Abstract
Background
Infant botulism is a rare toxicoinfectious disease caused by colonization of the infant’s intestine with botulinum neurotoxin-producing clostridia (i.e. Clostridium botulinum or neurotoxigenic strains of C. butyricum or C. baratii). Our goal was to examine data from laboratory-confirmed cases of infant botulism reported in Canada to summarize incidence over time, over geographic distribution by province or territory, and by sex, and to compare these parameters with data from the Canadian Notifiable Disease Surveillance System (CNDSS). The average age of onset, serotype of botulinum neurotoxin (BoNT), case outcomes, length of hospitalization and suitability of clinical specimens for laboratory confirmation were also determined.
Methods
We examined laboratory records from the Health Canada Botulism Reference Service and the British Columbia Centre for Disease Control (BCCDC) Public Health Laboratory. The Discharge Abstract Database (DAD) and the Hospital Morbidity Database (HMDB) of the Canadian Institute of Health Information (CIHI) were queried for data on hospitalization of infant botulism cases. The CNDSS was queried for data on reported cases of infant botulism.
Results
From 1979 to 2019, 63 laboratory-confirmed cases of infant botulism were confirmed by the Health Canada Botulism Reference Service and the BCCDC Public Health Laboratory for an annual rate of 4.30 cases per million live births. From 1983 to 2018, 57 cases of infant botulism were reported to the CNDSS. Of the 63 cases confirmed by the reference laboratories, the median age of onset was 16 weeks with a range of 2 to 52 weeks. The majority of cases were type A (76%) and B (21%), with single cases of type F and type AB. Of the 23 laboratory-confirmed cases with matched hospital records, 13 were transferred to special care and eight needed ventilator support; no deaths were reported.
Conclusion
Spores of C. botulinum are present naturally in the environment, thus diagnosis of infant botulism does not require a history of exposure to high-risk foods such as honey. Stool samples are the most useful diagnostic specimen.
Keywords: Clostridium botulinum, Clostridium butyricum, Clostridium baratii, incidence rate, geographic distribution, Canada, serotype, environmental source, botulinum neurotoxin
Introduction
Botulism is a neuroparalytic disease caused by exposure to botulinum neurotoxin (BoNT). Manifestations of botulism are classified according to the route of exposure to BoNTs. Foodborne botulism is an intoxication resulting from the ingestion of BoNT in food or beverages that supported the growth of Clostridium botulinum (1). Wound botulism and intestinal toxemia botulism occur when C. botulinum spores colonize an infected wound or the adult intestinal tract, respectively, and release BoNTs in situ (2,3).
Infant botulism, first described in 1976 (4,5), is a form of intestinal toxemia botulism that occurs in children younger than one year. Infants are particularly susceptible to C. botulinum intestinal colonization because of the immaturity of their gut microbiota, whereas children over one year old can ingest C. botulinum spores without colonization (6,7). In rare instances, infant botulism has been caused by BoNT-producing species C. butyricum type E (8–10) and C. baratii type F (11–14).
The clinical spectrum of infant botulism includes a wide range of severities from subclinical to fulminant. Symptoms may include, but are not limited to, hypotonia, weak suck, dysphagia, constipation, weak cry and diaphragm weakness that may require that the infant receives mechanical ventilation. Almost all cases of infant botulism are caused by Group I C. botulinum that produce BoNT type A or type B. Type A generally has a more severe clinical presentation (6,7).
Infant botulism is treated with Botulism Immune Globulin Intravenous (BIG-IV or BabyBIG) antitoxin that binds to and neutralizes circulating BoNT in the bloodstream (15). It is generally recommended that infants are treated with BabyBIG as soon as possible based on a physician’s differential diagnosis. Laboratory confirmation of infant botulism is based on detection of viable C. botulinum in stool or detection of BoNT in stool or serum.
There have been few published reports of infant botulism cases in Canada (16–20). Here we present a summary of infant botulism in Canada from 1979 to 2019, including incidence over reporting period, geographic distribution by province and territory, patient age and sex, serotype and group of C. botulinum involved, food or environmental source identification (where possible), as well as preferred clinical specimens for detection of BoNT and viable C. botulinum. Hospital records that match laboratory-confirmed cases demonstrate clinical outcomes of the disease.
Methods
Microbiology laboratory and national surveillance data
We examined two independent laboratory databases for laboratory-confirmed cases of infant botulism from 1979 to 2019. These databases are maintained by the Botulism Reference Service (BRS) for Canada at Health Canada, Ottawa, Ontario, and the British Columbia Centre for Disease Control (BCCDC) Public Health Laboratory, in Vancouver, British Columbia. The Health Canada BRS receives and tests clinical and food specimens associated with suspect cases of botulism from all provinces and territories when requested. The BCCDC laboratory maintains its capacity to test specimens from British Columbia, and has also tested specimens from the Yukon. Thus, these two databases do not overlap and, when combined, represent all the laboratory-confirmed cases of botulism in Canada.
We extracted information on patient age and sex, date and location of diagnosis, implicated source of C. botulinum, group of C. botulinum and BoNT serotype. The rates of disease per million live births were calculated using data from the United Nations Statistics Division and Statistics Canada (21,22).
We used the national case definition for confirmed cases of infant botulism to ensure consistency in data recording: “laboratory confirmation with symptoms compatible with botulism in a person less than one year of age [with] detection of botulinum toxin in stool or serum or isolation of C. botulinum from the patient’s stool or at autopsy” (23). Cases meeting this definition were extracted from the Canadian Notifiable Disease Surveillance System (CNDSS) and included the reporting year, province/territory, age group and sex. The CNDSS maintains basic surveillance on nationally notifiable diseases by collecting voluntarily submitted data from provinces and territories. CNDSS data was compared to laboratory data for completeness.
Laboratory confirmation of clinical cases
Detection of BoNT and isolation of viable C. botulinum from environmental and clinical specimens were performed according to Health Canada method MFHPB-16 (24). BoNT serotype was determined by neutralizing toxicity with serotype-specific antibodies provided by the United States (US) Centers for Disease Control. The group of C. botulinum (Group I or II) was determined based on proteolysis of cooked-meat media (CMM) pellets in pure culture (24). The California Department of Public Health (CDPH) identified the lone isolate of C. baratii type F based on this isolate’s ability to produce lecithinase, as demonstrated on egg yolk agar; its inability to produce lipase; and its ability to produce type F toxin in broth culture, detected using the mouse bioassay. API20 A was used to confirm the identification of C. baratii type F. All animal use followed protocols approved by institutional (Health Canada or CDPH) animal care and use committees.
Morbidity data
Records on patient clinical information were retrieved from the 2005–2018 Discharge Abstract Database (DAD) and the 2005–2010 Hospital Morbidity Database (HMDB) of the Canadian Institute for Health Information (CIHI) by querying all records currently available that listed botulism in the first 10 suspected diagnostic codes (25). These records were then matched to records by age, sex, date of admission, date of sample and province/territory of residence. Only laboratory-confirmed cases were included in the analyses of the DAD and HMDB data.
Ethics approval
Formal ethics approval was not required because this study used de-identified healthcare data that were obtained under an agreement with CIHI, and we report the results in aggregate form.
Results
While botulism has been nationally notifiable in Canada since 1933, the first case of infant botulism was reported in Canada in 1979 (16), three years after the first cases were described in the US (4,5). From 1979 to 2019, there were 63 laboratory-confirmed cases of infant botulism in Canada (Figure 1), that is, an average of 1.6 cases per year. The Health Canada BRS confirmed 44 cases, while the BCCDC confirmed 19 cases. These cases of infant botulism are further described as one complete dataset and will be referred to as “laboratory-confirmed”.
Figure 1.
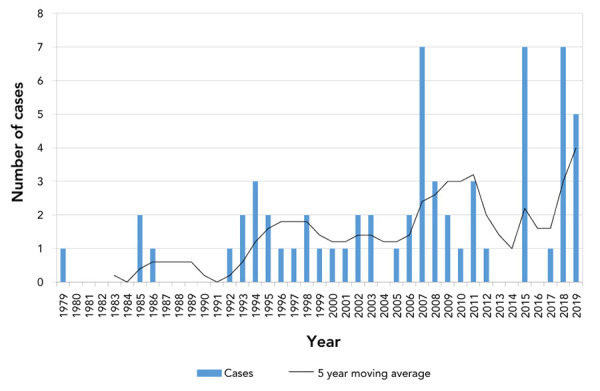
Number of laboratory-confirmed infant botulism cases in Canada, 1979–2019
Infant botulism is a rare disease; the average annual incidence was calculated at 4.3 cases per million live births in Canada during this time period (Table 1). Confirmed cases occurred in Ontario (n=21), British Columbia (n=19), Québec (n=12), Alberta (n=8), Newfoundland and Labrador (n=2) and Nova Scotia (n=1) (Table 1). Of the 58 records that indicated sex, 34 were female (59%) and 24 were male (41%).
Table 1. Laboratory-confirmed infant botulism cases by serotype and province/territory, 1979–2019.
| Province/territory | Cases by BoNT serotype | Total | Annual rate/million live birthsa | |||
|---|---|---|---|---|---|---|
| A | B | AB | F | |||
| Ontario | 11 | 9 | 1 | 0 | 21 | 3.75 |
| British Columbia | 19 | 0 | 0 | 0 | 19 | 10.86 |
| Québec | 9 | 2 | 0 | 1 | 12 | 3.63 |
| Alberta | 8 | 0 | 0 | 0 | 8 | 4.41 |
| Newfoundland and Labrador | 1 | 1 | 0 | 0 | 2 | 9.93 |
| Nova Scotia | 0 | 1 | 0 | 0 | 1 | 2.66 |
| Canadab | 48 | 13 | 1 | 1 | 63 | 4.30 |
Abbreviation: BoNT, botulinum neurotoxin
a Annual rate per million live births was calculated as the total case count divided by the average number of annual live births from 1991–2018 multiplied by 40 (years), divided by a million (21,22)
b No laboratory-confirmed cases of infant botulism in Manitoba, New Brunswick, Northwest Territories, Nunavut, Prince Edward Island, Saskatchewan or Yukon
In comparison to the laboratory-confirmed cases, the CNDSS reported 57 cases of infant botulism from 1983 to 2018, giving an average of 1.6 cases per year. Considering the single laboratory-confirmed case in 1979 and the five laboratory-confirmed cases in 2019, the total number of cases reported to the CNDSS matches the total number of 57 laboratory-confirmed cases from 1983 to 2018. However, the number of cases reported by the provinces and territories differed. Cases were reported to the CNDSS from Ontario (n=22), British Columbia (n=14), Québec (n=10), Alberta (n=9), Newfoundland and Labrador (n=1) and Nova Scotia (n=1). Of the 57 cases, 36 were female and 21 male. From 1983 to 2018, the laboratory-confirmed cases were from Ontario (n=19), British Columbia (n=17), Québec (n=11), Alberta (n=8), Newfoundland and Labrador (n=2), and Nova Scotia (n=1).
The age of onset follows a unimodal distribution with mean 17.8 weeks and median 16 weeks (Figure 2). The youngest infant was two weeks of age, and the oldest infant was 52 weeks old. BoNT type A constituted the majority of cases (n=48; 76%), followed by type B (n=13; 21%), type AB (n=1; 2%) and type F (n=1; 2%) (Table 1). The single type F case was identified as caused by C. baratii; this was the youngest infant, with an age of onset of two weeks. All type A strains of C. botulinum are Group I (proteolytic), while type B strains may be either Group I or II (non-proteolytic). Of the type B strains isolated and tested for proteolytic activity, all (n=10) were classified as Group I C. botulinum.
Figure 2.
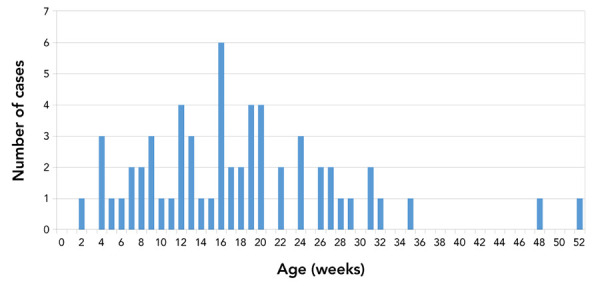
Age of onset for laboratory-confirmed infant botulism cases, Canada, 1979–2019
Of the 63 cases identified from 1979 to 2019, 29 (46%) had environmental or food samples submitted for testing and only six were matched with environmental samples, including honey (n=4) and crib dust (n=1), as well as a change mat swab and a washing tub swab (n=1) (Table 2). Of the 58 records that were available, stool was by far the best clinical specimen for detection of BoNT (n=55; 95%) and viable C. botulinum (n=58; 100%) (Table 3). BoNT was detected in only 3 of 33 (9%) serum specimens tested, while neither BoNT nor C. botulinum were detected in three specimens of gastric contents tested.
Table 2. Laboratory-confirmed source attribution of infant botulism.
| Year | Province/territory | Source |
|---|---|---|
| 1985 | Québec | Honey |
| 1993 | Alberta | Honey |
| 1995 | Québec | Honey |
| 2000 | British Columbia | Honey |
| 2005 | Ontario | Crib dust |
| 2009 | Québec | Change mat swab and washing tub swab |
Table 3. Laboratory detection of BoNT and viable Clostridium botulinum in clinical specimens.
| Specimen | Tested (n) | BoNT detected | C. botulinum detected | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Gastric contents | 3 | 0 | 0 | 0 | 0 |
| Serum | 33 | 3 | 9 | 0 | 0 |
| Stool | 58 | 55 | 95 | 58 | 100 |
Abbreviation: BoNT, botulinum neurotoxin
Of the 63 laboratory-confirmed infant botulism cases, 23 were cross-referenced to the 2005–2018 DAD and 2005–2010 HMDB. The mean (standard deviation [SD]) length of hospital stay of these patients was 20.3 (7.0) days (Table 4). Over a half (n=13) were transferred to special care units. These special care units are specifically designed, staffed and equipped for the continuous observation and treatment of patients who cannot be cared for in a general acute care unit. These include intensive care units and step-down units (25). The patients spent a mean (SD) of 12.2 (6.3) days in a special care unit; eight needed a ventilator (Table 4). Most were discharged to the patient’s home with either no support (n=9) or with the support of home care workers (n=10). Three were transferred to acute care during hospitalization; the discharge disposition of one patient was unknown (Table 5). No deaths were reported for the 23 cross-referenced cases.
Table 4. Hospital metrics for laboratory-confirmed hospitalization cases (n=23).
| Hospital metric | Mean | Standard deviation | Number of cases |
|---|---|---|---|
| Length of stay (days) | 20.3 | 7.0 | 23 |
| Time in special care (days) | 12.2 | 6.3 | 13 |
| Use of ventilator | N/A | N/A | 8 |
Abbreviation: N/A, not applicable
Table 5. Clinical outcome of laboratory-confirmed hospitalization cases (n=23).
| Discharge disposition | Number of cases |
|---|---|
| Home, no support | 9 |
| Home with support | 10 |
| Transferred to acute care | 3 |
| Unknown | 1 |
| Death | 0 |
Discussion
From 1979 to 2019 there were 63 laboratory-confirmed cases of infant botulism in Canada. From 1983 to 2018, 57 cases were reported to the CNDSS, which matched the total number of laboratory-confirmed cases during this time period, however the provinces and territories reporting the cases differed. The reasons for the discrepancies is unknown; however, this discrepancy is not unexpected, as the reference laboratories are directly involved in case diagnosis while public health authorities may not be involved in every sporadic case. In recent years, the BRS for Canada and the Public Health Agency of Canada have collaborated to ensure more complete reporting to the appropriate public health authorities.
The annual incidence rate of 4.3 cases per million live births in Canada is similar to incidence rates reported in Australia (4.4 cases per million live births), Italy (2.1 cases per million live births) and Denmark (6.7 cases per million live births) and less than the rates reported in the US (20.3 cases per million live births) and Argentina (24.1 cases per million live births) (26).
The average age at onset was 17.8 weeks in Canada. This is higher than the reported mean ages of onset of 13.8 weeks in the US and 14.3 weeks globally outside the US (26). The age of onset may correspond to changes in gut microbiota after weaning (27) that leaves the gut more susceptible to colonization. A recent study found that breast-fed infant botulism patients were older at onset than formula-fed patients (28), although the role of breast feeding in infant botulism is controversial (29,30).
The finding that 98% of the cases were types A, B or AB is consistent with a report that C. botulinum types A and B accounted for 98.7% of all recorded infant botulism cases worldwide from 1978 to 2006 (26). The single case of type F identified as being caused by C. baratii is rare but not unprecedented. Cases of infant botulism caused by toxigenic strains of C. butyricum type E (8–10) and C. baratii type F (11–14), as well as a single case caused by C. botulinum type E (31), have been described elsewhere. The predominance of C. botulinum type A in the western US correlates with the occurrence of only type A cases in the two western provinces, Alberta and British Columbia (Table 1) (32).
The sporadic nature of infant botulism and the ubiquity of C. botulinum spores in the environment, combined with what is likely a low infectious dose, makes identifying the source of C. botulinum a significant challenge. In contrast, foodborne botulism outbreaks tend to involve multiple cases, allowing epidemiological investigation and determination of possible food sources. For the 29 cases with environmental or food samples submitted for testing, the isolation of C. botulinum was a rare occurrence. Honey accounted for four cases, based on isolation of the same serotype from honey fed to the infant. In one instance, C. botulinum was isolated from a sample of dust from the crib. In another instance, a change mat and bath tub were found to be positive for C. botulinum. In these cases, it is unknown whether the environmental samples were the source of illness for the infant or whether they were contaminated by shedding from the infant’s stool. A case of infant botulism in Finland was linked to C. botulinum spores isolated from dust found in a vacuum cleaner (33). Absence of a history of honey consumption should not be considered reason to rule out infant botulism (18).
For laboratory detection of BoNT or isolation of viable C. botulinum, the proper collection and handling of clinical specimens is essential. Of the 58 records that were available, stool was by far the best clinical specimen for detection of BoNT (95%) and viable C. botulinum (100%). This is consistent with a previous report that stool or enema effluent are preferred diagnostic specimens for infant botulism (34).
Of the 23 infant botulism cases that were cross-referenced to the DAD and HMDB databases, 13 (57%) were transferred to special care and eight (35%) needed ventilator support. No deaths were reported. This is consistent with previous data indicating that infant botulism is a severe disease requiring intervention in the majority of cases. Outside the US, the percentage of reported cases who required ventilator or intubation support was 67% and deaths accounted for 1.1% of cases (26). In the US, 56% of hospitalized infant botulism cases required ventilator support or intubation during a trial of BabyBIG (15), while the mortality rate in the US is less than 1% (35).
Limitations
The narrow range of hospital records available from CIHI databases (2005–2018 for DAD and 2005–2010 for HMDB) reduced the number of cases that could be linked to the laboratory records and lowered the statistical power of data obtained from the hospital records, such as severity of illness and efficacy of treatment. Of note, treatment with BabyBIG antitoxin is not routinely recorded in the CIHI database; we recommend that this be included to properly evaluate the effect of this treatment.
Likewise, 28 instances from the CIHI database were coded as “botulism” based on differential diagnosis, yet samples were not sent to a laboratory for confirmation. Finally, there were several instances of laboratory-confirmed infant botulism cases that could not be identified in the CIHI databases, even within the reported years. Considering a recent summary of 1,345 infant botulism cases in California from 1976–2016 found that 99.3% of cases were hospitalized (36), it is likely that the missing hospital records for laboratory-confirmed cases in Canada were the result of a missing diagnostic code in the CIHI database. Improved communication between hospitals, public health officials and diagnostic laboratories would help to capture all cases of infant botulism in Canada and help in the diagnosis and evaluation of treatments. Physicians and hospital staff can refer to the Canadian botulism guide for healthcare professionals for instructions on differential diagnosis, proper sampling of clinical specimens and treatment with BabyBIG antitoxin (37).
Conclusion
Infant botulism is a rare disease in Canada. The unimodal distribution for age of onset suggests a temporal susceptibility to colonization by C. botulinum which peaks at 16 weeks. Although no deaths were reported in this time period, the average length of stay in the hospital and the number of cases transferred to special care demonstrate severe clinical outcomes for patients suffering from this disease. The effectiveness of the BabyBIG antitoxin could not be evaluated due to limitations in reporting and should be addressed in the future.
Acknowledgements
The authors wish to acknowledge J Barash and S Arnon of the California Department of Public Health Infant Botulism Treatment and Prevention Program (CDPH/IBTPP) for isolation and identification of C. baratii type F as the causative agent of the single case of type F infant botulism from a stool specimen sent to CDPH/IBTPP for research purposes.
Competing interests: None.
Funding: This work was supported by Health Canada, the British Columbia Centre for Disease Control (BCCDC) Public Health Laboratory, and the Public Health Agency of Canada.
References
- 1.Fleck-Derderian S, Shankar M, Rao AK, Chatham-Stephens K, Adjei S, Sobel J, Meltzer MI, Meaney-Delman D, Pillai SK. The epidemiology of foodborne botulism outbreaks: a systematic review. Clin Infect Dis 2017;66(suppl_1):S73–81. 10.1093/cid/cix846 [DOI] [PubMed] [Google Scholar]
- 2.Chatham-Stephens K, Fleck-Derderian S, Johnson SD, Sobel J, Rao AK, Meaney-Delman D. Clinical features of foodborne and wound botulism: a systematic review of the literature, 1932-2015. Clin Infect Dis 2017;66(suppl_1):S11–6. 10.1093/cid/cix811 [DOI] [PubMed] [Google Scholar]
- 3.Harris RA, Anniballi F, Austin JW. Adult intestinal toxemia botulism. Toxins (Basel) 2020;12(2):81. 10.3390/toxins12020081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pickett J, Berg B, Chaplin E, Brunstetter-Shafer MA. Syndrome of botulism in infancy: clinical and electrophysiologic study. N Engl J Med 1976;295(14):770–2. 10.1056/NEJM197609302951407 [DOI] [PubMed] [Google Scholar]
- 5.Midura TF, Arnon SS. Infant botulism. Identification of Clostridium botulinum and its toxins in faeces. Lancet 1976;308(7992):934–6. 10.1016/S0140-6736(76)90894-1 [DOI] [PubMed] [Google Scholar]
- 6.Arnon SS, Damus K, Chin J. Infant botulism: epidemiology and relation to sudden infant death syndrome. Epidemiol Rev 1981;3:45–66. 10.1093/oxfordjournals.epirev.a036239 [DOI] [PubMed] [Google Scholar]
- 7.Arnon SS. Infant botulism. Annu Rev Med 1980;31:541–60. 10.1146/annurev.me.31.020180.002545 [DOI] [PubMed] [Google Scholar]
- 8.Fenicia L, Da Dalt L, Anniballi F, Franciosa G, Zanconato S, Aureli P. A case if infant botulism due to neurotoxigenic Clostridium butyricum type E associated with Clostridium difficile colitis. Eur J Clin Microbiol Infect Dis 2002;21(10):736–8. 10.1007/s10096-002-0816-z [DOI] [PubMed] [Google Scholar]
- 9.McCroskey LM, Hatheway CL, Fenicia L, Pasolini B, Aureli P. Characterization of an organism that produces type E botulinal toxin but which resembles Clostridium butyricum from the feces of an infant with type E botulism. J Clin Microbiol 1986;23(1):201–2. 10.1128/jcm.23.1.201-202.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aureli P, Fenicia L, Pasolini B, Gianfranceschi M, McCroskey LM, Hatheway CL. Two cases of type E infant botulism caused by neurotoxigenic Clostridium butyricum in Italy. J Infect Dis 1986;154(2):207–11. 10.1093/infdis/154.2.207 [DOI] [PubMed] [Google Scholar]
- 11.Paisley JW, Lauer BA, Arnon SS. A second case of infant botulism type F caused by Clostridium baratii. Pediatr Infect Dis J 1995;14(10):912–4. 10.1097/00006454-199510000-00022 [DOI] [PubMed] [Google Scholar]
- 12.Barash JR, Tang TW, Arnon SS. First case of infant botulism caused by Clostridium baratii type F in California. J Clin Microbiol 2005;43(8):4280–2. 10.1128/JCM.43.8.4280-4282.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halpin AL, Khouri JM, Payne JR, Nakao JH, Cronquist A, Kalas N, Mohr M, Osborne M, O'Dell S, Luquez C, Klontz KC, Sobel J, Rao AK. Type F infant botulism: Investigation of recent clusters and overview of this exceedingly rare disease. Clin Infect Dis 2017;66(suppl_1):S92–4. 10.1093/cid/cix818 [DOI] [PubMed] [Google Scholar]
- 14.Moodley A, Quinlisk P, Garvey A, Kalas N, Barash JR, Khouri JM; Centers for Disease Control and Prevention (CDC). Notes from the field: infant botulism caused by Clostridium baratii type F - Iowa, 2013. MMWR Morb Mortal Wkly Rep 2015;64(14):400. [PMC free article] [PubMed] [Google Scholar]
- 15.Arnon SS, Schechter R, Maslanka SE, Jewell NP, Hatheway CL. Human botulism immune globulin for the treatment of infant botulism. N Engl J Med 2006;354(5):462–71. 10.1056/NEJMoa051926 [DOI] [PubMed] [Google Scholar]
- 16.McCurdy DM, Krishnan C, Hauschild AH. Infant botulism in Canada. Can Med Assoc J 1981;125(7):741–3. [PMC free article] [PubMed] [Google Scholar]
- 17.Roland EH, Ebelt VJ, Anderson JD, Hill A. Infant botulism: a rare entity in Canada? CMAJ 1986;135(2):130–1. [PMC free article] [PubMed] [Google Scholar]
- 18.Siu K, Rehan M, Austin JW, Ramachandran Nair R, Pernica J. It’s not all about the honey. Paediatr Child Health 2017;22(1):37–8. 10.1093/pch/pxx009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hauschild AH, Bowmer EJ, Gauvreau L. Infant botulism. Can Med Assoc J 1978;118(5):484. [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartz KL, Austin JW, Science M. Constipation and poor feeding in an infant with botulism. CMAJ 2012;184(17):1919–22. 10.1503/cmaj.120340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Statistics Canada. Census profile 2016 [Internet]. Ottawa (ON): Statistics Canada; (modified 2021-03-11; accessed 2020-09-28). https://www12.statcan.gc.ca/census-recensement/index-eng.cfm
- 22.United Nations Statistics Division. UNdata [Internet]. New York (NY): UNSD; (accessed 2020-09-28) http://data.un.org/en/iso/ca.html
- 23.National case definition: botulism [Internet]. Ottawa (ON): Government of Canada; (modified 2019-11-26; accessed 2020-12-22). https://www.canada.ca/en/public-health/services/diseases/botulism/professionals/national-case-definition.html
- 24.Austin J, Sanders G. HPB methods for the microbiological analysis of foods, Volume 2: detection of Clostridium botulinum and its toxins in suspect foods and clinical specimens. 2009 (modified 2018-05-09; accessed 2020-12-22). https://www.canada.ca/en/health-canada/services/food-nutrition/research-programs-analytical-methods/analytical-methods/compendium-methods/methods-microbiological-analysis-foods-compendium-analytical-methods.html
- 25.Canadian Institute for Health Information. Inpatient hospitalizations and average length of stay trends in Canada, 2003–2004 and 2004–2005. Analysis in brief. Ottawa (ON): CIHI; 2005. https://secure.cihi.ca/free_products/hmdb_analysis_in_brief_e.pdf
- 26.Koepke R, Sobel J, Arnon SS. Global occurrence of infant botulism, 1976-2006. Pediatrics 2008;122(1):e73–82. 10.1542/peds.2007-1827 [DOI] [PubMed] [Google Scholar]
- 27.Leong C, Haszard JJ, Lawley B, Otal A, Taylor RW, Szymlek-Gay EA, Fleming EA, Daniels L, Fangupo LJ, Tannock GW, Heath AM. Mediation analysis as a means of identifying dietary components that differentially affect the fecal microbiota of infants weaned by modified baby-led and traditional approaches. Appl Environ Microbiol 2018;84(18):e00914–8. 10.1128/AEM.00914-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Panditrao MV, Dabritz HA, Kazerouni NN, Damus KH, Meissinger JK, Arnon SS. Seven-year case-control study in California of risk factors for infant botulism. J Pediatr 2020;227:258–267.e8. 10.1016/j.jpeds.2020.07.014 [DOI] [PubMed] [Google Scholar]
- 29.Arnon SS, Damus K, Thompson B, Midura TF, Chin J. Protective role of human milk against sudden death from infant botulism. J Pediatr 1982;100(4):568–73. 10.1016/S0022-3476(82)80754-3 [DOI] [PubMed] [Google Scholar]
- 30.Spika JS, Shaffer N, Hargrett-Bean N, Collin S, MacDonald KL, Blake PA. Risk factors for infant botulism in the United States. Am J Dis Child 1989;143(7):828–32. 10.1001/archpedi.1989.02150190078026 [DOI] [PubMed] [Google Scholar]
- 31.Lúquez C, Dykes JK, Yu PA, Raphael BH, Maslanka SE. First report worldwide of an infant botulism case due to Clostridium botulinum type E. J Clin Microbiol 2010;48(1):326–8. 10.1128/JCM.01420-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith LD. The occurrence of Clostridium botulinum and Clostridium tetani in the soil of the United States. Health Lab Sci 1978;15(2):74–80. [PubMed] [Google Scholar]
- 33.Nevas M, Lindström M, Virtanen A, Hielm S, Kuusi M, Arnon SS, Vuori E, Korkeala H. Infant botulism acquired from household dust presenting as sudden infant death syndrome. J Clin Microbiol 2005;43(1):511–3. 10.1128/JCM.43.1.511-513.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Midura TF. Update: infant botulism. Clin Microbiol Rev 1996;9(2):119–25. 10.1128/CMR.9.2.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jackson KA, Mahon BE, Copeland J, Fagan RP. Botulism mortality in the USA, 1975-2009. Botulinum J 2015;3(1):6–17. 10.1504/TBJ.2015.078132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Panditrao MV, Dabritz HA, Kazerouni NN, Damus KH, Meissinger JK, Arnon SS. Descriptive epidemiology of infant botulism in California: the first 40 years. J Pediatr 2020;227:247–257.e3. 10.1016/j.jpeds.2020.08.013 [DOI] [PubMed] [Google Scholar]
- 37.Health Canada. Botulism – Guide for healthcare professionals [Internet]. Ottawa (ON): Health Canada; 2020 (accessed 2020-12-22). https://www.canada.ca/en/health-canada/services/food-nutrition/legislation-guidelines/guidance-documents/botulism-guide-healthcare-professionals-2012.html


