Abstract
Purpose
Individuals with diabetes/stress hyperglycemia carry an increased risk for adverse clinical outcome in case of SARS-CoV-2 infection. The purpose of this study was to evaluate whether this risk is, at least in part, modulated by an increase of thromboembolic complications.
Methods
We prospectively followed 180 hospitalized patients with confirmed COVID-19 pneumonia admitted to the Internal Medicine Units of San Raffaele Hospital. Data from 11 out of 180 patients were considered incomplete and excluded from the analysis. We analysed inflammation, tissue damage biomarkers, hemostatic parameters, thrombotic events (TEs) and clinical outcome according to the presence of diabetes/stress hyperglycemia.
Results
Among 169 patients, 51 (30.2%) had diabetes/stress hyperglycemia. Diabetes/stress hyperglycemia and fasting blood glucose (FBG) were associated with increased inflammation and tissue damage circulating markers, higher D-dimer levels, increased prothrombin time and lower antithrombin III activity. Forty-eight venous and 10 arterial TEs were identified in 49 (29%) patients. Diabetes/stress hyperglycemia (HR 2.71, p = 0.001), fasting blood glucose (HR 4.32, p < 0.001) and glucose variability (HR 1.6, p < 0.009) were all associated with an increased risk of thromboembolic complication. TEs significantly increased the risk for an adverse clinical outcome only in the presence of diabetes/stress hyperglycemia (HR 3.05, p = 0.010) or fasting blood glucose ≥7 mmol/L (HR 3.07, p = 0.015).
Conclusions
Thromboembolism risk is higher among patients with diabetes/stress hyperglycemia and COVID-19 pneumonia and is associated to poor clinical outcome. In case of SARS-Cov-2 infection patients with diabetes/stress hyperglycemia could be considered for a more intensive prophylactic anticoagulation regimen.
Abbreviation: aD-dimer, age-specific high D-dimer; ALT, alanine aminotransferase; AST, Aspartate transaminase; CRP, C-reactive protein; DIC, disseminated intravascular coagulation; DOACs, direct oral anticoagulants; DVT, deep vein thrombosis; FBG, fasting blood glucose; ICU, Intensive Care Unit; IQR, inter-quartile range; ISTH, International Society on Thrombosis and Hemostasis; HR, Hazard Ratios; LMWH, low-molecular-weight heparin; LDH, lactate dehydrogenase; LIPS, luciferase immunoprecipitation system; NP, nucleoprotein; OR, Odds Ratio; OAT, oral anticoagulant therapy; PE, pulmonary embolism; PT, prothrombin time; PTT, partial thromboplastin time; proBNP, pro-brain natriuretic peptide; RBD, Recombinant Receptor-Binding-Domain; S1 + S2, recombinant spike protein ectodomain; SISET, Italian Society on Thrombosis and Hemostasis; TEs, thrombotic events; VWF, von Willebrand factor
Keywords: COVID-19, Diabetes, Clinical outcome, Humoral response, SARS-CoV-2, Thromboembolism
1. Introduction
Diabetes has been confirmed as one of the most consistent risk factors for severe disease in case of SARS-CoV-2 infection [1,2]. In fact, the risks of admission to an Intensive Care Unit (ICU) and of in-hospital mortality are increased two to three fold by the presence of diabetes in patients with COVID 19 pneumonia [3]. Different pathophysiological mechanisms were suggested to explain the worse clinical outcome, including hyperglycemia, older age and the presence of comorbidities (i.e., hypertension, obesity, and cardiovascular disease) [4]. However, because of the syndromic nature of diabetes, additional potential causative factors should be considered, such as the increased susceptibility to hyperinflammation [5], the diminished immunological function [6] and the prothrombotic state [7] associated with hyperglycemia. We recently investigated whether diabetes or hyperglycemia are linked to a defect in the humoral immune response against SARS-CoV-2 [8,9]. Our data showed that the antibody response against multiple SARS-CoV-2 antigens in patients with diabetes is superimposable in terms of timing, persistence, classes, titers, and neutralizing activity to that of non-diabetic patients [9,10]. However, in our cohort of patients with SARS-CoV-2 pneumonia, we also observed a significant correlation between serum D-dimer levels and diabetes/hyperglycaemia, a finding confirmed also by others [[11], [12], [13]]. Elevated D-dimer levels are a direct consequence of increased fibrin formation and lysis and thus an indicator of increased thrombotic activity, such as disseminated intravascular coagulation (DIC) and thromboembolism [14]. COVID-19 is associated with an increased risk of arterial and venous thrombosis [[15], [16], [17]] because of a multitude of factors, including systemic inflammation, endothelial dysfunction, platelet activation, immobilization, mechanical ventilation and the use of central venous catheters [[18], [19], [20]]. Since diabetes is associated with a pro-thrombotic status [7] and elevated D-dimer levels [12], we hypothesized that diabetes is associated with an increased risk of thrombotic events (TEs) in patients with COVID-19 pneumonia. To prove this hypothesis we designed a prospective observational study in a cohort of 180 consecutively hospitalized patients with COVID-19 pneumonia, focusing on TEs which occurred during hospitalization and risk factors associated with these events.
2. Material and methods
2.1. Study population and data sources
The study population consisted of 180 adult patients (≥18 years) with confirmed COVID-19 pneumonia admitted to the Internal Medicine Units of San Raffaele Hospital, Milan, Italy from April to May 2020. Patients were included if they were diagnosed with COVID-19 as per the Chinese management guidelines and the World Health Organization interim guidance [21,22]. There was no exclusion criterion. A confirmed infection case was defined as a SARS-CoV-2-positive RT-PCR test from a nasal/throat swab, and/or signs, symptoms and radiological findings suggestive of COVID-19 pneumonia. Within 48 h from admission, we recorded demographic information, clinical features and laboratory exams on the day of admission on a dedicated data collection form. Data were recorded until hospital discharge or death, whichever occurred first. Data were cross-checked in blind and verified by data managers and clinicians for accuracy. We also recorded mortality beyond hospital discharge clinic: for patients non attending our dedicated outpatient follow-up clinic, we checked patient's vital status with either family members or family physician. The study was approved by the Ethics Committee Ospedale San Raffaele (protocol n 34/int/2020). A standard written informed consent was requested to all patients for their data use.
2.2. Thrombotic complications
The occurrence of any thrombotic event (TE) throughout the hospitalization was the primary outcome of the study. Thrombotic complications included deep vein thrombosis (DVT), pulmonary embolism (PE), and lower and upper limb ischemia, catheter-related thrombosis with deep vein involvement, mesenteric ischemia, stroke and myocardial infarction. A standard protocol to assess patients for thrombotic complications was implemented based on the position paper from the Italian Society on Thrombosis and Hemostasis (SISET) [23] and to the interim guidance to recognition and management of coagulopathy in COVID-19 from the International Society on Thrombosis and Hemostasis (ISTH) [24]. A close control of hemostasis parameters and clinical signs and symptoms was methodically pursued. Additional investigations, including CT scan and/or ultrasound, were performed on the basis of clinical suspicion of thromboembolic events: (i) elevated D-dimer levels and/or (ii) presence of respiratory failure and/or (iii) presence of symptoms suggestive of TEs. All patients received thromboprophylaxis with enoxaparin 4000 IU/day [adjusted to 6000 IU/day or 3000 IU/day in overweight (>100 kg) or underweight (<50 kg) subjects, respectively] or, alternatively, with mechanical compression of the lower limbs in case of anticoagulant contraindications (active bleeding and platelet count less than 25 × 109/l). If chronic oral anticoagulant therapy with direct oral anticoagulants (DOACs) or warfarin/acenocoumarol (oral anticoagulant therapy, OAT) was prescribed prior to admission, it was changed to low-molecular-weight heparin (LMWH) anticoagulant treatment. Thromboprophylaxis was administered on admission and during the entire duration of the hospital stay. Anti-Xa measurement was used to monitor anticoagulant treatment. There were no cases of heparin-induced thrombocytopenia. No major haemorrhagic event occurred in patients with thromboprophylaxis. The Padua Prediction Score and the IMPROVE Bleeding Risk Assessment Score were used at hospital admission for stratification of the venous thromboembolism and bleeding risks, respectively. A Padua score ≥ 4 identified patients at high risk for venous thromboembolism, an IMPROVE Bleeding Risk Assessment Score ≥ 7 identified patients at increased risk of bleeding. Overt DIC was defined when the ISTH diagnostic score was ≥5 [25].
2.3. Definition of diabetes/stress hyperglycemia
Study participants were defined as having diabetes/stress hyperglycaemia if they had a documented diagnosis before the hospital admission for COVID-19 pneumonia [Comorbid diabetes: fasting plasma glucose (FPG) ≥7.0 mmol/L or HbA1c ≥ 6.5% (48 mmol/mol), or prescription for diabetes medications] or if patients without a previous diagnosis of diabetes had a mean FPG ≥7.0 mmol/L. during the hospitalization for COVID-19 pneumonia (stress hyperglycaemia). We computed mean FPG and glucose variability (standard deviation) from all fasting laboratory glucose values measured during hospitalization. HbA1c levels during in hospital stay or in the year prior to hospital admission were recorded, where available. BMI was calculated at the admission time or in case of missing value it was derived by the clinical records of the three months prior to the patient's hospitalization for COVID, where available.
2.4. Laboratory variables
Routine blood tests encompassed serum biochemistry [including renal and liver function, lactate dehydrogenase (LDH) and electrolytes], complete blood count with differential, markers of myocardial damage [troponin T and pro-brain natriuretic peptide (proBNP)], inflammation markers [C-reactive protein (CRP), ferritin, interleukin-6 (IL-6)] and coagulation profile assessment [D-dimer, prothrombin time (PT), partial thromboplastin time (PTT)]. Specific antibodies to different SARS-CoV-2 antigens [SARS-COV-2 Recombinant Receptor-Binding-Domain (RBD), recombinant spike protein ectodomain (S1 + S2) and nucleoprotein (NP] were tested in a subset of patients by a luciferase immunoprecipitation system (LIPS) assay, as previously described [8]. Fibrinogen, antithrombin activity, von Willebrand factor (VWF), homocysteine, protein C and S. D-dimer levels were measured in a subset of patients through a STA-R® automatic coagulation analyser. Age-specific high D-dimer (aD-dimer) was defined as D-dimer levels above 0.5 μg/dL for patients with less than 50 years of age and above their age divided by 100 in patients older than 50 years [26].
2.5. Statistical analysis
Continuous variables were presented as median with inter-quartile range (IQR) in parenthesis. Categorical variables were reported as frequency or percent. Continuous variables were compared using the Wilcoxon rank sum or Kruskal-Wallis test. Categorical variables were compared using the Chi-square or Fischer's exact test, as appropriate. Imputation for missing data was not performed. Associations between baseline variables and diabetes were assessed by logistic regression. The effect estimates were reported as Odd Ratios (ORs). Survival was estimated according to Kaplan–Meier. The time-to-event was calculated from the date of symptom onset to the date of the event, or of last follow-up visit, whichever occurred first. We calculated univariate and multivariate Cox proportional hazards models to study the association between patient characteristics with time to thrombotic complication or time to adverse outcome (as defined by composite endpoint of transfer to ICU or death, whichever occurred first). In Cox proportional hazards models, the onset of a thrombotic complication was considered a time-varying covariate. The effect estimates were reported as Hazard Ratios (HRs) with the corresponding 95% CI, estimated according to the Wald approximation. Multivariate analyses were performed including variables significant at the level of <0.1 in the univariate analysis. Two-tailed P values are reported, with P value <0.05 indicating statistical significance. All confidence intervals are two-sided and not adjusted for multiple testing. Statistical analyses were performed with the SPSS 24 (SPSS Inc. /IBM), the R software version 3.4.0 (R Core Team (2020), and GraphPad Prism version 5.04.
3. Results
3.1. Study participants
A total of 180 consecutive patients with confirmed COVID-19 were prospectively enrolled. Data from 11 out of 180 (6.11%) patients were considered incomplete and excluded from the analysis. Among the 169 cases included in our study [median hospital stay 17 (8–31) days], 61 patients (36.1%) were treated with non-invasive ventilation and 23 (13.6%) accessed an ICU over the hospitalization period. As of January 25, 2021 the median follow-up time after symptoms onset was 222 (95% CI: 211–232) days. Thirty-five patients died during follow-up (20.7%). Fifty patients (29.6%) had an adverse in-hospital outcome, according to the composite endpoint of transfer to ICU or death, whichever occurred first.
3.2. Baseline characteristics of study population
The characteristics of study participants, according to diabetes status or glucose levels, are reported in Table 1 and Table 2 . Stress hyperglycaemia and comorbid diabetes accounted for 11.2% (n = 19) and 18.9% (n = 32) of the patients, respectively. Higher BMI [OR 1.106 (95% IC 1.02–1.2); p = 0.014], older age [OR 1.029 x year (95% IC 1.01–1.05); p = 0.013], and hypertension [OR 4.036 (1.04–3.98); p = 0.037] were all associated with diabetes/stress hyperglycaemia. As for diabetes treatment, 3.9% of subject with comorbid diabetes were being treated with lifestyle modifications, 11.8% with insulin, 39.2% with non-insulin oral or injectable anti-diabetes medications, 7.8% with insulin and oral diabetes medications, while patients with stress hyperglycaemia (37.3%) were untreated. The median time from symptoms onset to hospital admission was 7 (1–12.5) and 5 (1–8) days for patients without and with diabetes/stress hyperglycaemia, respectively (p = 0.330). On admission, 18.3% (n = 31) of the patients were taking ACE-inhibitors (25.5% vs 15.3%, p = 0.132 diabetes/stress hyperglycaemia vs no diabetes), 14.2% (n = 24) chronic antiplatelet therapy (21.6% vs 11%, p = 0.092), 16% (n = 27) statins (27.5% vs 11%, p = 0.011) and 18.3% (n = 31) anticoagulant treatments (23.5% vs 16.1%, p = 0.280).
Table 1.
Baseline characteristics of study population according to diabetes/stress hyperglycemia and median fasting plasm glucose.
| Characteristics | All | diabetes/stress hyperglycemia |
Median fasting glucose (mmol/l) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| No | Yes | p | <5.6 | 5.6–6.9 | ≥7 | p | Missing | ||
| N | 118 | 51 | 90 | 37 | 42 | ||||
| Age, years | 66 (53–77.5) | 62 (50–77.5) | 71 (61–78) | 0.014 | 62 (48.7–76.2) | 64 (55–79) | 72 (64–82.5) | 0.021 | 0 |
| Sex Male [N (%)] | 118 (69.8) | 75 (70.8) | 43 (68.3) | 0.73 | 55 (61.1) | 28 (75.7) | 23 (54.8) | 0.140 | 0 |
| Non Caucasian ethnicity [N (%)] | 32 (18.9) | 26 (22) | 6 (11.8) | 0.14 | 21 (23.3) | 5 (13.5) | 6 (14.3) | 0.300 | 0 |
| BMI (kg/m2) | 26.6 (23.7–30.4) | 25.8 (23.-29.4) | 27.8 (25.3–32.9) | 0.012 | 25.8 (22.9–30.4) | 26.9 (24.7–30.9) | 27.7 (24.5–30.5) | 0.230 | 23 |
| Smoke [N (%)] | 29 (17.3) | 23 (19.5) | 6 (12) | 0.27 | 19 (21.1) | 7 (18.9) | 3 (7.3) | 0.150 | 1 |
| Obesity | 46 (27.2) | 26 (22) | 20 (39.2) | 0.025 | 21 (23.3) | 11 (29.7) | 14 (33.3) | 0.450 | 0 |
| Previous venous thromboembolism [N (%)] | 9 (5.3) | 6 (5.1) | 3 (5.9) | 0.99 | 3 (3.3) | 3 (8.1) | 3 (7.1) | 0.460 | 0 |
| Previous bleeding [N (%)] | 8 (4.7) | 5 (4.2) | 3 (5.9) | 0.70 | 4 (4.4) | 2 (5.4) | 2 (4.8) | 0.970 | 0 |
| Previous cancer [N (%)] | 13 (7.7) | 9 (7.6) | 4 (7.8) | 0.99 | 9 (10) | 1 (2.7) | 3 (7.1) | 0.370 | 0 |
| Comorbidities [N (%)] | 0 | ||||||||
|
77 (45.6) | 47 (39.8) | 30 (58.8) | 0.029 | 36 (40) | 18 (48.6) | 23 (54.8) | 0.260 | |
|
51 (30.2) | 0 (0) | 51 (100) | – | 3 (3.3) | 6 (16.2) | 42 (100) | 0.001 | |
|
23 (13.6) | 16 (13.6) | 7 (13.7) | 0.99 | 12 (13.3) | 7 (18.9) | 4 (9.5) | 0.470 | |
|
20 (11.8) | 14 (11.9) | 6 (11.8) | 0.99 | 13 (14.4) | 3 (8.1) | 4 (9.5) | 0.520 | |
|
19 (11.2) | 12 (10.2) | 7 (13.7) | 0.60 | 8 (8.9) | 5 (13.5) | 6 (14.3) | 0.590 | |
|
18 (10.7) | 9 (7.6) | 9 (17.6) | 0.062 | 7 (7.8) | 3 (8.1) | 8 (19) | 0.130 | |
|
15 (8.9) | 11(9.3) | 4 (7.8) | 0.99 | 8 (8.9) | 3 (8.1) | 4 (9.5) | 0.980 | |
|
7 (4.1) | 3 (2.5) | 4 (7.8) | 0.2 | 1 (1.1) | 2 (5.4) | 4 (9.5) | 0.071 | |
|
6 (3.6) | 3 (2.5) | 3 (5.9) | 0.37 | 3 (3.3) | 0 (0) | 3 (7.1) | 0.230 | |
| Preadmission treatment [N (%)] | 0 | ||||||||
|
31 (18.3) | 18 (15.3) | 13 (25.5) | 0.132 | 16 (17.8) | 6 (16.2) | 9 (21.4) | 0.820 | |
|
24 (14.2) | 13 (11) | 11 (21.6) | 0.092 | 9 (10) | 7 (18.9) | 8 (19) | 0.250 | |
|
27 (16) | 13 (11) | 14 (27.5) | 0.011 | 12 (13.3) | 6 (16.2) | 9 (21.4) | 0.497 | |
|
31 (18.3) | 19 (16.1) | 12 (23.5) | 0.28 | 12 (13.3) | 8 (21.6) | 11 (26.2) | 0.170 | |
|
10 (5.9) | 6 (5.1) | 4 (7.8) | 0.49 | 6 (6.7) | 1 (2.7) | 3 (7.1) | 0.640 | |
|
5 (3) | 3 (2.5) | 2 (3.9) | 0.64 | 1 (1.1) | 2 (5.4) | 2 (4.8) | 0.310 | |
|
16 (9.5) | 10 (8.5) | 6 (11.8) | 0.57 | 5 (5.6) | 5 (13.5) | 6 (14.3) | 0.180 | |
|
4 (2.4) | 4 (3.4) | 0 (0) | 0.32 | 4 (4.4) | 0 (0) | 0 (0) | 0.170 | |
|
13 (7.7) | 11 (9.3) | 2 (3.9) | 0.35 | 9 (10) | 2 (5.4) | 2 (4.8) | 0.480 | |
|
|||||||||
|
20 (11.9) | 0 (0) | 20 (40) | <0.001 | 1 (1.1) | 6 (16.2) | 13 (31.7) | <0.001 | |
|
6 (3.6) | 0 (0) | 6 (12) | <0.001 | 1 (1.1) | 0 (0) | 5 (12.2) | 0.003 | |
| Gliclazide | 4 (2.4) | 0 (0) | 4 (8) | 0.007 | 0 (0) | 0 (0) | 4 (9.8) | 0.002 | |
| Glibenclamide | 1 (0.6) | 0 (0) | 1 (2) | 0.30 | 0 (0) | 0 (0) | 1 (2.4) | 0.210 | |
| Glimepiride | 1 (0.6) | 0 (0) | 1 (2) | 0.30 | 1 (1.1) | 0 (0) | 0 (0) | 0.650 | |
|
1 (0.6) | 0 (0) | 1 (2) | 0.30 | 0 (0) | 1 (2.7) | 0 (0) | 0.170 | |
|
3 (1.8) | 0 (0) | 3 (6) | 0.025 | 0 (0) | 0 (0) | 3 (7.3) | 0.009 | |
|
1 (0.6) | 0 (0) | 1 (2) | 0.30 | 0 (0) | 1 (2.7) | 0 (0) | 0.170 | |
|
10 (6) | 0 (0) | 10 (20) | <0.001 | 1 (1.1) | 0 (0) | 9 (22) | <0.001 | |
COPD chronic obstructive pulmonary disease; ACE angiotensin-converting enzyme; DOACs: direct oral anticoagulants; LMWH: Low-molecular-weight heparin; OAT: warfarin/acenocoumarol.
Table 2.
Outcomes and laboratory values according to diabetes/stress hyperglycemia and median fasting plasma glucose.
| Characteristics | All | diabetes/stress hyperglycemia |
Median fasting glucose (mmol/l) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| No | Yes | p | <5.6 | 5.6–6.9 | ≥7 | p | Missing | ||
| N | 118 | 51 | 90 | 37 | 42 | ||||
| Outcomes | |||||||||
| Median time from symptoms to admission | 6 (1−11) | 7 (1–12.5) | 5 (1–8) | 0.330 | 7 (0.75–12.2) | 7 (1–12.5) | 5 (1.7–7.5) | 0.770 | 0 |
| Median follow up, days (95%CI) | 222 (211−232) | 220 (209–230) | 231 (214–247) | 0.690 | 213 (192–-------------------------------------------------------- -------++++++233) | 231 (217–244) | 231 (215–246) | 0.620 | 0 |
| Hospital stay, days | 17 (8–31) | 14.5 (7–30) | 21 (12–35) | 0.040 | 13.5 (7–27.7) | 21 (8–30) | 22.5 (12.7–35.5) | 0.080 | 0 |
| Non-invasive ventilation | 61 (36.1) | 33 (28) | 28 (54.9) | 0.002 | 18 (20) | 20 (54.1) | 23 (54.8) | <0.001 | 0 |
| Invasive ventilation (ICU) | 23 (13.6) | 11 (9.3) | 12 (23.5) | 0.025 | 5 (5.6) | 7 (18.9) | 11 (26.2) | 0.003 | 0 |
| Death | 35 (20.7) | 15 (12.7) | 20 (39.2) | <0.001 | 9 (10) | 7 (18.9) | 19 (45.2) | <0.001 | 0 |
| Adverse outcome (death and/or Invasive ventilation) | 50 (29.6) | 24 (20.3) | 26 (51) | <0.001 | 13 (14.4) | 12 (32.4) | 25 (59.5) | <0.001 | 0 |
| Hospital treatmenta [N (%)] | |||||||||
| Antibiotic treatment | 114 (67.5) | 73 (61.9) | 41 (80.4) | 0.020 | 57 (63.3) | 23 (62.2) | 34 (81) | 0.098 | 0 |
| Hydroxychloroquine | 109 (64.5) | 78 (66.1) | 31 (60.8) | 0.600 | 56 (62.2) | 28 (75.7) | 25 (59.5) | 0.260 | 0 |
| Antiviral treatment | 56 (33.1) | 39 (33.1) | 17 (33.3) | 0.990 | 25 (27.8) | 17 (45.9) | 14 (33.3) | 0.140 | 0 |
| Steroids | 32 (19) | 23 (19.5) | 9 (18) | 0.990 | 18 (20) | 5 (13.5) | 9 (22) | 0.600 | 0 |
| Biologics | 27 (16) | 17 (14.4) | 10 (19.6) | 0.490 | 11 (12.2) | 6 (16.2) | 10 (23.8) | 0.250 | 0 |
| Oxygen | 115 (68) | 72 (61) | 43 (84.3) | 0.004 | 49 (54.4) | 29 (78.4) | 37 (88.1) | <0.001 | |
| Laboratory parameters | |||||||||
| Random fasting glycaemia (mmol/l) | |||||||||
|
97 (85–125) | 89 (81–100) | 147 (132–192) | <0.001 | 86 (80–94) | 111 (104–116) | 159.5 (139–197) | <0.001 | 0 |
|
116 (96–173) | 106 (91–122) | 209 (152–265) | <0.001 | 97.5 (87–113) | 128 (114–153) | 227 (187–282) | <0.001 | 0 |
|
81 (70–97) | 78.5 (68–88) | 108 (82–139) | <0.001 | 75 (675–82) | 90 (81–103) | 120 (88–150) | <0.001 | 0 |
|
16 (9–30) | 13 (7–18) | 46 (25–57) | <0.001 | 11 (6–16) | 20 (13–29) | 49 (32–72) | <0.001 | 0 |
|
3 (2–8) | 3 (2–7) | 3 (2–8) | 0.870 | 4 (2–8) | 3 (2–6) | 3 (2–8) | 0.810 | 0 |
| HbA1c (mmol/mol) | 43 (37.5–51) | 42.5 (35.7–44) | 51 (40.5–55.5) | 0.031 | 42.5 (34.7–44) | 38 (36–51) | 51 (41–56.2) | 0.017 | 130 |
| PaO2/FiO2 | 280 (200–368) | 300 (196–395) | 258 (204–349) | 0.250 | 305 (198–400) | 269 (195–363) | 258 (199–349) | 0.320 | 22 |
|
80 (54.4) | 52 (51) | 28 (62.2) | 0.220 | 37 (48.7) | 20 (58.8) | 23 (62.2) | 0.340 | 22 |
|
39 (26.5) | 30 (29.4) | 9 (20) | 0.310 | 21 (27.6) | 10 (29.4) | 8 (21.6) | 0.720 | 22 |
| Haemoglobin (g/L) | 128.5 (114–144) | 130 (114–146.2) | 126 (114–144) | 0.720 | 129.5 (113.7–144) | 126 (116–149.5) | 131 (114–144) | 0.900 | 1 |
| Lymphocytes (x109/L) | 1 (0.75–1.5) | 1.1 (0.8–1.55) | 1 (0.52–1.37) | 0.150 | 1.1 (0.7–1.6) | 1.1 (0.8–1.6) | 1 (0.8–1.6) | 0.034 | 4 |
| Creatinine (μmol/L) | 84.4 (65.4–111.4) | 82.2 (65.4–106.1) | 96.4 (65.4–152) | 0.039 | 77.8 (66.3–101.7) | 91.9 (72.5–109.6) | 97.2 (68–152.9) | 0.018 | 1 |
| Urea (mmol/L) | 13.9 (8.9–22.2) | 11.9 (8.66–19.6) | 18.6 (10.5–33.8) | 0.004 | 11 (8.6–17.8) | 16.4 (8.9–22.8) | 20 (11.4–40.3) | 0.001 | 3 |
| AST (μkat/L) | 0.68 (0.38–1.06) | 0.6 (0.43–0.91) | 0.82 (0.64–1.35) | 0.035 | 0.58 (0.42–1.07) | 0.83 (0.42–1.07) | 0.87 (0.6–1.39) | 0.005 | 10 |
| ALT (μkat/L) | 0.59 (0.35–0.89) | 0.57 (0.33–0.83) | 0.73 (0.45–0.97) | 0.077 | 0.57 (0.3–0.83) | 0.55 (0.40–0.85) | 0.75 (0.5–0.99) | 0.087 | 1 |
| GGT (μkat/L) | 1.14 (0.38–1.32) | 0.68 (0.33–1.25) | 0.83 (0.53–1.61) | 0.094 | 0.65 (0.32–1.17) | 0.92 (0.4–2.1) | 0.83 (0.5–1.25) | 0.210 | 23 |
| LDH (μkat/L) | 5.24 (3.91–7.95) | 5.04 (3.82–8.8) | 6.65 (4.33–8.8) | 0.006 | 0.83 (0.5–1.35) | 4.68 (3.64–6.93) | 5.63 (4.29–9.1) | 0.001 | 5 |
| Albumin (g/L) | 29.2 (24.5–33.37) | 29.2 (24.3–34.6) | 29.1 (25.9–31.1) | 0.630 | 30.7 (246–34.9) | 28.3 (24.3–34.6) | 27.2 (24.6–30.3) | 0.320 | 49 |
| Pro-BNP (ng/L) | 317 (5 (69.5–1573) | 193 (59–910) | 738 (193–2238) | 0.011 | 205 (55–733) | 232 (107–1788) | 826 (174–2306) | 0.035 | 45 |
| Troponin T (μg/L) | 15.7 (7.2–44.5) | 12.7 (6–42.6) | 19.5 (11.4–61.55) | 0.078 | 11.6 (5.4–35.3) | 20.6 (9.6–58.22) | 19.5 (9.9–67.45) | 0.038 | 36 |
AST aspartate transaminase; ALT alanine transaminase; LDH lactate dehydrogenase; pro-BNP precursor of the brain natriuretic peptide.
Antibiotic treatment: azithromycin, doxycycline, amoxicillin, amoxicillin-clavulanic acid, erythromycin, clarithromycin, ceftriaxone, ampicillin, gentamicin, benzylpenicillin, piperacillin/tazobactam, ceftriaxone, eftazidime/cefepime, vancomycin, meropenem, ampicillin, ceftriaxone/cefotaxime, cefuroxime, levofloxacin; antiviral treatment: lopinavir/ritonavir, remdesivir; steroids: dexamethasone, prednisone, methylprednisolone, or hydrocortisone; bilogics: tocilizumab, sarilumab, anakinra, eculizumab.
3.3. Hospital admission
On admission signs of respiratory insufficiency were evident in most patients [PaO2/FiO2 ratio 280 (200–368)] and a PaO2/FiO2 ratio < 200 was present in 20% and 29.4% of patients with or without diabetes/stress hyperglycemia, respectively (p = 0.310). Diabetes/stress hyperglycaemia was associated with worse kidney function [serum creatinine: 96.4 (65.4–152) vs 82.2 (65.4–106) μmol/L, p = 0.039; urea nitrogen 18.6 (10.5–33.8) vs 11.9 (8.66–19.6) mmol/L, p = 0.004], increased inflammation [CRP 87.5 (35.7–184) vs 53.5 (17.9–112) mg/L; p = 0.009)] and tissue damage markers [LDH 6.65 (4.33–8.8) vs 5.04 (3.82–8.8) μ kat/L, p = 0.006; AST 0.82 (0.64–1.35) vs 0.6 (0.43–0.91) μkat/L, p = 0.006; ALT 0.73 (0.45–0.97) vs 0.57 (0.33–0.83) μ kat/L, p = 0.077; pro-BNP 738 (193–2238) vs 193 (59–910) ng/L, p = 0.011; troponin T 19.5 (11.4–61.55) vs 12.7 (6–42.6) μg/L, p = 0.078]. The same changes were associated with progressively higher blood glucose levels (Table 2). Data regarding the IgG, IgM and IgA responses to the SARS-CoV-2 spike protein (RBD or S1 + S2) and IgG to NP (Table 3 ) were available for a subgroup of patients, as they were part of a previous cohort evaluated for the humoral response in the presence of diabetes (12). Marginal differences between patients with and without diabetes/stress hyperglycemia were evident. Over the hospitalization period antibiotic (80.4% vs 61.9%, p = 0.020) and oxygen (84.3% vs 61%, p = 0.004) treatments were more frequently used in patients with diabetes/stress hyperglycaemia while antiviral, immunomodulatory, steroid and biologic therapies were equally prescribed.
Table 3.
Hemostatic parameters, inflammation markers and SARS-Cov2 antibodies according to diabetes/stress hyperglycemia and median fasting plasma glucose.
| Characteristics | All | Diabetes/stress hyperglycemia |
Median fasting glucose (mmol/l) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| No | Yes | p | <5.6 | 5.6–6.9 | ≥7 | p | Missing | ||
| N | 118 | 51 | 90 | 37 | 42 | ||||
| Platelets (x109/L) | 225 (153–307) | 230 (157–309 | 212 (151–301) | 0.610 | 229 (157–379) | 200 (160–321) | 225 (146–324) | 0.900 | 1 |
| D-dimer (μg/ml) | 5.9 (2.8–16.6) | 4.2 (2.3–9.3) | 11.8 (5.5–12.4) | <0.001 | 4.16 (2.3–9) | 5.75 (2.35–11.4) | 15.1 (6–35.6) | <0.001 | 0 |
| Elevated aD-dimer [N (%)] | 106 (62.7) | 67 (56.8) | 39 (76.5) | 0.016 | 51 (56.7) | 21 (56.8) | 34 (81) | 0.019 | 0 |
| C reactive protein (mg/L) | 57.5 (20.7–128.6) | 53.5 (17.9–112) | 87.5 (35.7–184) | 0.009 | 35.4 (11.7–92.5) | 69.1 (54–130) | 117 (47.2–204) | <0.001 | 4 |
| PT-INR | 1.06 (0.99–1.2) | 1.04 (0.98–1.15) | 1.16 (1.02–1.25) | 0.001 | 1.04 (0.98–1.17) | 1.06 (0.97–1.16) | 1.17 (1.04–1.27) | 0.005 | 4 |
| PTT-R | 0.99 (0.94–1.05) | 1 (0.95—1.05) | 0.98 (0.91–1.06) | 0.520 | 0.99 (0.94–1.05) | 1.01 (0.95–1.1) | 0.97 (0.9–1.06) | 0.270 | 4 |
| Ferritin (μg/L) | 903 (387–1514) | 804 (331–1450) | 1058 (484–1689) | 0.170 | 700 (308–1115) | 1367 (447–2492) | 1092 (588–1821) | 0.003 | 29 |
| IL-6 (pg/mL | 43.3 (15.4–98.5) | 43.7 (13.2–95.85) | 41.5 (22–124) | 0.260 | 39.2 (9.5–86) | 74.3 (13.4–131) | 41.4 (26.7–153) | 0.120 | 44 |
| Fibrinogen (g/L) | 5.53 (4.19–6.39) | 5.46 (4.16–6.19) | 5.65 (4.18–7.34) | 0.240 | 5.11 (3.89–5.85) | 5.87 (5.57–6.59) | 5.75 (4.78–7.46) | 0.004 | 42 |
| von Willebrand factor (%) | 307 (190–379) | 306 (212–377) | 336 (165–420) | 0.920 | 307 (191–376) | 301 (176–372) | 358 (184–420) | 0.490 | 101 |
| Protein C (%) | 86 (72–106) | 86 (77–103) | 73.5 (53–111) | 0.240 | 87 (76–104) | 82.5 (72–93) | 78.5 (53–111) | 0.590 | 96 |
| Protein S (%) | 78 (60–98) | 82 (60–98) | 68 (56–94) | 0.360 | 84 (61.5–101.5) | 78.5 (59–94.5) | 68 (63–88.5) | 0.560 | 98 |
| Antithrombin III (%) | 98 (88–106) | 100 (92–106) | 91 (80–102) | 0.041 | 100 (88.25–105) | 98 (93–109) | 89 (79–104) | 0.230 | 95 |
| Homocysteine (μmol/L) | 14.8 (10.1–19.4) | 14.4 (10–18.4) | 16.5 (12.3–23.5) | 0.170 | 13.4 (10–16) | 19.4 (10–24) | 15.4 (9.9–21.7) | 0.140 | 102 |
| Padua score | 3 (2–5) | 3(1–5) | 3 (3–5) | 0.006 | 3 (1–5) | 3 (1.5–4.5) | 3 (3–5) | 0.049 | 0 |
| Padua score ≥ 4 [N (%)] | 59 (34.9) | 37 (31.4) | 22 (43.1) | 0.160 | 30 (33.3) | 11 (29.7) | 18 (42.9) | 0.430 | 0 |
| IMPROVE score | 2 (1–3) | 1.6 (0.97–2.9) | 2.2 (1.2–4.1) | 0.024 | 1.6 (0.9–2.9) | 1.5 (11–2.9) | 2.2 (1.42–4.02) | 0.150 | 0 |
| IMPROVE score ≥ 7 [N (%)] | 12 (7.1) | 7 (5.9) | 5 (9.8) | 0.350 | 7 (7.8) | 1 (2.7) | 4 (9.5) | 0.470 | 0 |
| DIC by ISTH definitions [N (%)] | 4 (2.4) | 1 (0.8) | 3 (5.9) | 0.083 | 0 (0) | 1 (2.7) | 3 (7.1) | 0.042 | 0 |
| IgG RBD [N (%)] | 45 (51.1) | 30 (47.6) | 15 (60) | 0.349 | 24 (50) | 8 (47.1) | 13 (56.5) | 0.817 | 81 |
| IgM RBD [N (%)] | 46 (52.3) | 33 (52.4) | 13 (52) | 0.990 | 24 (50) | 10 (58.8) | 12 (52.2) | 0.822 | 81 |
| IgA RBD [N (%)] | 44 (50) | 27 (42.9) | 17 (68) | 0.057 | 19 (39.6) | 9 (52.9) | 16 (69.6) | 0.059 | 81 |
| IgG S1/S2 | 53 (60.2) | 36 (57.1) | 17 (68) | 0.470 | 28 (58.3) | 10 (58.8) | 15 (65.2) | 0.850 | 81 |
| IgM S1/S2 | 61 (69.3) | 42 (66.7) | 19 (76) | 0.452 | 33 (68.8) | 11 (64.7) | 17 (73.9) | 0.816 | 81 |
| IgA S1/S2 | 66 (75) | 45 (71.4) | 21 (84) | 0.281 | 34 (70.8) | 13 (76.5) | 19 (82.6) | 0.556 | 81 |
| IgG NP | 58 (65.9) | 42 (66.7) | 16 (64) | 0.808 | 31 (64.6) | 12 (70.6) | 15 (65.2) | 0.901 | 81 |
DIC disseminated intravascular coagulation; INR: international normalized ratio ISTH: International Society of Thrombosis and Hemostasis; PT prothrombin time; PTT: partial thromboplastin time.
3.4. Hemostatic parameters
Upon admission patients with diabetes/stress hyperglycaemia exhibited significantly higher D-dimer levels [11.8 (5.5–12.4) vs 4.2 (2.3–9.3) μg/mL, p < 0.001] and increased prothrombin time (PT-INR 1.16 (1.02–1.25 vs 1.04 (0.98–1.15), p = 0.001) compared with patients without diabetes/stress hyperglycemia (Table 3). Concordantly, the percentage of patients with elevated age-specific D-dimer was significantly higher in the presence of diabetes/stress hyperglycemia (76.5% vs 56.8%, p = 0.016). Partial thromboplastin time, platelet count and fibrinogen were not affected by diabetes/stress hyperglycemia. Advanced markers of thrombophilia were available for a subgroup of patients (Table 3). Exploratory analysis did not show significant changes in coagulation factors levels or activity in subjects with diabetes, except for a lower antithrombin III activity [91% (80–120) vs 100% (92–106)]. The presence of diabetes/stress hyperglycemia did not significantly increase the proportion of patients who at baseline had a higher risk for venous thromboembolism based on the Padua score (Padua score ≥ 4: 43.1% vs 31.4%, p = 0.160), even if a marginal, although significant, difference in the absolute Padua score was evident [3 (3–5) vs 3 (1–5), p = 0.006)] (Table 3). Similarly, the proportion of patients at high baseline bleeding risk based on the IMPROVE score was not affected by the presence of diabetes/stress hyperglycemia (IMPROVE≥7: 9.8% vs 5.9%; p = 0.350), even if a marginal, although significant, difference in the IMPROVE score was evident [2.2 (1.2–4.1) vs 1.6 (0.97–2.9), p = 0.024)] (Table 1).
3.5. Thromboembolic complications
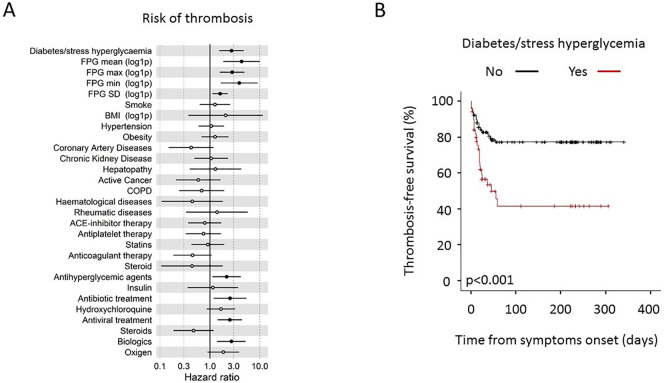
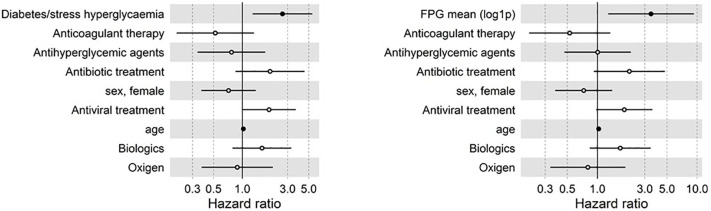
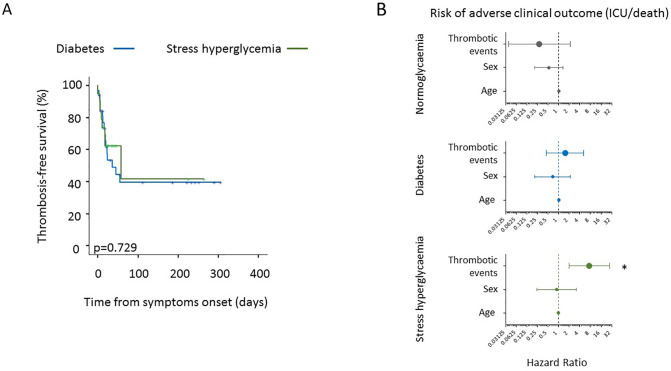
Forty-eight venous and 10 arterial TE were identified in 49 (29%) patients (Table 4 ). The median time from the onset of symptoms of COVID-19 pneumonia to the TE was 17 (10–24) days. Patients with diabetes/stress hyperglycaemia developed more frequently a thromboembolic complication (47.1% vs 21.2%, p = 0.001) than patients without diabetes/stress hyperglycaemia. Concordantly, the higher prevalence of thromboembolic complication was associated with higher fasting blood glucose levels during the hospitalization (Table 4). Regarding the thrombosis site, venous events (in particular deep vein thrombosis) contributed more significantly than arterial ones to the higher prevalence of thromboembolic complications in patients with diabetes/stress hyperglycaemia compared to those without diabetes/stress hyperglycaemia. In patients with and without diabetes/stress hyperglycaemia, 3 and 1 (5.9% vs 0.8%, p = 0.083) events were classified as overt DIC (≥5 points, according to the ISTH diagnostic criteria), respectively. The result of a Cox regression analysis for TE is presented in Fig. 1 . The Cox regression analysis adjusted for age and sex indicates that diabetes/stress hyperglycaemia (HR 2.71, CI 1.53–4.8; p = 0.001), fasting plasma glucose [FPG mean (log1p) HR 4.32, CI 1.86–10, p = 0.001] and glucose variability [FPG standard deviation (log1p) HR 1.6, CI 1.13–2.28, p = 0.009] were associated with a higher risk of thromboembolic complications. Concordantly the preadmission antihyperglycemic agent treatment, as a proxy of comorbid diabetes diagnosis, was associated with thromboembolic complications (HR 2.16, CI 1.12–4.16; p = 0.021). Differences in smoke habit, BMI, other comorbidities and preadmission antiplatelet or steroid were all statistically not significant. A trend towards a protective effect of preadmission anticoagulant therapy was evident (HR 0.45, CI 0.18–1.08; p = 0.075). Regarding the hospital therapy, antibiotic (HR 2.53, CI 1.18–5.4; p = 0.017), antiviral (HR 2.5, CI 1.42–4.4; p = 0.001) and biologic treatments (HR 2.7, CI 1.39–5.3; p = 0.003) were associated with a higher risk of thromboembolic complications and a trend was also evident for oxygen use (HR 1.85, CI 0.89–3.87; p = 0.099). A multivariate analysis including variables significant at the level of <0.1 in the univariate analysis (Fig. 2 ) confirmed diabetes/stress hyperglycaemia (HR 2.64, CI 1.28–5.44; p = 0.008) and fasting plasma glucose [FPG mean (log1p) HR 3.45, CI 1.3–9.3 p = 0.014] as independent predictors of thromboembolic complications. Comorbid diabetes and stress hyperglycaemia, when analysed separately, were equally associated with a higher prevalence of thromboembolic complication (52% vs 42.1% respectively, p = 0.770; Fig. 3a).
Table 4.
Thrombotic events (TEs).
| Diabetes/stress hyperglycemia |
Median fasting glucose (mmol/l) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All | No | Yes | p | Comorbid diabetes | Stress hyperglycemia | p | <5.6 | 5.6–6.9 | ≥ 7 | p | |
| N | 169 | 118 | 51 | 32 | 19 | 90 | 37 | 42 | |||
| Patients with at least one event [N (%)] | 49 (29) | 25 (21.2) | 24 (47.1) | 0.001 | 16 (50) | 8 (42.1) | 0.770 | 20 (22.2) | 9 (24.3) | 20 (47.6) | 0.009 |
| Median time from symptoms to thrombosis | 17 (10–24) | 11 (2−20) | 18 (8–28) | 0.110 | 21 (11−31) | 11 (0–28) | 0.440 | 13 (4–22) | 6 (0−12) | 18 (3−33) | 0.190 |
|
Arterial events [N (%)] |
|||||||||||
|
3 (1.8) | 1 (0.8) | 2 (3.9) | 0.220 | 2 (6.3) | 0 (0) | 0.520 | 0 (0) | 2 (5.4) | 1 (2.4) | 0.100 |
|
1 (0.6) | 0 (0) | 1 (2) | 0.300 | 1 (3.1) | 0 (0) | 0.990 | 0 (0) | 0 (0) | 1 (2.4) | 0.190 |
|
6 (3.6) | 4 (3.4) | 2 (3.9) | 0.990 | 1 (3.1) | 1 (5.3) | 0.990 | 4 (4.4) | 0 (0) | 2 (4.8) | 0.420 |
|
0 (0) | 0 (0) | 0 (0) | – | 0 (0) | 0 (0) | – | 0 (0) | 0 (0) | 0 (0) | – |
| Total patients with arterial event | 10 (5.9) | 5 (4.2) | 5 (9.8) | 0.170 | 4 (12.5) | 1 (5.3) | 0.640 | 4 (4.4) | 2 (5.4) | 4 (9.5) | 0.510 |
|
Venous events [N (%)] |
|||||||||||
|
17 (10.1) | 6 (5.1) | 11 (21.6) | 0.004 | 8 (25) | 3 (15.8) | 0.500 | 6 (6.7) | 3 (8.1) | 8 (19) | 0.080 |
|
15 (8.9) | 8 (6.8) | 7 (13.7) | 0.150 | 4 (12.5) | 3 (15.8) | 0.990 | 4 (4.4) | 5 (13.5) | 6 (14.3) | 0.096 |
|
8 (4.7) | 6 (5.1) | 2 (3.9) | 0.990 | 1 (3.1) | 1 (5.3) | 0.990 | 6 (6.7) | 0 (0) | 2 (4.8) | 0.270 |
|
8 (4.7) | 3 (2.5) | 5 (9.8) | 0.055 | 4 (12.5) | 1 (5.3) | 0.640 | 4 (4.4) | 1 (2.7) | 3 (7.1) | 0.640 |
| Total patients with venous events | 43 (25.4) | 22 (18.6) | 21 (48.8) | 0.004 | 13 (40.6) | 8 (42.1) | 0.990 | 18 (20) | 8 (21.6) | 17 (40.5) | 0.035 |
|
Arterial and venous events [N (%)] |
|||||||||||
|
1 (0.6) | 0 (0) | 1 (2) | 0.300 | 1 (3.1) | 0 (0) | 0.990 | 0 (0) | 1 (2.7) | 0 (0) | 0.170 |
|
0 (0) | 0 (0) | 0 (0) | – | 0 (0) | 0 (0) | – | 0 (0) | 0 (0) | 0 (0) | – |
|
3 (1.8 | 2 (1.7) | 1 (2) | 0.990 | 0 (0) | 1 (5.3) | 0.370 | 2 (2.2) | 0 (0) | 1 (2.4) | 0.650 |
| Total patients with arterial and venous events | 4 (2.4) | 2 (1.7) | 2 (3.9) | 0.580 | 1 (3.1) | 1 (5.3) | 0.990 | 2 (2.2) | 1 (2.7) | 1 (2.4) | 0.990 |
DVT: Deep vein thrombosis; PVT: Pulmonary vein thrombosis.
Fig. 1.
TEs in patients with COVID-19. Univariate Cox regression analysis for thrombosis risk adjusted for sex and age. The forest plots (panel a) show the Hazard Ratios (HR) for thrombosis for each factor tested. Dots represent the HR, lines represent 95% confidence interval (CI), and solid dots indicate P < 0.05. Kaplan-Meier thrombosis-free survival estimates for patients with COVID-19 pneumonia (panel b). Survival rate was estimated for the presence of diabetes. The log-rank test was used to test differences in the estimated survival rate. Crosses indicate censored patients (censoring for death end of follow-up data).
Fig. 2.
TEs in patients with COVID-19. Multivariate Cox regression analysis adjusted for sex and age including variables significant at the level of <0.1 in the univariate analysis. The forest plots show the Hazard Ratios (HR) for thrombosis for each factor tested. Dots represent the HR, lines represent 95% confidence interval (CI), and solid dots indicate P < 0.05.
Fig. 3.
Thrombosis-free survival and survival in the absence of adverse clinical outcome in patients with COVID-19 with or without TEs, according to diabetes and stress hyperglycemia. Kaplan-Meier thrombosis-free survival estimates for patients with COVID-19 pneumonia (panel a). Survival rate was estimated for the presence of diabetes or stress hyperglycemia. The log-rank test was used to test differences in the estimated survival rate. Crosses indicate censored patients (censoring for death end of follow-up data). The forest plots (panel b) show the hazard ratios for survival in the absence of adverse clinical outcome according to presence/absence of diabetes/stress hyperglycemia. The presence of thrombotic events was considered as a time-varying covariate in Cox proportional hazards models. The effect estimates were reported as Hazard Ratios with the corresponding 95% CI, estimated according to the Wald approximation. Cox regression analysis was adjusted for sex and age. Dots represent the HR, lines represent 95% confidence interval (CI), and * P < 0.01.
3.6. TEs and adverse clinical outcome in patients with and without diabetes/stress hyperglycaemia
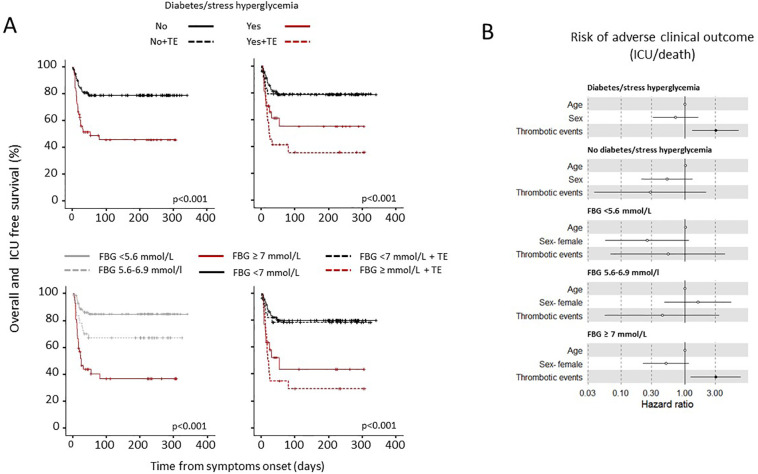
To assess whether the presence of TEs had an impact on patient outcome according to diabetes/stress hyperglycaemia or fasting glucose levels, we conducted a Kaplan-Meier estimator log-rank test and a Cox proportional hazards model for adverse clinical outcome (as defined by composite endpoint of transfer to ICU or death, whichever occurred first) (Fig. 4a). The Cox regression analysis adjusted for age and sex indicated that diabetes/stress hyperglycaemia (HR 2.99, CI 1.7–5.03; p < 0.001), fasting plasma glucose [FPG mean (log1p) HR 9.6, CI 4.59–20; p < 0.001] and glucose variability [FPG standard deviation (log1p) HR 2.02, CI 1.43–2.9; p < 0.001] were strongly associated with a higher risk of adverse clinical outcome (see also Table 2). TEs were not associated with an adverse clinical outcome in the absence of diabetes/stress hyperglycaemia (HR 0.29, CI 0.04–2.16; p = 0.225) or in the presence of FBG <7 mmol/l (HR 0.54, CI 0.07–4.35; p = 0.560), while they significantly increased the risk in the presence of diabetes/stress hyperglycaemia (HR 3.05, CI 1.31–7.09; p = 0.010) or FBG ≥ 7 mmol/l (HR 3.07, CI1.24–7.6; p = 0.015) (Fig. 4b) A separate analysis was also performed for diabetes and stress hyperglycaemia. Although the number of cases is inadequate for a definitive conclusion, the risk of adverse clinical outcome seems more associated with stress-related hyperglycemia than with diabetes (Fig. 3b).
Fig. 4.
Survival in the absence of adverse clinical outcome in patients with COVID-19 with or without TEs, according to diabetes/stress hyperglycemia or fasting glucose levels. Kaplan-Meier patient survival estimates for 169 patients with COVID-19 pneumonia (panel a). Survival rate in the absence of adverse clinical outcome (defined by composite endpoint of transfer to ICU or death, whichever occurred first) was estimated for the presence of any thrombotic event (TE) separately according diabetes/stress hyperglycemia or fasting glucose levels. The log-rank test was used to test differences in the estimated survival rate between groups. Crosses indicate censored patients (censoring for death or end of follow-up data). The forest plots (panel b) show the hazard ratios for survival in the absence of adverse clinical outcome according to presence/absence of diabetes/stress hyperglycemia or fasting glucose levels (FBG) categories. The presence of thrombotic events (TE) was considered as a time-varying covariate in Cox proportional hazards models. The effect estimates were reported as Hazard Ratios (HRs) with the corresponding 95% CI, estimated according to the Wald approximation. Cox regression analysis was adjusted for sex and age. Dots represent the HR, lines represent 95% confidence interval (CI), and solid dots indicate P < 0.05.
4. Discussion
There are few reports on the relationship between hyperglycemia and the rate of TEs in COVID-19 pneumonia and it is still unknown whether thrombosis affects the prognosis of patients with COVID-19 pneumonia in the presence of diabetes. Under the hypothesis of a relevant role for diabetes, we designed a prospective observational study focusing on TEs occurring during hospitalization and risk factors associated with thromboembolic complications in patients with COVID-19 pneumonia. Our study generated several interesting findings in those patients. First, diabetes/stress hyperglycemia, high fasting glycaemia and glycemic variability were strong risk factors for the development of thromboembolic complications. Second, the rate of venous thrombosis events (in particular deep vein thrombosis) was the most affected by the presence of diabetes/stress hyperglycemia. Third, thromboembolic complications had an adverse impact on clinical outcome exclusively in the presence of diabetes/stress hyperglycemia. While reasonable, these results could not have been taken for granted [27]. TEs have a higher incidence among patients with COVID-19 [[28], [29], [30]] and diabetes is per se characterized by a pro-thrombotic status [7]. We and others have previously reported an increase of D-dimer in patients with diabetes/stress hyperglycemia and COVID-19 pneumonia compared to those without [[11], [12], [13]]. However the clinical implications in term of thromboembolic risk of those findings were yet unclear. Moreover, data on the correlation of thromboembolic complications with clinical outcome were limited and contradictory, with some studies finding a higher risk of adverse outcome associated with TEs in hospitalized patients with COVID-19 [31], while others did not find any association [32]. The pathophysiological mechanisms related to the increased risk of TEs in patients with COVID-19 pneumonia and diabetes are still incompletely understood. In our study, diabetes/stress hyperglycemia was associated with both inflammation and coagulopathy (elevated C reactive protein and D-dimer levels, mild prolongation of the prothrombin time and decreased antithrombin III), suggesting that an hyperglycaemia-related amplification of the pathobiological mechanisms of immunothrombosis [33] could be responsible of the increased thrombotic risk. The reduced activity of antithrombin III is of particular interest in this context [34]. In fact, antithrombin III is a powerful natural anticoagulant which is regulated by inflammation [35]. Therefore, it can be speculated that hyperinflammation might have been triggering a decrease in antithrombin III levels and its physiological anticoagulant activity. Furthermore, since the clinical anticoagulant efficacy of heparin requires interaction with antithrombin III, an impaired levels/activity of antithrombin III may be associated with “heparin resistance” [35]. In agreement with this hypothesis, an association between antithrombin III levels and mortality in patients with COVID-19 pneumonia has already been reported [36]. An association between thromboembolic complications and the presence of antiphospholipid antibodies in patients with COVID-19 pneumonia has been previously reported in case reports, case series, cohort studies, and cross-sectional studies, although with contradictory results [37]. Unfortunately antiphospholipid antibody measurements were available only for a subgroup of our patients and, therefore, it was impossible to include them in a multivariate model to test for their contribution to thromboembolic risk.
An interesting result from out study is the fact that thromboembolism events did not happen after 100 days from symptom onset both in diabetes and non-diabetes patients. This supports the idea that thrombotic events are mainly sustained by the acute inflammatory phase of the disease while chronic inflammation or mechanisms possibly related to the activation of autoimmunity, which were suggest to be relevant for the post-acute COVID-19 syndrome, appear less relevant [38]. Of note, while comorbid diabetes and stress hyperglycaemia were both associated with a higher prevalence of thromboembolic complication, the risk of adverse clinical outcome seems more associated with stress-related hyperglycemia than with diabetes. Although there are not much enough cases for a definitive conclusion, this finding suggests that the new onset hyperglycemia is more dangerous than the established diabetes. Since the mean blood glucose during hospitalization was not different in patients with either new-onset or preexisting diabetes (data not shown), factors other than hyperglycemia should be considered to explain this differences.
Our study encompasses some limitations: first, our cohort was limited to hospitalized patients and results could be different in less severe COVID-19 disease. Second, even if the overall venous and arterial thromboembolism rate was similar to that described until now in various studies [15], our monocentric cohort was relatively small, and, therefore, a selection bias cannot be excluded. Third, we were unable to evaluate the specific role of some markers as predictors of thrombosis in multivariate models since a complete set of biochemical coagulation data was available only for a fraction of patients. Fourth, a role of a genetic thrombophilia predisposition within our cohort was not assessed. The presence of genetic polymorphisms related to higher risk for inherited thrombophilia did not emerge from any patient's medical history, however, genotyping was not performed during hospitalization and we cannot exclude its presence. However, since the prevalence of genetic thrombophilia susceptibility is not known to be higher in subjects with diabetes, it is very unlikely that it played a role in determining the outcome of our patients. Moreover, a recent study reported that the frequency of polymorphic biomarkers of thrombophilia [prothrombin gene F2 (rs1799963/G20210A), factor V Leiden F5 (rs6025/G1691A), and PAI-1 rs1799768] is not different between subject with severe coronavirus disease and healthy population [39]. Nevertheless, our study generated additional valuable knowledge about the role of diabetes/stress hyperglycemia in predicting TEs and in stratifying their prognostic significance.
5. Conclusions
Many evidences indicate that patients with diabetes, in case of COVID-19 pneumonia, carry a significant increased risk for adverse clinical outcome when compared with patients without diabetes. It is clear from our study that part of this excess risk is due to an increase in thromboembolic complications. These findings suggest that in in case of SARS-CoV-2 pneumonia, patients with diabetes/stress hyperglycemia should be considered for a more intensive prophylactic anticoagulation regimen.
Individual contributions
Stefania L Calvisi: Data Curation, Investigation, Writing - Review & Editing; Giuseppe A Ramirez Data Curation, Investigation, Writing - Review & Editing; Marina Scavini: Writing - Review & Editing, Formal analysis; Valentina Da Prat: Investigation; Giuseppe Di Lucca: Investigation; Andrea Laurenzi: Investigation; Gabriele Gallina: Investigation; Ludovica Cavallo: Investigation; Giorgia Borio: Investigation; Federica Farolfi: Investigation; Maria Pascali: Investigation; Jacopo Castellani: Investigation; Vito Lampasona: Resources, Formal analysis; Armando D'Angelo: Writing - Review & Editing; Giovanni Landoni: Resources; Fabio Ciceri: Funding acquisition; Patrizia Rovere Querini: Data Curation; Moreno Tresoldi: supervision; Lorenzo Piemonti: Conceptualization, Methodology, Formal analysis, Writing - Original Draft. . LP is the guarantor of this work and, as such, had full access to all the data presented in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The final manuscript has been read and approved by all named authors.
CRediT authorship contribution statement
Stefania L. Calvisi: Data curation, Investigation, Writing – review & editing. Giuseppe A. Ramirez: Data curation, Investigation, Writing – review & editing. Marina Scavini: Writing – review & editing, Formal analysis. Valentina Da Prat: Investigation. Giuseppe Di Lucca: Investigation. Andrea Laurenzi: Investigation. Gabriele Gallina: Investigation. Ludovica Cavallo: Investigation. Giorgia Borio: Investigation. Federica Farolfi: Investigation. Maria Pascali: Investigation. Jacopo Castellani: Investigation. Vito Lampasona: Resources, Formal analysis. Armando D'Angelo: Writing – review & editing. Giovanni Landoni: Resources. Fabio Ciceri: Funding acquisition. Patrizia Rovere Querini: Data curation. Moreno Tresoldi: Supervision. Lorenzo Piemonti: Conceptualization, Methodology, Formal analysis, Writing – original draft.
Declaration of competing interest
The authors have no conflict of interest to disclose in relation to the topic of this manuscript. The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
This work was funded by Program Project COVID-19 OSR-UniSR and COVID-2020-12371617 Ministero della Salute.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mantovani A., Byrne C.D., Zheng M.H., Targher G. Diabetes as a risk factor for greater COVID-19 severity and in-hospital death: a meta-analysis of observational studies. Nutr. Metab. Cardiovasc. Dis. 2020;30:1236–1248. doi: 10.1016/j.numecd.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Apicella M., Campopiano M.C., Mantuano M., Mazoni L., Coppelli A., Del Prato S. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020;8:782–792. doi: 10.1016/S2213-8587(20)30238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo W, Li M, Dong Y, Zhou H, Zhang Z, Tian C, et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020:e3319.1. [DOI] [PMC free article] [PubMed]
- 6.Geerlings S.E., Hoepelman A.I. Immune dysfunction in patients with diabetes mellitus (DM) FEMS Immunol. Med. Microbiol. 1999;26:259–265. doi: 10.1111/j.1574-695X.1999.tb01397.x. [DOI] [PubMed] [Google Scholar]
- 7.Vazzana N., Ranalli P., Cuccurullo C., Davi G. Diabetes mellitus and thrombosis. Thromb. Res. 2012;129:371–377. doi: 10.1016/j.thromres.2011.11.052. [DOI] [PubMed] [Google Scholar]
- 8.Secchi M., Bazzigaluppi E., Brigatti C., Marzinotto I., Tresoldi C., Rovere-Querini P., et al. COVID-19 survival associates with the immunoglobulin response to the SARS-CoV-2 spike receptor binding domain. J. Clin. Invest. 2020;130:6366–6378. doi: 10.1172/JCI142804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lampasona V., Secchi M., Scavini M., Bazzigaluppi E., Brigatti C., Marzinotto I., et al. Antibody response to multiple antigens of SARS-CoV-2 in patients with diabetes: an observational cohort study. Diabetologia. 2020;63:2548–2558. doi: 10.1007/s00125-020-05284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dispinseri S., Lampasona V., Secchi M., Cara A., Bazzigaluppi E., Negri D., et al. Robust neutralizing antibodies to SARS-CoV-2 develop and persist in subjects with diabetes and COVID-19 pneumonia. J. Clin. Endocrinol. Metab. 2021;106:1472–1481. doi: 10.1210/clinem/dgab055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan Y., Yang Y., Wang F., Ren H., Zhang S., Shi X., et al. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diabetes Res. Care. 2020;8 doi: 10.1136/bmjdrc-2020-001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mishra Y., Pathak B.K., Mohakuda S.S., Tilak T., Sen S. P H, et al. relation of D-dimer levels of COVID-19 patients with diabetes mellitus. Diabetes Metab Syndr. 2020;14:1927–1930. doi: 10.1016/j.dsx.2020.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sardu C., D’Onofrio N., Balestrieri M.L., Barbieri M., Rizzo M.R., Messina V., et al. Outcomes in patients with hyperglycemia affected by COVID-19: can we do more on glycemic control? Diabetes Care. 2020;43:1408–1415. doi: 10.2337/dc20-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soomro A.Y., Guerchicoff A., Nichols D.J., Suleman J., Dangas G.D. The current role and future prospects of D-dimer biomarker. Eur Heart J Cardiovasc Pharmacother. 2016;2:175–184. doi: 10.1093/ehjcvp/pvv039. [DOI] [PubMed] [Google Scholar]
- 15.Malas M.B., Naazie I.N., Elsayed N., Mathlouthi A., Marmor R., Clary B. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: a systematic review and meta-analysis. EClinicalMedicine. 2020;29:100639. doi: 10.1016/j.eclinm.2020.100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang C., Zhang H., Zhou M., Cheng Y., Ye L., Chen J., et al. Prognosis of COVID-19 in patients with vein thrombosis: a systematic review and meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2020;24:10279–10285. doi: 10.26355/eurrev_202010_23252. [DOI] [PubMed] [Google Scholar]
- 17.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Connors J.M., Levy J.H. Thromboinflammation and the hypercoagulability of COVID-19. J. Thromb. Haemost. 2020;18:1559–1561. doi: 10.1111/jth.14849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bozzani A., Arici V., Tavazzi G., Franciscone M.M., Danesino V., Rota M., et al. Acute arterial and deep venous thromboembolism in COVID-19 patients: risk factors and personalized therapy. Surgery. 2020;168:987–992. doi: 10.1016/j.surg.2020.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu S.X., Tyagi T., Jain K., Gu V.W., Lee S.H., Hwa J.M., et al. Thrombocytopathy and endotheliopathy: crucial contributors to COVID-19 thromboinflammation. Nat. Rev. Cardiol. 2020;18:194–209. doi: 10.1038/s41569-020-00469-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.China NHCo The diagnosis and treatment plan for the novel coronavirus disease. The seventh edition. 2020;2020 [Google Scholar]
- 22.World_Health_Organization Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. Interim Guidance. 2020 https://www.who.int/publications/i/item/10665-332299 [Google Scholar]
- 23.Marietta M., Ageno W., Artoni A., De Candia E., Gresele P., Marchetti M., et al. COVID-19 and haemostasis: a position paper from Italian society on thrombosis and Haemostasis (SISET) Blood Transfus. 2020;18:167–169. doi: 10.2450/2020.0083-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thachil J., Tang N., Gando S., Falanga A., Cattaneo M., Levi M., et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J. Thromb. Haemost. 2020;18:1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor F.B., Jr., Toh C.H., Hoots W.K., Wada H., Levi M. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb. Haemost. 2001;86:1327–1330. [PubMed] [Google Scholar]
- 26.Righini M., Van Es J., Den Exter P.L., Roy P.M., Verschuren F., Ghuysen A., et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. Jama. 2014;311:1117–1124. doi: 10.1001/jama.2014.2135. [DOI] [PubMed] [Google Scholar]
- 27.Wijaya I., Andhika R., Huang I. Hypercoagulable state in COVID-19 with diabetes mellitus and obesity: is therapeutic-dose or higher-dose anticoagulant thromboprophylaxis necessary? Diabetes Metab Syndr. 2020;14:1241–1242. doi: 10.1016/j.dsx.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lodigiani C., Iapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T., et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan. Italy Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X., et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ali M.A.M., Spinler S.A. COVID-19 and thrombosis: from bench to bedside. Trends Cardiovasc Med. 2021;31:143–160. doi: 10.1016/j.tcm.2020.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang L., Feng X., Zhang D., Jiang C., Mei H., Wang J., et al. Deep vein thrombosis in hospitalized patients with COVID-19 in Wuhan, China: prevalence, risk factors, and outcome. Circulation. 2020;142:114–128. doi: 10.1161/CIRCULATIONAHA.120.046702. [DOI] [PubMed] [Google Scholar]
- 32.Hippensteel J.A., Burnham E.L., Jolley S.E. Prevalence of venous thromboembolism in critically ill patients with COVID-19. Br. J. Haematol. 2020;190:e134–e137. doi: 10.1111/bjh.16908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loo J., Spittle D.A., Newnham M. COVID-19, immunothrombosis and venous thromboembolism: biological mechanisms. Thorax. 2021;76:412–420. doi: 10.1136/thoraxjnl-2020-216243. [DOI] [PubMed] [Google Scholar]
- 34.Mir N., D’Amico A., Dasher J., Tolwani A., Valentine V. Understanding the andromeda strain - the role of cytokine release, coagulopathy and antithrombin III in SARS-CoV2 critical illness. Blood Rev. 2020;100731 doi: 10.1016/j.blre.2020.100731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levy J.H., Sniecinski R.M., Welsby I.J., Levi M. Antithrombin: anti-inflammatory properties and clinical applications. Thromb. Haemost. 2016;115:712–728. doi: 10.1160/TH15-08-0687. [DOI] [PubMed] [Google Scholar]
- 36.Gazzaruso C., Paolozzi E., Valenti C., Brocchetta M., Naldani D., Grignani C., et al. Association between antithrombin and mortality in patients with COVID-19. A possible link with obesity. Nutr. Metab. Cardiovasc. Dis. 2020;30:1914–1919. doi: 10.1016/j.numecd.2020.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El Hasbani G., Taher A.T., Jawad A., Uthman I. COVID-19, antiphospholipid antibodies, and catastrophic antiphospholipid syndrome: a possible association? Clin Med Insights Arthritis Musculoskelet Disord. 2020;13 doi: 10.1177/1179544120978667. 1179544120978667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., et al. Post-acute COVID-19 syndrome. Nat. Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kiraz A., Guzeldag S., Eren E., Goksu M. Investigation of the relationship between inherited thrombophilia and novel coronavirus pneumonia; Future Virology: 2021. Bayram A. [Google Scholar]