Abstract
The prevalence of obesity is rising worldwide. Adipose tissue exerts anatomic and physiological effects with significant implications for critical illness. Changes in respiratory mechanics cause expiratory flow limitation, atelectasis, and V̇/Q̇ mismatch with resultant hypoxemia. Altered work of breathing and obesity hypoventilation syndrome may cause hypercapnia. Challenging mask ventilation and peri-intubation hypoxemia may complicate intubation. Patients with obesity are at increased risk of ARDS and should receive lung-protective ventilation based on predicted body weight. Increased positive end expiratory pressure (PEEP), coupled with appropriate patient positioning, may overcome the alveolar decruitment and intrinsic PEEP caused by elevated baseline pleural pressure; however, evidence is insufficient regarding the impact of high PEEP strategies on outcomes. Venovenous extracorporeal membrane oxygenation may be safely performed in patients with obesity. Fluid management should account for increased prevalence of chronic heart and kidney disease, expanded blood volume, and elevated acute kidney injury risk. Medication pharmacodynamics and pharmacokinetics may be altered by hydrophobic drug distribution to adipose depots and comorbid liver or kidney disease. Obesity is associated with increased risk of VTE and infection; appropriate dosing of prophylactic anticoagulation and early removal of indwelling catheters may decrease these risks. Obesity is associated with improved critical illness survival in some studies. It is unclear whether this reflects a protective effect or limitations inherent to observational research. Obesity is associated with increased risk of intubation and death in SARS-CoV-2 infection. Ongoing molecular studies of adipose tissue may deepen our understanding of how obesity impacts critical illness pathophysiology.
Key Words: adiposity, artificial respiration, logistics, patient outcome assessment, physiology
Abbreviations: AKI, acute kidney injury; CKD, chronic kidney disease; HFNC, high flow nasal cannula; LA, left atrial; LV, left ventricular; NIV, noninvasive ventilation; OHS, obesity hypoventilation syndrome; PEEP, positive end-expiratory pressure; PGD, primary graft dysfunction; Vd, volume of distribution; VV-ECMO, venovenous extracorporeal membrane oxygen
One-third of adults in the United States and 13% worldwide meet the World Health Organization definition of obesity (BMI ≥ 30 kg/m2).1,2 A substantial body of literature details the impact of obesity on critical illness pathophysiology and management.3, 4, 5 In this state-of-the-art concise review, we will highlight clinically relevant and recent studies to equip physicians with an understanding of obesity’s effects on pathophysiology, logistics, and outcomes in the critical care setting.
General Pathophysiology of Obesity
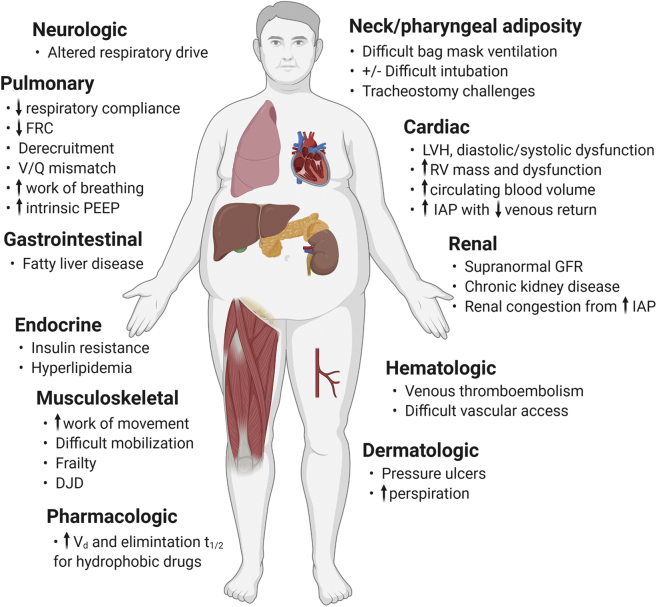
Obesity may exert physical, metabolic, and molecular effects across multiple organ systems, and is associated with numerous comorbidities (eg, diabetes mellitus, hypertension, chronic kidney disease [CKD], hepatic steatosis, OSA).6 This underlying pathophysiological milieu has both direct and indirect impacts in the setting of critical illness (Fig 1). Obesity, however, is a heterogeneous disease. Some effects of obesity may only become relevant for patients with very high BMI (≥ 40-50 kg/m2). Others may depend on adipose distribution or contribution of lean muscle mass to BMI. Excess visceral adipose tissue is associated with a chronic inflammatory state and insulin resistance.6,7 Circulating adipokines (eg, leptin, resistin, visfatin, adiponectin) have pleiotropic immunomodulatory effects that could impact acute organ dysfunction syndromes, but adipokine concentrations are not explained solely by BMI.7 Therefore, in the system-based discussion that follows, it is of paramount importance to remember that pathophysiological effects of obesity may vary substantially across the obese population.
Figure 1.
Summary of pathophysiological and management challenges relevant to critically ill patients with obesity. Some challenges are relevant across the obesity severity spectrum, whereas others may predominantly affect patients with more severe obesity (BMI > 40-50 kg/m2). Figure created with BioRender.com. DJD = degenerative joint disease; FRC = functional residual capacity; GFR = glomerular filtration rate; IAP = intraabdominal pressure; LVH = left ventricular hypertrophy; PEEP = positive end-expiratory pressure; RV = right ventricle; t1/2 = half-life; Vd = volume of distribution.
Respiratory Pathophysiology and Management
Obesity has well-described effects on respiratory anatomy and physiology that may impact baseline and sick-state gas exchange and airway and ventilator management. Increased airway resistance from parapharyngeal adipose tissue renders the upper airway susceptible to collapse, as seen in OSA.8 Increased baseline pleural pressure from abdominal and chest wall adiposity results in reduced expiratory reserve volume and functional residual capacity.9, 10, 11 Patients with obesity are therefore susceptible to collapse of peripheral-dependent airways, atelectasis, and tidal ventilation below the lower inflection point of the inspiratory pressure-volume curve, with a corresponding decrease in lung compliance.10,11 Because dependent perfusion is increased in some patients with obesity, concomitant basilar atelectasis may cause V̇/Q̇ mismatch and resultant hypoxemia.12 Severe obesity may also substantially increase the metabolic demand of breathing: one study showed that approximately one-half of the 60% increase in resting oxygen consumption among patients with obesity (mean BMI, 53 kg/m2) compared with control subjects was caused by respiratory muscle demand.13 This increased ventilatory load results in a compensatory increase in neural respiratory drive,14 a mechanism that fails in obesity hypoventilation syndrome (OHS) with consequent decreased neural drive, hypercapnia, and hypoxemia.15
Intubation
Obesity is associated with difficult mask ventilation, including the need for two-provider ventilation or airway adjuncts, likely because of mask fit challenges, increased upper airway resistance, and reduced respiratory system compliance.16 Data are less clear as to BMI’s specific impact on difficult tracheal intubation. Large operating room studies suggest small or no risk differences in patients with BMI ≥ 35 to 40 kg/m2.17,18 In a multicenter study of patients intubated in the ICU, however, De Jong et al19 showed that obesity was 1.5 to 2 times more prevalent in those with difficult intubation. Both operating room and ICU studies show that modified Mallampati class III or IV, which is associated with obesity, is a more important independent predictor of difficult intubation than BMI.17,19 Peri-intubation hypoxemia, already common in ICU patients, is of particular concern in patients with obesity because they have shown more rapid and severe oxygen desaturation during preoperative intubation.20 Operating room interventions shown to improve peri-intubation Pao2 in patients with BMI > 40 kg/m2 include 30° reverse Trendelenburg or 25° head up positioning, noninvasive ventilation (NIV) during preoxygenation, and postintubation recruitment maneuver.20,21 One randomized trial of patients with BMI > 35 kg/m2 found that high flow nasal cannula (HFNC) alone resulted in lower nadir end-tidal oxygen saturation during the 2 min after preoperative intubation compared with NIV.22 A systematic review highlighted trials in critically ill patients showing that preintubation NIV, potentially with addition of apneic oxygenation with HFNC, may decrease the depth of peri-intubation desaturation.23 These trials included patients with normal and elevated BMI, and impact of the interventions on patient-centered outcomes remains unclear.
ARDS
ICU patients with obesity have elevated risk of ARDS, possibly related to baseline / mismatch, atelectrauma from alveolar collapse during tidal ventilation, or even a proinflammatory response from adipose tissue.7,24 As with all patients with ARDS, patients with high BMI should be ventilated with tidal volumes normalized to ARDSnet protocol-predicted body weight based on height and sex.10,25 Although a plateau pressure goal ≤ 30 cm H2O remains standard, it is important for physicians to understand that higher plateau pressure in patients with obesity may not entirely reflect lung-injurious increased transpulmonary pressure—elevated baseline pleural pressure related to adiposity may also contribute to this finding.11
Several studies have explored optimal positive end-expiratory pressure (PEEP) titration for patients with ARDS with class III obesity, based on earlier perioperative studies suggesting that higher extrinsic PEEP may improve respiratory system compliance, oxygenation, and expiratory flow limitation.26,27 In a series of crossover studies in patients with ARDS with mean BMI range 48 to 57 kg/m2, average PEEP of 20 to 22 cm H2O was found to optimize end-expiratory lung volumes, / matching and oxygenation, lung compliance, and ventilation homogeneity without overdistention, far above the average of 12 to 13 cm H2O set by physicians based on the ARDSnet low PEEP table.25,28, 29, 30 Hemodynamics were preserved even when recruitment maneuvers were used prior to PEEP titration, and a corresponding experiment in a swine model of obese ARDS found that pulmonary vascular resistance and pressure decreased with this method.31 Should patients with ARDS with obesity be managed with this approach? First, the studies were small (< 20 patients each). Second, this approach increased mortality in a broad population of patients with moderate to severe ARDS.32 Finally, accurate assessment of lung compliance and driving pressure, used to optimize PEEP settings, may be complicated by end-expiratory complete airway closure, present in up to two-thirds of patients with ARDS with BMI ≥ 40 kg/m2.33 At PEEP below airway opening pressure, standard end-expiratory airway pressure measurements may therefore underestimate alveolar pressure and overestimate driving pressure and respiratory system elastance. There is a sound physiological basis, however, for a higher PEEP approach in patients with very high BMI, and trials testing clinical outcomes using this strategy in the obese population are clearly warranted.
Prone positioning is feasible and, despite slightly conflicting observational data,34,35 likely to benefit patients with obesity who develop ARDS and severe hypoxemia. Notably, the average BMI in the Proning Severe ARDS Patients (PROSEVA) randomized controlled trial was 28 to 29 kg/m2.36 Prone positioning is also known to improve oxygenation and lung compliance of patients with obesity in the perioperative setting.37 Clinical teams may benefit from additional planning to address logistical challenges involved in turning patients with very high BMI, particularly if needed rapidly, in the setting of clinical instability.
Obesity should not be considered an absolute contraindication to venovenous extracorporeal membrane oxygen (VV-ECMO) in patients with severe forms of ARDS. Historically, the use of VV-ECMO in patients with obesity was discouraged because of concerns regarding technical difficulties with cannulation, obtaining sufficient flow indexed to body surface area, greater comorbidity burden, and challenges with early mobilization.38 Multiple observational studies, however, suggest that VV-ECMO in patients with high BMI is safe, feasible, and associated with similar39 or improved40 outcomes compared with nonobese patients. VV-ECMO has also been safely used to transport patients with obesity who have refractory respiratory failure to quaternary care centers.38 ECMO to Rescue Lung Injury in Severe ARDS (EOLIA), an international multicenter randomized controlled trial of VV-ECMO in severe ARDS, had BMI means of approximately 28.5 kg/m2 in both VV-ECMO and control groups (despite excluding patients with BMI ≥ 45 kg/m2), suggesting that many study subjects were overweight or obese.41 No subgroup or safety analyses were performed by BMI. When managing patients with obesity receiving VV-ECMO support, additional venous drainage cannulas may be necessary to provide enough extracorporeal blood flow to meet oxygenation needs when cardiac output is high,38 and close attention should be paid to the circuit for clot formation and cannula site infections. Given the limitations in the existing data, particularly for those with BMI > 50 kg/m2, providers should consider local expertise in choosing candidates for cannulation.
Other Respiratory Failure Syndromes
Obesity also impacts respiratory failure beyond ARDS. Higher BMI is a risk factor for primary graft dysfunction (PGD) after lung transplantation, potentially for reasons similar to the factors previously noted that may contribute to ARDS risk.42 A study found that CT-quantified abdominal subcutaneous adipose tissue was associated with PGD.43 The finding that adipose correlated with circulating levels of the immune-modulating adipokine leptin and vascular endothelial markers raises the possibility of a molecular link between adiposity and PGD. Obesity is a sine qua non of OHS, which may progress to acute on chronic hypercapnic respiratory failure in the ICU. Physicians must consider current and prior available data on partial pressure of CO2, serum bicarbonate concentration, and other measures of acid-base status to distinguish acute and chronic components. Cohort studies have demonstrated that NIV can be used to treat acute respiratory failure in patients with OHS with intubation avoidance rates of 83% to 94% and adjusted mortality rates comparable with patients with COPD exacerbations.44 Patients with OHS may require higher pressures, management in a sitting position, and extended initial NIV to significantly improve blood gas parameters.
Ventilator Weaning
A study by O’Brien et al45 showed in a risk-adjusted analysis of 508 patients that patients with obesity in the medical ICU had shorter time to successful extubation than those with BMI < 25 kg/m2, with no difference in reintubation rates. These findings remain more convincing than prior studies, which were limited by lack of appropriate adjustment for confounders. Interestingly, obesity was not a typical criterion in studies supporting a consensus recommendation for extubation to NIV in high-risk patients.46 As in nonobese patients, patients with obesity who have hypercapnia during spontaneous breathing trials may be good candidates for this approach. HFNC has also been studied in the prevention of respiratory failure after cardiac surgery in patients with obesity, with some data suggesting similar efficacy to NIV, whereas other data suggest no improvement in atelectasis compared with standard nasal cannula.47,48 Although a number of older studies found associations of severe obesity with tracheostomy complications,49 more recent studies using extralong tracheostomy tubes and bronchoscopy- or ultrasound-aided percutaneous dilatational tracheostomy suggest an overall low complication rate in patients with obesity.50 Of note, however, the percutaneous approach may not be feasible when anatomic landmarks on the neck are obscured.
Cardiovascular Considerations
Obesity results in multiple cardiovascular changes including increased blood volume, stroke volume, and cardiac output; increased left ventricular (LV) and left atrial (LA) filling pressures; LV hypertrophy and LA enlargement; and increased risk for LV dysfunction.51,52 These factors likely increase the risk of atrial fibrillation, as may sleep-disordered breathing, which results in autonomic changes that may be arrhythmogenic.53,54 Patients with OSA or OHS may also develop pulmonary hypertension and right ventricular dysfunction.55 Intensivists should correspondingly have an increased index of suspicion for these conditions while realizing that many patients with obesity have normal cardiac function.
How to account for higher BMI in fluid resuscitation remains unclear. Although initial fluid regimens for sepsis, for example, are indexed to weight,56 obesity-related blood volume increases plateau at the upper extremes of BMI.51 In trauma patients who were largely managed with non-weight-based initial fluid resuscitation, Winfield et al57 showed that metabolic acidosis was slower to resolve in patients with BMI ≥ 40 kg/m2 than in those with normal BMI, raising the possibility that such an approach might underresuscitate patients with obesity. Acknowledging the limitations of current evidence and potential risks of excess fluid administration, a weight-based approach to resuscitation, with modification at very high BMI and heightened attention to both perfusion goals and early signs of fluid overload, may be most prudent.
Renal Considerations
Obesity is a risk factor for CKD and acute kidney injury (AKI) in critical illness populations.58,59 Mechanisms proposed for the obesity-AKI link include subclinical CKD, intraabdominal hypertension, and alteration in baseline and evoked circulating inflammatory mediators and adipokines.59 A recent study in critically ill trauma patients found that the association of BMI with AKI risk was in part explained by creatine kinase levels, raising the possibility that higher BMI could predispose patients to rhabdomyolysis-mediated kidney injury.60 This finding may be specific to trauma patients, given the frequency of rhabdomyolysis and a demographic including young, healthy individuals whose high BMI may sometimes reflect increased muscle mass rather than adiposity.
Consensus criteria define AKI in part by urine output (mL/kg/h).61 This may bias classification in favor of AKI, particularly in patients with very high weight; however, there is currently no consensus to index urine output to adjusted or ideal weight estimates such as those using height and sex.62 Creatinine criteria for AKI are less susceptible to this bias, and novel AKI biomarkers more specific to kidney injury may ultimately supplant conventional definitions. For estimating glomerular filtration rate in patients with obesity and stable kidney function, the Chronic Kidney Disease Epidemiology Collaboration equation is best validated; however, its use of race is a point of active, ongoing discussion within the nephrology community.63
Pharmacology
Medication dosing regimens are often determined by cohorts of normal weight participants, raising questions about their applicability to patients with obesity in whom clearance and volume of distribution (Vd) may be substantially different. Weight-based dosing guidelines often do not specify the use of actual body weight vs ideal (based on height and sex) or adjusted (typically between actual and ideal) weight estimates,62 and multiple additional factors impacted by obesity must be considered for appropriate dosing. Diabetes may lead to glomerular hyperfiltration in some patients with obesity, whereas CKD or AKI (see Renal Considerations section) may decrease glomerular filtration in others. This variability may complicate dose selection for renally cleared medications. Hepatic steatosis may decrease clearance of hepatically metabolized medications. Vd, calculated by dividing the total amount of drug in the body by the plasma concentration, is influenced by medication lipophilicity, molecular size, and protein binding, which alter a drug’s ability to move between blood and tissues. Lipophilic medications in particular may have a larger Vd in patients with obesity, requiring higher loading doses but also increasing elimination half-life.64 A recent review details the impact of obesity on dosing of many common ICU medications.65 ICU physicians should focus on basic pharmacodynamic and pharmacokinetic principles when dosing medications in patients with obesity, with special attention to (1) renal and hepatic function, (2) medication lipophilicity, (3) recommended dosing weight, and (4) observability of medication effects. Particular care should be taken with medications that have a narrow therapeutic window (eg, low-molecular-weight heparins) and for which the detection of harm may be delayed (eg, antibiotics). Pharmacists should be involved in medication and dose selection as much as possible.
Complications and Logistics
Thrombosis
Obesity is associated with increased risk of VTE in both the general population and hospitalized patients.66 This may be because of increased circulating procoagulant factors,67 slowed venous return related to increased intraabdominal pressure,68 or inadequate dosing of prophylactic anticoagulation.69 Diagnosis of VTE on ultrasound in obesity may be challenging because increased subcutaneous tissue can make visualizing deeper proximal veins difficult.70 Establishing venous compressibility with significant subcutaneous tissue may be difficult, potentially resulting in false-positive results.70 Physicians should incorporate a careful physical examination and clinical impression into the interpretation of ultrasound results in critically ill patients with obesity and suspected VTE.
Pressure Ulcers
Whether obesity contributes to excess pressure ulcers in hospitalized patients remains unclear.71 Studies are limited by failure to account for differences in nursing care intensity for patients with high BMI, and often do not distinguish pressure ulcer sites and stages. Pressure ulcer mechanisms specific to patients with obesity include increased difficulty repositioning, increased tensile pressure on skin, greater sweat production within more skin folds, and impaired microcirculation. At this time, standard care is recommended for pressure ulcer prevention including regular repositioning and frequent checks for early pressure injury.
Bed Size
Bariatric hospital beds are typically at least 120 cm wide, have greater weight capacity, and may help with positioning and mobility in patients with a BMI > 40 kg/m2.72 Increased bed width allows caregivers to roll patients to both sides without pushing or lifting. Furthermore, patients with obesity who want to reposition themselves in narrow hospital beds must use abdominal muscles to sit up, whereas wider beds allow them to roll over and push to a seated position, allowing greater movement and independence.
Infections
Obesity may be a risk factor for bloodstream infections, pneumonia, and soft tissue infections in hospitalized and critically ill patients.73,74 Potential contributors include altered cellular immunity,75 increased use of central venous catheters because of difficulties with peripheral access,73,74 prolonged urinary catheter use,73,74 and inadequate antibiotic dosing.76 Physicians should focus on frequent assessment and prompt removal of all in-dwelling catheters and appropriate antibiotic dose selection to mitigate infection risk.
Logistics
Incorrect BP cuff size may affect accuracy of BP measurement and lead to inappropriate care in the ICU. Physicians should confirm that the appropriate cuff is being used in patients with obesity.
Vascular Access
The placement of central venous catheters may be more challenging in the presence of increased subcutaneous adipose tissue. Ultrasound guidance should mitigate the increased challenges in identifying and cannulating vessels; however, dilation and catheter placement through greater subcutaneous tissue, particularly with a femoral approach, may still be more challenging. Central venous catheters may be more prone to infection in patients with more skin folds, a large pannus, and greater local sweat production. As in all patients, central venous catheters should be used only when necessary, assessed frequently, and removed for any signs of infection.
Radiology
Increased soft tissue density and upward displacement of the diaphragm may make interpretation of radiographs in patients with obesity more challenging. CT scans in such patients may exhibit increased noise because of radiation scatter caused by subcutaneous adipose tissue. This is particularly problematic when a larger field of view is required as in abdominal and pelvic imaging. Increased radiation dose may mitigate some of these effects.77 Bedside ultrasound may provide additional diagnostic information; however, quality may be limited because greater adipose tissue leads to decreased penetration of sound waves, difficulty identifying landmarks because of beam attenuation, and difficulty adequately positioning patients. Whether this alters diagnostic accuracy of lung ultrasound in particular has not been reported.
Obesity and Outcomes in Critical Illness
Many physicians think patients with obesity have worse ICU survival than those without. However, the preponderance of data suggests that this is unlikely. Meta-analyses78,79 have largely overcome the significant statistical heterogeneity limiting earlier studies. Most of the more recent studies, including an analysis of > 50,000 patients at 139 US hospitals,80 demonstrate that obesity is associated with similar or decreased mortality in mixed ICU populations,81 sepsis,79 and ARDS.82 In-depth comparisons of these studies can be found in systematic reviews.78,79
There are multiple potential explanations for this obesity paradox. Obesity may have protective physiological effects that contribute to improved ICU outcomes (see the Research and Emerging Literature section). Additionally, differences in fluid management, vasopressor dosing, and other aspects of treatment may differ systematically between critically ill patients with and without obesity, potentially impacting survival. It is also possible, however, that the obesity paradox reflects limitations inherent in observational studies of critical illness. Preexisting beliefs that patients with obesity are more ill or in need of closer monitoring than nonobese patients with comparable derangements may favor ICU admission of less ill patients with obesity, on average, leading to collider bias, in which one of the study selection variables (ICU admission) is linked to both the predictor (BMI) and outcome (survival).83 Furthermore, residual confounding may persist in all retrospective analyses, particularly those from large databases. Reviews of the obesity paradox in critical illness are available for additional details on this topic.78,84
Research and Emerging Literature
Whether changes in adipose tissue influence survival from critical illness remains unclear. Serum from critically ill patients, regardless of body mass, stimulates proliferation and accumulation of small adipocytes.85 These new adipocytes lead to the accumulation of antiinflammatory macrophages,86 which facilitate lipid storage while improving insulin sensitivity86 and protecting mitochondrial function.87,88 Adipose tissue may also serve as an energy reservoir in critical illness. In both mice and humans, obesity was associated with less muscle mass and function loss during critical illness because of greater mobilization of triglycerides from adipose tissue and less utilization of ectopically stored lipids and proteins.89,90 Further work is needed to identify how early in the course of critical illness these adipose tissue changes occur and whether they influence survival.
Adipokines regulate multiple immune cells and inflammatory pathways, but associations with organ dysfunction and survival are inconsistent. Circulating leptin has been associated with higher, similar, and lower survival in critical illness.91, 92, 93 Higher adiponectin levels were associated with higher mortality in sepsis and ARDS from extrapulmonary causes,94,95 but with lower Sequential Organ Function Assessment score on ICU admission.96 Resistin and visfatin concentrations have demonstrated more consistent associations with decreased survival in critical illness.97,98 Reviews of adipokines and adipose tissue in critical illness are recommended for in-depth summaries.7,98,99
Obesity and COVID-19
Obesity is associated with an increased risk of testing positive,100 developing severe disease,101 and dying102 from SARS-CoV-2 infection after adjustment for age, sex, and comorbidities. These associations may be modified by age with a stronger association between obesity and respiratory failure, ICU admission, or death among patients < 60 to 65 years of age.101,102
Obesity has been associated with similar or decreased risk of death in critical illness (see Obesity and Outcomes in Critical Illness section), which differs from findings in COVID-19.101,102 These disparate findings may reflect the study of a single homogeneous disease, differences in the allocation of pandemic-limited resources (eg, ventilators, prone positioning, extracorporeal membrane oxygen), or pathophysiological mechanisms specific to SARS-CoV-2 infection. For example, the gene for the SARS-CoV-2-binding ACE2 receptor is upregulated in adipose tissue,103 suggesting that adipose may serve as a reservoir for the SARS-CoV-2 virus; however, evidence for this is currently lacking.104
Conclusions
Obesity remains a common but unique challenge in critical illness. Studies have extended understanding of obesity-related pulmonary pathophysiology from the perioperative to the ICU setting. Obesity’s impact on multiple organ systems, medication dosing, and ICU complications and logistics is essential for physicians to consider in management decisions (Table 1).105, 106, 107, 108 Experience managing patients with obesity and severe SARS-CoV-2 infection has highlighted the importance of ongoing work to understand molecular mechanisms underlying both beneficial and injurious effects of obesity in critical illness.
Table 1.
Management Considerations in Specific Clinical Scenarios for Patients With Obesity
| Clinical Scenario | Management Considerations in Patients With Obesity | Key References |
|---|---|---|
| Intubation |
|
Dixon et al20 Futier et al21 Cabrini et al23 |
| ARDS |
|
Brower et al25 De Jong et al34Erstad and Barletta65 |
| Refractory hypoxemia |
|
Salna et al38 Schmidt et al105 |
| OHS + respiratory failure |
|
Bahammam106 |
| Extubation |
|
El Solh et al107 Ouellette et al46 |
| Hypotension |
|
Stelfox et al108 Lemmens et al51 |
| Pressure ulcer |
|
Wiggerman et al72 |
| Hospital-acquired infection |
|
Bochicchio et al74 Dossett et al73 |
| DVT |
|
Sebaaly and Covert69 Cascio et al70 |
ECMO = extracorporeal membrane oxygenation; HOB = head of bed; LV = left ventricular; NIV = noninvasive ventilation; OHS = obesity hypoventilation syndrome; PEEP = positive end-expiratory pressure; RV = right ventricular; VV-ECMO = venovenous extracorporeal membrane oxygenation.
Acknowledgments
Author contributions: M. G. S. S. takes responsibility for the content of the manuscript. M. R. A. and M. G. S. S. determined the scope and structure of the review, conducted the literature review, and drafted and made critical revisions to the manuscript. Both authors have given final approval of the manuscript.
Financial/nonfinancial disclosures: None declared.
Role of sponsor: The views expressed in this review do not communicate an official position of the National Institutes of Health.
Footnotes
FUNDING/SUPPORT: M. R. Anderson was supported by the National Institutes of Health [Grant K23-HL150280]. M. G. S. Shashaty was supported by the National Institutes of Health [Grant R01-DK111638].
References
- 1.Flegal K.M., Carroll D., Kit B.K., Ogden C.L. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307(5):491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization Obesity and overweight. Accessed March 10, 2021. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
- 3.Umbrello M., Fumagalli J., Pesenti A., Chiumello D. Pathophysiology and management of acute respiratory distress syndrome in obese patients. Semin Respir Crit Care Med. 2019;40(1):40–56. doi: 10.1055/s-0039-1685179. [DOI] [PubMed] [Google Scholar]
- 4.Schetz M., De Jong A., Deane A.M., et al. Obesity in the critically ill: a narrative review. Intensive Care Med. 2019;45(6):757–769. doi: 10.1007/s00134-019-05594-1. [DOI] [PubMed] [Google Scholar]
- 5.Mittwede P.N., Clemmer J.S., Bergin P.F., Xiang L. Obesity and critical illness: insights from animal models. Shock. 2016;45(4):349–358. doi: 10.1097/SHK.0000000000000512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heymsfield S.B., Wadden T.A. Mechanisms, pathophysiology, and management of obesity. N Engl J Med. 2017;376(3):254–266. doi: 10.1056/NEJMra1514009. [DOI] [PubMed] [Google Scholar]
- 7.Marques M.B., Langouche L. Endocrine, metabolic, and morphologic alterations of adipose tissue during critical illness. Crit Care Med. 2013;41(1):317–325. doi: 10.1097/CCM.0b013e318265f21c. [DOI] [PubMed] [Google Scholar]
- 8.Busetto L., Enzi G., Inelmen E.M., et al. Obstructive sleep apnea syndrome in morbid obesity: effects of intragastric balloon. Chest. 2005;128(2):618–623. doi: 10.1378/chest.128.2.618. [DOI] [PubMed] [Google Scholar]
- 9.Pelosi P., Croci M., Ravagnan I., Vicardi P., Gattinoni L. Total respiratory system, lung, and chest wall mechanics in sedated-paralyzed postoperative morbidly obese patients. Chest. 1996;109(1):144–151. doi: 10.1378/chest.109.1.144. [DOI] [PubMed] [Google Scholar]
- 10.Jones R.L., Nzekwu M.M.U. The effects of body mass index on lung volumes. Chest. 2006;130(3):827–833. doi: 10.1378/chest.130.3.827. [DOI] [PubMed] [Google Scholar]
- 11.Behazin N., Jones S.B., Cohen R.I., Loring S.H. Respiratory restriction and elevated pleural and esophageal pressures in morbid obesity. J Appl Physiol. 2010;108(1):212–218. doi: 10.1152/japplphysiol.91356.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pelosi P., Croci M., Ravagnan I., et al. Respiratory system mechanics in sedated, paralyzed, morbidly obese patients. J Appl Physiol. 1997;82(3):811–818. doi: 10.1152/jappl.1997.82.3.811. [DOI] [PubMed] [Google Scholar]
- 13.Kress J.P., Pohlman A.S., Alverdy J., Hall J.B. The impact of morbid obesity on oxygen cost of breathing (V̇O(2RESP)) at rest. Am J Respir Crit Care Med. 1999;160(3):883–886. doi: 10.1164/ajrccm.160.3.9902058. [DOI] [PubMed] [Google Scholar]
- 14.Steier J., Jolley C.J., Seymour J., Roughton M., Polkey M.I., Moxham J. Neural respiratory drive in obesity. Thorax. 2009;64(8):719–725. doi: 10.1136/thx.2008.109728. [DOI] [PubMed] [Google Scholar]
- 15.Lourenço R.V. Diaphragm activity in obesity. J Clin Invest. 1969;48(9):1609–1614. doi: 10.1172/JCI106126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kheterpal S., Han R., Tremper K.K., et al. Incidence and predictors of difficult and impossible mask ventilation. Anesthesiology. 2006;105(5):885–891. doi: 10.1097/00000542-200611000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Lundstrøm L.H., Møller A.M., Rosenstock C., Astrup G., Wetterslev J. High body mass index is a weak predictor for difficult and failed tracheal intubation: a cohort study of 91,332 consecutive patients scheduled for direct laryngoscopy registered in the danish anesthesia database. Anesthesiology. 2009;110(2):266–274. doi: 10.1097/ALN.0b013e318194cac8. [DOI] [PubMed] [Google Scholar]
- 18.Moon T.S., Fox P.E., Somasundaram A., et al. The influence of morbid obesity on difficult intubation and difficult mask ventilation. J Anesth. 2019;33(1):96–102. doi: 10.1007/s00540-018-2592-7. [DOI] [PubMed] [Google Scholar]
- 19.De Jong A., Molinari N., Terzi N., et al. Early identification of patients at risk for difficult intubation in the intensive care unit. Am J Respir Crit Care Med. 2013;187(8):832–839. doi: 10.1164/rccm.201210-1851OC. [DOI] [PubMed] [Google Scholar]
- 20.Dixon B.J., Dixon J.B., Carden J.R., et al. Preoxygenation is more effective in the 25° head-up position than in the supine position in severely obese patients: a randomized controlled study. Anesthesiology. 2005;102(6):1110–1115. doi: 10.1097/00000542-200506000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Futier E., Constantin J.M., Pelosi P., et al. Noninvasive ventilation and alveolar recruitment maneuver improve respiratory function during and after intubation of morbidly obese patients: a randomized controlled study. Anesthesiology. 2011;114(6):1354–1363. doi: 10.1097/ALN.0b013e31821811ba. [DOI] [PubMed] [Google Scholar]
- 22.Vourc'h M., Baud G., Feuillet F., et al. High-flow nasal cannulae versus non-invasive ventilation for preoxygenation of obese patients: the PREOPTIPOP randomized trial. EClinicalMedicine. 2019;13:112–119. doi: 10.1016/j.eclinm.2019.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cabrini L., Landoni G., Baiardo Redaelli M., et al. Tracheal intubation in critically ill patients: a comprehensive systematic review of randomized trials. Crit Care. 2018;22(1):6. doi: 10.1186/s13054-017-1927-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gong M.N., Bajwa E.K., Thompson B.T., Christiani D.C. Body mass index is associated with the development of acute respiratory distress syndrome. Thorax. 2010;65(1):44–50. doi: 10.1136/thx.2009.117572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brower R.G., Matthay M.A., Morris A., Schoenfeld D., Thompson B.T., Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 26.Pelosi P., Ravagnan I., Gíuratí G., et al. Positive end-expiratory pressure improves respiratory function in obese but not in normal subjects during anesthesia and paralysis. Anesthesiology. 1999;91(5):1221–1231. doi: 10.1097/00000542-199911000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Koutsoukou A., Koulouris N., Bekos B., et al. Expiratory flow limitation in morbidly obese postoperative mechanically ventilated patients. Acta Anaesthesiol Scand. 2004;48(9):1080–1088. doi: 10.1111/j.1399-6576.2004.00479.x. [DOI] [PubMed] [Google Scholar]
- 28.Pirrone M., Fisher D., Chipman D., et al. Recruitment maneuvers and positive end-expiratory pressure titration in morbidly obese ICU patients. Crit Care Med. 2016;44(2):300–307. doi: 10.1097/CCM.0000000000001387. [DOI] [PubMed] [Google Scholar]
- 29.Fumagalli J., Berra L., Zhang C., et al. Transpulmonary pressure describes lung morphology during decremental positive end-expiratory pressure trials in obesity. Crit Care Med. 2017;45(8):1374–1381. doi: 10.1097/CCM.0000000000002460. [DOI] [PubMed] [Google Scholar]
- 30.Fumagalli J., Santiago R.R.S., Teggia Droghi M., et al. Lung recruitment in obese patients with acute respiratory distress syndrome. Anesthesiology. 2019;130(5):791–803. doi: 10.1097/ALN.0000000000002638. [DOI] [PubMed] [Google Scholar]
- 31.De Santis Santiago R., Teggia Droghi M., Fumagalli J., et al. High pleural pressure prevents alveolar overdistension and hemodynamic collapse in ARDS with class III obesity. Am J Respir Crit Care Med. 2020;203(5):575–584. doi: 10.1164/rccm.201909-1687OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Writing Group for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial (ART) Investigators. Cavalcanti A.B., Suzumura É.A., et al. Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs low PEEP on mortality in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2017;318(14):1335–1345. doi: 10.1001/jama.2017.14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coudroy R., Vimpere D., Aissaoui N., et al. Prevalence of complete airway closure according to body mass index in acute respiratory distress syndrome: pooled cohort analysis. Anesthesiology. 2020;133(4):867–878. doi: 10.1097/ALN.0000000000003444. [DOI] [PubMed] [Google Scholar]
- 34.De Jong A., Molinari N., Sebbane M., et al. Feasibility and effectiveness of prone position in morbidly obese patients with ARDS: a case-control clinical study. Chest. 2013;143(6):1554–1561. doi: 10.1378/chest.12-2115. [DOI] [PubMed] [Google Scholar]
- 35.Weig T., Schubert M.I., Gruener N., et al. Abdominal obesity and prolonged prone positioning increase risk of developing sclerosing cholangitis in critically ill patients with influenza A-associated ARDS. Eur J Med Res. 2012;17(1):30. doi: 10.1186/2047-783X-17-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guérin C., Reignier J., Richard J.C., et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 37.Pelosi P., Croci M., Calappi E., et al. Prone positioning improves pulmonary function in obese patients during general anesthesia. Anesth Analg. 1996;83(3):578–583. doi: 10.1097/00000539-199609000-00025. [DOI] [PubMed] [Google Scholar]
- 38.Salna M., Chicotka S., Biscotti M., III, et al. Morbid obesity is not a contraindication to transport on extracorporeal support. Eur J Cardiothorac Surg. 2018;53(4):793–798. doi: 10.1093/ejcts/ezx452. [DOI] [PubMed] [Google Scholar]
- 39.Swol J, Buchwald D, Strauch JT, Schildhauer TA, Ull C. Effect of body mass index on the outcome of surgical patients receiving extracorporeal devices (VV ECMO, pECLA) for respiratory failure [published online ahead of print April 20, 2017]. Int J Artif Organs.https://doi.org/10.5301/ijao.5000572. [DOI] [PubMed]
- 40.Galvagno S.M., Jr., Pelekhaty S., Cornachione C.R., et al. Does weight matter? Outcomes in adult patients on venovenous extracorporeal membrane oxygenation when stratified by obesity class. Anesth Analg. 2020;131(3):754–761. doi: 10.1213/ANE.0000000000004454. [DOI] [PubMed] [Google Scholar]
- 41.Combes A., Hajage D., Capellier G., et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378(21):1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 42.Lederer D.J., Kawut S.M., Wickersham N., et al. Obesity and primary graft dysfunction after lung transplantation: the Lung Transplant Outcomes Group Obesity Study. Am J Respir Crit Care Med. 2011;184(9):1055–1061. doi: 10.1164/rccm.201104-0728OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson M.R., Udupa J.K., Edwin E., et al. Adipose tissue quantification and primary graft dysfunction after lung transplantation: The Lung Transplant Body Composition study. J Heart Lung Transplant. 2019;38(12):1246–1256. doi: 10.1016/j.healun.2019.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carrillo A., Ferrer M., Gonzalez-Diaz G., et al. Noninvasive ventilation in acute hypercapnic respiratory failure caused by obesity hypoventilation syndrome and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(12):1279–1285. doi: 10.1164/rccm.201206-1101OC. [DOI] [PubMed] [Google Scholar]
- 45.O'Brien J.M., Jr., Philips G.S., Ali N.A., Aberegg S.K., Marsh C.B., Lemeshow S. The association between body mass index, processes of care, and outcomes from mechanical ventilation: a prospective cohort study. Crit Care Med. 2012;40(5):1456–1463. doi: 10.1097/CCM.0b013e31823e9a80. [DOI] [PubMed] [Google Scholar]
- 46.Ouellette D.R., Patel S., Girard T.D., et al. Liberation From Mechanical Ventilation in Critically Ill Adults: An Official American College of Chest Physicians/American Thoracic Society Clinical Practice Guideline: Inspiratory Pressure Augmentation During Spontaneous Breathing Trials, Protocols Minimizing Sedation, and Noninvasive Ventilation Immediately After Extubation. Chest. 2017;151(1):166–180. doi: 10.1016/j.chest.2016.10.036. [DOI] [PubMed] [Google Scholar]
- 47.Stéphan F., Bérard L., Rézaiguia-Delclaux S., Amaru P. High-flow nasal cannula therapy versus intermittent noninvasive ventilation in obese subjects after cardiothoracic surgery. Respir Care. 2017;62(9):1193–1202. doi: 10.4187/respcare.05473. [DOI] [PubMed] [Google Scholar]
- 48.Corley A., Bull T., Spooner A.J., Barnett A.G., Fraser J.F. Direct extubation onto high-flow nasal cannulae post-cardiac surgery versus standard treatment in patients with a BMI ≥30: a randomised controlled trial. Intensive Care Med. 2015;41(5):887–894. doi: 10.1007/s00134-015-3765-6. [DOI] [PubMed] [Google Scholar]
- 49.El Solh A.A., Jaafar W. A comparative study of the complications of surgical tracheostomy in morbidly obese critically ill patients. Crit Care. 2007;11(1):R3. doi: 10.1186/cc5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dennis B.M., Eckert M.J., Gunter O.L., Morris J.A., Jr., May A.K. Safety of bedside percutaneous tracheostomy in the critically ill: evaluation of more than 3,000 procedures. J Am Coll Surg. 2013;216(4):858–867. doi: 10.1016/j.jamcollsurg.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 51.Lemmens H.J.M., Bernstein D.P., Brodsky J.B. Estimating blood volume in obese and morbidly obese patients. Obes Surg. 2006;16(6):773. doi: 10.1381/096089206777346673. [DOI] [PubMed] [Google Scholar]
- 52.Kenchaiah S., Evans J.C., Levy D., et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347(5):305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 53.Vyas V., Lambiase P. Obesity and atrial fibrillation: epidemiology, pathophysiology and novel therapeutic opportunities. Arrhythm Electrophysiol Rev. 2019;8(1):28–36. doi: 10.15420/aer.2018.76.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang T.J., Parise H., Levy D., et al. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292(20):2471–2477. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 55.Chahal H., McClelland R.L., Tandri H., et al. Obesity and right ventricular structure and function: the MESA-right ventricle study. Chest. 2012;141(2):388–395. doi: 10.1378/chest.11-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rhodes A., Evans L.E., Alhazzani W., et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017;43(3):304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 57.Winfield R.D., Delano M.J., Lottenberg L., et al. Traditional resuscitative practices fail to resolve metabolic acidosis in morbidly obese patients after severe blunt trauma. J Trauma. 2010;68(2):317–328. doi: 10.1097/TA.0b013e3181caab6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ejerblad E., Fored C.M., Lindblad P., Fryzek J., McLaughlin J.K., Nyrén O. Obesity and risk for chronic renal failure. J Am Soc Nephrol. 2006;17(6):1695–1702. doi: 10.1681/ASN.2005060638. [DOI] [PubMed] [Google Scholar]
- 59.Soto G.J., Frank A.J., Christiani D.C., Gong M.N. Body mass index and acute kidney injury in the acute respiratory distress syndrome. Crit Care Med. 2012;40(9):2601–2608. doi: 10.1097/CCM.0b013e3182591ed9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vasquez C.R., DiSanto T., Reilly J.P., et al. Relationship of body mass index, serum creatine kinase, and acute kidney injury after severe trauma. J Trauma Acute Care Surg. 2020;89(1):179–185. doi: 10.1097/TA.0000000000002714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kellum J.A., Lameire N., Aspelin P., et al. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney International Supplements. 2012;2(1):1–138. [Google Scholar]
- 62.Erstad B.L. In: Critical Care Management of the Obese Patient. El Solh A.A., editor. John Wiley & Sons; 2012. Drug dosing in the critically ill obese patient.https://onlinelibrary.wiley.com/doi/book/10.1002/9781119962083 [Google Scholar]
- 63.Levey A.S., Stevens L.A., Schmid C.H., et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Casati A., Putzu M. Anesthesia in the obese patient: pharmacokinetic considerations. J Clin Anesth. 2005;17(2):134–145. doi: 10.1016/j.jclinane.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 65.Erstad B.L., Barletta J.F. Drug dosing in the critically ill obese patient-a focus on sedation, analgesia, and delirium. Crit Care. 2020;24(1):315. doi: 10.1186/s13054-020-03040-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Puurunen M.K., Gona P.N., Larson M.G., Murabito J.M., Magnani J.W., O'Donnell C.J. Epidemiology of venous thromboembolism in the Framingham Heart Study. Thromb Res. 2016;145:27–33. doi: 10.1016/j.thromres.2016.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rosito G.A., D'Agostino R.B., Massaro J., et al. Association between obesity and a prothrombotic state: the Framingham Offspring Study. Thromb Haemost. 2004;91(4):683–689. doi: 10.1160/th03-01-0014. [DOI] [PubMed] [Google Scholar]
- 68.Willenberg T., Schumacher A., Amann-Vesti B., et al. Impact of obesity on venous hemodynamics of the lower limbs. J Vasc Surg. 2010;52(3):664–668. doi: 10.1016/j.jvs.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 69.Sebaaly J., Covert K. Enoxaparin dosing at extremes of weight: literature review and dosing recommendations. Ann Pharmacother. 2018;52(9):898–909. doi: 10.1177/1060028018768449. [DOI] [PubMed] [Google Scholar]
- 70.Cascio V., Hon M., Haramati L.B., et al. Imaging of suspected pulmonary embolism and deep venous thrombosis in obese patients. Br J Radiol. 2018;91(1089):20170956. doi: 10.1259/bjr.20170956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hyun S., Li X., Vermillion B., et al. Body mass index and pressure ulcers: improved predictability of pressure ulcers in intensive care patients. Am J Crit Care. 2014;23(6):494–500. doi: 10.4037/ajcc2014535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wiggermann N., Smith K., Kumpar D. What bed size does a patient need? The relationship between body mass index and space required to turn in bed. Nurs Res. 2017;66(6):483–489. doi: 10.1097/NNR.0000000000000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dossett L.A., Dageforde L.A., Swenson B.R., et al. Obesity and site-specific nosocomial infection risk in the intensive care unit. Surg Infect (Larchmt) 2009;10(2):137–142. doi: 10.1089/sur.2008.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bochicchio G.V., Joshi M., Bochicchio K., Nehman S., Tracy J.K., Scalea T.M. Impact of obesity in the critically ill trauma patient: a prospective study. J Am Coll Surg. 2006;203(4):533–538. doi: 10.1016/j.jamcollsurg.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 75.Green W.D., Beck M.A. Obesity impairs the adaptive immune response to influenza virus. Ann Am Thorac Soc. 2017;14(suppl 5):S406–S409. doi: 10.1513/AnnalsATS.201706-447AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Roe J.L., Fuentes J.M., Mullins M.E. Underdosing of common antibiotics for obese patients in the ED. Am J Emerg Med. 2012;30(7):1212–1214. doi: 10.1016/j.ajem.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 77.Buckley O., Ward E., Ryan A., Colin W., Snow A., Torreggiani W.C. European obesity and the radiology department. What can we do to help? Eur Radiol. 2009;19(2):298–309. doi: 10.1007/s00330-008-1154-z. [DOI] [PubMed] [Google Scholar]
- 78.Zhao Y., Li Z., Yang T., Wang M., Xi X. Is body mass index associated with outcomes of mechanically ventilated adult patients in intensive critical units? A systematic review and meta-analysis. PLoS One. 2018;13(6) doi: 10.1371/journal.pone.0198669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pepper D.J., Sun J., Welsh J., Cui X., Suffredini A.F., Eichacker P.Q. Increased body mass index and adjusted mortality in ICU patients with sepsis or septic shock: a systematic review and meta-analysis. Crit Care. 2016;20(1):181. doi: 10.1186/s13054-016-1360-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pepper D.J., Demirkale C.Y., Sun J., et al. Does obesity protect against death in sepsis? A retrospective cohort study of 55,038 adult patients. Crit Care Med. 2019;47(5):643–650. doi: 10.1097/CCM.0000000000003692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Acharya P., Upadhyay L., Qavi A., et al. The paradox prevails: outcomes are better in critically ill obese patients regardless of the comorbidity burden. J Crit Care. 2019;53:25–31. doi: 10.1016/j.jcrc.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 82.Ni Y.N., Luo J., Yu H., et al. Can body mass index predict clinical outcomes for patients with acute lung injury/acute respiratory distress syndrome? A meta-analysis. Crit Care. 2017;21(1):36. doi: 10.1186/s13054-017-1615-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Decruyenaere A., Steen J., Colpaert K., Benoit D.D., Decruyenaere J., Vansteelandt S. The obesity paradox in critically ill patients: a causal learning approach to a casual finding. Crit Care. 2020;24(1):485. doi: 10.1186/s13054-020-03199-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Karampela I., Chrysanthopoulou E., Christodoulatos G.S., Dalamaga M. Is there an obesity paradox in critical illness? Epidemiologic and metabolic considerations. Curr Obes Rep. 2020;9(3):231–244. doi: 10.1007/s13679-020-00394-x. [DOI] [PubMed] [Google Scholar]
- 85.Goossens C., Vander Perre S., Van den Berghe G., Langouche L. Proliferation and differentiation of adipose tissue in prolonged lean and obese critically ill patients. Intensive Care Med Exp. 2017;5(1):16. doi: 10.1186/s40635-017-0128-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Langouche L., Marques M.B., Ingels C., et al. Critical illness induces alternative activation of M2 macrophages in adipose tissue. Crit Care. 2011;15(5):R245. doi: 10.1186/cc10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vanhorebeek I., Ellger B., De Vos R., et al. Tissue-specific glucose toxicity induces mitochondrial damage in a burn injury model of critical illness. Crit Care Med. 2009;37(4):1355–1364. doi: 10.1097/CCM.0b013e31819cec17. [DOI] [PubMed] [Google Scholar]
- 88.Thiessen S.E., Vanhorebeek I., Derese I., Gunst J., Van den Berghe G. FGF21 response to critical illness: effect of blood glucose control and relation with cellular stress and survival. J Clin Endocrinol Metab. 2015;100(10):E1319–E1327. doi: 10.1210/jc.2015-2700. [DOI] [PubMed] [Google Scholar]
- 89.Goossens C., Marques M.B., Derde S., et al. Premorbid obesity, but not nutrition, prevents critical illness-induced muscle wasting and weakness. J Cachexia Sarcopenia Muscle. 2017;8(1):89–101. doi: 10.1002/jcsm.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Goossens C., Weckx R., Derde S., et al. Adipose tissue protects against sepsis-induced muscle weakness in mice: from lipolysis to ketones. Crit Care. 2019;23(1):236. doi: 10.1186/s13054-019-2506-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bornstein S.R., Licinio J., Tauchnitz R., et al. Plasma leptin levels are increased in survivors of acute sepsis: associated loss of diurnal rhythm in cortisol and leptin secretion. J Clin Endocrinol Metab. 1998;83(1):280–283. doi: 10.1210/jcem.83.1.4610. [DOI] [PubMed] [Google Scholar]
- 92.Koch A., Weiskirchen R., Zimmermann H.W., Sanson E., Trautwein C., Tacke F. Relevance of serum leptin and leptin-receptor concentrations in critically ill patients. Mediators Inflamm. 2010;2010:473540. doi: 10.1155/2010/473540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bracho-Riquelme R.L., Reyes-Romero M.A., Pescador N., Flores-García A.I. A leptin serum concentration less than 10 ng/ml is a predictive marker of outcome in patients with moderate to severe secondary peritonitis. Eur Surg Res. 2008;41(2):238–244. doi: 10.1159/000136480. [DOI] [PubMed] [Google Scholar]
- 94.Koch A., Sanson E., Voigt S., Helm A., Trautwein C., Tacke F. Serum adiponectin upon admission to the intensive care unit may predict mortality in critically ill patients. J Crit Care. 2011;26(2):166–174. doi: 10.1016/j.jcrc.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 95.Walkey A.J., Demissie S., Shah D., Romero F., Puklin L., Summer R.S. Plasma adiponectin, clinical factors, and patient outcomes during the acute respiratory distress syndrome. PLoS One. 2014;9(9) doi: 10.1371/journal.pone.0108561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hillenbrand A., Knippschild U., Weiss M., et al. Sepsis induced changes of adipokines and cytokines - septic patients compared to morbidly obese patients. BMC Surg. 2010;10:26. doi: 10.1186/1471-2482-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee K., Huh J.W., Lim C.M., Koh Y., Hong S.B. Clinical role of serum pre-B cell colony-enhancing factor in ventilated patients with sepsis and acute respiratory distress syndrome. Scand J Infect Dis. 2013;45(10):760–765. doi: 10.3109/00365548.2013.797600. [DOI] [PubMed] [Google Scholar]
- 98.Hajri T., Gharib M., Kaul S., Karpeh M.S., Jr. Association between adipokines and critical illness outcomes. J Trauma Acute Care Surg. 2017;83(3):507–519. doi: 10.1097/TA.0000000000001610. [DOI] [PubMed] [Google Scholar]
- 99.Karampela I., Christodoulatos G.S., Dalamaga M. The role of adipose tissue and adipokines in sepsis: inflammatory and metabolic considerations, and the obesity paradox. Curr Obes Rep. 2019;8(4):434–457. doi: 10.1007/s13679-019-00360-2. [DOI] [PubMed] [Google Scholar]
- 100.de Lusignan S., Dorward J., Correa A., et al. Risk factors for SARS-CoV-2 among patients in the Oxford Royal College of General Practitioners Research and Surveillance Centre primary care network: a cross-sectional study. Lancet Infect Dis. 2020;20(9):1034–1042. doi: 10.1016/S1473-3099(20)30371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Anderson M.R., Geleris J., Anderson D.R., et al. Body mass index and risk for intubation or death in SARS-CoV-2 infection: a retrospective cohort study. Ann Intern Med. 2020;173(10):782–790. doi: 10.7326/M20-3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tartof S.Y., Qian L., Hong V., et al. Obesity and mortality among patients diagnosed with COVID-19: results from an integrated health care organization. Ann Intern Med. 2020;173(10):773–781. doi: 10.7326/M20-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Al-Benna S. Association of high level gene expression of ACE2 in adipose tissue with mortality of COVID-19 infection in obese patients. Obes Med. 2020;19:100283. doi: 10.1016/j.obmed.2020.100283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Safari S., Keyvani H., Alamdari N.M., et al. Abdominal surgery in patients with COVID-19: detection of SARS-CoV-2 in abdominal and adipose tissues. Ann Surg. 2020;272(3):e253–e256. doi: 10.1097/SLA.0000000000004165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schmidt M., Bailey M., Sheldrake J., et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am J Respir Crit Care Med. 2014;189(11):1374–1382. doi: 10.1164/rccm.201311-2023OC. [DOI] [PubMed] [Google Scholar]
- 106.Bahammam A. Acute ventilatory failure complicating obesity hypoventilation: update on a ‘critical care syndrome.’. Curr Opin Pulm Med. 2010;16(6):543–551. doi: 10.1097/MCP.0b013e32833ef52e. [DOI] [PubMed] [Google Scholar]
- 107.El Solh A., Aquilina A.A., Pineda L., Dhanvantri V., Grant B., Bouquin P. Noninvasive ventilation for prevention of post-extubation respiratory failure in obese patients. Eur Respir J. 2006;28(3):588–595. doi: 10.1183/09031936.06.00150705. [DOI] [PubMed] [Google Scholar]
- 108.Stelfox H.T., Ahmed S.B., Ribeiro R.A., Gettings E.M., Pomerantsev E., Schmidt U. Hemodynamic monitoring in obese patients: the impact of body mass index on cardiac output and stroke volume. Crit Care Med. 2006;34(4):1243–1246. doi: 10.1097/01.CCM.0000208358.27005.F4. [DOI] [PubMed] [Google Scholar]