Abstract
Cancer vaccines are promising adjuvant immunotherapies that can stimulate the immune system to recognize tumor-associated antigens and eliminate the residual or recurring disease. The aberrant and restricted expression of highly immunogenic cancer testis antigen NY-ESO-1 in several malignancies, including triple-negative breast cancer, melanoma, myelomas, and ovarian cancer, makes NY-ESO-1 an attractive antigenic target for cancer vaccines. This study describes a NY-ESO-1 vaccine based on a bio-inspired nanomaterial platform technology, specifically a plant virus nanoparticle. The 30 nm icosahedral plant virus cowpea mosaic virus (CPMV) displaying multiple copies of human HLA-A2 restricted peptide antigen NY-ESO-1157–165 exhibited enhanced uptake and activation of antigen-presenting cells and stimulated a potent CD8+ T cell response in transgenic human HLA-A2 expressing mice. CD8+ T cells from immunized mice exhibited antigen-specific proliferation and cancer cell cytotoxicity, highlighting the potential application of a CPMV-NY-ESO-1 vaccine against NY-ESO-1+ malignancies.
Keywords: viral nanotechnology, cancer vaccine, plant virus, cowpea mosaic virus, NY-ESO-1 antigen, cancer testis antigen
Graphical Abstract
INTRODUCTION
Cancer vaccines targeted to tumor-associated antigens can improve disease-free survival through immune system-mediated elimination of the residual or recurring disease.1,2 With its aberrant expression in several malignancies, including triple-negative breast cancer, melanomas, myeloma, and ovarian cancer, the cancer testis antigen NY-ESO-1 is an attractive target for immunotherapeutic intervention as evident from over 30+ ongoing vaccine clinical trials.3–5 NY-ESO-1+ malignancies are characterized by the presence of low levels of spontaneous antigen-specific humoral and cellular immune responses, including elevated CD8+ T cell infiltration and IFN-γ levels.3,6 However, these spontaneous responses are highly diminished by the immunosuppressive tumor microenvironment, which prevents immunological clearance of cancer.7–10 Therefore, cancer vaccines provide an opportunity to initiate or amplify pre-existing antitumor immunity to clear primary tumors and metastasis and prime immune memory against recurring cancers.
Currently, several NY-ESO-1 vaccines are under evaluation, including dendritic cell (DC)-based vaccines, recombinant proteins or peptide subunit vaccines, and mammalian viral vectors. These approaches are hampered by numerous limitations: DC-based vaccines need to be custom made for each patient and can be technically challenging and expensive.11,12 Recombinant proteins may be costly to synthesize and suffer from inefficient or nonspecific responses arising due to misfolded proteins and inefficient presentation of epitopes.11,13,14 Mammalian viral vector expression systems carry antigen-coding RNA and depend on protein expression, proteolytic processing, and presentation of appropriate antigenic peptides to stimulate a cellular immune response.15,16 Subunit peptide vaccines offer the simplest form of vaccine that can incorporate a wide spectrum of antigenic determinants derived from tumor-associated antigens (TAAs). Short peptides derived from tumor antigens overcome the need for expression and processing of protein antigen prior to loading of epitopes on MHC-I/MHC-II molecules. Using rapidly evolving prediction methods, neoantigens can be identified and peptide fragments thereof can be selected for customized immune response.17–19 However, short peptides are poorly immunogenic and require adjuvants for enhanced and prolonged immune response.20 In addition, short antigenic peptides also rely on delivery systems to protect against degradation and transport to desired tissues/cells.21,22 Many of conventional adjuvants are therefore delivered together with peptide antigens for improved vaccine efficacies.23,24
In this study, we exploited a plant virus epitope display platform technology—cowpea mosaic virus (CPMV)—to develop an NY-ESO-1 vaccine. Here, CPMV serves the dual purpose of a delivery system and an adjuvant. The 30 nm icosahedral ssRNA viral nanoparticle of CPMV has been previously established as a highly potent antigenic carrier25 and immune stimulant.26 The potency of CPMV as an immune stimulant is derived from its highly organized three-dimensional (3D) protein architecture with its encapsidated nucleic acid and an intrinsic immune cell tropism.27 We have previously established that CPMV can facilitate efficient delivery of tumor antigens to antigen-presenting cells (APCs) and provide the additional immune stimulus for effective processing and presentation of these antigens.28 Based on these features, we have previously developed a human epidermal growth factor receptor 2 (HER2) breast cancer vaccine using the CPMV platform. The CPMV-HER2 vaccine primed an effective anti-HER2 antibody response leading to delayed tumor progression and improved survival in mice challenged with HER2+ tumors.25,29 Here, the motivation was to develop a CPMV-based vaccine to stimulate an antigen-specific cellular immune response. To generate an effective cytotoxic T cell response against cancer antigen using a cancer vaccine, exogenous peptide epitopes must be delivered to the cytosol of antigen-presenting cells (APCs) for cross-presentation.30,31 Particulate antigen delivery platforms including virus-like particles (VLPs) and plant viruses are known to facilitate such antigen cross-presentation of exogenous antigens.32–34 Here, CPMV was chemically modified to display multiple copies of the HLA-A2-restricted NY-ESO-1157–165 peptide. The potency CPMV-NY-ESO-1 vaccine to stimulate an antigen-specific CTL response was then tested in transgenic human HLA-A2 expressing mice.
RESULTS AND DISCUSSION
CPMV is a 30 nm icosahedral viral particle with a pseudo T = 3 symmetry and consists of 60 copies of a 24 kDa small coat protein (S-CP) and 42 kDa large coat protein (L-CP) (Figure 1A). A CPMV capsid offers 300 reactive lysines available for bioconjugation using the N-hydroysuccinimide (NHS) chemistry. CPMV was produced in Vigna unguiculata plants with yields of 50–100 mg of virus/100 g of infected leaves. NY-ESO-1157–165 (SLLMWITQC) is a validated immunodominant MHC-I epitope that has been extensively studied for the development of subunit vaccines. NY-ESO-1 peptide with a C165V substitution (as previously described35), flexible LSPG linker, and a terminal cysteine (SLLMWITQV-LSPG-C) was conjugated to CPMV using a two-step protocol through a bifunctional NHS-maleimide (SM-PEG12) linker (Figure 1B). Conjugation of NY-ESO-1 peptide on CPMV was confirmed by denaturing sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) of purified CPMV-NY-ESO-1 particles, which revealed the presence of higher molecular weight protein bands for both the S and L proteins. This is as expected because the Lys side chains are available on both CPMV capsid proteins. The higher molecular weight band above the 24 kDa small coat protein (S-CP) band of CPMV was better resolved, which allowed us to quantify the bands using the ImageJ band analysis tool (Figure 1C). Density analysis indicated ~45% of S-CP modified with the NY-ESO-1 peptide (or 27 NY-ESO-1 peptides per S-CP); the measurement on the L-CP was not conclusive because the bands of free and modified L-CP were not sufficiently separated. Therefore, we estimate that on an average 30–60 peptides were displayed per CPMV. While using over a thousand fold molar excess of reacting ligands maximizes uniform conjugation densities, VNP heterogeneity cannot be totally ruled out. Alternatively, genetic engineering methods can be used for the expression of epitopes on CPMV.36,37 However, genetic engineering and chemical bioconjugation each have their advantages: for initial development, chemical bioconjugation is the preferred method because of its ease and speed. While some degree of heterogeneity cannot be ruled out, there is no indication that this is a barrier for clinical development as several VLPs formulated using these principles have been in clinical trials.38,39 Genetic engineering may allow for a greater degree of homogeneity; however, the development timeline is longer and more cumbersome. Finally, homogeneity is not guaranteed, as previous studies indicated that inserted peptides could be cleaved resulting in loss of the peptides.40
Figure 1.

Synthesis and characterization of the CPMV-NY-ESO-1 nanoparticles. (A) Structure of CPMV created with UCSF Chimera (v1.12) using the Protein Data Bank entry 1NY7 shows icosahedral morphology. The two protein subunits are colored red (S) and blue (L). The reactive Lysine residues on the capsid and the asymmetric unit are highlighted in green. (B) NY-ESO-1 peptide with the flexible LSPG linker with a C-terminal Cys was conjugated through the solvent-exposed Lys residues using NHS-chemistry via the bifunctional linker SM (PEG)12. (C) Denaturing 4–12% Nu-PAGE gel stained with GelCode Blue Safe protein stain was used to confirm the successful conjugation of the peptide to the CPMV coat proteins (S-CP and L-CP). (D) Transmission electron microscopic (TEM) images of the negatively stained CPMV-NY-ESO-1 nanoparticles show particle integrity post-conjugation.
Fluorescent NY-ESO-1TMR peptide was similarly conjugated to CPMV and characterized using native and denaturing gel electrophoresis and size exclusion chromatography (Figure S1, Supporting Information). The association of fluorescent peptide with the capsid protein was confirmed by the native gel and SDS-PAGE, where CPMV capsid/coat protein bands are fluorescently tagged. The co-elution of 550 nm fluorescent peak with the 260/280 peaks representing the CPMV also confirmed the association of NY-ESO-1-TMR peptide with CPMV capsid. TEM images confirmed the structural integrity of the purified CPMV-NY-ESO-1 particles (Figure 1D).
As an epitope display platform, the highly ordered 3D architecture of plant virus CPMV offers several unique features. The proteinaceous scaffold allows for a multivalent and repetitive display of the antigenic epitope, which activates pathogen-associated molecular pattern (PAMP) recognition pathways leading to the induction of stronger and longer-lasting antigen-specific immune responses. The viral nucleocapsid itself engages several pattern recognition receptors (PRRs) on immune cells, thereby enhancing the immunological visibility of the vaccine and providing additional immune stimulus.41 Based on these features, we anticipated that CPMV would be a suitable platform technology for the NY-ESO-1 display to launch a potent CD8+ CTL response.
We first evaluated the delivery of the NY-ESO-1 peptide antigen to APCs with and without the CPMV carrier and compared their activation. RAW 264.7 macrophages were incubated with CPMV-NY-ESO-1TMR or equivalent concentration of soluble NY-ESO-1TMR peptide, and cellular uptake was compared using confocal microscopy. As evident from imaging data, a significantly higher uptake of CPMV-conjugated NY-ESO-1TMR was observed over soluble non-conjugated NY-ESO-1TMR peptide (Figure 2A,B). The data corroborated previously observed enhanced delivery of epitopes to APCs by CPMV nanocarriers.28 Earlier studies have revealed a natural tropism of CPMV for APCs.42,43 We have also demonstrated that subcutaneously injected CPMV particles form local depots at the site of administration for sustained trafficking to the draining lymph nodes, thereby extending antigen sampling by peripheral APCs and improving the delivery of antigen to the draining lymph nodes.28 Together with efficient trafficking, the cellular uptake of the CPMV carrier improves the delivery of tumor antigens to APCs for effective processing and presentation.
Figure 2.

CPMV-NY-ESO-1 is taken up by the antigen-presenting cells and triggers activation. (A, B) Confocal microscopy was used to verify the uptake of fluorescent CPMV-NY-ESO-1TMR vaccine particles and soluble NY-ESO-1TMR peptides by murine macrophage RAW 264.7 cells. Cell nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (blue), the cell membrane was stained with WGA-A555 (red), and fluorescent TMR (green) was used for monitoring the peptide/CPMV-peptide. (C) Immunostimulation of bone marrow-derived dendritic cells (BMDCs) derived from the transgenic HLA-A2 mice by the CPMV-NY-ESO-1 vaccine was examined by incubating 500 000 BMDCs with the CPMV-NY-ESO-1 vaccine and the soluble NY-ESO-1 peptide for 24 h and quantifying the cytokines (ILβ1, TNF-α, IL-6, and IL-12p70) released in the culture supernatant using enzyme-linked immunosorbent assays (ELISAs). The results were compared using one-way analysis of variance (ANOVA) (with **** = p < 0.0001).
To investigate the potency of the CPMV-NY-ESO-1 vaccine to activate APCs, we measured the release of cytokines TNF-α, IL-1β, IL-6, and IL-12p70 by mouse BMDCs upon stimulation with CPMV-NY-ESO-1 versus soluble NY-ESO-1 peptide antigen. Following 24 h incubation with the CPMV-NY-ESO-1 vaccine or equivalent amount of soluble peptide, culture supernatant from mouse BMDCs was analyzed for above-mentioned cytokines using ELISAs. NY-ESO-1 peptide and the CPMV-NY-ESO-1 vaccine both resulted in increased production of cytokines over nonstimulated BMDCs, highlighting the potency of the NY-ESO-1 antigen. However, CPMV-NY-ESO-1 significantly enhanced the cytokine levels, with nearly 2-fold higher levels of TNF-α, 1.5-fold higher levels of IL-1β, 1.7-fold higher IL-6, and ~1.2-fold higher levels of IL-12p70 over the soluble NY-ESO-1 peptide (Figure 2C).
DCs play a central role in initiating an antigen-specific immune response by presenting antigens to T cells and providing the required immune stimulus through cell-to-cell contact and secreted cytokines. TNF-α plays an important role in maturation and migration of DCs to lymph nodes after sampling antigens and thus is critical for subsequent presentation of the antigens to T cells.44 TNF-α also enhances local inflammatory responses and plays an essential role in the inhibition of tumor growth. IL-1β release by DCs is induced by the activation of the NLRP3 inflammasome, an intracellular multiprotein signaling complex assembled as an inflammatory response to internalization of certain types of particulate antigens in dendritic cells.45 IL-1β signaling activates innate immune cells and is critical for T cell priming by dendritic cells.46 Proinflammatory cytokines IL-6 and IL-12 produced by activated DCs provide the necessary signal to induce the development of CTL effector functions. IL-6 plays a key role in promoting T cell trafficking to lymph nodes for activation and to tumor sites for effector functions.47 IL-12 links innate and adaptive immune responses. IL-12 released by APCs induces activation and proliferation of NK cells and T cells, polarizes T cells to a type 1 helper T (Th1) effector cell phenotype, and induces production of IFN-γ as primary antitumor response.48
We next investigated the efficacy of the CPMV-NY-ESO-1 vaccine to elicit an antigen-specific T cell response using transgenic mice expressing human HLA-A2, as NY-ESO-1157–165 is an HLA-A2-restricted antigen. Soluble peptides are poorly immunogenic in vivo, therefore the efficacy of the CPMV-NY-ESO-1 vaccine was compared to the equivalent amount of NY-ESO-1 peptide administered with a commercial adjuvant Complete Freund’s Adjuvant (CFA). Female HLA-A2 mice were immunized subcutaneously with CPMV-NY-ESO-1 or CFA + NY-ESO-1 at day 0 and with a booster dose of CPMV-NY-ESO-1 or IFA (Incomplete Freund’s Adjuvant) + NY-ESO-1 on day 14 (Figure 3A). Two weeks after the second immunization, spleens were harvested from immunized mice and CD8+ T cells were isolated from the splenocytes. To probe antigen specificity, CD8+ T cell proliferation and activation were evaluated in the presence of NY-ESO-1 peptide-pulsed BMDCs isolated from naïve HLA-A2 mice (Figure 3B). CD8+ T cells incubated with nonpulsed BMDCs or with NY-ESO-1 peptide were used as controls. Improved antigen trafficking, APC uptake, and activation facilitated by CPMV translated into an effective cellular immune response. Thus, immunizations with the CPMV-NY-ESO-1 vaccine significantly increased the NY-ESO-1-specific CD8+ T cell population in spleens as evident from the enhanced proliferation and elevated IFN-γ secretion by CD8+ T cells cultured with peptide-pulsed BMDCs as compared to CD8+ T cells incubated with peptide alone or with nonpulsed BMDCs (Figure 3C,D). We also observed high-peptide-specific CD8+ T cell proliferation and IFN-γ secretion for the group immunized with NY-ESO-1 peptide + CFA, an adjuvant which is known to induce a strong Th1-dominated inflammatory response.49 However, the CD8+ T cells from CPMV-NY-ESO-1-immunized mice displayed significantly higher proliferation and IFN-γ secretion compared to those from CFA + NY-ESO-1-immunized mice, suggesting enhanced potency of the CPMV-based vaccine. Furthermore, CD8+ T cell from CPMV-NY-ESO-1 also showed ~6-fold higher IFN-γ levels when incubated with the NY-ESO-1-pulsed BMDCs as compared to an irrelevant HER2-derived P4 peptide-pulsed BMDCs, suggesting antigen specificity (Figure 3E).
Figure 3.

Immunogenicity of the CPMV-NY-ESO-1 vaccine. (A) Transgenic HLA-A2 mice (n = 5) were immunized subcutaneously 2× biweekly with 50 μg of the CPMV-NY-ESO-1 vaccine or equivalent amount of NY-ESO-1 peptide mixed with the CFA adjuvant (CFA + NY-ESO-1); the IFA adjuvant was used for the booster dose on day 14. Spleens were harvested from immunized mice 2 weeks following the booster dose and CD8+ T cells were isolated. (B) Schematic representation of assays used to determine antigen-specific CD8+ T cell proliferation. (C, D) T cell proliferation was measured using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays (C) and quantification of secreted IFN-γ (D) using ELISAs to determine epitope-specific immunostimulation. CD8+ T cells harvested from immunized mice were co-cultured with NY-ESO-1 peptide-pulsed BMDCs from naïve HLA-A2 mice. Nonpulsed BMDCs and NY-ESO-1 peptide (without BMDCs) were used as controls. (E) Antigen specificity of T cell response was determined by co-culturing CD8+ T cells from CPMV-NY-ESO-1-immunized mice with BMDCs pulsed with NY-ESO-1 peptide or irrelevant HER2 peptide P4 and comparing IFN-γ secretion. The results were compared using one-way ANOVA (with **** = p < 0.0001).
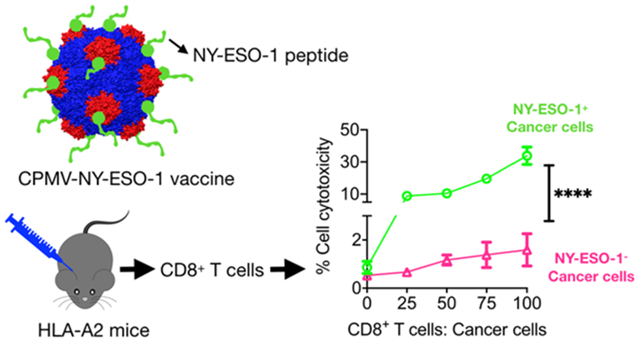
We next evaluated the cytotoxicity of CD8+ T cells toward NY-ESO-1-expressing cancer cells. The elevated levels of peptide-specific IFN-γ translated into significantly higher effector/target cell ratio-dependent cancer cell cytotoxicity. Thus, CD8+ T cells from immunized mice (both the CPMV-NY-ESO-1 vaccine and CFA + NY-ESO-1 groups) showed significant cancer cell cytotoxicity when co-cultured with NY-ESO-1+/HLA-A2-expressing A375 human melanoma cells as compared to NY-ESO-1− mouse B16F10-OVA melanoma cells, which also did not express the HLA-A2 antigen (Figure 4). Similar studies with a CPMV-OVA vaccine formulation revealed an antigen-specific cellular immune response in C57BL6 mice. Here, CD8+ T cells from immunized mice displayed antigen-specific IFN-γ secretion when co-cultured with OVA-pulsed BMDCs from naïve C57BL6 mice and resulted in B16F10-OVA cell lysis (Figure S2, Supporting Information).
Figure 4.

Antigen-specific cancer cell lysis. (A) CD8+ T cells from CPMV-NY-ESO-1 and CFA + NY-ESO-1-immunized mice were cultured for 3 days with NY-ESO-1 peptide. Activated CD8+ T cells were then co-cultured at varying effector/target cell ratios with NY-ESO-1+ A375 cancer cells or NY-ESO-1− B16F10-OVA cells. (B) Cytotoxicity of CD8+ T cells was determined using MTT assays. The results were compared using one-way ANOVA (with **** = p < 0.0001).
Our results are comparable to those obtained with the protein nanoparticle pyruvate dehydrogenase (E2 nanoparticle)-based NY-ESO-1 vaccine that has been shown to improve DC activation and antigen cross-presentation.50,51 However, while the E2 nanoparticle-based NY-ESO-1 vaccine required an additional immune stimulant in the form of the TLR9 agonist CpG oligonucleotide (ODN) 1826 for efficacy, the CPMV viral nanoparticle itself was able to achieve comparable outcomes.
We also observed a comparable antigen-specific cancer cell cytotoxicity with the CFA + NY-ESO-1 vaccine, suggesting similar potency of CPMV and CFA as adjuvants. Similar to other adjuvants, CFA is likely to stimulate the immune system via multiple mechanisms including depot effect, recruiting APCs to the site of injection, enhancing antigen uptake, APC activation, and migration of activated APCs to draining lymph nodes.52 While CFA has been proven an effective Th1 adjuvant for preclinical research, its use is associated with strong long-lasting and possibly painful local inflammation.53 Therefore, the application of CFA as an adjuvant is restricted by numerous regulatory guidelines.20,54 IFA, used for booster immunizations, has been tested in clinical trials but was discontinued as a vaccine adjuvant in humans due to associated severe side effects.55,56 Therefore, there is a need for the development of novel adjuvants, and our data support the need for further development of the CPMV platform for cancer immunotherapy applications. Other clinically approved adjuvants such as monophosphoryl lipid A (MPL), MF59, and alum have been previously evaluated for cancer vaccines, often requiring additional immunostimulatory molecules to achieve a Th1 immune response (for example, CpG ODN).23,57,58 CPMV, on the other hand, serves the dual purpose of a carrier and an adjuvant, with established Th1 immune response.29 Thus, the CPMV-delivering platform obviates the need for additional immunostimulants.
CONCLUSIONS
In conclusion, we have developed an NY-ESO-1 cancer vaccine based on the plant viral nanoparticle CPMV as the epitope display platform. The CPMV platform enhanced uptake of NY-ESO-1 peptides into antigen-presenting cells and led to improved activation of immune cells. The CPMV-NY-ESO-1 vaccine triggered a potent CD8+ T cell response in transgenic HLA-A2 mice and demonstrated antigen-specific lysis of NY-ESO-1+ cancer cells. The efficacy of this vaccine can be attributed to the inherent immunogenicity, cellular tropism toward immune cells, and efficient lymphatic trafficking as described previously.28
We incorporated the adjuvant CFA in our studies to demonstrate the feasibility and comparable efficacy of the CPMV-based vaccine approach. Future studies comparing the CPMV-based cancer vaccine with other adjuvants such as MPL or MF5923,59 will help us further evaluate the mechanism and efficacy of our approach. A vaccine-based immunotherapy approach could particularly benefit patients with NY-ESO-1+ malignancies such as triple-negative breast cancer (TNBC) that frequently exhibits local and regional recurrence and metastatic relapse within 5 years following surgical resection of the primary tumor and have no tumor-specific treatment options.60,61
The plant virus-based approach to vaccines offers several advantages. Genetic engineering could be used to express epitopes on the viral capsid leading to a homogenous formulation and mitigate heterogeneity of the chemical conjugation methods.62 Such genetically engineered vaccines can also be propagated in and purified from host plants using molecular farming, thereby reducing downstream processing and cost.63,64 Furthermore, plant virus-based vaccines could also be incorporated into polymeric implants and devices, which improves the shelf life of the product and will enable the extended release of the antigen.65 Along with its immunostimulatory properties and added layer of safety associated with plant viruses, such advantages make CPMV an attractive plant viral platform for vaccine applications.
EXPERIMENTAL SECTION
Production of Cowpea Mosaic Virus (CPMV).
CPMV was propagated in V. unguiculata plants and purified from infected leaves using previously described methods.66 Post-purification CPMV concentrations were determined by ultraviolet/visible (UV/vis) spectroscopy (CPMV specific extinction coefficient ε260nm = 8.1 mg−1 mL cm−1). Particle integrity was verified by the elution profile determined by size exclusion chromatography using a Superose6 column on the ÄKTA Explorer chromatography system (GE Healthcare, Pittsburgh, PA) and the 260:280 ratio (for intact CPMV the 260:280 ratio is 1.8).
Synthesis of CPMV-NY-ESO-1 Vaccine.
NY-ESO-1 peptide (NY-ESO-1157–165) with a terminal cysteine and a flexible LSPG linker—SLLMWITQV-LSPG-C, or its fluorescent version—tetrame-thylrhodamine (TMR)-conjugated peptide NY-ESO-1TMR (Genscript, Piscataway, NJ), was conjugated to CPMV using a two-step protocol through a bifunctional N-hydroxysuccinimide-PEG12-maleimide (SM-PEG12) linker (Thermo Fisher Scientific, Waltham, MA). CPMV in 0.1 M sodium phosphate (KP) buffer pH 7.4 with constant mixing was first reacted with 2000 molar excess of the SM-PEG12 linker at room temperature for 2 h at 1 mg/mL protein concentration. Next, 3000 molar excess of the peptide was reacted overnight with SM-PEG12-modified CPMV. The CPMV-NY-ESO-1 formulation was purified by spin filtration (10 000 rpm/5 min; 100 kDa molecular weight cutoff filters, Amicon Ultra, Millipore Sigma, Burlington, MA).
Conjugation of NY-ESO-1 peptides on CPMV was quantified using SDS-PAGE gels. Ten micrograms of CPMV and CPMV-NY-ESO-1 was mixed with SDS running buffer (Thermo Fisher Scientific), heated at 100 °C for 5 min, and then loaded on precast Nu-PAGE 4–12% Bis–Tris protein gels (Thermo Fisher Scientific). Electrophoresis was performed for 40 min at 200 V. Gels were stained using GelCode Blue Safe protein stain (Thermo Fisher Scientific) to visualize the protein bands corresponding to molecular weight ladders; the degree of peptide modification was quantified using lane density analysis (Fiji software). Particle integrity was verified by transmission electron microscopy (TEM). CPMV-NY-ESO-1 particles (0.5 mg/mL) were loaded on 400-mesh copper grids bearing the Formvar support film, stained with 2% (w/v) uranyl acetate, and visualized using the FEI Tecnai Spirit G2 BioTWIN microscope (FEI, Hillsboro, OR). CPMV-NY-ESO-1-TMR formulation was characterized using denaturing and native gel electrophoresis. Native gel electrophoresis (100 V for 40 min) of CPMV and CPMV-NY-ESO-1-TMR particles (10 μg in 6× loading dye) was performed on agarose gel (1.2% w/v) containing 1 μL of GelRed Nucleic Acid Stain for RNA visualization (GoldBio, St Louis, MO) in Tris borate EDTA (TBE) buffer. The native gel was visualized under UV light for nucleic acid, using 534 nm light source for TMR dye and after staining with Coomassie Brilliant Blue (0.25% w/v) (Sigma, St Louis, MO) to visualize the capsid protein; denaturing gel was visualized under 534 nm for fluorescence and white light for stained proteins. CPMV-NY-ESO-1-TMR was also characterized using size exclusion chromatography using a Superose6 column on the ÄKTA Explorer chromatography system (GE Healthcare, Marlborough, MA).
A CPMV-OVA vaccine was similarly synthesized by conjugating the H2-Kb-restricted OVA peptide with the GPSL linker and a terminal cysteine C-LSPG-SIINFEKL (Genscript) to CPMV via the SM-PEG12 linker.
Mice.
All animal experiments were carried out in accordance with Case Western Reserve University’s Institutional Animal Care and Use Committee (IACUC). Transgenic C57BL/-Mcph1 Tg (HLA-A2.1)-1Enge/J mice, expressing the human HLA-A2 gene (HLA-A2 mice), were obtained from the Jackson Laboratory (Bar Harbor, ME). Six–eight week old female HLA-A2 mice were immunized subcutaneously twice at 14 days intervals with 50 μg of CPMV-NY-ESO-1 vaccine in 100 μL of phosphate-buffered saline (PBS) or equivalent dose of the NY-ESO-1 peptide mixed with Complete Freud’s Adjuvant (CFA) for first injection/Incomplete Freund’s Adjuvant (IFA) for booster injection (Invivogen, San Diego, CA) in 100 μL of PBS. To evaluate the CPMV-OVA vaccine, 8-week-old female C57BL6 mice (Jackson Laboratory) were immunized with 50 μg of CPMV-OVA or 1 μg of OVA peptide, as described for the NY-ESO-1 antigen. Two weeks after the last immunization, mice were sacrificed and spleens were harvested to isolate CD8+ T cells.
Cell Lines.
A375, an HLA-A2+ NY-ESO-1+ human malignant melanoma cell line, was purchased from ATCC (Manassas, VA) and cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS) (Atlanta Biologicals, Minneapolis, MN) and 1% (v/v) penicillin/streptomycin (Pen/Strep) (Thermo Fisher Scientific). Murine macrophage cell lines RAW 264.7, mouse melanoma cells B16F10 (ATCC), and B16F10-OVA (gift from Dr. Steve N. Fiering, Dartmouth College, NH) were maintained on the DMEM medium described above.
Macrophage Uptake.
Macrophage uptake of CPMV-ESO-1TMR particles and soluble ESO-1TMR peptides was compared using confocal microscopy. Thirty thousand RAW 264.7 macrophage cells in 0.5 mL media were seeded overnight in 24-well plates on circular glass coverslips. Cells were incubated with 5 μg of CPMV-NY-ESO-1TMR particles or 1 μg of NY-ESO-1TMR peptides for 2 h at 37 °C. Cells were washed, fixed in 5% (v/v) paraformaldehyde/0.3% (v/v) glutaraldehyde in Dulbecco’s phosphate-buffered saline (DPBS) for 10 min, stained for the cell membrane with Wheat Germ Agglutinin-AlexaFluor 488 (Thermo Fisher Scientific), and diluted 1:1000 in 5% (v/v) goat serum (Thermo Fisher Scientific) in DPBS. Nuclei were stained with DAPI in the mounting medium (Vector Laboratories, Burlingame, CA). The stained cells were imaged on a Leica TCS SPE confocal microscope with a 63× oil immersion objective and images were analyzed with Fiji software.
Bone Marrow-Derived Dendritic Cells (BMDCs).
BMDCs were isolated from a single-cell suspension of whole bone marrow cells harvested from the femurs and tibias of female HLA-A2 or C57BL6 mice. The cells were washed with PBS, and red blood cells were lysed using RBC lysis buffer (Thermo Fisher Scientific) at 37 °C for 5 min. The cells were then centrifuged, washed, and resuspended at 3 × 106 cells/mL in the T cell medium: Roswell Park Memorial Institute (RPMI) (Thermo Fisher Scientific) supplemented with 10% (v/v) GemCell FBS (Gemini Bio-Products, West Sacramento, CA), 1% (w/v) Pen/Strep, 1 mM sodium pyruvate (Thermo Fisher Scientific), and 50 mM β-mercaptoethanol (Millipore Sigma) supplemented with 10 ng/mL mouse IL-4 and 15 ng/mL mouse GM-CSF (both Peprotech, Rocky Hill, NJ). The media was removed and replaced with fresh T cell media supplemented with IL-4 and GM-CSF on day 3 and then again on day 5. Cells were harvested on day 7 and used for BMDC activation and antigen-presentation studies.
BMDC Activation and Cytokine Secretion.
BMDCs harvested on day 7 were plated at 1 × 106 cells/100 μL medium and incubated with 10 μg of CPMV-NY-ESO-1 particles and 2 μg of the NY-ESO-1 peptide (10× equivalent NY-ESO-1 peptide compared to CPMV-NY-ESO-1) at 37 °C for 24 h in cytokine-free T cell media. Bacterial LPS (100 ng/mL, eBioscience, Thermo Fisher Scientific) was used as a positive control. Following incubation, cell supernatants were collected and analyzed for cytokines TNF-α, IL-6, IL-12, and IL-1β using ELISA kits (BioLegend, San Diego, CA) as per instructions from the manufacturer.
CD8+ T Cell Proliferation and Stimulation.
CD8+ T cells were isolated from single-cell suspension obtained from the spleens of mice immunized with CPMV-NY-ESO-1 and CFA + NY-ESO-1 peptides. Spleens were homogenized and passed through a 40 μm cell strainer in ice-cold PBS and centrifuged at 500g for 5 min. RBCs were depleted with RBC lysing buffer (eBioscience, Thermo Fisher Scientific), and CD8+ T cells were isolated using the RoboSep CD8+ T cell negative isolation kit (STEMCELL Technologies, Cambridge, MA) according to the manufacturer’s instructions.
CD8+ T cells were co-cultured with antigen-pulsed BMDCs to measure proliferation and IFN-γ secretion. Specifically, BMDCs isolated from naïve HLA-A2 mice were pulsed with increasing concentrations of NY-ESO-1 peptide (10, 20, 30 μg/mL) for 4 h at 37 °C. Cells were washed twice with PBS to remove excessive peptides. CD8+ T cells isolated from immunized mice (CPMV-NY-ESO-1 and CFA + NY-ESO-1 groups) were co-cultured with antigen-pulsed and nonpulsed BMDCs (stimulating cells) at a 1:10 (T cells/BMDCs) ratio in a 96-well plate in triplicate at 37 °C for 48 h in an atmosphere of 5% CO2. CD8+ T cells incubated with CD8+ T cells incubated with the NY-ESO-1 peptide alone were used as controls. T cell proliferation was measured using MTT assays, performed as per the manufacturer’s recommendation. A Tecan microplate reader was used for readout. The % cell proliferation was calculated as experimental proliferation/control proliferation × 100, where experimental proliferation is the proliferation of co-cultured cells minus proliferation of BMDCs only minus proliferation of T cells only and control proliferation is the proliferation of T cells only. For measurements of secreted IFN-γ, culture supernatants were collected and assayed with the Mouse IFN-γ ELISA kits (BioLegend) as per instructions from the manufacturer.
To determine the epitope specificity, CD8+ T cells from CPMV-NY-ESO-1-immunized mice were co-cultured with BMDCs pulsed with NY-ESO-1 peptide or irrelevant HER2 peptide P4 (PESFDGD-PASNTAPLQPEQLQ). Secreted IFN-γ levels were compared, as described above.
CD8+ T cells from CPMV-OVA-immunized mice were similarly harvested and secreted IFN-γ levels were measured by co-culturing CD8+ T cells with OVA/ P4-pulsed BMDCs from naïve C57BL6 mice.
CD8+ T Cell-Mediated Cancer Cell Cytotoxicity.
CD8+ T cells from immunized mice were plated in 96-well plates in RPMI at 5 × 106 cells/mL and incubated overnight at 37 °C. On day 1, 10 μg/mL NY-ESO-1 was added to the cells and incubated at 37 °C. Cells were collected on day 3 to perform the cytotoxicity assay. To examine antigen-specific cytotoxicity, NY-ESO-1+ target cancer cells A375 and NY-ESO-1− control cell line B16F10-OVA were co-cultured with CD8+ T cells at effector-to-target ratios of 100:1, 75:1, 50:1, 25:1, 0:1 and cytotoxicity evaluated using the MTT assay. Percentage cytotoxicity was determined as experimental cytotoxicity/control toxicity × 100, where experimental cytotoxicity is cytotoxicity for co-culture (cytotoxicity of CD8+ T cells only + Cytotoxicity of cancer cells only) and control cytotoxicity is the cytotoxicity of cancer cells only under identical culture conditions. Cytotoxicity of CD8+ T cells from CPMV-OVA/CFA + OVA-immunized mice was similarly evaluated against OVA+B16F10-OVA and OVA−B16F10 cells.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded in part by a grant from the Case Western Reserve University (Center For Clinical Research and Technology 2016 Team Science Award 16176 to SS and AL) and a grant from the NIH (R01CA224605 to NFS).
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsabm.0c00259.
Characterization of the CPMV-NY-ESO-1TMR particles by native gel electrophoresis, denaturing gel electrophoresis and size exclusion chromatography indicating conjugation of TMR-tagged peptide with CPMV capsid; CPMV-OVA vaccine, immunization schedule, antigen-specific CD8+ T cell activation and cancer cell cytotoxicity (PDF)
Complete contact information is available at: https://pubs.acs.org/10.1021/acsabm.0c00259
The authors declare no competing financial interest.
Contributor Information
Bindi K. Patel, Department of Biomedical Engineering, Case Western Reserve University, Cleveland, Ohio 44106, United States
Chao Wang, Department of NanoEngineering, University of California-San Diego, La Jolla, California 92093, United States.
Braulio Lorens, Department of Molecular Biology and Microbiology, Case Western Reserve University, Cleveland, Ohio 44106, United States.
Alan D. Levine, Department of Molecular Biology and Microbiology and Medicine, Pediatrics Pathology, and Pharmacology, Case Western Reserve University, Cleveland, Ohio 44106, United States
Nicole F. Steinmetz, Department of Biomedical Engineering, Case Western Reserve University, Cleveland, Ohio 44106, United States;; Department of NanoEngineering, Department of Bioengineering, Department of Radiology, Moores Cancer Center, and Center for Nano-ImmunoEngineering, University of California-San Diego, La Jolla, California 92093, United States;
Sourabh Shukla, Department of Biomedical Engineering, Case Western Reserve University, Cleveland, Ohio 44106, United States;; Department of NanoEngineering, University of California-San Diego, La Jolla, California 92093, United States;
REFERENCES
- (1).Hu Z; Ott PA; Wu CJ Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat. Rev. Immunol 2018, 18, 168–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Hollingsworth RE; Jansen K Turning the corner on therapeutic cancer vaccines. npj Vaccines 2019, 4, No. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Grigoriadis A; Caballero OL; Hoek KS; da Silva L; Chen YT; Shin SJ; Jungbluth AA; Miller LD; Clouston D; Cebon J; Old LJ; Lakhani SR; Simpson AJ; Neville AM CT-X antigen expression in human breast cancer. Proc. Natl. Acad. Sci. U.S.A 2009, 106, 13493–13498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Caballero OL; Chen YT Cancer/testis (CT) antigens: potential targets for immunotherapy. Cancer Sci. 2009, 100, 2014–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Curigliano G; Viale G; Ghioni M; Jungbluth AA; Bagnardi V; Spagnoli GC; Neville AM; Nole F; Rotmensz N; Goldhirsch A Cancer-testis antigen expression in triple-negative breast cancer. Ann. Oncol 2011, 22, 98–103. [DOI] [PubMed] [Google Scholar]
- (6).Mattarollo SR; Loi S; Duret H; Ma Y; Zitvogel L; Smyth MJ Pivotal role of innate and adaptive immunity in anthracycline chemotherapy of established tumors. Cancer Res. 2011, 71, 4809–4820. [DOI] [PubMed] [Google Scholar]
- (7).Fridman WH; Pages F; Sautes-Fridman C; Galon J The immune contexture in human tumours: impact on clinical outcome. Nat. Rev. Cancer 2012, 12, 298–306. [DOI] [PubMed] [Google Scholar]
- (8).Gross S; Walden P Immunosuppressive mechanisms in human tumors: why we still cannot cure cancer. Immunol. Lett 2008, 116, 7–14. [DOI] [PubMed] [Google Scholar]
- (9).Schreiber RD; Old LJ; Smyth MJ Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570. [DOI] [PubMed] [Google Scholar]
- (10).Zou W Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat. Rev. Cancer 2005, 5, 263–274. [DOI] [PubMed] [Google Scholar]
- (11).Ordaz ML; Larmonier N; Lybarger L DC-expressed MHC class I single-chain trimer-based vaccines prime cytotoxic T lymphocytes against exogenous but not endogenous antigens. Cell. Immunol 2010, 262, 141–149. [DOI] [PubMed] [Google Scholar]
- (12).Cruz LJ; Rueda F; Simon L; Cordobilla B; Albericio F; Domingo JC Liposomes containing NYESO1/tetanus toxoid and adjuvant peptides targeted to human dendritic cells via the Fc receptor for cancer vaccines. Nanomedicine 2014, 9, 435–449. [DOI] [PubMed] [Google Scholar]
- (13).Ebert LM; MacRaild SE; Zanker D; Davis ID; Cebon J; Chen W A cancer vaccine induces expansion of NY-ESO-1-specific regulatory T cells in patients with advanced melanoma. PLoS One 2012, 7, No. e48424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Nicholaou T; Ebert LM; Davis ID; McArthur GA; Jackson H; Dimopoulos N; Tan B; Maraskovsky E; Miloradovic L; Hopkins W; Pan L; Venhaus R; Hoffman EW; Chen W; Cebon J Regulatory T-cell-mediated attenuation of T-cell responses to the NY-ESO-1 ISCOMATRIX vaccine in patients with advanced malignant melanoma. Clin. Cancer Res 2009, 15, 2166–2173. [DOI] [PubMed] [Google Scholar]
- (15).Garcia Casado J; Janda J; Wei J; Chapatte L; Colombetti S; Alves P; Ritter G; Ayyoub M; Valmori D; Chen W; Levy F Lentivector immunization induces tumor antigen-specific B and T cell responses in vivo. Eur. J. Immunol 2008, 38, 1867–1876. [DOI] [PubMed] [Google Scholar]
- (16).Odunsi K; Matsuzaki J; Karbach J; Neumann A; Mhawech-Fauceglia P; Miller A; Beck A; Morrison CD; Ritter G; Godoy H; Lele S; duPont N; Edwards R; Shrikant P; Old LJ; Gnjatic S; Jager E Efficacy of vaccination with recombinant vaccinia and fowlpox vectors expressing NY-ESO-1 antigen in ovarian cancer and melanoma patients. Proc. Natl. Acad. Sci. U.S.A 2012, 109, 5797–5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Hirayama M; Nishimura Y The present status and future prospects of peptide-based cancer vaccines. Int. Immunol 2016, 28, 319–328. [DOI] [PubMed] [Google Scholar]
- (18).Skwarczynski M; Toth I Peptide-based synthetic vaccines. Chem. Sci 2016, 7, 842–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Parmiani G; Castelli C; Dalerba P; Mortarini R; Rivoltini L; Marincola FM; Anichini A Cancer immunotherapy with peptide-based vaccines: what have we achieved? Where are we going? J. Natl. Cancer Inst 2002, 94, 805–818. [DOI] [PubMed] [Google Scholar]
- (20).Apostólico JDS; Lunardelli VA; Coirada FC; Boscardin SB; Rosa DS Adjuvants: Classification, Modus Operandi, and Licensing. J. Immunol. Res 2016, 2016, No. 1459394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Hou C; Yi B; Jiang J; Chang YF; Yao X Up-to-date vaccine delivery systems: robust immunity elicited by multifarious nanomaterials upon administration through diverse routes. Biomater. Sci 2019, 7, 822–835. [DOI] [PubMed] [Google Scholar]
- (22).Schwendener RA Liposomes as vaccine delivery systems: a review of the recent advances. Ther. Adv. Vaccines 2014, 2, 159–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Yang M; Yan Y; Fang M; Wan M; Wu X; Zhang X; Zhao T; Wei H; Song D; Wang L; Yu Y MF59 formulated with CpG ODN as a potent adjuvant of recombinant HSP65-MUC1 for inducing anti-MUC1+ tumor immunity in mice. Int. Immunopharmacol 2012, 13, 408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Wang N; Chen M; Wang T Liposomes used as a vaccine adjuvant-delivery system: From basics to clinical immunization. J. Controlled Release 2019, 303, 130–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Shukla S; Jandzinski M; Wang C; Gong X; Bonk KW; Keri RA; Steinmetz NF A Viral Nanoparticle Cancer Vaccine Delays Tumor Progression and Prolongs Survival in a HER2+ Tumor Mouse Model. Adv. Ther 2019, 2, No. 1800139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Lizotte PH; Wen AM; Sheen MR; Fields J; Rojanasopondist P; Steinmetz NF; Fiering S In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer. Nat. Nanotechnol 2016, 11, 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Wen AM; Le N; Zhou X; Steinmetz NF; Popkin DL Tropism of CPMV to Professional Antigen Presenting Cells Enables a Platform to Eliminate Chronic Infections. ACS Biomater. Sci. Eng 2015, 1, 1050–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Shukla S; Myers JT; Woods SE; Gong X; Czapar AE; Commandeur U; Huang AY; Levine AD; Steinmetz NF Plant viral nanoparticles-based HER2 vaccine: Immune response influenced by differential transport, localization and cellular interactions of particulate carriers. Biomaterials 2017, 121, 15–27. [DOI] [PubMed] [Google Scholar]
- (29).Cai H; Shukla S; Wang C; Masarapu H; Steinmetz NF Heterologous Prime-Boost Enhances the Antitumor Immune Response Elicited by Plant-Virus-Based Cancer Vaccine. J. Am. Chem. Soc 2019, 141, 6509–6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Joffre OP; Segura E; Savina A; Amigorena S Cross-presentation by dendritic cells. Nat. Rev. Immunol 2012, 12, 557–569. [DOI] [PubMed] [Google Scholar]
- (31).Rock KL; Reits E; Neefjes J Present Yourself! By MHC Class I and MHC Class II Molecules. Trends Immunol. 2016, 37, 724–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Win SJ; Ward VK; Dunbar PR; Young SL; Baird MA Cross-presentation of epitopes on virus-like particles via the MHC I receptor recycling pathway. Immunol. Cell Biol 2011, 89, 681–688. [DOI] [PubMed] [Google Scholar]
- (33).Leclerc D; Beauseigle D; Denis J; Morin H; Pare C; Lamarre A; Lapointe R Proteasome-independent major histocompatibility complex class I cross-presentation mediated by papaya mosaic virus-like particles leads to expansion of specific human T cells. J. Virol 2007, 81, 1319–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Makarkov AI; Golizeh M; Ruiz-Lancheros E; Gopal AA; Costas-Cancelas IN; Chierzi S; Pillet S; Charland N; Landry N; Rouiller I; Wiseman PW; Ndao M; Ward BJ Plant-derived virus-like particle vaccines drive cross-presentation of influenza A hemagglutinin peptides by human monocyte-derived macrophages. npj Vaccines 2019, 4, No. 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Chen JL; Dunbar PR; Gileadi U; Jager E; Gnjatic S; Nagata Y; Stockert E; Panicali DL; Chen YT; Knuth A; Old LJ; Cerundolo V Identification of NY-ESO-1 peptide analogues capable of improved stimulation of tumor-reactive CTL. J. Immunol 2000, 165, 948–955. [DOI] [PubMed] [Google Scholar]
- (36).Durrani Z; McInerney TL; McLain L; Jones T; Bellaby T; Brennan FR; Dimmock NJ Intranasal immunization with a plant virus expressing a peptide from HIV-1 gp41 stimulates better mucosal and systemic HIV-1-specific IgA and IgG than oral immunization. J. Immunol. Methods 1998, 220, 93–103. [DOI] [PubMed] [Google Scholar]
- (37).Sainsbury F; Saxena P; Aljabali AA; Saunders K; Evans DJ; Lomonossoff GP Genetic engineering and characterization of Cowpea mosaic virus empty virus-like particles. Methods Mol. Biol 2014, 1108, 139–153. [DOI] [PubMed] [Google Scholar]
- (38).Bachmann MF; Jennings GT Therapeutic vaccines for chronic diseases: successes and technical challenges. Philos. Trans. R. Soc. London, Ser. B 2011, 366, 2815–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Kundig TM; Senti G; Schnetzler G; Wolf C; Prinz Vavricka BM; Fulurija A; Hennecke F; Sladko K; Jennings GT; Bachmann MF Der p 1 peptide on virus-like particles is safe and highly immunogenic in healthy adults. J. Allergy Clin. Immunol 2006, 117, 1470–1476. [DOI] [PubMed] [Google Scholar]
- (40).Taylor KM; Lin T; Porta C; Mosser AG; Giesing HA; Lomonossoff GP; Johnson JE Influence of three-dimensional structure on the immunogenicity of a peptide expressed on the surface of a plant virus. J. Mol. Recognit 2000, 13, 71–82. [DOI] [PubMed] [Google Scholar]
- (41).Albakri MM; Veliz FA; Fiering SN; Steinmetz NF; Sieg SF Endosomal toll-like receptors play a key role in activation of primary human monocytes by cowpea mosaic virus. Immunology 2020, 159, 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Gonzalez MJ; Plummer EM; Rae CS; Manchester M Interaction of Cowpea mosaic virus (CPMV) nanoparticles with antigen presenting cells in vitro and in vivo. PLoS One 2009, 4, No. e7981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Plummer EM; Manchester M Endocytic uptake pathways utilized by CPMV nanoparticles. Mol. Pharm 2013, 10, 26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Trevejo JM; Marino MW; Philpott N; Josien R; Richards EC; Elkon KB; Falck-Pedersen E TNF-alpha-dependent maturation of local dendritic cells is critical for activating the adaptive immune response to virus infection. Proc. Natl. Acad. Sci. U.S.A 2001, 98, 12162–12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Moossavi M; Parsamanesh N; Bahrami A; Atkin SL; Sahebkar A Role of the NLRP3 inflammasome in cancer. Mol. Cancer 2018, 17, No. 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Ghiringhelli F; Apetoh L; Tesniere A; Aymeric L; Ma Y; Ortiz C; Vermaelen K; Panaretakis T; Mignot G; Ullrich E; Perfettini JL; Schlemmer F; Tasdemir E; Uhl M; Genin P; Civas A; Ryffel B; Kanellopoulos J; Tschopp J; Andre F; Lidereau R; McLaughlin NM; Haynes NM; Smyth MJ; Kroemer G; Zitvogel L Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat. Med 2009, 15, 1170–1178. [DOI] [PubMed] [Google Scholar]
- (47).Fisher DT; Appenheimer MM; Evans SS The two faces of IL-6 in the tumor microenvironment. Semin. Immunol 2014, 26, 38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Tugues S; Burkhard SH; Ohs I; Vrohlings M; Nussbaum K; Vom Berg J; Kulig P; Becher B New insights into IL-12-mediated tumor suppression. Cell Death Differ. 2015, 22, 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Wiedemann F; Link R; Pumpe K; Jacobshagen U; Schaefer HE; Wiesmüller K-H; Hummel R-P; Jung G; Bessler W; Böltz T Histopathological studies on the local reactions induced by complete fReund’s adjuvant (CFA), bacterial lipopolysaccharide (LPS), and synthetic lipopeptide (P3C) conjugates. J. Pathol 1991, 164, 265–271. [DOI] [PubMed] [Google Scholar]
- (50).Molino NM; Anderson AK; Nelson EL; Wang SW Biomimetic protein nanoparticles facilitate enhanced dendritic cell activation and cross-presentation. ACS Nano 2013, 7, 9743–9752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Neek M; Tucker JA; Kim TI; Molino NM; Nelson EL; Wang SW Co-delivery of human cancer-testis antigens with adjuvant in protein nanoparticles induces higher cell-mediated immune responses. Biomaterials 2018, 156, 194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Awate S; Babiuk LA; Mutwiri G Mechanisms of action of adjuvants. Front. Immunol 2013, 4, No. 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Billiau A; Matthys P Modes of action of Freund’s adjuvants in experimental models of autoimmune diseases. J. Leukocyte Biol 2001, 70, 849–860. [PubMed] [Google Scholar]
- (54).Leenaars PP; Hendriksen CF; de Leeuw WA; Carat F; Delahaut P; Fischer R; Halder M; Hanly WC; Hartinger J; Hau J; Lindblad EB; Nicklas W; Outschoorn IM; Stewart-Tull DE The Production of Polyclonal Antibodies in Laboratory Animals. The Report and Recommendations of ECVAM Workshop 35. Altern. Lab. Anim 1999, 27, 79–102. [DOI] [PubMed] [Google Scholar]
- (55).Miller LH; Saul A; Mahanty S Revisiting Freund’s incomplete adjuvant for vaccines in the developing world. Trends Parasitol. 2005, 21, 412–414. [DOI] [PubMed] [Google Scholar]
- (56).Alving CR Design and selection of vaccine adjuvants: animal models and human trials. Vaccine 2002, 20, S56–S64. [DOI] [PubMed] [Google Scholar]
- (57).Tandrup Schmidt S; Foged C; Korsholm KS; Rades T; Christensen D Liposome-Based Adjuvants for Subunit Vaccines: Formulation Strategies for Subunit Antigens and Immunostimulators. Pharmaceutics 2016, 8, No. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Tian Y; Li M; Yu C; Zhang R; Zhang X; Huang R; Lu L; Yuan F; Fan Y; Zhou B; Men K; Xu H; Yang L The novel complex combination of alum, CpG ODN and HH2 as adjuvant in cancer vaccine effectively suppresses tumor growth in vivo. Oncotarget 2017, 8, 45951–45964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Ko EJ; Kang SM Immunology and efficacy of MF59-adjuvanted vaccines. Hum. Vaccines Immunother 2018, 14, 3041–3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Oakman C; Viale G; Di Leo A Management of triple negative breast cancer. Breast 2010, 19, 312–321. [DOI] [PubMed] [Google Scholar]
- (61).Cleator S; Heller W; Coombes RC Triple-negative breast cancer: therapeutic options. Lancet Oncol. 2007, 8, 235–244. [DOI] [PubMed] [Google Scholar]
- (62).Kumar S; Ochoa W; Singh P; Hsu C; Schneemann A; Manchester M; Olson M; Reddy V Tomato bushy stunt virus (TBSV), a versatile platform for polyvalent display of antigenic epitopes and vaccine design. Virology 2009, 388, 185–190. [DOI] [PubMed] [Google Scholar]
- (63).Lebel ME; Chartrand K; Leclerc D; Lamarre A Plant Viruses as Nanoparticle-Based Vaccines and Adjuvants. Vaccines 2015, 3, 620–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Balke I; Zeltins A Recent Advances in the Use of Plant Virus-Like Particles as Vaccines. Viruses 2020, 12, No. 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Lee PW; Shukla S; Wallat JD; Danda C; Steinmetz NF; Maia J; Pokorski JK Biodegradable Viral Nanoparticle/Polymer Implants Prepared via Melt-Processing. ACS Nano 2017, 11, 8777–8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Wellink J Comovirus isolation and RNA extraction. Methods Mol. Biol 1998, 81, 205–209. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


