Abstract
Background
The relationship of vitamin D status and other biochemical parameters with the risk of SARS-CoV-2 infection remains inconclusive, especially in regions with high solar incidence. Therefore, we aimed to associate the 25-hydroxyvitamin D (25(OH)D) concentrations and lipid profile prior to the SARS-CoV-2 tests in a population from a sunny region in Brazil (5 degrees S, 35 degrees W).
Methods
This retrospective cohort study enrolled 1634 patients tested for SARS-CoV-2 of a private medical laboratory with 25(OH)D concentration and lipid profile measured ≥ 7 days before the date of the first SARS-CoV-2 RT-PCR test and were categorized according to 25(OH)D sufficiency (≥30 ng/mL) or insufficiency (<30 ng/mL). Multiple logistic regression analyses were performed to assess risk factors associated with positive tests for SARS-CoV-2.
Results
Average serum 25(OH)D was 33.6 ng/mL. Vitamin D deficiency (<20 ng/mL) was only found in 2.6% of the participants. Multivariate analysis demonstrated that patients > 49 y with insufficient 25(OH)D (<30 ng/mL) presented increased odds to test positive for SARS-CoV-2 (OR: 2.02, 95 %CI: 1.15 to 3.55, P = 0.015). The same is observed among those with total cholesterol > 190 mg/dL (OR: 1.90, 95 %CI: 1.10 to 3.28, P = 0.020).
Conclusions
Previous insufficient 25(OH)D (<30 ng/mL) concentration and high total cholesterol were associated with SARS-CoV-2 infection among adults > 48 y in the study population. Further studies should be conducted to confirm whether measurement of 25(OH)D and lipid profile could be useful to identify patients who are more susceptible to COVID-19.
Keywords: COVID-19, Cholecalciferol, Lipid Profile, SARS-CoV-2
1. Introduction
Vitamin D is an immunomodulatory hormone which performs metabolic functions related to adaptive and innate immunity with proven efficacy against various upper respiratory infections [1], [2], [3]. Its deficiency is gaining prominence in the current pandemic caused by the SARS-CoV-2 virus [4], [5].
Some studies have shown an association between insufficient vitamin D status and major symptom complications in COVID-19 due to impaired immune function [6], [7], [8]. An adequate serum concentration was associated with decreased ‘cytokine storm’, a severe manifestation of COVID-19 [9] which occurs through lysosomal enzyme expression and nitric oxide release, mitigating the hyperinflammatory response [10]. Vitamin D also influences the expression of toll-like receptors which recognize pathogenic proteins and articulate the immune response [11].
There are also discussions about the potential role of vitamin D in reducing the risk of infection and the development of COVID-19 [12], [13]. This antiviral function is related to mechanisms that block viral entry into cells, suppress viral replication and cell autophagy, and strengthen the body’s immune defense against viral infection [14], [15].
Although vitamin D deficiency is commonly seen among patients with COVID-19 [16], [17], [18], the predictor role of 25-hydroxyvitamin D (25(OH)D) status for disease risk remains inconclusive. Some retrospective studies observed that lower 25(OH)D concentrations were associated with a worse prognosis in COVID-19 hospitalized patients [19], [20], [21]. Furthermore, the threat of severe COVID-19 in patients with impaired metabolic health (such as dyslipidemia) is much higher than that of the general population [22], [23], and whether metabolic health combined with prolonged vitamin D insufficiency predispose individuals to virus infection remains unknown.
However, caution is needed when interpreting 25(OH)D values among hospitalized patients, since vitamin D concentrations may be subject to the effect of critical illness [24], [25]. Thus, research involving 25(OH)D analysis before being tested for SARS-CoV-2 infection is essential for this understanding [26], [27].
2. Methods
2.1. Study population
SARS-CoV-2 patients tested at the DNA Center Laboratory (Natal, Brazil) from April 1st to December 31st, 2020, with serum 25(OH)D and lipid profile performed from April 1st to June 30th, 2020, were recruited for a retrospective study. Natal is a city in northeastern Brazil located at 5 degrees south latitude, and has a high solar radiation intensity [28]. Patients who had their data available in the DNA Center Laboratory database (SoftLab© program - ND Engenharia e Software) were included in the analysis. This study was reviewed and approved by the Ethics Committee of the Federal University of Rio Grande do Norte, with a consent waiver for the use of non-identifiable data. No data to allow personal identification of the participants was made available. Retrospective data was collected from 25(OH)D, total cholesterol, high-density lipoprotein (HDL), non-HDL cholesterol (nHDL), and triglyceride serum concentration.
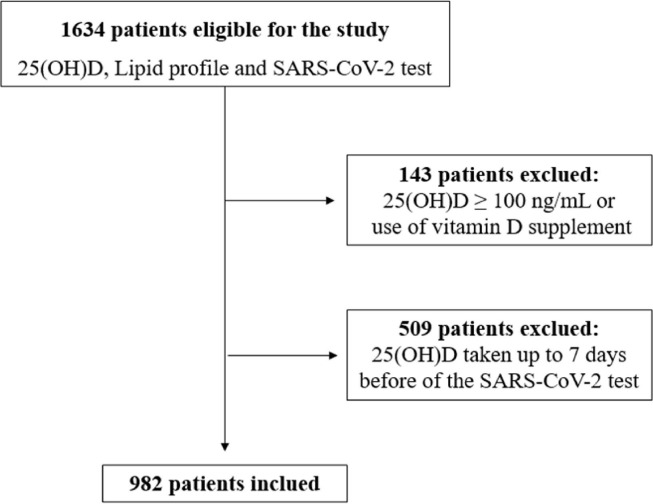
Retrospective data were collected from 1634 patients tested for SARS-CoV-2. Data regarding 25(OH)D and lipid profile were obtained from the most recent collection before the SARS-CoV-2 test, regardless of positive or negative result. Patients who had serum analysis taken up to 7 days before the date of the first SARS-CoV-2 test were excluded from the study to avoid possible confounding due to potential early manifestations of infection. Patients who reported the use of any vitamin D supplement and those who had 25(OH)D ≥ 100 ng/mL (risk of vitamin D toxicity) [29] were also excluded. After the exclusions, data from 982 individuals were included in this study (Fig. 1 ).
Fig. 1.
Flow diagram of retrospective study inclusion.
2.2. Measurements
The SARS-CoV-2 infection diagnosis was determined by real-time reverse-transcription-polymerase-chain-reaction assay (RT-PCR) with nasopharyngeal and oropharyngeal swab according to the protocol from the Centers for Disease Control and Prevention, USA. Biopur Fast® (Mobius Life Science) reagent was used to extract viral RNA from SARS-CoV-2. The equipment used to heat the sample was Veriti™ 96-Well Thermal Cycler (Thermo Fisher Scientific). The analyses were performed according to the recommendations of the manufacturers. Real-time PCR (RT-PCR) analysis of viral RNA was performed using the XGEN MASTER COVID-19 Kit (Mobius Life Science). The amplification of the ORFI1ab (FAM) and N (ROX) genes (belonging to the SARS-CoV-2 virus) was performed in an automated ABI 7500 Fast (Applied Biosystems) system. The reactions were unitary and the data analyzed using the System 7500 v 2.0.5 software program (Applied Biosystems). The samples which presented Ct (Cycle Threshold) < 38 and amplification for the genes ORF1ab (FAM) and N (ROX) were considered positive for the presence of the SARS-CoV-2 virus. Serum 25(OH)D concentrations were evaluated by automated chemiluminescence using VITROS® MicroWell Technology from VITROS® 5600 Integrated System (Ortho Clinical Diagnostics) according to manufacturer’s instructions.
Vitamin D insufficiency was defined as 25(OH)D < 30 ng/mL, and vitamin D sufficiency as 25(OH)D ≥ 30 ng/mL [29]. Total cholesterol, high density lipoprotein cholesterol (HDL-C), and triglycerides were measured using VITROS® MicroSlide from VITROS® 5600 Integrated System (Ortho Clinical Diagnostics). Low density lipoprotein cholesterol (LDL-C) concentrations were calculated according to the Martin [30] formula. The non-high density lipoprotein cholesterol (non-HDL-C) concentrations were calculated by the formula: non-HDL-C = total cholesterol minus HDL-C [31]. Total cholesterol, HDL-C, non-HDL-C, LDL-C and triglycerides were categorized according to the V Brazilian Guideline for Dyslipidemias and Prevention of Atherosclerosis [32].
2.3. Statistical analysis
Statistical analysis was performed using SPSS® 22.0 software program (and R version 3.6.1 using the ggplot2 package [33]. Normal distribution was evaluated using the Kolmogorov–Smirnov test. All variables are presented as the median and interquartile range (25% − 75%) and were compared using Mann-Whitney tests. Categorical variables were compared by the chi-squared test. The evaluation of odds factors associated with SARS-CoV-2 test results was conducted using the multivariate model of logistic regression following the stepwise method to select the model variables. Odds Ratio (OR) were estimated with a 95% confidence interval (95% CI). A p < 0.05 was considered significant.
3. Results
A total of 982 patients (70.7% female, mean age 45 ± 16 years) were enrolled, and 258 (26.3%) tested positive for SARS-CoV-2 (Table 1 ). The frequency of individuals with 25(OH)D < 20 ng/mL was 2.6% (26 patients).
Table 1.
Characteristics of study population based on 25(OH)D status.
| Total (n = 982) | 25(OH)D ≥ 30 ng/mL(n = 650) | 25(OH)D <30 ng/mL(n = 332) | P-value | |
|---|---|---|---|---|
| Age, median (IQR), y | 41 (33–55) | 41 (34–57) | 39 (33–52) | 0.002 |
| Sex, female (% within stratum) | 70.7 (694) | 70.0 (454) | 72.3 (240) | NS |
| SARS-CoV-2 positive test (% within stratum) | 26.3 (258) | 26.2 (171) | 26.4 (87) | NS |
| 25(OH)D, median (IQR), ng/mL | 33.6 (28.0–40.0) | 37.3 (33.6–44.1) | 25.7 (23.1–28.0) | ND |
| Total cholesterol, median (IQR), mg/dL | 194 (166–224) | 192 (161–221) | 198 (174–228) | 0.006 |
| Non-HDL cholesterol, median (IQR), mg/dL | 141 (113–172) | 136 (109–166) | 146 (119–177) | <0.001 |
| LDL cholesterol, median (IQR), mg/dL | 115.8 (91.0–142.4) | 112.3 (88.7–139.4) | 118.1 (97.9–146.6) | 0.010 |
| HDL cholesterol, median (IQR), mg/dL | 51 (42–61) | 52 (43–62) | 49 (41–61) | NS |
| Triglycerides, median (IQR), mg/dL | 126 (86–180) | 119 (84–168) | 146 (96–204) | <0.001 |
Abbreviations: ND, not determined; NS, non-significant; 25(OH)D, 25-hydroxyvitamin D; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2.
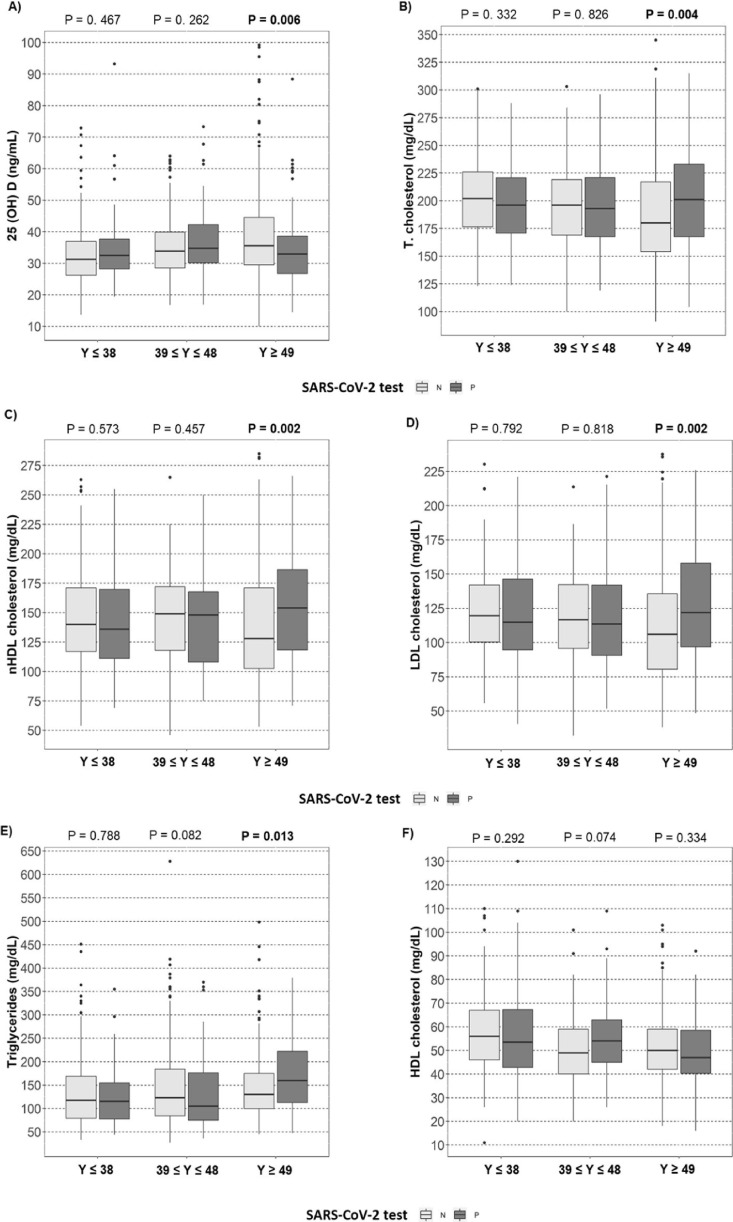
25(OH)D status was associated with age and serum lipid profile, but not associated with the SARS-CoV-2 test (Table 1). After stratification by age (in tertiles), insufficient 25(OH)D concentrations (<30 ng/mL) were associated with SARS-CoV-2 positivity in the third tertile of age (>49 years) (Fig. 2 A).
Fig. 2.
Association of age tertiles with 25(OH)D and lipid profile in negative and positive SARS-CoV-2 test. A) Plasma concentrations of 25(OH)D (vertical axis), B) Plasma concentrations of total-cholesterol, C) Plasma concentrations of HDL cholesterol, D) Plasma concentrations of LDL cholesterol, E) Plasma concentrations of triglycerides, F) Plasma concentrations of HDL cholesterol, and 3 tertiles (≤38, 39 ≤ y ≥ 48, ≥49 y) of age (horizontal axis), among patients with positive or negative SARS-Cov-2 test. The significant values (P < 0.05) are shown in bold. Abbreviations: 25(OH)D, 25-hydroxyvitamin D; nHDL cholesterol, non-HDL Cholesterol; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2;
Multivariate analysis demonstrated that patients > 49 years with 25(OH)D < 30 ng/mL presented increased odds for a positive SARS-CoV-2 test (OR: 2.02, 95 %CI: 1.15–3.55, P = 0.015, Table 2 ). The same is observed among those with Total Cholesterol > 190 mg/dL (OR: 1.90, 95 %CI: 1.10–3.28, P = 0.020, Table 2). The cumulative odds for SARS-CoV-2 infection is 3.92 (Table 2). A second multivariate analysis was performed without males and > 49 years. Female patients > 49 years with 25(OH)D < 30 ng/mL and Total Cholesterol > 190 mg/dL presented cumulative odds for SARS-CoV-2 infection of 5.35 (Table S1).
Table 2.
Multivariable logistic regression between 25(OH)D and lipid profile with SARS-CoV-2 positive test among patients in the third tertile of age (>49 y).
| Models | Variables in equation | OR | 95 %CI | P-value |
|---|---|---|---|---|
| Model 01 | 25(OH)D (<30 ng/mL) | 1.91 | 1.07–3.41 | 0.027 |
| Total cholesterol (>190 mg/dL) | 1.53 | 0.71–3.30 | NS | |
| Non-HDL cholesterol (>160 mg/dL) | 1.14 | 0.46–2.85 | NS | |
| LDL cholesterol (>160 mg/dL) | 1.33 | 0.56–3.14 | NS | |
| Triglycerides (>150 mg/dL) | 1.26 | 0.71–2.23 | NS | |
| Model 02 | 25(OH)D (<30 ng/mL) | 1.91 | 1.08–3.42 | 0.027 |
| Total cholesterol (>190 mg/dL) | 1.64 | 0.88–3.03 | NS | |
| LDL cholesterol (>160 mg/dL) | 1.41 | 0.67–3.01 | NS | |
| Triglycerides (>150 mg/dL) | 1.28 | 0.73–2.24 | NS | |
| Model 03 | 25(OH)D (<30 ng/mL) | 2.01 | 1.14–3.55 | 0.015 |
| Total cholesterol (>190 mg/dL) | 1.65 | 0.89–3.05 | NS | |
| LDL cholesterol (>16 mg/dL) | 1.48 | 0.70–3.12 | NS | |
| Model 04 | 25(OH)D (<30 ng/mL) | 2.02 | 1.15–3.55 | 0.015 |
| Total cholesterol (>190 mg/dL) | 1.90 | 1.10–3.28 | 0.020 |
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; NS, non-significant.
4. Discussion
Our study was performed in a region with high solar radiation intensity and shows the association of prior vitamin D status with SARS-CoV-2 infection. We demonstrated that concentrations of 25(OH)D < 30 ng/mL and total cholesterol > 190 mg/dL were positively associated with greater odds of a positive SARS-CoV-2 test in patients > 49 y.
Studies examining the relationship between vitamin D concentrations and COVID-19 have produced controversial results, and many of them assessed 25(OH)D at the time of illness. The assessment time may impact the results of 25(OH)D because vitamin D binding protein may be subject to an effect of the systemic inflammatory response [34]. This reinforces the importance of our finding since our results reflect the status of vitamin D without the interference of the inflammation caused by COVID-19.
Some other studies have also similarly analyzed the 25(OH)D results preceding the SARS-CoV-2 test. Kaufman et al. [35] performed a cohort of 191,779 patients from all 50 states and the District of Columbia (USA) and observed that SARS-CoV-2 positivity is strongly and inversely associated with circulating 25(OH)D concentrations. They showed that those with 25(OH)D < 20 ng/mL had a 54% higher positivity rate compared to those with 25(OH)D between 30 and 34 ng/mL, and the risk of SARS-CoV- 2 positivity continued to decline until the serum concentrations reached 55 ng/mL.
Similar data were observed by Meltzer et al. [36] in their retrospective cohort study using data from the electronic health records at the University of Chicago Medicine. They concluded that Black individuals with 25(OH)D < 40 ng/mL had an increased risk for COVID-19. No significant associations were observed for White individuals. Although we did not evaluate ethnic characteristics, recent studies [37], [38] on the population of the Brazilian Northeast, the region of our study, demonstrate multiple origins of founding groups - a factor which qualifies our population as mixed.
In disagreement with our findings, Raisi-Estabragh et al. [39] evaluated 4510 UK Biobank participants tested for COVID-19 in a hospital setting and showed that male sex, Black, Asian and Minority Ethnicities (Chinese, Mixed and other ethnicities), higher body mass index, and greater household size were associated with significantly greater odds of a positive result, but not 25(OH)D concentrations. However, the vitamin D concentrations were analyzed between 10 and 14 y before the COVID-19 diagnosis and may not reflect the actual vitamin D status shortly before infection. Szeto et al. [24] evaluated the association of 25(OH)D concentrations pre-hospitalization with COVID-19 clinical outcomes of 93 patients admitted to Columbia University Irving Medical Center in New York City, but significant results were not found. These conflicting findings reinforce the importance of further studies evaluating the relationship between vitamin D and COVID-19.
Vitamin D might play a critical role in regulating the immunological response. Studies have found the modulating role of vitamin D in immune function through effects on dendritic and T cells [40], which may promote viral clearance, reduce inflammatory responses, and decreased interleukin-6 concentrations, which are targets for controlling cytokine storm in COVID-19 [41], [42].
The serum threshold for vitamin D deficiency and sufficiency is still divergent in the literature, and an international consensus is lacking [43]. In our study, we used the serum threshold recommended by the European Society [29] and the Brazilian Society of Endocrinology and Metabolism [44], but an optimal value for preventing infectious diseases has not been established. Our findings show a low prevalence of vitamin D deficiency (<20 ng/mL) when compared to other studies [35], [36], which is probably explained by the significant exposure to sunlight [45] since our study was carried out in a city 5 degrees south of the equator and with high solar radiation concentrations throughout the year [28].
Nevertheless, we observed a relationship between insufficient vitamin D and high total cholesterol with the incidence of SARS-CoV-2 infection in older people (>49 y). Cholesterol is being recognized as a molecule involved in regulating the entry of the SARS-CoV-2 virus into the host cell. Cholesterol is necessary for forming lipid rafts, where a greater amount of angiotensinogen converting enzyme (ACE2) receptors are concentrated, which are the gateway to the virus. This in turn affects membrane permeability, signaling and transport [46].
An experimental study [47] evaluated in vitro cellular activity of the entry of SARS-CoV-2 in those charged and not loaded with cholesterol. Lung tissue (cells expressing ACE 2) from C57BL/6J wild type young (8 weeks) and older (28 weeks) mice were subsequently loaded with cholesterol, where cholesterol dependence for virus entry and the influence of tissue age was confirmed since older mice had significantly higher cholesterol concentrations (P < 0.01). With aging, average cell cholesterol concentrations in the lung increase, thus increasing the number and size of virus entry points [47]. This may explain why older patients have a higher risk of infection.
Although cholesterol plays an important role in COVID-19, we do not evaluate cholesterol concentrations in the plasma membrane and they do not directly correlate with plasma cholesterol concentrations. In a study in Wenzhou/China [48], patients with SARS-CoV-2 infection had significantly lower concentrations of total cholesterol (TC) (143.08 ± 3.48 mg/dL), HDL-cholesterol (45.63 ± 1.16 mg/dL) and LDL-cholesterol (70.38 ± 3.09 mg/dL) compared to control (non-infected patients). Lower cholesterol concentrations can be explained due to inhibition of cholesterol efflux proteins in peripheral tissue, increasing cholesterol concentration in monocytes during infection [47].
The serum lipid profile (total, LDL, non-HDL, and HDL cholesterol) of patients included in a Spanish cohort database were evaluated before and after SARS-CoV-2 infection [49]. Patients with severe COVID-19 evolution had lower HDL [48.25 mg/dL (40.92–57.13); P = 0.007)] and higher triglycerides [127.30 mg/dL (93.70–175.92); P < 0.001] before infection. The lipid profile measured during hospitalization also showed that a severe outcome was associated with lower concentrations of HDL cholesterol [28.18 mg/dL (22.77–37.83); P < 0.001] and higher triglycerides [171.5 mg/dL (122.88–254.6); P < 0.001] [49]. Our study contrasts with the literature since patients with lower 25(OH)D and higher total cholesterol (>190 mg/dL) had a 3.92 cumulative odds to test positive for SARS-CoV-2.
This study has several limitations. First, the SARS-CoV-2 infection outcomes were only evaluated by viral RNA detection, and no other detection methods (viral antigen detection and serum antibody detection) nor clinical symptoms. Second, the RT-PCR is the gold standard to detect SARS-CoV-2 nucleic acids present in nasopharyngeal fluids; however, the false-negative rate for SARS-CoV-2 by RT-PCR testing is highly variable, as it is highest within the first 5 days after exposure (up to 67%), and lowest on day 8 after exposure (21%) [50]. Clinical data were not evaluated in our study, so it was not considered which day after exposure or symptoms the patient collected swabs for the SARS-CoV-2 test. Third, because the patients usually had several comorbidities and were subjected to a diverse medication regimen, the results could have been affected by the heterogeneity of the sample. Fourth, the relatively low sample size in this study may have inadequate power to exclude small but meaningful laboratory differences between the groups. Further prospective studies should be conducted to confirm the existence of a causal relationship between vitamin D, cholesterol, and SARS-CoV-2 infection. Moreover, large multicenter studies are necessary to determine whether 25(OH)D concentrations and lipid profile could be useful to identify patients who are more susceptible to COVID-19.
5. Conclusion
Insufficient 25(OH)D status and high total cholesterol previous to the virus infection were associated with positive SARS-CoV-2 test among adults > 49 y in a population resident in a region with high solar radiation intensity. These results could indicate vitamin D, dyslipidemia, and older age as potential risk factors for COVID-19. Further studies should be conducted to confirm our hypotheses and whether the measure of 25(OH)D and lipid profile could be useful to identify patients who are more susceptible to SARS-CoV-2 infection.
Funding statement
This work was funded by the National Council for Scientific and Technological Development (CNPq)-Brazil, and JFPM and RCSDK are recipients of a fellowship from Coordination for the Improvement of Higher Education Personnel (CAPES)-Brazil. The funding entities had no role in the study design; the collection, analyses, or interpretation of data; the writing of the manuscript; or the decision to publish the results.
Author contributions
VNS and HGR designed and executed the study;
HGR, RCSDK, JFPM, MCCC, and BZR wrote the paper;
ADL performed statistical analysis of the data;
VLS contributed to the execution of the study;
VNS, ADL, and BZR revised the final draft of the manuscript.
All authors read and approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cca.2021.08.003.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Mohan M., Cherian J.J., Sharma A. Exploring links between vitamin D deficiency and COVID-19. PLoS Pathog. 2020;16(9) doi: 10.1371/journal.ppat.1008874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zdrenghea M.T., Makrinioti H., Bagacean C., Bush A., Johnston S.L., Stanciu L.A. Vitamin D modulation of innate immune responses to respiratory viral infections. Rev. Med. Virol. 2017;27 doi: 10.1002/rmv.1909. [DOI] [PubMed] [Google Scholar]
- 3.Greiller C.L., Martineau A.R. Modulation of the immune response to respiratory viruses by vitamin D. Nutrients. 2015;7:4240–4270. doi: 10.3390/nu7064240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bilezikian J.P., Bikle D., Hewison M., Lazaretti-Castro M., Formenti A.M., Gupta A., Giustina A. Mechanisms in endocrinology: vitamin D and COVID-19. Eur. J. Endocrinol. 2020;183(5):R133–R147. doi: 10.1530/EJE-20-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou Y.F., Luo B.A., Qin L.L. The association between vitamin D deficiency and community-acquired pneumonia: a meta-analysis of observational studies. Medicine. 2019;98 doi: 10.1097/MD.0000000000017252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hastie C.E., Mackay D.F., Ho F., Celis-Morales C.A., Katikireddi S.V., Niedzwiedz C.L., et al. Vitamin D concentrations and COVID-19 infection UK Biobank. Diabet. Metab. Syndr. Clin. Res. Rev. 2020;14:561–565. doi: 10.1016/j.dsx.2020.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daneshkhah A., Agrawal V., Eshein A., Subramanian H., Roy H.K., Backman V. The possible role of vitamin D in suppressing cytokine storm and associated mortality in COVID-19 patients. Infect. Dis. (except HIV/AIDS) 2020 doi: 10.1007/s40520-020-01677-y. [DOI] [Google Scholar]
- 8.Lau F.H., Majumder R., Torabi R., Saeg F., Hoffman R., Cirillo J.D., et al. Vitamin D insufficiency is prevalent in severe COVID-19. Infect. Dis. (exceptHIV/AIDS) 2020 doi: 10.1101/2020.04.24.20075838. [DOI] [Google Scholar]
- 9.Jose R.J., Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir. Med. 2020 doi: 10.1016/S2213-2600(20)30216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sly L.M., Lopez M., Nauseef W.M., Reiner N.E. 1alpha,25-dihydroxyvitamin D3-induced monocyte antimycobacterial activity is regulated by phosphatidylinositol 3-kinase and mediated by the NADPH-dependent phagocyte oxidase. J. Biol. Chem. 2001;276(38):35482–35493. doi: 10.1074/jbc.M102876200. [DOI] [PubMed] [Google Scholar]
- 11.Liu P.T., Stenger S., Li H., Wenzel L., Tan B.H., Krutzik S.R., et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–2177. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 12.Panarese A., Shahini E. Letter: Covid-19, and vitamin D. Aliment. Pharmacol. Ther. 2020;51:993–999. doi: 10.1111/apt.15752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ilie PC, Stefanescu S, Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin. Exp. Res. 2020. http://doi.org/10.1007/s40520-020-01570-8. [DOI] [PMC free article] [PubMed]
- 14.Barlow P.G., Svoboda P., Mackellar A., Nash A.A., York I.A., Pohl J., Davidson D.J., Donis R.O. Antiviral activity and increased host defense against influenza infection elicited by the human cathelicidin LL-37. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0025333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed A., Siman-Tov G., Hall G., Bhalla N., Narayanan A. Human antimicrobial peptides as therapeutics for viral infections. Viruses. 2019;11:704. doi: 10.3390/v11080704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernández J.L., Nan D., Fernandez-Ayala M., García-Unzueta M., Hernández-Hernández M.A., López-Hoyos M., Martínez-Taboada V.M. Vitamin D status in hospitalized patients with SARS-CoV-2 infection. J. Clin. Endocrinol. Metab. 2021;106(3):e1343–e1353. doi: 10.1210/clinem/dgaa733. [DOI] [PubMed] [Google Scholar]
- 17.Pizzini A., Aichner M., Sahanic S., Böhm A., Egger A., Hoermann G., Löffler-Ragg J. Impact of vitamin d deficiency on COVID-19—A prospective analysis from the CovILD registry. Nutrients. 2020;12(9):2775. doi: 10.3390/nu12092775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cereda E., Bogliolo L., Klersy C., Lobascio F., Masi S., Crotti S., Di Terlizzi F. Vitamin D 25OH deficiency in COVID-19 patients admitted to a tertiary referral hospital. Clin. Nutr. 2020 doi: 10.1016/j.clnu.2020.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo X., Liao Q., Shen Y., Li H., Cheng L. Vitamin D deficiency is inversely associated with covid-19 incidence and disease severity in Chinese people. J. Nutr. 2021;151(1):98–103. doi: 10.1093/jn/nxaa332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abrishami A., Dalili N., Torbati P.M., Asgari R., Arab-Ahmadi M., Behnam B., Sanei-Taheri M. Possible association of vitamin D status with lung involvement and outcome in patients with COVID-19: a retrospective study. Eur. J. Nutr. 2020:1–9. doi: 10.1007/s00394-020-02411-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hars M., Mendes A., Serratrice C., Herrmann F.R., Gold G., Graf C., Trombetti A. Sex-specific association between vitamin D deficiency and COVID-19 mortality in older patients. Osteoporos. Int. 2020;31(12):2495–2496. doi: 10.1007/s00198-020-05677-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu H., Chen S., Liu M., Nie H., Lu H. Comorbid Chronic Diseases are Strongly Correlated with Disease Severity among COVID-19 Patients: A Systematic Review and Meta-Analysis. Aging Dis. 2020;11:668–678. doi: 10.14336/AD.2020.0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stefan N., Birkenfeld A.L., Schulze M.B., Ludwig D.S. Obesity and impaired metabolic health in patients with COVID-19. Nat. Rev. Endocrinol. 2020;16:341–342. doi: 10.1038/s41574-020-0364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szeto B., Zucker J.E., LaSota E.D., Rubin M.R., Walker M.D., Yin M.T., Cohen A. Vitamin D Status and COVID-19 Clinical Outcomes in Hospitalized Patients. Endocr. Res. 2020;30:1–8. doi: 10.1080/07435800.2020.1867162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silva M.C., Furlanetto T.W. Does serum 25-hydroxyvitamin D decrease during acute-phase response? A systematic review. Nutr. Res. 2015;35(2):91–96. doi: 10.1016/j.nutres.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Meltzer DO, et al. Association of vitamin D status and other clinical characteristics with COVID-19 test results. JAMA Netw Open 2020;3(9):e2019722-e2019722. http://doi.org/10.1001/jamanetworkopen.2020.19722. [DOI] [PMC free article] [PubMed]
- 27.Raisi-Estabragh Z., McCracken C., Bethell M.S., Cooper J., Cooper C., Caulfield M.J., Petersen S.E. Greater risk of severe COVID-19 in Black, Asian and Minority Ethnic populations is not explained by cardiometabolic, socioeconomic or behavioural factors, or by 25 (OH)-vitamin D status: study of 1326 cases from the UK Biobank. J. Public Health. 2020;42(3):451–460. doi: 10.1093/pubmed/fdaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Institute for Space Research (NISR). Natal UV radiation. Natal (RN/Brazil): Laboratory of tropical environmental variables, Ministry of Science, Technology and Innovation 2012.
- 29.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, 512 Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D 513 deficiency: An endocrine society clinical practice guideline. J. Clin. Endocrinol. 514 Metab 2011;96:1911–30. http://doi.org/10.1210/jc.2011-0385. [DOI] [PubMed]
- 30.Martin S.S., et al. Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol concentrations from the standard lipid profile. JAMA. 2013;310(19):2061–2068. doi: 10.1001/jama.2013.280532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Virani S.S. Non-HDL cholesterol as a metric of good quality of care: opportunities and challenges. Tex. Heart Inst. J. 2011;38(2):160–162. [PMC free article] [PubMed] [Google Scholar]
- 32.Faludi AA, et al. Update of the Brazilian Dyslipidemia and Atherosclerosis Prevention Directive. Arq. Bras. Cardiol. 2017;109(2 Supl 1):1-76. http://doi.org/10.5935/abc.20170121.
- 33.Wickham H. Springer-Verlag; New York, USA: 2016. Ggplot2: Elegant Graphics for Data Analysis. [Google Scholar]
- 34.Jassil N.K., Sharma A., Bikle D., Wang X. Vitamin d binding protein and 25-hydroxyvitamin d concentrations: emerging clinical applications. Endocr. Pract. 2017;23(5):605–613. doi: 10.4158/EP161604.RA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaufman H.W., Niles J.K., Kroll M.H., Bi C., Holick M.F. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D concentrations. PLoS ONE. 2020;15(9) doi: 10.1371/journal.pone.0239252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meltzer D.O., Best T.J., Zhang H., Vokes T., Arora V.M., Solway J. Association of Vitamin D Concentrations, Race/Ethnicity, and Clinical Characteristics With COVID-19 Test Results. JAMA Netw. Open. 2021;4(3) doi: 10.1001/jamanetworkopen.2021.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schaan A.P., Gusmaõ L., Jannuzzi J., Modesto A., Amador M., Marques D., et al. New insights on intercontinental origins of paternal lineages in Northeast Brazil. BMC Evol. Biol. 2020;20(1):1–9. doi: 10.1186/s12862-020-1579-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schaan A.P., Costa L., Santos D., Modesto A., Amador M., Lopes C., et al. MtDNA structure: The women who formed the Brazilian Northeast. BMC Evol. Biol. 2017;17(1):1–12. doi: 10.1186/s12862-017-1027-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raisi-Estabragh Z, McCracken C, Bethell MS, Cooper J, Cooper C, Caulfield MJ, Munroe PB, Harvey NC, Petersen SE. Greater risk of severe COVID-19 in Black, Asian and Minority Ethnic populations is not explained by cardiometabolic, socioeconomic or behavioural factors, or by 25(OH)-vitamin D status: study of 1326 cases from the UK Biobank. J Public Health (Oxf) 2020;18;42(3):451-460. http://doi.org/10.1093/pubmed/fdaa095. [DOI] [PMC free article] [PubMed]
- 40.Yin K., Agrawal D.K. Vitamin D and inflammatory diseases. J. Inflamm. Res. 2014;7:69–87. doi: 10.2147/JIR.S63898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roffe-Vazquez DN, Huerta-Delgado AS, Castillo EC, et al. Correlation of vitamin D with inflammatory cytokines, atherosclerotic parameters, and lifestyle factors in the setting of heart failure: a 12-month follow-up study. Int J Mol Sci. 2019;20(22):5811. http://doi.org/10.3390/ijms20225811. [DOI] [PMC free article] [PubMed]
- 42.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lips P., et al. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: a position statement of the European Calcified Tissue Society. Eur. J. Endocrinol. 2019;180(4):23–54. doi: 10.1530/EJE-18-0736. [DOI] [PubMed] [Google Scholar]
- 44.Moreira C.A., Ferreira C.E.S., Madeira M., Silva B.C.C., Maeda S.S., Batista M.C., et al. Reference values of 25-hydroxyvitamin D revisited: a position statement from the Brazilian Society of Endocrinology and Metabolism (SBEM) and the Brazilian Society of Clinical Pathology/Laboratory Medicine (SBPC) Arch. Endocrinol. Metab. 2020;64(4):462–478. doi: 10.20945/2359-3997000000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holick M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocrine Metab. Disord. 2017;18:153–165. doi: 10.1007/s11154-017-9424-1. [DOI] [PubMed] [Google Scholar]
- 46.Kočar E., Režen T., Rozman D. Cholesterol, lipoproteins, and COVID-19: Basic concepts and clinical applications. Biochim. Biophys. Acta – Mol. Cell Biol. Lipids. 2021;1866(2) doi: 10.1016/j.bbalip.2020.158849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang H., Yuan Z., Pavel M.A., Hobson R., Hansen S.B. The role of high cholesterol in age-related COVID19 lethality. BioRxiv. 2020:1–17. doi: 10.1101/2020.05.09.086249. [DOI] [Google Scholar]
- 48.Hu X., Chen D., Wu L., He G., Ye W. Declined serum high density lipoprotein cholesterol is associated with the severity of COVID-19 infection. Clin. Chim. Acta. 2020;510:105–110. doi: 10.1016/j.cca.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Masana L., Correig E., Ibarretxe D., Anoro E., Arroyo J.A., Jericó C., et al. Low HDL and high triglycerides predict COVID-19 severity. Sci. Rep. 2021;11(1):7217. doi: 10.1038/s41598-021-86747-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kucirka L.M., Lauer S.A., Laeyendecker O., Boon D., Lessler J. Variation in false-negative rate of reverse transcriptase polymerase chain reaction–based SARS-CoV-2 tests by time since exposure. Ann. Intern. Med. 2020;173(4):262–267. doi: 10.7326/M20-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.