plasma proteins in spinal fluid as markers for brain damage and severity of stroke. Stroke. 1984;15:138–144. doi: 10.1161/01.STR.15.1.138. [DOI] [PubMed] [Google Scholar]
- 39.Jha RM, Puccio AM, Chou SH, Chang CH, Wallisch JS, Molyneaux BJ, Zusman BE, Shutter LA, Poloyac SM, Janesko-Feldman KL, Okonkwo DO, Kochanek PM. Sulfonylurea receptor-1: a novel biomarker for cerebral edema in severe traumatic brain injury. Crit Care Med. 2017;45:e255–e264. doi: 10.1097/CCM.0000000000002079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martínez-Morillo E, Childs C, García BP, Álvarez Menéndez FV, Romaschin AD, Cervellin G, Lippi G, Diamandis EP. Neurofilament medium polypeptide (NFM) protein concentration is increased in CSF and serum samples from patients with brain injury. Clin Chem Lab Med. 2015;53:1575–1584. doi: 10.1515/cclm-2014-0908. [DOI] [PubMed] [Google Scholar]
- 41.Bellander BM, Olafsson IH, Ghatan PH, Bro Skejo HP, Hansson LO, Wanecek M, Svensson MA. Secondary insults following traumatic brain injury enhance complement activation in the human brain and release of the tissue damage marker S-100β. Acta Neurochir (Wien) 2011;153:90–100. doi: 10.1007/s00701-010-0737-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grossetete M, Phelps J, Arko L, Yonas H, Rosenberg GA. Elevation of matrix metalloproteinases 3 and 9 in cerebrospinal fluid and blood in patients with severe traumatic brain injury. Neurosurgery. 2009;65:702–708. doi: 10.1227/01.NEU.0000351768.11363.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cardali S, Maugeri R. Detection of alphaII-spectrin and breakdown products in humans after severe traumatic brain injury. J Neurosurg Sci. 2006;50:25–31. [PubMed] [Google Scholar]
- 44.Farkas O, Polgár B, Szekeres-Barthó J, Dóczi T, Povlishock JT, Büki A. Spectrin breakdown products in the cerebrospinal fluid in severe head injury-preliminary observations. Acta Neurochir (Wien) 2005;147:855–861. doi: 10.1007/s00701-005-0559-6. [DOI] [PubMed] [Google Scholar]
- 45.Kay AD, Petzold A, Kerr M, Keir G, Thompson EJ, Nicoll JA. Cerebrospinal fluid apolipoprotein E concentration decreases after traumatic brain injury. J Neurotrauma. 2003;20:243–250. doi: 10.1089/089771503321532824. [DOI] [PubMed] [Google Scholar]
- 46.Franz G, Beer R, Kampfl A, Engelhardt K, Schmutzhard E, Ulmer H, Deisenhammer F. Amyloid beta 1–42 and tau in cerebrospinal fluid after severe traumatic brain injury. Neurology. 2003;60:1457–1461. doi: 10.1212/01.WNL.0000063313.57292.00. [DOI] [PubMed] [Google Scholar]
- 47.Raby CA, Morganti-Kossmann MC, Kossmann T, Stahel PF, Watson MD, Evans LM, Mehta PD, Spiegel K, Kuo YM, Roher AE, Emmerling MR. Traumatic brain injury increases beta-amyloid peptide 1–42 in cerebrospinal fluid. J Neurochem. 1998;71:2505–2509. doi: 10.1046/j.1471-4159.1998.71062505.x. [DOI] [PubMed] [Google Scholar]
- 48.Nathoo N, Chetty R, van Dellen JR, Barnett GH. Genetic vulnerability following traumatic brain injury: the role of apolipoprotein E. Mol Pathol. 2003;56:132–136. doi: 10.1136/mp.56.3.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chesnut RM, Marshall LF, Klauber MR, Blunt BA, Baldwin N, Eisenberg HM, Jane JA, Marmarou A, Foulkes MA. The role of secondary brain injury in determining outcome from severe head injury. J Trauma. 1993;34:216–222. doi: 10.1097/00005373-199302000-00006. [DOI] [PubMed] [Google Scholar]
- 50.Sonnemann KJ, Bement WM. Wound repair: Toward understanding and integration of single-cell and multicellular wound responses. Annu Rev Cell Dev Biol. 2011;27:237–263. doi: 10.1146/annurev-cellbio-092910-154251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hadass O, Tomlinson BN Gooyit M, Chen S, Purdy JJ, Walker JM, Zhang C, Giritharan AB, Purnell W, Robinson CR 2nd, Shin D, Schroeder VA, Suckow MA, Simonyi A, Sun GY, Mobashery S, Cui J, Chang M, Gu Z. Selective inhibition of matrix metalloproteinase-9 attenuates secondary damage resulting from severe traumatic brain injury. PLoS One. 2013;8:e76904. [DOI] [PMC free article] [PubMed]
- 52.Gerber J, Tumani H, Kolenda H, Nau R. Lumbar and ventricular CSF protein, leukocytes, and lactate in suspected bacterial CNS infections. Neurology. 1998;51:1710–1714. doi: 10.1212/WNL.51.6.1710. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.