INTRODUCTION
Cardiovascular (CV) disease complicates an estimated 1% to 4% of all pregnancies and is the leading cause of death in pregnant and postpartum women in the United States.1,2 Valvular heart disease is a common cause of CV disease that affects women of childbearing age.3,4 Congenital heart disease is the leading cause of valvular heart disease in the United States; however, rheumatic heart disease is a prevalent condition especially among immigrant populations.5,6 Most women with valvular heart disease will do well during pregnancy, but high-risk conditions such as severe mitral stenosis (MS) or aortic stenosis (AS), can be associated with significant maternal morbidity and mortality. Management of anticoagulation of pregnant women with mechanical heart valves presents unique challenges to reduce the risk of maternal and fetal complications. Women with valvular heart disease who are pregnant or considering pregnancy should be managed by a multidisciplinary Pregnancy Heart Team consisting of cardiologists and high-risk obstetricians.
Hemodynamic changes start early in pregnancy. Cardiac output increases 30% to 50% and peaks between the second and third trimesters.7,8 Changes in cardiac output are driven by an increase in stroke volume in the first half of pregnancy followed by a gradual rise in heart rate. As a result of placental maturation, systemic vascular resistance and blood pressure decrease in the first and second trimesters and returns to pre-pregnancy levels in the third trimester. Women with valvular heart disease, especially left-sided obstructive lesions, may have limited cardiac reserve to accommodate these hemodynamic changes. As a result, close serial monitoring during pregnancy is necessary to assess for clinical decompensation. The changes in flow can lead to increases in mitral and aortic transvalvular gradients and an overestimation of lesion severity. Direct valve planimetry for patients with AS or MS may more accurately reflect the degree of valve stenosis, especially for patients newly diagnosed during pregnancy. The hypercoagulable state of pregnancy increases the risk of thromboembolic events during pregnancy and the first 6 to 12 weeks postpartum, further complicating the anticoagulation management of women with mechanical valves.9
Labor and delivery is associated with sudden hemodynamic changes and increases in oxygen consumption. After delivery, dramatic changes in hemodynamics occur as a result of autotransfusion of uterine blood volume, relief of caval pressure, and mobilization of dependent edema. The sudden increase in preload can lead to clinical decompensation and women with high-risk lesions will need to be followed closely immediately after delivery and in the subsequent days post-delivery.
PRECONCEPTION COUNSELING
Reproductive age women with valvular heart disease should undergo counseling before conception by a collaborative Pregnancy Heart Team consisting of a maternal fetal medicine (MFM) specialist and cardiologist with experience in caring for pregnant women with heart disease.10 The goal of preconception counseling is to review and individualize the maternal and fetal risk of pregnancy. Baseline cardiac function should be assessed with an electrocardiogram and echocardiogram to start. Exercise stress testing can be an important tool to assess exercise capacity, development of arrhythmias and symptomatic response, which may guide risk stratification and treatment before conception. Additional imaging modalities such as cardiac MRI or computed tomography may be used to further assess valvular function, anatomy of structures not well seen by echocardiogram, and associated aortopathies.
For women planning pregnancy, medications should be reviewed for safety during pregnancy. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are teratogenic and can be changed to medications with a better safety profile during pregnancy. Bosentan and statins are also considered teratogenic and should be stopped before pregnancy. For women with mechanical valves taking warfarin, shared decision making will help guide the appropriate choice of anticoagulation in the first trimester. Beta blockers are generally considered safe in pregnancy. Women may frequently present during pregnancy with newly diagnosed or newly symptomatic valvular heart disease and collaborative care between MFM, cardiology, anesthesia, and other specialists is needed to reduce ongoing maternal, obstetric, and fetal risk.
RISK ASSESSMENT
The most common maternal complications of valvular heart disease during pregnancy are heart failure, arrhythmias, and thromboembolic complications. Postpartum hemorrhage can be a common complication for women on anticoagulation. Cardiac symptoms can be managed in many women with diuresis, medical therapy, and reducing level of physical activity. If symptoms are refractory to conservative management, valve intervention during pregnancy may be necessary. Percutaneous balloon valvuloplasty performed by experienced operators is preferred for stenotic lesions. Ideally these interventions should be performed after the fourth month in the second trimester to minimize radiation exposure during organogenesis.6 Valve surgery with cardiopulmonary bypass performed during pregnancy is associated with rates of fetal death up to 30%, especially when surgery is emergent and/or performed at early gestational age.11,12 If surgery is needed, however, the second trimester is the preferred time frame with use of high flow on cardiopulmonary bypass to provide adequate placental perfusion.13
Maternal cardiac risk can be estimated using the lesion specific modified World Health Organization (WHO) classification (Table 1).6 Women with severe MS and severe symptomatic AS are considered to be at extremely high risk of maternal morbidity or mortality (WHO IV) and pregnancy is contraindicated. Most other types of valvular heart disease in pregnancy are considered to be moderate to high risk (WHO II-III). Those with regurgitant lesions such as aortic regurgitation and mitral regurgitation usually tolerate pregnancy well due to the decreased systemic afterload during pregnancy. Individualized risk can be further estimated using pregnancy-specific risk indices developed in large cohorts, including the CARPREG II and the ZAHARA models.4,14,15 Contraception should be discussed with all women with valvular heart disease but highly effective contraception should be particularly recommended for women at high risk of pregnancy complications. Estrogen-containing contraception increases the risk of venous and arterial thrombosis and hypertension and should be avoided in women with cardiac disease, especially those at increased thrombotic risk. In such patients, long-acting progesterone-only methods are recommended, such as an intrauterine device or subdermal implants.16
Table 1.
Modified World Health Organization (WHO) classification of pregnancy risk
| Maternal Risk | |
|---|---|
| WHO I Mild pulmonary stenosis Small patents ductus arteriosus (PDA) Mitral valve prolapse with mild mitral regurgitation Repaired simple lesions: ASD, VSD, PDA, anomalous pulmonary venous drainage Isolated atrial or ventricular ectopic beats |
• Morbidity: little to no increased risk • Mortality: no increased risk |
| WHO II Uncorrected ASD or VSD Repaired Tetralogy of Fallot Most arrhythmias |
• Morbidity: moderately increased risk • Mortality: mildly increased risk |
| WHO II-III Mild LV impairment (EF >45%) Hypertrophic cardiomyopathy Valvular heart disease not considered WHO I or IV Marfan syndrome, aorta <40 mm Bicuspid aortic valve, aorta <45 mm Repaired aortic coarctation |
Risk varies based on individual patient • Morbidity: moderately to severely increased risk • Mortality: intermediate increased risk |
| WHO III Mechanical valve Moderate LV dysfunction (EF 30%–45%) PPCM with recovered LV function(EF ≥ 50%) Systemic right ventricle Fontan circulation Unrepaired Tetralogy of Fallot Marfan syndrome, aorta 40–45 mm Bicuspid aortic valve, aorta 45–50 mm Unrepaired cyanotic heart disease Other complex congenital heart disease |
• Morbidity: severely increased risk • Mortality: significantly increased risk |
| WHO IV Severe mitral stenosis Severe symptomatic aortic stenosis Pulmonary arterial hypertension Severe LV dysfunction (EF <30% or NYHA class III-IV) PPCM with persistent LV dysfunction (EF <50%) Uncorrected severe aortic coarctation Marfan syndrome, aorta >45 mm Bicuspid aortic valve, aorta >50 mm |
• Pregnancy is contraindicated due to extremely high risk of maternal mortality or severe maternal morbidity |
Abbreviations: ASD, atrial septal defect; EF, ejection fraction; LV, left ventricle; NYHA, New York Heart Association; PPCM, peripartum cardiomyopathy; VSD, ventricular septal defect.
Adapted from Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J 2018;39(34):3165–3241; with permission.
DELIVERY CONSIDERATIONS
Unless indicated for obstetric indications, a vaginal delivery is preferred for most women with valvular heart disease.1 Vaginal delivery is associated with less blood loss, more rapid recovery, and less thrombogenic and infectious risk. Patients at elevated risk of complications should discuss a delivery plan in consultation with a multidisciplinary team consisting of a MFM specialist, cardiologist, and obstetric anesthesiologist. Women with stable cardiac disease can undergo full-term delivery at 39 weeks of gestation.1 Good pain control with regional anesthesia during vaginal delivery can minimize the catecholamine release associated with sudden increases in heart rate and stroke volume. Epidural is preferred over spinal anesthesia due to lower rates of hypotension. Women with moderate to severe left-sided obstructive lesions may benefit from an assisted second stage of labor using forceps or vacuum, which shortens the time to delivery and minimizes the frequency and intensity of maternal effort with Valsalva maneuver, which transiently drops cardiac output. Cesarean delivery should be considered in women with severe heart failure (New York Heart Association [NYHA] class III-IV), high-risk aortic disease, and severe forms of pulmonary hypertension.6 For women requiring delivery while fully anticoagulated on warfarin, Cesarean delivery should be considered to minimize the risk of fetal intracranial hemorrhage. Telemetry is recommended during labor and delivery and up to 24 hours after delivery in women at risk for developing arrhythmias. Women with severely stenotic or symptomatic valvular disease may require monitoring in a cardiac care or telemetry unit for at least 24 hours after delivery, with close monitoring of hemodynamics and volume status.
SPECIFIC VALVE LESIONS
Mitral Stenosis
MS is the most common valvular lesion managed during pregnancy and its prevalence is more common in areas of the world with a higher burden of rheumatic heart disease.17 In some cases, MS may be congenital and due to a dysplastic valve or as a result of valve stenosis following an earlier intervention during childhood, as may be the case in patients with surgical repair of atrio-ventricular (AV) septal defects. During pregnancy, the physiologic increase in stroke volume and heart rate lead to higher gradients across the stenosed mitral valve and an increase in left atrial pressure. These changes can lead to worsening heart failure symptoms or the development of new symptoms in women who were previously asymptomatic. The hemodynamic changes of pregnancy can also lead to atrial arrhythmias, including atrial fibrillation, which can in turn precipitate pulmonary congestion.18 In up to a quarter of women, pregnancy may be the first time a diagnosis of MS is made.17,19
Maternal outcomes
MS is associated with an increased risk of heart failure and atrial arrhythmias during pregnancy. Women with moderate or severe stenosis (mitral valve area (MVA) <1.5 cm2), baseline maternal NYHA class III or IV, or a history of cardiac complications before pregnancy represent the groups at highest risk of maternal complications.17,18,20 Most complications can be managed medically and rates of mitral valve intervention and maternal mortality are low, especially in North American and European cohorts.
Many women with MS will develop or experience progression of symptoms during pregnancy. Among 44 women with MS representing 46 pregnancies treated in California, 74% advanced ≥ 1 NYHA class during pregnancy.19 Women with moderate or severe MS had high rates of developing heart failure or atrial arrhythmias, whereas women with mild MS had maternal outcomes similar to women without valvular disease (Fig. 1). In a Canadian cohort of 74 women representing 80 pregnancies, 31% developed pulmonary edema and 11% developed arrhythmias, with risk proportional to severity of stenosis.18 Heart failure was managed medically in both cohorts and no maternal deaths were reported.
Fig. 1.
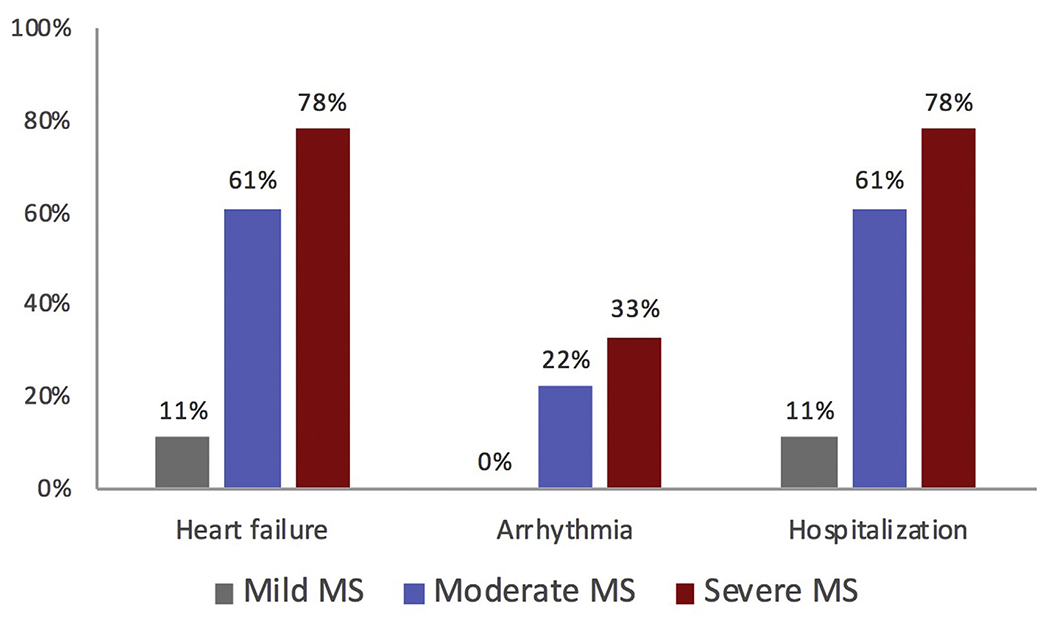
Cardiac complication of MS according to severity. (Adapted from Hameed A, Karaalp IS, Tummala PP, Wani OR, Canetti M, Akhter MW, Goodwin M, Zapadinsky N, Elkayam U. The effect of valvular heart disease on maternal and fetal outcome of pregnancy. J Am Coll Cardiol. 2001;37:893–899; with permission.)
Similar rates of maternal complications were reported among the 273 women with MS participating in the International Registry of Pregnancy and Cardiac Disease (ROPAC).17 In this cohort, 15 patients (5.9%) underwent mitral valve intervention including percutaneous balloon mitral commissurotomy (n = 14) and surgical valve replacement (n = 1). Most interventions occurred in women who were symptomatic before pregnancy. One woman with severe MS died during pregnancy and 2 died postpartum. Women with moderate and severe MS have high rates of preterm delivery and intrauterine growth restriction (IUGR).19
Management
The medical management of women who become symptomatic during pregnancy consists of beta blockers, diuretics, and activity restriction. Beta blockers slow the heart rate, lengthen diastolic filling time, and lower left atrial pressure.21 Beta-1 selective agents, such as metoprolol, are preferred so as to avoid interfering with beta-2 mediated uterine relaxation. Furosemide should be used in patients with pulmonary edema or ongoing symptoms despite beta blockers. Women who develop atrial fibrillation should be anticoagulated, usually with low molecular weight heparin, unfractionated heparin, or warfarin depending on the trimester and clinical context. Anticoagulation should also be considered in women with severe MS and other risk factors for stroke, such as spontaneous echocardiographic contrast in the left atrium, large left atrium (≥60 mL/m2), or congestive heart failure.1 Rate control with beta blockers or digoxin should be used as an initial strategy, though many women will ultimately undergo electrical cardioversion (which is considered safe in pregnancy) due to ongoing symptoms, poor rate control, or hemodynamic instability.
Women who remain severely symptomatic despite adequate medical therapy and activity restriction may need to undergo mitral valve intervention during pregnancy. Percutaneous mitral balloon valvotomy (PMBV) can be safely performed during pregnancy and result in improved valve area and gradients.11,22 Due to risk of ionizing radiation to the fetus, PMBV should be avoided during the first trimester, if possible, and performed by experienced operators. Surgical mitral valve replacement may be considered in women with refractory symptoms who are not candidates for PMBV but is associated with high rates of fetal mortality, estimated at 20% to 30%.11,12
Most women with MS can undergo a vaginal delivery with regional anesthesia, with preference for epidural placement.23 An assisted second stage should be considered for women with moderate to severe stenosis. Cesarean delivery is reserved for obstetric indications and decompensated heart failure. Due to the hemodynamic shifts that occur postpartum, monitoring in a special care unit for at least 24 hours after delivery is recommended.
Careful preconception counseling of women with MS is critical in order to identify severity of stenosis, symptoms, and need for intervention before pregnancy. Similar to non-pregnant patients, the 2014 American Heart Association (AHA)/American College of Cardiology (ACC) Valvular Heart Disease Guidelines recommends PMBV, when feasible, in patients with severe symptomatic MS (Class I recommendation) before pregnancy. In order to avoid clinical decompensation and need for intervention during pregnancy. The AHA/ACC Guidelines also recommend PMBV in patients with severe MS who are asymptomatic (Class I recommendation). The decision to intervene in asymptomatic women before pregnancy should depend on valve area, exercise tolerance, and the presence of pulmonary hypertension, especially among women who are not candidates for PMBV.6,23,24
Aortic Stenosis
AS in pregnancy is most often caused by congenital bicuspid aortic valve and less commonly other congenital abnormalities or rheumatic heart disease.24,25 Pregnancy is well tolerated in women with mild and moderate AS. Women with severe AS are at higher risk of developing cardiac complications, such as heart failure or atrial arrhythmias, however the risk of maternal mortality and need for aortic valve intervention during pregnancy is low. Women with congenital bicuspid valve or Marfan syndrome may have an associated aortopathy which further increases maternal risk and warrants additional monitoring before and during pregnancy. Pregnancy is contraindicated in women with bicuspid aortic valve when aortic dilation is >50 mm and in women with Marfan syndrome when aortic dilation is >45 mm.6
In a Canadian cohort of 39 women representing 49 pregnancies, cardiac complications, including heart failure or arrhythmias, were observed in 10% of women with severe AS. Only 1 woman required aortic valve intervention during pregnancy and no maternal deaths were reported.25 Other series have reported that heart failure occurs in 3.8% to 44% of patients, with the highest rate observed in the smallest (n = 12) cohort.19,26,27 Maternal complications are associated with severity of AS, especially when symptomatic, and maternal age >30 years.26,27 Maternal mortality in contemporary cohorts and need for valvular intervention during pregnancy is low. Valve deterioration and need for aortic valve intervention may be higher in women with severe AS after pregnancy, although the causes for this are not well-understood.25,26 Women with severe AS experience higher rates of preterm delivery, low birth weight, and fetal death.19,26
Management
Women who become symptomatic should be managed with activity restriction. Diuretics should be carefully used in women who develop pulmonary edema so as to avoid a sudden drop in preload. Women who remain symptomatic despite conservative management may need valvular intervention during pregnancy with a preference for percutaneous aortic balloon valvuloplasty if the valve anatomy is favorable and an experienced team is available. Percutaneous transcatheter aortic valve replacement for bicuspid severe AS has been successfully performed during pregnancy, and may be preferred over valvuloplasty if significant aortic regurgitation is present.28 Women who develop severe symptoms early in pregnancy may consider pregnancy termination. Similar to patients with MS, vaginal delivery is the preferred mode of delivery with an assisted second stage for women with moderate to severe stenosis, though Cesarean delivery may be considered for patients with severe symptoms.6,24 Regional anesthesia with an epidural is preferred for pain control but hemodynamics should be monitored closely to avoid a sudden drop in preload and systemic vascular resistance, which may poorly tolerated.
MITRAL REGURGITATION AND AORTIC REGURGITATION
The most common causes of mitral regurgitation (MR) during pregnancy are rheumatic heart disease and mitral valve prolapse. Patients with previously repaired (or unrepaired) AV septal defects may also have significant left-sided AV valve regurgitation. In contrast, aortic regurgitation (AR) is more commonly associated with congenital bicuspid aortic valve or aortopathy, and less commonly rheumatic heart disease. Both MR and AR are well tolerated during pregnancy, even if severe, due to the fall in systemic vascular resistance and blood pressure. Surgical intervention before pregnancy is reserved for women meeting routine indications for surgery, including severe symptomatic valve disease. Exercise testing before pregnancy can be considered to assess for exercise tolerance and symptoms.29 Women who develop heart failure symptoms or left ventricular dysfunction can be treated with diuretics and vasodilators, such as hydralazine or nitrates, with care to avoid hypotension which can lead to placental hypoperfusion. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are contraindicated during pregnancy.
PULMONIC STENOSIS
Pulmonic stenosis (PS) is most commonly a result of congenital valve disease but may also occur as a result of homograft calcification after a Ross procedure or prosthetic valve stenosis in patients with repaired tetralogy of Fallot. Mild and moderate PS are well tolerated during pregnancy. Severe PS is associated with high rates of hypertensive disorders, such as preeclampsia, preterm delivery, and thromboembolic complications.30 Although severe PS may be well tolerated during pregnancy, some women may experience right ventricular heart failure or arrhythmias. As a result, women with severe PS, even if asymptomatic, should be considered for balloon valvuloplasty, surgical val-votomy, or percutaneous valve replacement before pregnancy.31
PULMONIC REGURGITATION
Pulmonic regurgitation (PR) may be secondary to prior tetralogy of Fallot repair, balloon valvuloplasty for isolated PS, or develop in patients with a prior right ventricle to pulmonary artery conduit. PR is generally well tolerated during pregnancy. Similar to the systemic vascular resistance, pulmonary vascular resistance also decreases during pregnancy. However, the increased plasma volume and CO associated with pregnancy can lead to right-sided heart failure symptoms in women with severe PR, especially in the presence of underlying right ventricular (RV) dysfunction, RV hypertrophy, or additional obstructive lesions such as branch pulmonary artery stenosis.32,33 Right-sided heart failure can often be treated with diuretics and activity restriction. Valve intervention is rarely needed during pregnancy.6 In women with severe PR before pregnancy who are symptomatic or have progressive RV dilatation or dysfunction, pulmonary valve replacement is recommended.31
TRICUSPID REGURGITATION
Isolated tricuspid regurgitation (TR) in young women is uncommon and, when present, occurs in the setting of Ebstein anomaly, rheumatic heart disease, or endocarditis. Patients with AV septal defects commonly have right-sided AV valve regurgitation. The hemodynamic changes of pregnancy are usually well tolerated in women with TR, even if severe. Ebstein anomaly is associated with atrial septal defect and Wolff-Parkinson-White syndrome. As a result, pregnancy may be associated with progressive cyanosis and/or arrhythmias in women at risk.34 Ebstein anomaly is also associated with increased risk of preterm delivery.35 Secondary TR can occur as a result of RV pressure or volume overload as a result of left-sided heart disease and pulmonary hypertension, cardiac conditions associated with significantly elevated maternal risk during pregnancy.
PROSTHETIC VALVES
Pregnancy is a prothrombotic state and is associated with an increased risk of valve thrombosis in women with prosthetic heart valves. Pregnant women with mechanical heart valves require careful anticoagulation management to prevent severe maternal morbidity while minimizing anticoagulation-related risk to the fetus. Although hypercoagulability risk increases throughout pregnancy and peaks in the immediate postpartum period, valve thrombosis frequently occurs in the first trimester and may be related to sub-therapeutic anticoagulation, underscoring the importance of preconception counseling.36,37
Anticoagulation
Warfarin is the standard of care for mechanical valves in non-pregnant patients to prevent thromboembolic complications. However, warfarin crosses the placenta and is associated with an embryopathy, consisting of nasal hypoplasia, stippled epiphyses, and choanal atresia, when exposure occurs between 6 and 12 weeks of gestation.37 Later exposure is associated with central nervous system abnormalities and intracranial hemorrhage. The most common fetal adverse even is miscarriage and fetal demise can occur at any gestational age.
Warfarin has a dose-dependent effect on fetal outcomes with the highest risk associated with daily warfarin doses >5 mg,38 though lower risk with lower doses has not been demonstrated in all studies.36 In a 2017 meta-analysis, the rate of livebirths among women taking ≤5 mg compared with >5 mg of warfarin daily was 83.6% versus 43.9%, respectively.39 The rate of embryopathy/fetopathy was 2.3% with lower dose (≤5 mg) and 12.4% with higher dose (>5 mg) of warfarin. Women treated with low molecular weight heparin (LMWH) alone during pregnancy had the highest rate of livebirth at 92%.
LMWH does not cross the placenta and is therefore not associated with congenital malformations. Weight-based dosing is administered twice daily and cleared by the kidneys. Dose adjustment in response to peak anti-Xa levels is needed due to changes in renal clearance and volume of distribution over the course of pregnancy.40 In contemporary studies, dose-adjusted LMWH is still associated with thromboembolic complication in 4% to 17% of pregnancies.39,41,42
Thromboembolic complications occur throughout pregnancy and may be related to sub-therapeutic anticoagulation during transition of anticoagulants, especially in the first trimester, or sub-therapeutic LMWH levels. Fixed dose LMWH is associated with significantly higher thromboembolic complications compared with dose-adjusted regimens.43 The measurement of peak anti-Xa levels may not sufficiently assure adequate anticoagulation. Among pregnant women with peak anti-Xa levels within the recommended range of 0.8 to 1.2 U/mL, 57% had sub-therapeutic trough levels (<0.6 U/ml).44 Low trough levels were still observed among women with peak anti-Xa levels at the upper range of 1.0 to 1.2 U/ml. Several small series have demonstrated favorable thromboembolic outcomes among women treated with close monitoring of both peak and trough anti-Xa levels, with peak levels targeted to 1 to 1.2 U/mL.37
Comparing Anticoagulation Strategies
Four anticoagulation strategies were compared in a meta-analysis of contemporary studies representing 800 pregnancies between 1974 and 2014.42 Studies were excluded if fixed dose LMWH or unfractionated heparin (UFH) were used or if ball-in-cage valves were present in greater than 10% of reported pregnancies. Maternal risk was lowest in women using vitamin K antagonist (VKA) throughout pregnancy and 3-times-higher in women using alternative strategies, see Table 2. Maternal deaths were rare and adverse events were driven by systemic thromboembolism or valve thrombosis. Fetal risk was lowest in women using LMWH throughout pregnancy or LMWH plus VKA. Differences in fetal outcomes were driven by spontaneous abortions; congenital defects were uncommon. Women taking low-dose VKA throughout pregnancy had similar fetal outcomes compared with women taking LMWH or LMWH plus VKA. A similar metaanalysis (see Table 2) demonstrated that women treated with VKA throughout pregnancy had the lowest proportion of livebirths compared with women treated with LMWH (64.5% vs 92%) but had a lower risk of thromboembolic complications (2.7% vs 8.7).39
Table 2.
Comparison of maternal and fetal risk with different anticoagulation strategies among women with mechanical valves
| Steinberg et al42 |
D’Souza et al39 |
|||
|---|---|---|---|---|
| Maternal risk,a % | Fetal risk,b % | Maternal TE event,c % | Livebirths, % | |
| VKA only | 5 | 39 | 2.7 | 64.5 |
| Low-dose VKA only | 5 | 15 | 83.6 | |
| LMWH + VKA | 16 | 16 | 8.3 | 89.5 |
| UFH + VKA | 16 | 34 | 6.1 | 72.4 |
| LMWH only | 15 | 14 | 8.7 | 92.0 |
Abbreviations: LMWH, low molecular weight heparin; TE, thromboembolic; UFH, unfractionated heparin; VKA, vitamin K antagonist.
Maternal death, systemic TE, or valve failure resulting in heart failure, arrhythmia, or surgery.
Spontaneous abortion, fetal death, or congenital defect.
Valve thrombus or extravalvular TE event.
Data from D’Souza R, Ostro J, Shah PS, Silversides CK, Malinowski A, Murphy KE, Sermer M, Shehata N. Anticoagulation for pregnant women with mechanical heart valves: a systematic review and meta-analysis. Eur Heart J. 2017;38:1509–1516 and Steinberg ZL, Dominguez-Islas CP, Otto CM, Stout KK, Krieger EV. Maternal and Fetal Outcomes of Anticoagulation in Pregnant Women With Mechanical Heart Valves. J Am Coll Cardiol. 2017;69:2681–2691.
Management
Women with bioprosthetic and mechanical valves should be treated with a baby aspirin during the second and third trimesters. For women with mechanical valves, warfarin continued throughout pregnancy offers the lowest risk of maternal thromboembolic complications but carries a higher risk of miscarriage and embryopathy, as described previously. The 2014 ACC/AHA Valvular Heart Disease Guidelines and the 2018 ESC Pregnancy and Heart Disease Guidelines recommend continuing warfarin at doses ≤5 mg/d during the first trimester and transitioning to dose-adjusted LMWH or intravenous (IV) UFH when the daily dose is >5 mg/d, as summarized in Table 3.6,45 Regardless of anticoagulant choice in the first trimester, treatment with warfarin is usually recommended in the 2nd and 3rd trimesters. Discontinuation of warfarin and starting IV UFH before planned vaginal delivery is recommended. Women who are therapeutically anticoagulated on warfarin and need to be delivered should undergo Cesarean delivery to minimize traumatic fetal hemorrhagic.
Table 3.
Recommendations regarding anticoagulation strategy for mechanical valves during pregnancy
| 1st Trimester | 2nd and 3rd Trimesters | Peripartum | |
|---|---|---|---|
| AHA/ACC guidelines45 | |||
| Warfarin dose ≤5 mg | Warfarin (IIa) or LMWH (IIb) or IV UFH (IIb) |
Warfarin (I) | IV UFH (I) |
| Warfarin dose >5 mg | LMWH (IIa) or IV UFH (IIa) |
Warfarin (I) | IV UFH (I) |
| •Aspirin is routinely recommended starting in 2nd trimester •Target anti-Xa peak level: 0.8–1.2 U/ml 4–6 h post-dose (I) | |||
| ESC guidelines6 | |||
| Warfarin dose ≤5 mg | Warfarin (IIa) or LMWH (IIb) or IV UFH (IIb) |
Warfarin (I) | IV UFH (I) |
| Warfarin dose >5 mg | Warfarin (IIb) or LMWH (IIa) or IV UFH (IIa) |
Warfarin (IIa) or LMWH (IIb) |
IV UFH (I) |
| •Aspirin is not routinely recommend •Target anti-Xa peak level: 1.0–1.2 U/mL (mitral and right-sided valves) or 0.8–1.2 U/mL (aortic valves) 4–6 h post-dose (I). Target anti-Xa trough level: >0.6 U/mL (IIb) | |||
Both LMWH and IV UFH refer to dose-adjusted rather than fixed dosing.
Abbreviations: AHA/ACC, American Heart Association/American College of Cardiology; IV UFH, intravenous unfractionated heparin; LMWH, low molecular weight heparin.
Data from Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39:3165–3241 and Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:e57–e185.
The AHA/ACC guidelines recommend targeting a peak anti-Xa level of 0.8 to 1.2 U/mL 4 to 6 hours after dosing for women treated with LMWH during pregnancy. Given the higher risk of thromboembolic complications in women with sub-therapeutic anticoagulation, aiming for peak levels in the 1.0 to 1.2 U/mL range with trough levels greater than 0.6 U/mL may be reasonable, and is recommended in the 2018 ESC pregnancy and heart disease guidelines (see Table 3).6 Because the safety profile of low-dose warfarin is based on a small number of studies and the risk of fetal loss is present throughout pregnancy, even at lower warfarin doses, some investigators advocate for using LMWH throughout pregnancy with closely monitored anti-Xa levels.46 Favorable clinical outcomes have been demonstrated in women treated with this strategy, but high levels of medication adherence and patient engagement are needed. This strategy may be desirable for women who are at otherwise low risk of thromboembolic complications (eg, mechanical valve in aortic position) or women who place higher value on avoiding potential fetal risk than maternal complications.
Valve thrombosis during pregnancy should be confirmed with transesophageal echocardiogram and treated first with heparin and, if needed, thrombolytic therapy for women with small thrombus and mild symptoms. Tissue-type plasminogen activator is associated with hemorrhagic complications but has been successfully used in pregnant women.47
Women presenting with large thrombus burden and more severe symptoms may require emergent surgery, which is associated with adverse maternal and fetal outcomes.
Choosing Prosthetic Valve Type Before Pregnancy
Mechanical heart valves offer superior hemodynamic profile and durability compared with bioprosthetic valves. Younger age at bioprosthetic valve implantation is associated with accelerated valve degeneration, which further shortens durability in women of reproductive age.48 Preconception counseling regarding valve choice and implications for maternal and fetal risk in future pregnancies, especially for mechanical valves, is critically important and should be performed by a cardiologist familiar with treating pregnant patients with heart disease.
KEY POINTS.
Pregnancy is well tolerated in most women with valvular heart disease. Cardiac output increases up to 50% and can lead to clinical decompensation in high-risk women.
Women with severe mitral stenosis and symptomatic severe aortic stenosis are at high risk of poor outcomes and should be evaluated for valvular intervention before conception.
Women with mechanical heart valves need careful management of anticoagulation during pregnancy to minimize maternal and fetal risks.
Vaginal delivery with epidural anesthesia is recommended for most women with stable valvular heart disease.
All women with valvular heart disease should be managed by a multidisciplinary Pregnancy Heart Team before and during pregnancy.
SUMMARY.
Pregnancy in the setting of mild to moderate valvular heart disease is often well tolerated. Patients with severe mitral or severe symptomatic AS are at increased risk of severe maternal morbidity and mortality and pregnancy may be prohibitively high risk unless valve intervention is performed. Care by a multidisciplinary Pregnancy Heart Team consisting of MFM specialists and cardiologists can improve preconception counseling and coordinated pregnancy and postpartum care to minimize maternal and fetal complications.
Footnotes
DISCLOSURE
The authors have nothing to disclose.
REFERENCES
- 1.ACOG practice bulletin No. 212: pregnancy and heart disease. Obstet Gynecol 2019;133:e320–56. [DOI] [PubMed] [Google Scholar]
- 2.Petersen EE, Davis NL, Goodman D, et al. Vital Signs: pregnancy-related deaths, United States, 2011-2015, and strategies for prevention, 13 states, 2013-2017. MMWR Morb Mortal Wkly Rep 2019;68: 423–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roos-Hesselink J, Baris L, Johnson M, et al. Pregnancy outcomes in women with cardiovascular disease: evolving trends over 10 years in the ESC Registry of Pregnancy and Cardiac disease (RO-PAC). Eur Heart J 2019;40:3848–55. [DOI] [PubMed] [Google Scholar]
- 4.Siu SC, Sermer M, Colman JM, et al. , Investigators on behalf of the CD in P (CARPREG). Prospective multicenter study of pregnancy outcomes in women with heart disease. Circulation 2001. Available at: 10.1161/hc3001.093437. [DOI] [PubMed] [Google Scholar]
- 5.Nanna M, Stergiopoulos K. Pregnancy complicated by valvular heart disease: an update. J Am Heart Assoc 2014;3:e000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J 2018;39:3165–241. [DOI] [PubMed] [Google Scholar]
- 7.Sanghavi M, Rutherford JD. Cardiovascular physiology of pregnancy. Circulation 2014;130:1003–8. [DOI] [PubMed] [Google Scholar]
- 8.Robson SC, Hunter S, Boys RJ, et al. Serial study of factors influencing changes in cardiac output during human pregnancy. Am J Physiol 1989;256:H1060–5. [DOI] [PubMed] [Google Scholar]
- 9.Kamel H, Navi BB, Sriram N, et al. Risk of a thrombotic event after the 6-week postpartum period. N Engl J Med 2014;370:1307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta LS, Warnes CA, Bradley E, et al. American Heart Association Council on Clinical Cardiology; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; and Stroke Council. Cardiovascular Considerations in caring for pregnant patients: A Scientific Statement from the American Heart Association. Circulation 2020;141:e884–903. [DOI] [PubMed] [Google Scholar]
- 11.de Souza JA, Martinez EE, Ambrose JA, et al. Percutaneous balloon mitral valvuloplasty in comparison with open mitral valve commissurotomy for mitral stenosis during pregnancy. J Am Coll Cardiol 2001;37:900–3. [DOI] [PubMed] [Google Scholar]
- 12.John AS, Gurley F, Schaff HV, et al. Cardiopulmonary bypass during pregnancy. Ann Thorac Surg 2011;91:1191–6. [DOI] [PubMed] [Google Scholar]
- 13.Canobbio Mary M, Warnes Carole A, Aboulhosn J, et al. Management of pregnancy in patients with complex congenital heart disease: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2017;135:e50–87. [DOI] [PubMed] [Google Scholar]
- 14.Silversides CK, Grewal J, Mason J, et al. Pregnancy outcomes in women with heart disease: the CARPREG II study. J Am Coll Cardiol 2018; 71:2419–30. [DOI] [PubMed] [Google Scholar]
- 15.Drenthen W, Boersma E, Balci A, et al. Predictors of pregnancy complications in women with congenital heart disease. Eur Heart J 2010;31: 2124–32. [DOI] [PubMed] [Google Scholar]
- 16.Roos-Hesselink JW, Cornette J, Sliwa K, et al. Contraception and cardiovascular disease. Eur Heart J 2015;36:1728–34. [DOI] [PubMed] [Google Scholar]
- 17.van Hagen IM, Thorne SA, Taha N, et al. , ROPAC Investigators and EORP Team. Pregnancy outcomes in women with rheumatic mitral valve disease: results from the Registry of pregnancy and cardiac disease. Circulation 2018;137:806–16. [DOI] [PubMed] [Google Scholar]
- 18.Silversides CK, Colman JM, Sermer M, et al. Cardiac risk in pregnant women with rheumatic mitral stenosis. Am J Cardiol 2003;91:1382–5. [DOI] [PubMed] [Google Scholar]
- 19.Hameed A, Karaalp IS, Tummala PP, et al. The effect of valvular heart disease on maternal and fetal outcome of pregnancy. J Am Coll Cardiol 2001;37:893–9. [DOI] [PubMed] [Google Scholar]
- 20.Leśniak-Sobelga A, Tracz W, KostKiewicz M, et al. Clinical and echocardiographic assessment of pregnant women with valvular heart diseases–maternal and fetal outcome. Int J Cardiol 2004;94:15–23. [DOI] [PubMed] [Google Scholar]
- 21.al Kasab SM, Sabag T, al Zaibag M, et al. Beta-adrenergic receptor blockade in the management of pregnant women with mitral stenosis. Am J Obstet Gynecol 1990;163:37–40. [DOI] [PubMed] [Google Scholar]
- 22.Joshi HS, Deshmukh JK, Prajapati JS, et al. Study of effectiveness and safety of percutaneous balloon mitral valvulotomy for treatment of pregnant patients with severe mitral stenosis. J Clin Diagn Res 2015;9:OC14–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elkayam U, Goland S, Pieper PG, et al. High-risk cardiac disease in pregnancy. J Am Coll Cardiol 2016;68:396–410. [DOI] [PubMed] [Google Scholar]
- 24.Elkayam U, Bitar F. Valvular heart disease and pregnancy: Part I: native valves. J Am Coll Cardiol 2005;46:223–30. [DOI] [PubMed] [Google Scholar]
- 25.Silversides CK, Colman JM, Sermer M, et al. Early and intermediate-term outcomes of pregnancy with congenital aortic stenosis. Am J Cardiol 2003;91:1386–9. [DOI] [PubMed] [Google Scholar]
- 26.Yap S-C, Drenthen W, Pieper PG, et al. , ZAHARA Investigators. Risk of complications during pregnancy in women with congenital aortic stenosis. Int J Cardiol 2008;126:240–6. [DOI] [PubMed] [Google Scholar]
- 27.Orwat S, Diller G-P, van Hagen IM, et al. Risk of pregnancy in moderate and severe aortic stenosis. J Am Coll Cardiol 2016;68:1727–37. [DOI] [PubMed] [Google Scholar]
- 28.Hodson R, Kirker E, Swanson J, et al. Transcatheter aortic valve replacement during pregnancy. Circ Cardiovasc Interv 2016;9:e004006. [DOI] [PubMed] [Google Scholar]
- 29.2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary. :53. [Google Scholar]
- 30.Drenthen W, Pieper PG, Roos-Hesselink JW, et al. , ZAHARA Investigators. Non-cardiac complications during pregnancy in women with isolated congenital pulmonary valvar stenosis. Heart 2006;92:1838–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stout KK, Daniels CJ, Aboulhosn JA, et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: a Report of the American College of Cardiology/American Heart Association Task Force on clinical Practice guidelines. Circulation 2019;139:e698–800. [DOI] [PubMed] [Google Scholar]
- 32.Greutmann M, Von Klemperer K, Brooks R, et al. Pregnancy outcome in women with congenital heart disease and residual haemodynamic lesions of the right ventricular outflow tract. Eur Heart J 2010;31:1764–70. [DOI] [PubMed] [Google Scholar]
- 33.Khairy P, Ouyang DW, Fernandes SM, et al. Pregnancy outcomes in women with congenital heart disease. Circulation 2006;113:517–24. [DOI] [PubMed] [Google Scholar]
- 34.Connolly HM, Warnes CA. Ebstein’s anomaly: outcome of pregnancy. J Am Coll Cardiol 1994;23:1194–8. [DOI] [PubMed] [Google Scholar]
- 35.Lima F, Nie L, Yang J, et al. Postpartum cardiovascular outcomes among women with heart disease from A Nationwide study. Am J Cardiol 2019;123: 2006–14. [DOI] [PubMed] [Google Scholar]
- 36.van Hagen IM, Roos-Hesselink JW, Ruys TPE, et al. , ROPAC Investigators and the EURObservational Research Programme (EORP) Team*. Pregnancy in women with a mechanical heart valve: data of the European Society of Cardiology Registry of Pregnancy and Cardiac Disease (ROPAC). Circulation 2015;132:132–42. [DOI] [PubMed] [Google Scholar]
- 37.Alshawabkeh L, Economy KE, Valente AM. Anticoagulation during pregnancy. J Am Coll Cardiol 2016; 68:1804–13. [DOI] [PubMed] [Google Scholar]
- 38.Vitale N, De Feo M, De Santo LS, et al. Dose-dependent fetal complications of warfarin in pregnant women with mechanical heart valves. J Am Coll Cardiol 1999;33:1637–41. [DOI] [PubMed] [Google Scholar]
- 39.D’Souza R, Ostro J, Shah PS, et al. Anticoagulation for pregnant women with mechanical heart valves: a systematic review and meta-analysis. Eur Heart J 2017;38:1509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quinn J, Von Klemperer K, Brooks R, et al. Use of high intensity adjusted dose low molecular weight heparin in women with mechanical heart valves during pregnancy: a single-center experience. Haematologica 2009;94:1608–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhagra CJ, D’Souza R, Silversides CK. Valvular heart disease and pregnancy part II: management of prosthetic valves. Heart 2017;103:244–52. [DOI] [PubMed] [Google Scholar]
- 42.Steinberg ZL, Dominguez-Islas CP, Otto CM, et al. Maternal and fetal outcomes of anticoagulation in pregnant women with mechanical heart valves. J Am Coll Cardiol 2017;69:2681–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oran B, Lee-Parritz A, Ansell J. Low molecular weight heparin for the prophylaxis of thromboembolism in women with prosthetic mechanical heart valves during pregnancy. Thromb Haemost 2004; 92:747–51. [DOI] [PubMed] [Google Scholar]
- 44.Goland S, Schwartzenberg S, Fan J, et al. Monitoring of anti-Xa in pregnant patients with mechanical prosthetic valves receiving low-molecular-weight heparin: peak or trough levels? J Cardiovasc Pharmacol Ther 2014;19:451–6. [DOI] [PubMed] [Google Scholar]
- 45.Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a Report of the American College of Cardiology/American Heart Association Task Force on Practice guidelines. J Am Coll Cardiol 2014;63:e57–185. [DOI] [PubMed] [Google Scholar]
- 46.Elkayam U Anticoagulation therapy for pregnant women with mechanical prosthetic heart valves: how to improve safety? J Am Coll Cardiol 2017;69:2692–5. [DOI] [PubMed] [Google Scholar]
- 47.Özkan M, Çakal B, Karakoyun S, et al. Thrombolytic therapy for the treatment of prosthetic heart valve thrombosis in pregnancy with low-dose, slow infusion of tissue-type plasminogen activator. Circulation 2013;128:532–40. [DOI] [PubMed] [Google Scholar]
- 48.Pibarot P, Dumesnil JG. Prosthetic heart valves: selection of the optimal prosthesis and long-term management. Circulation 2009;119:1034–48. [DOI] [PubMed] [Google Scholar]


