The coronavirus disease 2019 (COVID-19) pandemic has raised concern about the influence of smoking and alcohol drinking behaviour on the susceptibility to coronavirus infection and its severity. Widely debated, the connection remains highly controversial. Conclusions derived from observational studies commonly suffer from limited ability to discern causes and effects.
Short abstract
A one standard deviation increase in cigarettes smoked per day is associated with 2.5-fold increased risk for very severe COVID-19 and 2-fold increased risk for hospitalisation with COVID-19 https://bit.ly/2UuVO1o
To the Editor:
The coronavirus disease 2019 (COVID-19) pandemic has raised concern about the influence of smoking and alcohol drinking behaviour on the susceptibility to coronavirus infection and its severity. Widely debated, the connection remains highly controversial. Conclusions derived from observational studies commonly suffer from limited ability to discern causes and effects.
We used two-sample Mendelian randomisation (MR) to investigate individual causal relationships between multiple traits of smoking and alcohol intake and COVID-19 outcomes, and meta-analysis to evaluate overall causal effects of smoking or alcohol consumption on COVID-19 outcomes. We analysed summary results of genome-wide association studies, with seven datasets on smoking, including four quantitative datasets, providing the age of smoking (AOS; the age at which an individual started smoking cigarettes regularly, 341 427 participants) from Liu et al. [1], cigarettes per day from Liu et al. [1] (CPD1; 337 334 participants) and Erzurumluoglu et al. [2] (CPD2; 134 316 participants) [2], and cigarette pack-years (CPY, 622 409 participants) [2], and three datasets reporting the smoking status in binary form (regular smoker (current or former) versus participant who reported never being a regular smoker), namely, these by Liu et al. [1] (SMK1, 1 232 091 participants), Erzurumluoglu et al. [2] (SMK2, 353 630 participants), and Karlsson Linnér et al. [3] (SMK3, 518 633 participants). To analyse alcohol intake, three datasets were employed, including alcohol drinks per week from Liu et al. [1] (DPW1, 941 280 participants) and Karlsson Linnér et al. [3] (DPW2, 414 343 participants), and alcohol intake per day from Evangelou et al. [4] (DPD, 480 843 participants). For COVID-19, three datasets were obtained from the COVID-19 Host Genetic Initiative (round 4) [5], including three separate COVID-19 outcomes: severe COVID-19 (4438 very severe respiratory COVID-19 cases and 718 232 controls), COVID-19 hospitalisation (6406 hospitalised COVID-19 cases and 902 088 controls), and SARS-CoV-2 infection (14 134 cases with reported SARS-CoV-2 infection and 1 284 876 controls). The controls in the COVID-19 datasets were from genetically ancestry-matched samples without known SARS-CoV-2 infection. The participants of MR analysis should come from the same population across studies. All or a majority of the participants in the datasets were of European origins.
The main analyses were performed using the inverse-variance weighted (IVW) method and complemented with the weighted median and MR-Egger methods implemented in TwoSampleMR [6]. The intercept from the MR-Egger model was used as a measure of directional pleiotropy (a single-nucleotide polymorphism (SNP) influencing both the exposure and outcome through independent pathways). SNPs associated with smoking at genome-wide significance (p<5×10–8) were selected as instrumental variants and further pruned using a clumping r2 cutoff of 0.01. The p-value threshold of 1×10–5 was used for the CPY dataset due to the number of instrumental variants being less than five.
We performed meta-analyses for the causal effects in two quantitative smoking datasets (CPD1 and CPD2), three categorical smoking status datasets (SMK1, SMK2 and SMK3), and three alcohol drinking datasets on each of the three COVID-19 conditions, separately. A meta-analysis of the MR results was conducted using a random-effect model implemented in metafor [7].
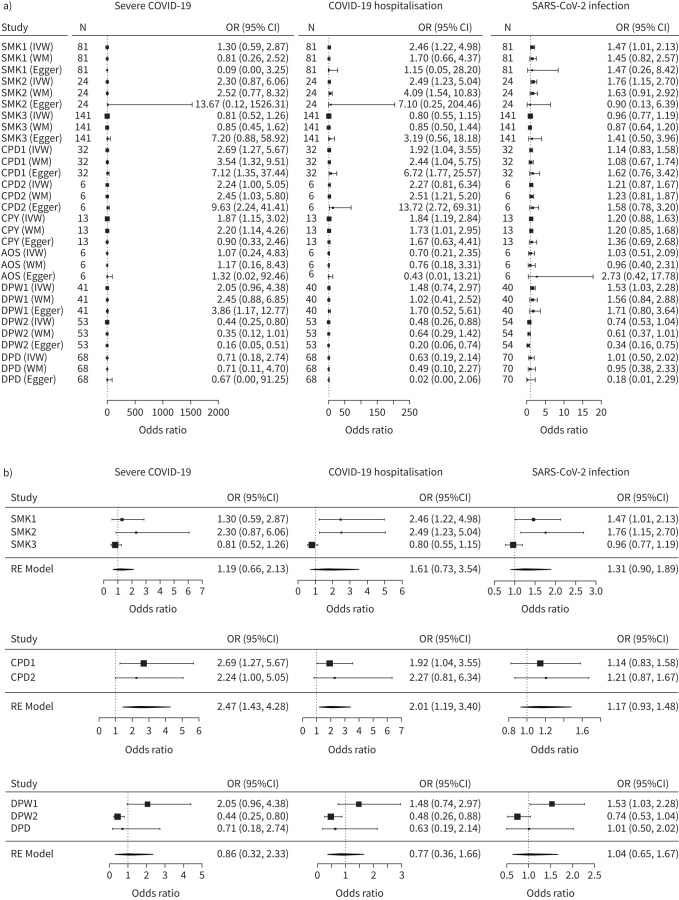
As shown in figure 1a, our MR analysis detected eight causal associations between smoking traits and the COVID-19 outcomes: CPD1 (OR 2.69, 95% CI 1.27–5.67) and CPY (OR 1.87, 95% CI 1.15–3.02) with severe COVID-19; CPD1 (OR 1.92, 95% CI 1.04–3.55), CPY (OR 1.84, 95% CI 1.19–2.85), SMK1 (OR 2.46, 95% CI 1.22–4.98) and SMK2 (OR 2.49, 95% CI 1.23–5.04) with COVID-19 hospitalisation; SMK1 (OR 1.47, 95% CI 1.01–2.13 and SMK2 (OR 1.76, 95% CI 1.15–2.70) with SARS-CoV-2 infection. Smoking status displayed mixed associations with COVID-19 outcomes, with smoking-related features in SMK1 and SMK2 tending to increase the risk for COVID-19 hospitalisation and in SMK3 tending to decrease this risk.
FIGURE 1.
Causal associations between coronavirus disease 2019 (COVID-19) and smoking and alcohol drinking. CPD1: cigarettes per day from Liu et al. [1]; CPD2: cigarettes per day from Erzurumluoglu et al. [2]; CPY: cigarette pack-years from Erzurumluoglu et al. [2]; AOS: the age at which an individual started smoking cigarettes regularly from Liu et al. [1]; SMK1: smoking from Liu et al. [1]; SMK2: smoking from Erzurumluoglu et al. [2]; SMK3: smoking from Karlsson Linnér et al. [3]; DPW1: alcohol drinks per week from Liu et al. [1]; DPW2: alcohol drinks per week from Karlsson Linnér et al. [3]; DPD: alcohol drinking per day from Evangelou et al. [4]; IVW: inverse variance weighted; WM: weighted mean; Egger: MR Egger; RE: random effects. a) Mendelian randomisation analysis. Rows are exposures with different methods and columns are outcomes. b) Meta-analysis of the causal effects from IVW model. Rows are exposures with different methods and columns are outcomes.
For alcohol traits, our MR analysis across the three alcohol datasets yielded inconsistent results. Within the Karlsson Linnér et al. [3] dataset (DPW2), alcohol consumption has shown a protective effect both on severe COVID-19 (OR 0.44, 95% CI 0.25–0.80) and COVID-19 hospitalisation (OR 0.48, 95% CI 0.26–0.88), while the analysis of the Liu et al. [1] dataset (DPW1) indicated that consumption of alcohol may increase risk for COVID-19 susceptibility (OR 1.53, 95% CI 1.03–2.28). No causal effects were detected when the Evangelou et al. [4] dataset was analysed.
The sensitivity analyses suggested that directions of causal effect estimates across the methods were predominantly consistent (figure 1a). Tests of MR-Egger regression intercepts did not support the directional pleiotropy of the genetic instrumental variables.
Our meta-analysis indicated that incremental increases in smoking intensity are positively associated with increased risk for severe COVID-19 (OR 2.47, 95% CI 1.43–4.28; p=1.26×10−3) and hospitalised COVID-19 (OR 2.01, 95% CI 1.19–3.40; p=9.56×10−3), while the binary smoking status and all the alcohol drinking traits had no associations with any kind of COVID-19 outcomes (figure 1b).
Our study reveals that the amount of smoking causally and positively influences the risk of COVID-19 severity, presumably due to reduced lung function caused by smoking of the tobacco, which is proportional to cigarette pack-years. However, the binary defined smoking status does not show any effect on susceptibility to COVID-19 or its severity. This inconsistency may reflect a balanced effect of possible protective effects of cigarette smoking as such, including intermittent ones, on susceptibility to COVID-19 and the extent of smoking-related lung damage which is evident in heavy smokers. Our study suggests that heavy smokers have an increased risk for the development of severe outcomes after SARS-CoV-2 infection. For heavy smokers, attention should be paid to avoidance of contact with the virus.
Our study reveals that the individual effects of alcohol consumption on COVID-19 susceptibility and severity are mixed, and may be cohort-specific. It is possible that in certain populations, the immunosuppressive effect of alcohol [8] may provide some protection, while in others, alcohol-related toxicity may outweigh this putative benefit. Overall, our study did not support a causal effect of alcohol consumption on COVID-19.
Several limitations are also to be acknowledged. Pleiotropy is a potential source of bias capable of threatening the validity of any MR study. However, our results were consistent in all analyses when performed by different MR methods, with no statistical indications of directional pleiotropy revealed in the case of smoking and COVID-19 connections.
In conclusion, our study indicated that a one standard deviation increase in cigarettes smoked per day is associated with 2.5-fold increased risk for severe COVID-19 and 2-fold increased risk for hospitalised COVID-19, while the smoking status and alcohol drinking traits have no associations with any kind of COVID-19 outcomes.
Shareable PDF
Acknowledgements
We thank members of the HGI and other teams, who generously shared the GWAS data.
Footnotes
Author contributions: F. Zhang conceived the study and performed the analyses; F. Zhang and A. Baranova drafted the manuscript. Both authors assisted with interpretation, commented on drafts of the manuscript. Both authors approved the final version.
Conflict of interest: F. Zhang has nothing to disclose.
Conflict of interest: A. Baranova has nothing to disclose.
Support statement: This work was supported by the National Natural Science Foundation of China (81471364). The funders had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
References
- 1.Liu M, Jiang Y, Wedow R, et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet 2019; 51: 237–244. doi: 10.1038/s41588-018-0307-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erzurumluoglu AM, Liu M, Jackson VE, et al. Meta-analysis of up to 622,409 individuals identifies 40 novel smoking behaviour associated genetic loci. Mol Psychiatry 2020; 25: 2392–2409. doi: 10.1038/s41380-018-0313-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karlsson Linner R, Biroli P, Kong E, et al. Genome-wide association analyses of risk tolerance and risky behaviors in over 1 million individuals identify hundreds of loci and shared genetic influences. Nat Genet 2019; 51: 245–257. doi: 10.1038/s41588-018-0309-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evangelou E, Gao H, Chu C, et al. New alcohol-related genes suggest shared genetic mechanisms with neuropsychiatric disorders. Nat Hum Behav 2019; 3: 950–961. doi: 10.1038/s41562-019-0653-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.COVID-19 Host Genetics Initiative. The COVID-19 Host Genetics Initiative, a global initiative to elucidate the role of host genetic factors in susceptibility and severity of the SARS-CoV-2 virus pandemic. Eur J Hum Genet 2020; 28: 715–718. doi: 10.1038/s41431-020-0636-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hemani G, Zheng J, Elsworth B, et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife 2018; 7: e34408. doi: 10.7554/eLife.34408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Software 2010; 36: 1–48. doi: 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 8.Zhang P, Bagby GJ, Happel KI, et al. Pulmonary host defenses and alcohol. Front Biosci 2002; 7: d1314–d1330. doi: 10.2741/A842 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This one-page PDF can be shared freely online.
Shareable PDF ERJ-01273-2021.Shareable (275.1KB, pdf)