Abstract
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread worldwide and has seriously threatened public health by causing significant morbidity and mortality. Patients with coronavirus disease (COVID-19) with preexisting endothelial dysfunction caused by aging, diabetes, hypertension, and obesity are at high risk for life-threatening thromboembolic complications. This suggests a possibility that reduced endothelial nitric oxide (NO) production and NO bioavailability could be a common underlying pathology for the progression of COVID-19. Increasingly, evidence from experimental and clinical studies of SARS-CoV-2 infection shows that NO inhibits the pathogenesis of COVID-19, including virus entry into host cells, viral replication, host immune response, and subsequent thromboembolic complications. Restoring NO bioavailability may have the potential to be a preventive or early-treatment option for COVID-19. This review aims to provide in-depth discussion of NO bioavailability to prevent SARS-CoV-2 infection, particularly by focusing on lifestyle factors such as nitrate-rich diets, physical exercise, and nasal breathing, which could be easily performed on a daily basis to boost NO bioavailability.
Keywords: SARS-CoV-2, COVID-19, Nitric oxide, Lifestyle, Endothelial dysfunction, ARDS
Abbreviations: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; COVID-19, coronavirus disease; NO, nitric oxide; ARDS, acute respiratory distress syndrome; cGMP, cyclic guanosine-3′,5′-monophosphate; ACE2, angiotensin-converting enzyme 2; TMPRSS2, transmembrane protease serine 2; 3CL, 3-chymotrypsin-like; NO+, nitrosonium ion; PRR, pattern-recognition receptor; IFN, interferon; NF-κB, nuclear factor-κB; eNOS, NO synthase; AT1R, Ang-Ⅱ type 1 receptor; N2O3, dinitrogen trioxide
1. Introduction
The novel coronavirus disease (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has swept worldwide and has seriously threatened public health by causing significant morbidity and mortality [1]. Despite commencing vaccination, with initially satisfactory efficacy, recent emergence of variant strains necessitates further investigation to reevaluate vaccine efficacy and safety [2]. Therefore, confirmatory polymerase chain reaction tests to identify SARS-CoV-2 infection and basic preventive measures, such as social distancing and wearing a mask, remain important precautions against COVID-19 [3]. SARS-CoV-2 primarily infects the respiratory tract and often progresses rapidly to acute respiratory distress syndrome (ARDS), with subsequent systemic viral spread and cytokine storm to cause endotheliitis of the vasculature of the intestine, kidney, heart, and brain [4]. Healthy endothelial cells naturally produce and release nitric oxide (NO), which regulates vascular tone and inhibits platelet aggregation and coagulation to maintain vascular integrity [5]. However, persistent endothelial injury in severe COVID-19 decreases endothelial NO generation, thereby disrupting vascular integrity and increasing systemic thromboembolic complications. In particular, COVID-19 patients with preexisting endothelial dysfunction (i.e., aging, diabetes, hypertension, and obesity) are likely to physically worsen than younger and healthy individuals [[6], [7], [8]]. Furthermore, epidemiological studies showed that SARS-CoV-2 infection causes greater morbidity and mortality in males than in females due to less estrogen-mediated vascular endothelial protection in the former [9]. These observations suggest that endothelial dysfunction could be a common underlying pathology in patients at risk for COVID-19 [10]. Recent studies have shown that lifestyle factors, such as diet and physical exercise, have a considerable effect on increasing NO bioavailability and can prevent lifestyle-related diseases by compensating for impaired vascular endothelial NO production [5]. Therefore, restoring NO bioavailability may be a potential preventive or early-treatment option for COVID-19. This review aims to provide an in-depth discussion of lifestyle factors to prevent SARS-CoV-2 infection and its complications by boosting NO bioavailability.
2. Pleiotropic inhibitory effects of NO on the pathogenesis of COVID-19
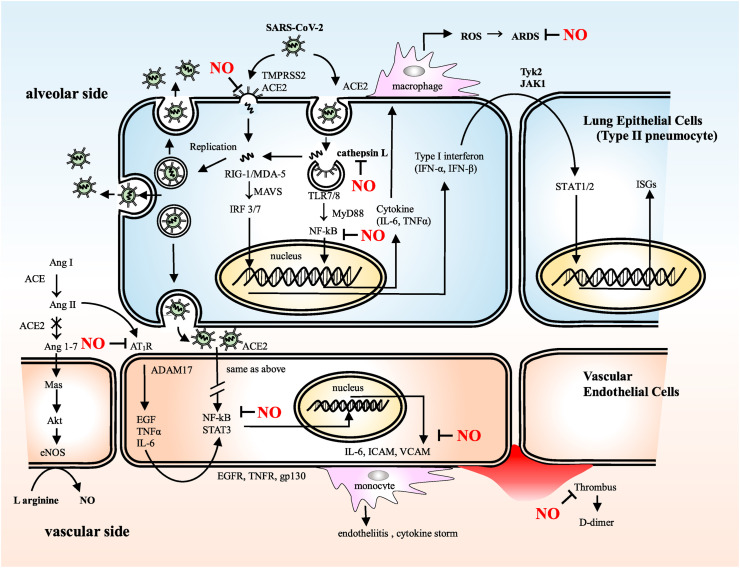
In a cyclic guanosine-3′,5′-monophosphate (cGMP)-dependent and -independent manner that inhibits viral enzymes and cellular host factors essential for the virus life cycle and subsequent host inflammatory response, NO inhibits most stages of the inflammatory process underlying COVID-19 (Fig. 1 ).
Fig. 1.
SARS-CoV-2 entry to host cells, replication, recognition by innate immune system and subsequent inflammatory process
The SARS-CoV-2 life cycle in the host cells and subsequent inflammatory process are described in detail in the text.
SARS-CoV-2: severe acute respiratory syndrome coronavirus 2, TMPRSS2: transmembrane protease serine 2, ACE2: angiotensin-converting enzyme 2, ROS: reactive oxygen species, ARDS: acute respiratory distress syndrome, RIG-Ⅰ: retinoic acid-inducible gene-Ⅰ, MDA-5: melanoma differentiation-associated protein 5, MAVS: mitochondrial antiviral-signaling protein, IRF: interferon regulatory factor, TLR7/8: Toll-like receptor 7/8, MyD88: myeloid differentiation primary response 88, NF-kB: nuclear factor-kappa B, IL-6: interleukin-6, TNFα: tumor necrosis factor α, Ang Ⅰ: angiotensin Ⅰ:, Ang Ⅱ: angiotensin Ⅱ, Ang1-7: angiotensin 1-7, eNOS: endothelial nitric oxide synthase, AT1R: angiotensin II type I receptor, ADAM17: a disintegrin and metalloprotease 17, EGF: epidermal growth factor, EGFR: epidermal growth factor receptor, TNFR: tumor necrosis factor receptor, STAT3: signal transducer and activator of transcription 3, gp130: glycoprotein 130, ICAM: intercellular adhesion molecule, VCAM: vascular cell adhesion molecule, ISGs: interferon-stimulated genes.
2.1. Viral entry to the host cells
In the respiratory tract, NO provides a first-line of defense against micro-organisms via its antiviral and antimicrobial activities. In airborne viral transmission to the airway, NO can physically remove the viral particles from the upper respiratory tract by activating ciliary movement [11] and mucus secretion in the airway epithelium [11,12]; aging and habitual smoking may eliminate this defensive ability and facilitate the viral invasion of the lower respiratory tract, where the virus can infect multiple cell types, including alveolar airway epithelial cells, alveolar macrophages, and vascular endothelial cells (Fig. 1). In the case of SARS-CoV-2, the viral spike (S)-protein, which is required for viral entry, has two regions, S1 and S2. S1 has a receptor-binding domain that mediates direct contact with the host cellular receptor, angiotensin-converting enzyme 2 (ACE2), whereas S2 is involved in subsequent membrane fusion [13]. Following receptor binding at the host cell membrane to form the S-protein–ACE2 complex, the S-protein is proteolytically cleaved at the S1/S2 and S2’ sites by the cellular protease, transmembrane protease serine 2 (TMPRSS2) [14,15], followed by a conformational change that may cause a close apposition of S-protein to the cellular membrane. In this setting, the membrane fusion and viral entry are facilitated due to increasing lipophilicity when the cysteine residues of S-protein are palmitoylated. However, NO interferes with the fusion between the viral S-protein and the host cellular membrane [16]. In vitro studies of SARS-CoV-1 have shown that in the presence of the NO donor, S-nitroso-N-acetylpenicillamine (SNAP), the exposed cysteine residues of the S-protein, following enzymatic priming by TMPRSS2, are competitively nitrosylated with palmitoylation, leading to the inhibition of membrane fusion and viral entry to the host cells [17]. Moreover, it may be possible that endosomal cathepsin L, an acid-activated cysteine protease and alternative enzyme for the membrane fusion following endocytosis, could be nitrosylated at the cysteine residue in the active site of this enzyme to inhibit the ensuing viral replication and immune reaction (Fig. 1) [18].
2.2. Viral replication and innate immune response
After SARS-CoV-2 enters the target cells, viral replication and the innate immune response are initiated, and SARS-CoV-2 is disassembled to release nucleocapsid and viral RNA into the cytosol for translation and replication (Fig. 1). Translated viral proteins are then assembled in the endoplasmic reticulum to form new virions, which are then, released from the Golgi membrane system by exocytosis into the extracellular compartment for the propagation of infection in other target cells [19].
The SARS-CoV-1 genome encodes several non-structural proteins that are essential for the life cycle of the virus. Among them, the viral 3-chymotrypsin-like (3CL) cysteine protease controls coronavirus replication [17]. Similar to SARS-CoV-1, in SARS-CoV-2, the SARS-CoV-2 3CL cysteine protease is an essential factor for viral replication in the host cell. Akaberi et al. showed that SARS-CoV-2 3CL cysteine protease, which cleaves viral polyproteins into functional polypeptides that are responsible for viral replication, is a possible target for S-nitrosylation, suggesting a potential mechanism of inhibitory action of NO on this cysteine protease to interrupt the viral life cycle. The in vitro study by Akaberi showed that the SARS-CoV-2 3CL recombinant protease was covalently inhibited by SNAP through the transfer of nitrosonium ion (NO+) to the protease cysteine residue; the observed reduction in SARS-CoV-2 protease activity was consistent with S-nitrosylation of the enzyme active site cysteine [20]. These observations suggest that S-nitrosylation of pathogenic cysteine protease plays an important role in host defenses if its catalytic site includes a cysteine residue [17].
On the other hand, in parallel with ongoing viral replication, the viral RNA genome released into the cytosol is detected by intracellular pattern-recognition receptors (PRRs), innate immune sensors such as endosomal Toll-like receptor, and cytosolic retinoic acid-inducible gene I-like receptor. Following PRR activation, molecular signaling cascades culminate in the activation of downstream transcription factors, such as nuclear factor-κB (NF-κB) and interferon (IFN) regulatory factors, to produce proinflammatory mediators and type I/III IFNs (Fig. 1) [19].
Innate immune responses mediated by PRRs following SARS-CoV-2 infection activate NF-κB, which translocates to the nucleus and initiates transcription of adhesion molecules such as intercellular adhesion molecule-1, vascular cell adhesion molecule-1, E-selectin, and P-selectin, and inflammatory cytokines, such as interleukin-6 (IL-6). The serum levels of these adhesion molecules and cytokines are significantly increased in severe COVID-19, suggesting that NF-κB is the main regulator of the inflammatory process of this infection [21].
Regulation of NF-κB activation by NO has been well investigated for its involvement in various physiological and pathological conditions [22]. NF-κB is the p50-p65 heterodimer and is sequestered in the cytoplasm by IκB inhibitors. When IκB is phosphorylated by the IκB-kinase complex (comprising three subunits, IKKα, IKKβ, and IKKγ), NF-κB is translocated to the nucleus for DNA binding, which initiates the production of multiple proinflammatory mediators. S-nitrosylation of IKKβ at Cys179 and p50 at Cys62 inhibits NF-κB-dependent DNA binding, promoter activity, and gene transcription, leading to the subsequent inflammatory response [22]. In this context, NO-mediated inhibition of NF-κB pathway might be a potential therapeutic option for COVID-19 [21].
2.3. 3. ACE2-angiotensin (1–7)-Mas axis
Another notable pathway for COVID-19 progression is the ACE2-angiotensin (1–7)-Mas axis (Fig. 1). ACE2 catalyzes the conversion of angiotensin-Ⅱ (Ang-Ⅱ) to Ang (1–7), which stimulates a transmembrane receptor, Mas, and initiates the Akt phosphorylation cascade for the activation of endothelial NO synthase (eNOS) to increase NO production [23]. SARS-CoV-2 infection downregulates ACE2 expression by internalizing it with virus particles from the host cell surface and fails to catalyze the conversion of Ang-Ⅱ to Ang (1–7), resulting in Ang-Ⅱ accumulation, resultant increased reactive oxygen species production through the activation of nicotinamide adenine dinucleotide phosphate oxidase, and generation of peroxynitrite by consuming eNOS-derived NO. The accumulation of Ang-Ⅱ under these conditions can induce vasoconstriction and act as a proinflammatory cytokine when bound to the Ang-Ⅱ type 1 receptor (AT1R). This Ang Ⅱ–AT1R signaling axis activates a disintegrin and metalloprotease 17, which digests and releases the membrane forms of epidermal growth factor family members, interleukin-6 receptor, and tumor necrosis factor-α, followed by gene expression for a broad range of proinflammatory cytokines and chemokines, adhesion molecules, and acute-phase protein via the triggering of transcriptional factors, such as NF-κB and signal transducer and activator of transcription-3 (Fig. 1) [24].
The in vitro study by Leclerc et al. showed that the NO donor, sodium nitroprusside, dose-dependently inhibited the binding affinity of AT1R with Ang Ⅱ by S-nitrosylation of AT1R at cysteine 289 [25]. Furthermore, Pinherio reported that treatment of rats with oral nitrite increased circulating S-nitrosothiol levels and reduced Ang-Ⅱ-induced vasoconstriction with increased aortic protein kinase C (PKC) nitrosylation [26]. These results suggest that the potential beneficial effects of NO may outweigh the deleterious effects of Ang-Ⅱ in COVID-19 progression.
2.4. NO as vasodilator and antiplatelet agent
In the progressive stage of COVID-19, vascular endothelial dysfunction followed by thrombus formation and life-threatening blood coagulation are the underlying pathologies [10]. Inorganic nitrate/nitrite ingestion, via diet or supplementation, inhibits platelet reactivity and aggregation via cGMP-mediated reduction of platelet P-selectin expression in vitro and in vivo [27]. Srihirun et al. further suggest that deoxygenated erythrocytes reduce nitrite in the circulation, and subsequent NO-mediated activation of soluble guanylyl cyclase modulates platelet activation and blood clotting within the various parts of the vascular bed [28]. Moreover, NO can lower pulmonary vascular resistance and decrease pulmonary edema due to its potent and selective pulmonary vasodilation, which enhances ventilation/perfusion matching and may improve respiratory symptoms [27].
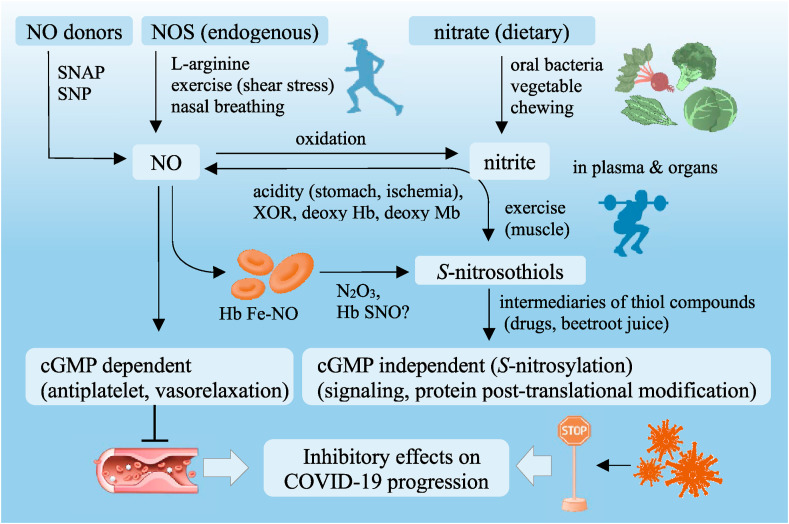
3. Lifestyle factors that boost NO bioavailability
Based on the proposed inhibitory effects of NO on COVID-19, therapeutic strategies using NO may be strongly recommended, particularly for COVID-19 patients with endothelial dysfunction. NO-mediated prevention of SARS-CoV-2 infection could be facilitated by lifestyle factors, such as nitrate-rich diets, physical exercise, and nasal breathing [[29], [30], [31]], which are eventually integrated in cGMP-dependent and -independent pathways (Fig. 2 ). The cGMP-independent pathway constitutes posttranslational protein modification (S-nitrosylation) through the covalent attachment of NO+ to reactive cysteine residues of target proteins such as receptors, enzymes, and transcription factors that mediate COVID-19 pathogenesis. However, the cGMP-dependent pathway is mediated by binding of NO to the heme moiety of its effector (soluble guanylyl cyclase), followed by subsequent activation of cGMP-dependent signaling, leading to vascular dilatation and antithrombotic properties [5].
Fig. 2.
Lifestyle factors boost NO bioavailability to prevent COVID-19 progression
Inhibitory effects of lifestyle factors on COVID-19 progression are transmitted via NO-mediated signaling and finally integrated to cGMP-dependent and -independent pathways.
NO: nitric oxide, SNAP: S-nitroso-N-acetylpenicillamine, SNP: sodium nitroprusside, NOS: NO synthase, Hb: hemoglobin, Mb: myoglobin, XOR: xanthine oxidoreductase,Fe–NO: nitrosyl iron, N2O3: dinitrogen trioxide, SNO: S-nitrosothiol, cyclic cGMP: cyclic guanosine-3′, 5′-monophosphate, COVID-19: coronavirus disease.
3.1. Enterosalivary nitrate–nitrite–NO pathway
Apart from the enzymatic l-arginine/NOS pathway, the enterosalivary nitrate–nitrite–NO pathway is an alternative for gastric and systemic NO generation [32]. Abundant dietary nitrate in green leafy vegetables is absorbed from the upper gastrointestinal tract into the systemic circulation, wherein approximately 60% of nitrate is excreted renally within 48 h [33] and 25% is actively absorbed from the circulation by the salivary gland and secreted into saliva. Dietary and salivary-derived nitrates are reduced to nitrite in the oral cavity by commensal bacteria residing on the lingual surface [34]. Upon deglutition into the stomach, the nitrite is reduced by H+ in the gastric juice to form nitrous acid, which decomposes to NO and other nitrogen oxides, such as dinitrogen trioxide (N2O3) and NO2. N2O3 can donate an NO + for subsequent S-transnitrosylation in gastric and systemic NO physiology. Pinherio et al. reported that antiseptic mouthwash disrupts the enterosalivary nitrate circulation, thereby decreasing the levels of nitrite and S-nitrosothiol in the plasma and vascular tissue that diminishes the blood pressure-lowering effect of dietary nitrate in hypertensive rat models [35]. Montenegro et al. reported results from a human study that an increase in gastric pH induced by pretreatment with proton-pump inhibitor not only blunts intragastric NO generation following nitrate/nitrite ingestion but also decreases plasma levels of S-nitrosothiol and its hypotensive effect that reflects a downstream NO signaling pathway [36]. These observations suggest that nitrate-rich diets (i.e., beetroot, spinach, etc.), saliva secretion during frequent chewing, oral commensal bacteria, and gastric acidic environment are essential to boost NO bioavailability through the enterosalivary nitrate/nitrite/NO pathway [[37], [38], [39]].
Furthermore, beetroot juice, which contains nitrate and antioxidant polyphenols [40], and co-ingestion of nitrate/nitrite with drugs containing structural sulfhydryl compounds (i.e., ACE inhibitor, captopril [30], and antiplatelet drug, thienopyridine [29,41]) can increase the plasma levels of S-nitrosothiols, which function as an intermediary receiver of NO+ that is generated in the stomach to nitrosylate target proteins and peptides, such as albumin and glutathione, that contain cysteine residues to boost peripheral NO bioavailability [42].
On the other hand, nitrate and nitrite contents in drinking water and foods have been limited by regulations in many countries because of their acute and chronic toxicities such as methemoglobinemia and cancer. However, the report on methemoglobinemia is a very limited case of infants fed formula milk prepared with bacterially contaminated well water [43]. Chronic exposure to nitrate in foods (e.g., high red meat intake) has been linked to increased risk of cancer, whereas, the World Cancer Research Fund Continuous Update Report in 2015 reported no consistent epidemiological evidence of an increased risk of human cancer due to nitrate consumption (e.g., fruits and vegetables) [44].
Taken together, these physiological, nutritional, and pharmacological factors involved in the enterosalivary pathway could possibly mediate the S-nitrosylation of the viral proteins and cellular host factors essential for the virus life cycle to prevent SARS-CoV-2 infection.
3.2. Physical exercise
Shear stress induced by pulsatile mechanical stimulus to the vascular endothelium, particularly during exercise, enhances transcriptional and protein levels of eNOS, which increases NO production to regulate vascular tone in response to endurance exercise, and maintains the integrity of the vascular endothelial barrier [45]. Therefore, aerobic physical exercise (e.g., walking and jogging) possibly inhibits SARS-CoV-2 infection through the NO-mediated mechanism shown in Fig. 1 [46,47].
In addition to vascular eNOS-derived NO generation during aerobic exercise, resistance exercise creates a shift toward a different biological mechanism for NO generation by non-enzymatic reduction of nitrate/nitrite stored in skeletal muscle [48], which might facilitate NO-mediated prevention of SARS-CoV-2 infection, particularly during anaerobic resistance exercise (e.g., weight lifting and sprinting). Basal and postprandial (particularly nitrate-rich foods) concentrations of nitrate are substantially higher in human skeletal muscle tissue than in plasma following active transport from the circulating blood, suggesting that skeletal muscle tissue may serve as an endogenous nitrate reservoir [49]. Moreover, oxidation of NO produced by muscular nNOS may contribute to the increased levels of nitrate stores in the skeletal muscle. Piknova et al. found that a very large decrease in muscle nitrate levels immediately after exercise is accompanied by a transient increase in the muscle tissue nitrite level, which is then reduced to NO formation at least partially by xanthine oxidoreductase under conditions of slight lowering of pH and hypoxia that is induced by resistance exercise [50] and contributing to basal vascular endothelial physiology and NO-mediated mitochondrial ergogenic effect [51,52]. Furthermore, this would generate the possibility of NO-mediated cytoprotection against SARS-CoV-2 during exercise.
Erythrocytes can store nitrate/nitrite derived from diet and endothelium-derived NO oxidation. Nitrite consumption in blood from artery to vein is observed at rest, and is further enhanced during hypoxia and exercise, suggesting that nitrite generates NO following exercise but even at resting in the presence of deoxyhemoglobin due to its reductase activity; however, in the presence of oxyhemoglobin, this phenomenon is markedly reduced due to the rapid NO-scavenging characteristics of oxyhemoglobin [53]. However, the question remains as to how the NO produced by deoxyhemoglobin can escape scavenging by oxyhemoglobin; some hypotheses were proposed to explain how this might occur. The reaction of nitrite and deoxyhemoglobin generates less reactive intermediates, such as N2O3 and/or S-nitroso-hemoglobin, which allow escape from erythrocyte and subsequent dissociation to release NO+ that is responsible not only for physiological effects, such as vasodilatation and antiplatelet action [54], but also possibly for an inhibitory effect against SARS-CoV-2 infection.
3.3. NO inhalation therapy and nasal breathing
NO inhalation therapy (≥80 ppm) for patients with SARS-CoV-induced ARDS was previously performed during the 2002–2004 SARS outbreak, and preliminary studies showed that NO inhalation showed a promising potency to improve oxygenation and decrease lung infiltration by lowering pulmonary vascular resistance and alveolar edema via improved ventilation/perfusion mismatch and inhibition of neutrophil activation and subsequent proinflammatory cytokine release [55]. Moreover, inhaled NO has a wide range of systemic effects as an airborne messenger via cGMP-dependent and cGMP-independent mechanisms leading to decreased systemic vascular tone, reduced risk of thrombosis, and prevention of abnormal leukocyte adhesion to the endothelium [39]. Given the common pathological processes of ARDS between SARS and COVID-19, NO inhalation is expected to be effective for COVID-19 ARDS. Recently, a small portable NO inhalation device, which is convenient to carry, was invented to prevent SARS-CoV-2 infection from the upper respiratory tract, particularly for healthcare workers who are in close contact with COVID-19 patients [56]. This clinical trial is currently ongoing to test its efficacy and safety against SARS-CoV-2.
Thus, nasal breathing may be considered an additional lifestyle factor to boost NO bioavailability for preventing SARS-CoV-2 infection in the upper airways [31]. The nasal cavity fulfills important physiological functions by filtering, warming, and humidifying inhaled air and produces NO in high quantities in the upper airways. Tornberg et al. reported that nasal breathers have higher NO levels in the respiratory tract than mouth breathers [57]. Although the NO values in the exhaled air to be measured depend on the method used and varies among laboratories, recent studies have shown that oral NO ranges from 4 to 160 ppb, whereas nasal NO concentrations vary from 200 to 2000 ppb. Paranasal sinuses are the major source of NO, sometimes having NO concentrations of 5–20 ppm in healthy adults. However, NO concentrations apparently decrease after entry into the nasal cavity through the communicating ostia during nasal inhalation. Despite lower nasal NO concentrations than those used for NO inhalation therapy for ARDS patients, clear effects of NO bioavailability on arterial oxygenation and pulmonary arterial pressure using inhaled NO concentrations of 10–100 ppb have been reported [[58], [59], [60]] with similar NO levels as those that we breathe through the nose. Furthermore, Weitzberg and Lundberg reported that humming causes a 15-fold increase in nasal NO compared with quiet exhalation (252 ± 63 ppb vs. 17 ± 3 ppb) due to humming-induced air oscillation between the sinuses and nasal cavity [61], suggesting that nasal breathing provides potential benefits for the early prevention of SARS-CoV-2 infection.
4. Conclusion
To summarize, the preventive strategies against COVID-19 that are proposed herein are lifestyle factors (nitrate-rich diets, exercise, and nasal breathing) that can be easily followed every day. These strategies potentially provide additional benefits for preventing lifestyle-related diseases (i.e., diabetes, hypertension, metabolic syndrome) and should be analyzed for their preventive efficacy against COVID-19, and if the efficacy is confirmed, they should be implemented in the clinical setting in the near future.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Role of the funding source
None.
Declaration of competing interest
None.
Data statement
I declare that the data supporting the findings of this study are available within the paper.
Acknowledgements
I would like to thank Editage (www.editage.com) for English language editing.
References
- 1.Abrams E.M., Szefler S.J. COVID-19 and the impact of social determinants of health. Lancet Respir. Med. 2020;8:659–661. doi: 10.1016/S2213-2600(20)30234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwok H.F. Review of Covid-19 vaccine clinical trials-A puzzle with missing pieces. Int. J. Biol. Sci. 2021;17:1461. doi: 10.7150/ijbs.59170. https://pubmed.ncbi.nlm.nih.gov/33907509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y., Tian H., Zhang L., Zhang M., Guo D., Wu W., MacIntyre C.R. Reduction of secondary transmission of SARS-CoV-2 in households by face mask use, disinfection and social distancing: a cohort study in Beijing, China. BMJ Global Health. 2020;5 doi: 10.1136/bmjgh-2020-002794. https://pubmed.ncbi.nlm.nih.gov/32467353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calabretta E., Moraleda J.M., Iacobelli M., Jara R., Vlodavsky I., O'Gorman P., Richardson P. COVID‐19‐induced endotheliitis: emerging evidence and possible therapeutic strategies. Br. J. Haematol. 2021;193:43. doi: 10.1111/bjh.17240. https://onlinelibrary.wiley.com/doi/10.1111/bjh.17240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kobayashi J., Ohtake K., Uchida H. NO-rich diet for lifestyle-related diseases. Nutrients. 2015;7:4911–4937. doi: 10.3390/nu7064911. https://www.ncbi.nlm.nih.gov/pubmed/26091235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banerjee A., Pasea L., Harris S., Gonzalez-Izquierdo A., Torralbo A., Shallcross L., Noursadeghi M., Pillay D., Sebrie N., Holmes C., Pagel C., Wong W.K., Langenberg C., Williams B., Denaxas S., Hemingway H. Estimating excess 1-year mortality associated with the COVID-19 pandemic according to underlying conditions and age: a population-based cohort study. Lancet. 2020;395:1715–1725. doi: 10.1016/S0140-6736(20)30854-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gandhi R.T., Lynch J.B., Del Rio C. Mild or moderate covid-19. N. Engl. J. Med. 2020;383:1757–1766. doi: 10.1056/NEJMcp2009249. https://www.nejm.org/doi/full/10.1056/NEJMcp2009249 [DOI] [PubMed] [Google Scholar]
- 8.Li H., Tian S., Chen T., Cui Z., Shi N., Zhong X., Qiu K., Zhang J., Zeng T., Chen L., Zheng J. Newly diagnosed diabetes is associated with a higher risk of mortality than known diabetes in hospitalized patients with COVID-19. Diabetes Obes. Metabol. 2020;22:1897–1906. doi: 10.1111/dom.14099. https://pubmed.ncbi.nlm.nih.gov/32469464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ozdemir B., Yazici A. Could the decrease in the endothelial nitric oxide (NO) production and NO bioavailability be the crucial cause of COVID-19 related deaths? Med. Hypotheses. 2020;144:109970. doi: 10.1016/j.mehy.2020.109970. https://pubmed.ncbi.nlm.nih.gov/32534341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nägele M.P., Haubner B., Tanner F.C., Ruschitzka F., Flammer A.J. Endothelial dysfunction in COVID-19: current findings and therapeutic implications. Atherosclerosis. 2020;314:58–62. doi: 10.1016/j.atherosclerosis.2020.10.014. https://pubmed.ncbi.nlm.nih.gov/33161318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Runer T., Cervin A., Lindberg S., Uddman R. Nitric oxide is a regulator of mucociliary activity in the upper respiratory tract. Otolaryngol. Head Neck Surg. 1998;119:278–287. doi: 10.1016/S0194-5998(98)70063-4. https://pubmed.ncbi.nlm.nih.gov/9743084 [DOI] [PubMed] [Google Scholar]
- 12.Nagaki M., Shimura S., Irokawa T., Sasaki T., Shirato K. Nitric oxide regulation of glycoconjugate secretion from feline and human airways in vitro. Respir. Physiol. 1995;102:89–95. doi: 10.1016/0034-5687(95)00042-C. [DOI] [PubMed] [Google Scholar]
- 13.Li F., Berardi M., Li W., Farzan M., Dormitzer P.R., Harrison S.C. Conformational states of the severe acute respiratory syndrome coronavirus spike protein ectodomain. J. Virol. 2006;80:6794–6800. doi: 10.1128/JVI.02744-05. https://pubmed.ncbi.nlm.nih.gov/16809285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shulla A., Heald-Sargent T., Subramanya G., Zhao J., Perlman S., Gallagher T.A. A transmembrane serine protease is linked to the severe acute respiratory syndrome coronavirus receptor and activates virus entry. J. Virol. 2011;85:873–882. doi: 10.1128/JVI.02062-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartenian E., Nandakumar D., Lari A., Ly M., Tucker J.M., Glaunsinger B.A. The molecular virology of coronavirus. J. Biol. Chem. 2020;295:12910–12934. doi: 10.1074/jbc.REV120.013930. https://pubmed.ncbi.nlm.nih.gov/32661197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffman M., Kleine-Weber H., Schroder S., Kruger N., Herrier T., Erichen S S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACS2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. https://pubmed.ncbi.nlm.nih.gov/32142651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Åkerström S., Gunalan V., Keng C.T., Tan Y.J., Mirazimi A. Dual effect of nitric oxide on SARS-CoV replication: viral RNA production and palmitoylation of the S protein are affected. Virology. 2009;395:1–9. doi: 10.1016/j.virol.2009.09.007. https://core.ac.uk/download/pdf/82609608.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.G. Simmons, D.N. Gosalia, A.J. Rennekamp, J.D. Reeves, S.L. Diamond, Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry, PNAS. 102 (200) 11876-11881. https://pubmed.ncbi.nlm.nih.gov/16081529. [DOI] [PMC free article] [PubMed]
- 19.Harrison A.G., Lin T., Wang P. Mechanisms of SARS-CoV-2 transmission and pathogenesis. Trends Immunol. 2020;41:1100–1115. doi: 10.1016/j.it.2020.10.004. https://pubmed.ncbi.nlm.nih.gov/33132005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akaberi D., Krambrich J., Ling J., Luni C., Hedenstierna G., Järhult J.D., Lennersteand J., Lundkvist Å. Mitigation of the replication of SARS-CoV-2 by nitric oxide in vitro. Redox Biol. 2020;37:101734. doi: 10.1016/j.redox.2020.101734. https://pubmed.ncbi.nlm.nih.gov/33007504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kircheis R., Haasbach E., Lueftenegger D., Heyken W.T., Ocker M., Planz O. NF-κB pathway as a potential target for treatment of critical stage COVID-19 patients. Front. Immunol. 2020;11:1–12. doi: 10.3389/fimmu.2020.598444. https://pubmed.ncbi.nlm.nih.gov/33362782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hess D.T., Matsumoto A., Kim S.O., Marshall H.E., Stamler J.S. Protein S-nitrosylation: purview and parameters. Nat. Commun. 2005;6:150–166. doi: 10.1038/nrm1569. https://pubmed.ncbi.nlm.nih.gov/15688001 [DOI] [PubMed] [Google Scholar]
- 23.Kidde J., Sahebkar A. From foe to friend in COVID-19: renin-angiotensin system inhibitors. J. Infect. Dis. 2021;223:174–175. doi: 10.1093/infdis/jiaa629. https://academic.oup.com/jid/article/223/1/174/5917667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirano T., Murakami M. COVID-19: a new virus, but a familiar receptor and cytokine release syndrome. Immunity. 2020;52:731–733. doi: 10.1016/j.immuni.2020.04.003. https://pubmed.ncbi.nlm.nih.gov/32325025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leclerc P.C., Lanctot P.M., Auger-Messier M., Escher E., Leduc R., Guillemette G. S-nitrosylation of cysteine 289 of the AT1 receptor decreases its binding affinity for angiotensin Ⅱ. Br. J. Pharmacol. 2006;148:306–313. doi: 10.1038/sj.bjp.0706725. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1751562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pinherio L.C., Oliveira-Paula G.H., Ferreria G.C., Dal-Cin de Paula T., Duarte D.A., Costa-Neto C.M., Tanus-Santos J.E. Oral nitrite treatment increases S-nitrosylation of vascular protein kinase C and attenuates the response to angiotensin Ⅱ. Redox Biol. 2021;38:1–11. doi: 10.1016/j.redox.2020.101769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fang W., Jiang J., Su L., Shu T., Liu H., Lai S., Ghiladi R.A., Wang J. The role of NO in COVID-19 and potential therapeutic strategies. Free Radic. Boil. Med. 2021;163:153–162. doi: 10.1016/j.freeradbiomed.2020.12.008. https://pubmed.ncbi.nlm.nih.gov/33347987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Srihirun S., Piknova B., Sibmooh N., Schechter A.N. Phosphorylated vasodilator-stimulated phosphoprotein (P-VASP Ser239) in platelets is increased by nitrite and partially deoxygenated erythrocytes. PloS One. 2018;13(3) doi: 10.1371/journal.pone.0193747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burnley-Hall N., Abdul F., Androshchuk V., Morris K., Ossei-Gerning N., Anderson R., Rees D.A., James P.E. Dietary nitrate supplementation reduces circulating platelet-derived extracellular vesicles in coronary artery disease patients on clopidogrel therapy: a randomized, double-blind, placebo-controlled study. Thromb. Haemostasis. 2018;118:112–122. doi: 10.1160/TH17-06-0394. [DOI] [PubMed] [Google Scholar]
- 30.Zhou Y., Lin M., Wang J., Chen F., Li F., Chen W., Han L., Wang C., Chen J., Shao J.W., Jia L. A novel S-nitrosocaptopril monohydrate for pulmonary arterial hypertension: H2O and -SNO intermolecular stabilization chemistry. Free Radic. Biol. Med. 2020;129:107–115. doi: 10.1016/j.freeradbiomed.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 31.Martel J., Ko Y.F., Young J.D., Ojcius D.M. Could nasal nitric oxide help to mitigate the severity of COVID-19? Microb. Infect. 2020;22:168–171. doi: 10.1016/j.micinf.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kapil V., Milsom A.B., Okorie M., Maleki-Toyserkani S., Akram F., Rehman F., Arghandawi S., Pearl V., Benjamin N., Loukogeorgakis S., Macallister R., Hobbs A.J., Webb A.J., Ahluwalia A. Inorganic nitrate supplementation lowers blood pressure in humans: role for nitrite-derived NO. Hypertension. 2010;56:274–281. doi: 10.1161/HYPERTENSIONAHA.110.153536. https://pubmed.ncbi.nlm.nih.gov/20585108 [DOI] [PubMed] [Google Scholar]
- 33.Wargner D.A., Schultz D.S., Deen W.M., Young V.R., Tannenbaum S.R. Metabolic fate of an oral dose of 15N-labeled nitrate in humans: effect of diet supplementation with ascorbic acid. Canc. Res. 1983;43:1921–1925. https://pubmed.ncbi.nlm.nih.gov/6831427 [PubMed] [Google Scholar]
- 34.Lundberg J.O., Weitzberg E., Lundberg J.M., Alving K. Intragastric nitric oxide production in humans: measurements in expelled air. Gut. 1994;35:1543–1546. doi: 10.1136/gut.35.11.1543. https://pubmed.ncbi.nlm.nih.gov/7828969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinherio L.C., Ferreria G.C., Amaral J.H., Portella R.L., Tella S.O.C., Passos M.A., Tanus-Santos J.E. Oral nitrite circumvents antiseptic mouthwash-induced disruption of enterosalivary circuit of nitrate and promotes nitrosation and blood pressure lowering effect. Free Radic. Biol. Med. 2016;101:226–235. doi: 10.1016/j.freeradbiomed.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 36.Montenegro M.F., Sundqvist M.L., Larsen F.J., Zhuge Z., Carlström M., Weitzberg E., Lundberg J.O. Blood pressure-lowering effect of orally ingested nitrite is abolished by a proton pump inhibitor. Hypertension. 2017;69:23–31. doi: 10.1161/HYPERTENSIONAHA.116.08081. https://pubmed.ncbi.nlm.nih.gov/27802417 [DOI] [PubMed] [Google Scholar]
- 37.Lundberg J.O., Weitzberg E., Gladwin M.T. The nitrate–nitrite–nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. https://pubmed.ncbi.nlm.nih.gov/18167491 [DOI] [PubMed] [Google Scholar]
- 38.Kobayashi J. Chewing well during meals may benefit health via the enterosalivary nitrate-nitrite-nitric oxide pathway. J Gastroenterol. Hepatol. Res. 2019;8:2882–2885. ghrnet.org/index.php/joghr/article/view/2615/2907 [Google Scholar]
- 39.Kobayashi J., Murata I. Nitric oxide inhalation as an interventional rescue therapy for COVID-19-induced acute respiratory distress syndrome. Ann. Intensive Care. 2020;10:1–2. doi: 10.1186/s13613-020-00681-9. https://pubmed.ncbi.nlm.nih.gov/32436029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mcllvenna L.C., Monaghan C., Liddle L., Fernandez B.O., Feelisch M., Muggeridge D.J., Easton C. Beetroot juice versus chard gel: a pharmacokinetic and pharmacodynamics comparison of nitrate bioavailability. Nitric Oxide. 2017;64:61–67. doi: 10.1016/j.niox.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 41.Bundhoo S.S., Anderson R.A., Sagan E., Hassan N., Pinder A.G., Rogers S.C., Morris K., James P.E. Direct formation of thienopyridine-derived nitrosothiol—just add nitrite! Eur. J. Pharmacol. 2011;670:534–540. doi: 10.1016/j.ejphar.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 42.Pinherio L.C., Amaral J.H., Ferreria G.C., Portella R.L., Ceron C.S., Montenegro M.F., Toledo J.C., Jr., Tanus-Santos J.E. Gastric S-nitrosothiol formation drives the antihypertensive effects of oral sodium nitrite and nitrate in a rat model of renovascular hypertension. Free Radic. Biol. Med. 2015;87:252–262. doi: 10.1016/j.freeradbiomed.2015.06.038. https://pubmed.ncbi.nlm.nih.gov/26159506 [DOI] [PubMed] [Google Scholar]
- 43.Fawns H.T., Aldridge A.G. Methaemoglobinaemia due to nitrates and nitrites in drinking-water. Br. Med. J. 1954;2:575–576. doi: 10.1136/bmj.2.4887.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mills C.E., Khatri J., Maskell P., Odongerel C., Webb A.J. It is rocket science - why dietary nitrate is hard to ‘beet’! Part II: further mechanisms and therapeutic potential of the nitrate-nitrite-NO pathway. Br. J. Clin. Pharmacol. 2017;83:140–151. doi: 10.1111/bcp.12918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davis M.E., Cai H., Drummond G.R., Harrison D.G. Shear stress regulates endothelial nitric oxide synthase expression through c-Src by divergent signaling pathways. Circ. Res. 2001;89:1073–1080. doi: 10.1161/hh2301.100806. https://pubmed.ncbi.nlm.nih.gov/11717166 [DOI] [PubMed] [Google Scholar]
- 46.Fernández-Lázaro D., González-Bernal J.J., Sánchez-Serrano N., Navascués L.J., Ascaso-del-Río A., Mielgo-Ayuso J. Physical exercise as a multimodal tool for COVID-19: could it be used as a preventive strategy? Int. J. Environ. Res. Publ. Health. 2020;17:8496. doi: 10.3390/ijerph17228496. https://pubmed.ncbi.nlm.nih.gov/33212762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim B., Arany Z. Could shear stress mimetics delay complications in COVID-19? Trends Cardiovasc. Med. 2021 doi: 10.1016/j.tcm.2021.01.004. https://pubmed.ncbi.nlm.nih.gov/33515686 In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wylie L.J., Park J.W., Vanhatalo A., Kadach S., Black M.I., Stoyanov Z., Schechter A.N., Jones A.M., Piknova B. Human skeletal muscle nitrate store: influence of dietary nitrate supplementation and exercise. J. Physiol. 2019;597:5565–5576. doi: 10.1113/JP278076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qin L., Liu X., Sun Q., Fan Z., Xia D., Ding G., Ong H.L., Adams D., Gahl W.A., Zheng C., Qi S., Jin L., Zhang C., Gu L., He J., Deng D., Ambudkar I.S., Wang S. Sialin (SCL17A5) functions as a nitrate transporter in the plasma membrane. Proc. Natl. Acad. Sci. U.S.A. 2012;109:13434–13439. doi: 10.1073/pnas.1116633109. https://www.pnas.org/content/pnas/109/33/13434.full.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Piknova B., Park J.W., Lam K.K., Schechter A.N. Nitrate as a source of nitric oxide during exercise hyperemia in rat skeletal muscle. Nitric Oxide. 2016:55–56. doi: 10.1016/j.niox.2016.03.005. https://pubmed.ncbi.nlm.nih.gov/27000467 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Larsen F.J., Schiffer T.A., Borniquel S., Sahlin K., Ekblom B., Lundberg J.O., Weitzberg E. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metabol. 2011;13:149–159. doi: 10.1016/j.cmet.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 52.Hernandez A., Schiffer T.A., Ivarsson N., Cheng A.J., Bruton J.D., Lundberg J.O., Weitzberg E., Westerblad H. Dietary nitrate increases tetanic [Ca2+]i and contractile force in mouse fast-twich muscle. J. Physiol. 2012;590:3575–3583. doi: 10.1113/jphysiol.2012.232777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cosby K., Partovi K.S., Crawford J.H., Patel R.P., Reiter C.D., Martyr S., Yang B.K., Waclawiw M.A., Zalos G., Xu X., Huang K.T., Shields H., Kim-Shapiro D.B., Schechter A.N., Cannon Ⅲ R.O., Gladwin M.T. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 54.Khambata V. Kapil R.S., Jones D.A., Rathod K., Primua C., Massimo G., Fukuto J.M., Ahluwalia A. The noncanonical pathway for in vivo nitric oxide generation: the nitrate-nitrite-nitric oxide pathway. Pharmacol. Rev. 2020;72:692–766. doi: 10.1124/pr.120.019240. [DOI] [PubMed] [Google Scholar]
- 55.Chen L., Liu P., Gao H., Sun B., Chao D., Wang F., Zhu Y., Hedenstierna G., Wang C.G. Inhalation of nitric oxide in the treatment of severe acute respiratory syndrome: a rescue trial in Beijing. Clin. Infect. Dis. 2004;39:1531–1535. doi: 10.1086/425357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gianni S., Fakhr B.S., Morais C.C.A., Di Fenza R., Larson G., Pinciroli R., Houle T., Mueller A.L., Bellavia A., Kacmarek R., Carroll R., Berra L L. medRxiv; 2020. Nitric Oxide Gas Inhalation to Prevent COVID-19 in Healthcare Providers. [DOI] [Google Scholar]
- 57.Tornberg D.C., Marteus H., Schedin U., Alving K., Lundberg J.O., Weitzberg E. Nasal and oral contribution to inhaled and exhaled nitric oxide: a study in tracheotomized patients. Eur. Respir. J. 2002;19:859–864. doi: 10.1183/09031936.02.00273502. [DOI] [PubMed] [Google Scholar]
- 58.Puybasset L., Rouby J.J., Mourgeon E., Stewart T.E., Cluzel P., Arthaud M., Poete P., Bondin L., Korinek A.M., Viars P. Inhaled nitric oxide in acute respiratory failure: dose-response curve. Intensive Care Med. 1994;20:319–327. doi: 10.1007/BF01720903. [DOI] [PubMed] [Google Scholar]
- 59.Gerlach H., Rossaint R., Pappert D., Knorr M., Falke K.J. Autoinhalation of nitric oxide after endogenous synthesis in nasopharynx. Lancet. 1994;343:518–519. doi: 10.1016/S0140-6736(94)91465-6. [DOI] [PubMed] [Google Scholar]
- 60.Lum L.C.S., Tan P.S.K., Saville A., Venkataraman S.T., Pinsky M.R. Occult nitric oxide inhalation improves oxygenation in mechanically ventilated children. J. Pediatr. 1998;133:613–616. doi: 10.1016/S0022-3476(98)70099-X. [DOI] [PubMed] [Google Scholar]
- 61.Weitzberg E., Lundberg J.O. Humming greatly increases nasal nitric oxide. Am. J. Respir. Crit. Care Med. 2002;166:145. doi: 10.1164/rccm.200202-138BC. 145. [DOI] [PubMed] [Google Scholar]