Supplemental Digital Content is available in the text.
Keywords: COVID-19, COVID-19 vaccines, mRNA vaccine, myocarditis, pericarditis, SARS-CoV-2, vaccination
Abstract
Myocarditis has been recognized as a rare complication of coronavirus disease 2019 (COVID-19) mRNA vaccinations, especially in young adult and adolescent males. According to the US Centers for Disease Control and Prevention, myocarditis/pericarditis rates are ≈12.6 cases per million doses of second-dose mRNA vaccine among individuals 12 to 39 years of age. In reported cases, patients with myocarditis invariably presented with chest pain, usually 2 to 3 days after a second dose of mRNA vaccination, and had elevated cardiac troponin levels. ECG was abnormal with ST elevations in most, and cardiac MRI was suggestive of myocarditis in all tested patients. There was no evidence of acute COVID-19 or other viral infections. In 1 case, a cardiomyopathy gene panel was negative, but autoantibody levels against certain self-antigens and frequency of natural killer cells were increased. Although the mechanisms for development of myocarditis are not clear, molecular mimicry between the spike protein of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and self-antigens, trigger of preexisting dysregulated immune pathways in certain individuals, immune response to mRNA, and activation of immunologic pathways, and dysregulated cytokine expression have been proposed. The reasons for male predominance in myocarditis cases are unknown, but possible explanations relate to sex hormone differences in immune response and myocarditis, and also underdiagnosis of cardiac disease in women. Almost all patients had resolution of symptoms and signs and improvement in diagnostic markers and imaging with or without treatment. Despite rare cases of myocarditis, the benefit-risk assessment for COVID-19 vaccination shows a favorable balance for all age and sex groups; therefore, COVID-19 vaccination is recommended for everyone ≥12 years of age.
There is now increasing evidence for myocarditis and myopericarditis as rare complications of coronavirus disease 2019 (COVID-19) mRNA vaccinations, especially in young adult and adolescent males. Here we provide further details about this phenomenon and its potential underlying mechanisms. We also discuss the balance of risk of myocarditis with vaccination versus cardiac and other risks from COVID-19 viral infection.
Epidemiology and Clinical Presentation of Myocarditis After COVID-19 Vaccination
Historically, postvaccination myocarditis has been reported as a rare adverse event after vaccinations, especially smallpox vaccination, influenza, hepatitis B, or other vaccinations.1 In the general population, myocarditis is diagnosed in approximately 10 to 20 individuals per 100 000 per year,2 and occurs more commonly and at younger ages in males compared with females.3
In the pre–COVID-19 era, among 620 195 reports filed at the Vaccine Adverse Event Reporting System (VAERS) between 1990 and 2018, 0.1% were attributable to myopericarditis.1 Of those myopericarditis reports, 79% were in males.1 However, VAERS is primarily a safety signal detection and hypothesis-generating system and cannot be used to determine if a vaccine caused an adverse event.4 Through this passive reporting, the Centers for Disease Control and Prevention (CDC) and the US Food and Drug Administration conduct postlicensure vaccine safety monitoring.4 This approach is not specific, and most VAERS events are typically not actually linked to vaccinations. Instead, various methods and statistical techniques are used to analyze VAERS data, which the CDC and Food and Drug Administration use to guide further safety evaluations, such as Vaccine Safety Datalink, and inform decisions around vaccine recommendations and regulatory action. Therefore, VAERS data must be interpreted with caution because of the inherent limitations of passive surveillance.4 VAERS is subject to reporting bias, including both under- and overreporting of adverse events or stimulated reporting that might occur in response to intense media attention and increased public awareness.4
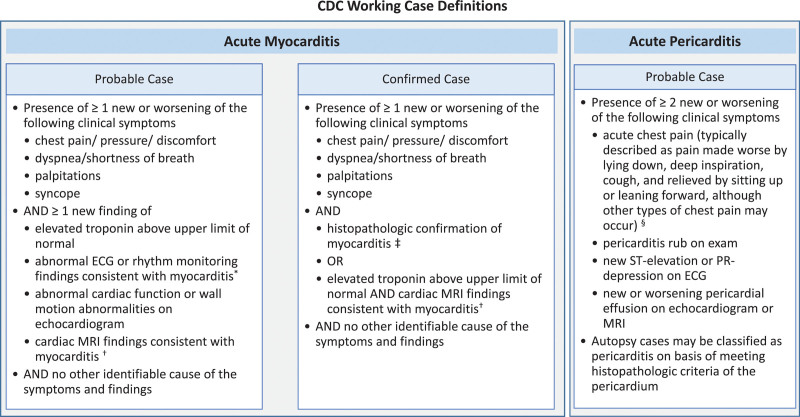
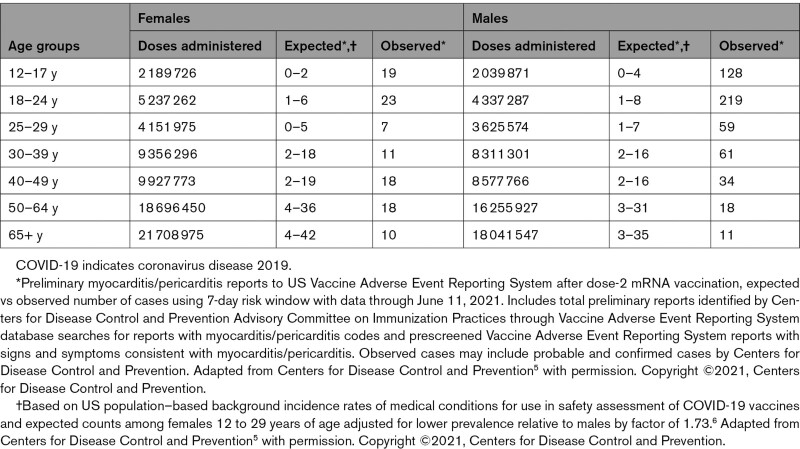
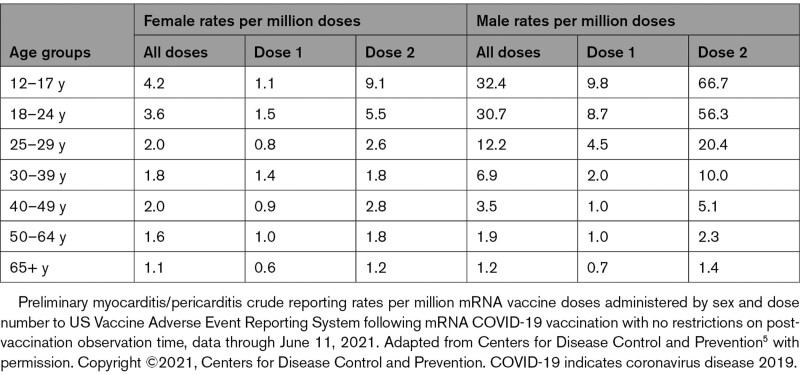
Recently, a CDC Advisory Committee on Immunization Practices identified a likely association between the 2 COVID-19 mRNA vaccines from Pfizer-BioNTech and Moderna and cases of myocarditis and pericarditis.5 Patient reports in VAERS were categorized according to CDC work case definitions as probable myocarditis, confirmed myocarditis, or acute pericarditis5 (Figure 1). According to the Advisory Committee on Immunization Practices, after ≈300 million COVID-19 mRNA vaccine doses administered through June 11, 2021, there were 1226 reports of probable myocarditis/pericarditis cases in VAERS, 67% of which followed the second dose.5 Seventy-nine percent were in males, with the majority in individuals <30 years of age with a median age of 24. Time to onset of symptoms was a median of 3 days, with the highest rate at day 2 after vaccination and among patients 16 to 18 years of age. In 484 probable myocarditis/pericarditis cases among patients ≤29 years of age that were reviewed and characterized by the CDC,5 86% had reports of chest pain on presentation, 61% had reports of ST- or T-wave changes on ECG, 64% had reports of elevated cardiac enzymes, and 17% had reports of abnormal cardiac imaging.5 In 323 of the reports that met the CDC definition of confirmed myocarditis/pericarditis, 96% were hospitalized, but most were discharged with a resolution of symptoms.5 The observed myocarditis/pericarditis reports were higher than expected case rates for males compared with females, and higher at younger ages compared with older ages (Tables 1 and 2).5
Figure 1.
Centers for Disease Control and Prevention working case definitions for acute myocarditis and acute pericarditis. Adapted from Centers for Disease Control and Prevention5 with permission. Copyright ©2021, Centers for Disease Control and Prevention.
Table 1.
Expected Versus Observed Number of Myocarditis/Pericarditis Cases in 7-Day Risk Window After Dose 2 of mRNA Covid-19 Vaccination*
Table 2.
Crude Reporting Rates of Myocarditis/Pericarditis Cases per Million Doses After mRNA COVID-19 Vaccination
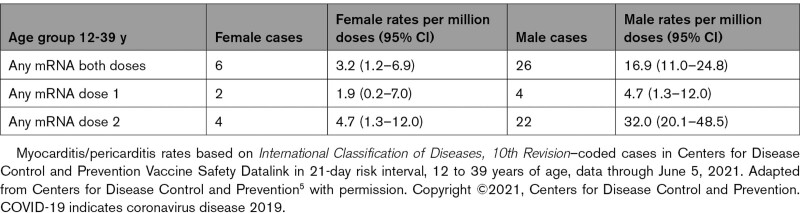
Additional analyses of CDC Vaccine Safety Datalink with data from 9 participating integrated health care organizations revealed an increased risk of myocarditis/pericarditis events among individuals 12 to 39 years of age in the 7-day risk interval after vaccination with mRNA COVID-19 vaccines compared with unvaccinated individuals or individuals vaccinated with non-mRNA COVID-19 vaccines on the same calendar days (rate ratio of 10.8 [95% CI, 3.2–49.0], adjusted for site, age, sex, race/ethnicity, and calendar date).5 The estimated myocarditis/pericarditis chart-confirmed rate was 12.6 cases per million doses with second-dose mRNA vaccine among individuals 12 to 39 years of age.5 The rates based on International Classification of Diseases, 10th Revision–coded cases were also higher in males than in females5 (Table 3). All chart-confirmed cases with follow-up had resolution of symptoms; and among those who had follow-up ECG/echocardiography and laboratory testing, most had returned to normal or baseline.5 On this basis, the Food and Drug Administration will add a warning to the product label of both mRNA vaccines regarding the risk of myocarditis.7
Table 3.
Myocarditis/Pericarditis Rates Based on International Classification of Diseases, 10th Revision Codes
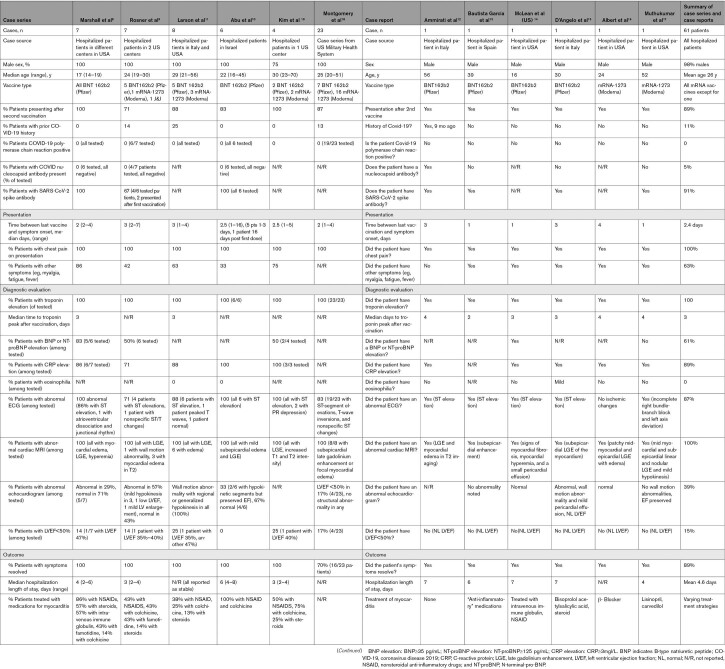
Several myocarditis cases after COVID-19 vaccination have been published in peer-reviewed journals,8–19 with reports predominantly after the second dose of mRNA COVID-19 vaccines (BNT162b2 mRNA-Pfizer-BioNTech and the mRNA-1273-Moderna; Table 4). Patients in these reports invariably presented with chest pain, usually 2 to 3 days after a second dose of mRNA vaccination, some preceded with fever and myalgia 1 day after vaccination. These were predominantly young males requiring hospitalization for myocarditis and without a history of COVID-19 or comorbidities. All tested negative for current COVID-19 by polymerase chain reaction testing. A majority had spike antibody levels for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) suggesting effective immunization. All had elevated cardiac troponin, the highest level peaking usually 3 days after vaccination (Table 4). ECG was abnormal with ST elevations in most presentations. An echocardiogram was abnormal in only 40%, with only a small percentage having a left ventricular ejection fraction<50% on presentation. Cardiac MRI was abnormal in all tested patients, with findings suggestive of myocarditis such as late gadolinium enhancement and myocardial edema. B-type natriuretic peptide or N-terminal pro-B-type natriuretic peptide levels were only mildly elevated in approximately two-thirds of the patients when measured. C-reactive protein levels were elevated in most and decreased along with troponin through the hospital stay. Almost all patients had resolution of symptoms and signs and improvement in diagnostic markers and imaging with or without treatment (Table 4).
Table 4.
Case Reports and Case Series of Myocarditis after COVID-19 Vaccination
The Israeli Ministry of Health also reported 148 myocarditis cases among 10.4 million vaccinated individuals occurring within 30 days of mRNA vaccination, a majority after a second dose, mostly in males 16 to 30 years of age.20 Most cases required hospitalization up to 4 days but were considered mild. The report suggested a probable link between the second-dose mRNA vaccine and myocarditis among men 16 to 30 years of age,20 with a stronger link for age 16 to 19, and decreasing association with older age.16,20 The prevalence of myocarditis was 1/20 000 for the 16- to 30-year group compared with 1/100 000 in the general population receiving the same vaccine. Similarly, the US Department of Defense reported 23 male military personnel diagnosed with myocarditis after 2.8 million doses of COVID-19 vaccinations administered in the Military Health System, mostly after the second dose of mRNA COVID-19 vaccination, reflecting higher than expected numbers of myocarditis cases.18
COVID-19–Associated Myocarditis
With the emergence of COVID-19 in Hubei Province, China, there was an expectation that the SARS-CoV-2 would cause predominantly respiratory illness, similar to that seen with severe acute respiratory syndrome (SARS) in 2002 to 2003.21 However, with the next phase of the COVID-19 epidemic in Southern Europe and later New York City, it became apparent that there were cardiovascular involvement and thromboembolic complications.22 Therefore, COVID-19 emerged as a virus pathogen affecting the vasculature and resulting in myocardial injury, requiring far different therapeutic approaches compared with SARS.22,23 Historically, pre–COVID-19, coronaviruses have not been commonly associated with significant myocardial damage. SARS infected >8000 individuals without significant incidence of myocarditis. In 1 autopsy series, SARS-CoV-1 was polymerase chain reaction amplifiable in 7 of 20 (35%) hearts, but was not associated with lymphocytic myocarditis, the hallmark of classic viral myocarditis.24 Similarly, Middle East respiratory syndrome coronavirus infected >2000 individuals, with only 1 case report of MRI-diagnosed Middle East respiratory syndrome coronavirus myocarditis.25 On the other hand, epidemiological data suggest that ≈12% to 20% of hospitalized patients with COVID-19 have evidence of cardiac injury as indicated by elevated levels of cardiac troponin.23,26 Furthermore in young athletes recovering from COVID-19 infection,27 cardiac MRI abnormalities consistent with myocarditis have been reported at a higher prevalence than expected, in ≈1% to 3% of the athletes.28–32 It was also recognized that COVID-19 can result in a multisystem inflammatory syndrome in children and younger adults. This rare but serious condition is defined by an excessive hyperinflammatory response that can affect multiple organs including the lungs, kidneys, brain, skin, eyes, the gastrointestinal system, and the cardiovascular system, resulting in ventricular dysfunction, coronary aneurysms, and shock.33,34
Although some investigators have proposed direct virus invasion as the most likely mechanism, others focus more on host inflammatory cell responses. Emerging data indicate that a maladaptive host immune response fueled by excessive activation of innate immune pathways along with proinflammatory cytokine surge, deregulated thromboinflammation, thrombotic microangiopathy, and endothelial dysfunction may play a role in pathogenesis of cardiac injury related to COVID-19.35,36 Other hypothesized mechanisms include demand ischemia, and stress- and hypoxia-induced myocardial injury.23 Baseline comorbidities including metabolic syndrome, hypertension, and cardiovascular disease likely also play a role.
Although SARS-CoV-2 can enter the cardiomyocyte through an angiotensin-converting enzyme 2–mediated entry and SARS-CoV-2 copies have been detected in heart tissue,37–39 cardiac histopathology studies have reported the absence of diffuse lymphocytic myocarditis traditionally seen in viral myocarditis or confluent myocyte necrosis expected in fulminant myocarditis.38,40–43 Hearts of patients who died of COVID-19 have revealed a greater number and diffuse distribution of CD68+ cells compared with matched control or other myocarditis hearts, indicating that cells of monocyte/macrophage lineage rather than lymphocytes may be dominant in this setting.35 Other studies revealed that interstitial cells, pericytes, and macrophages in the myocardium contain SARS-COV-2 RNA by in situ hybridization, and that pericytes infected by SARS-CoV-2 may play a role in capillary endothelial cell or microvascular dysfunction and individual cell necrosis.39,42,44 It is important to note that macrophages can mediate both local and systemic responses to viral infection, are also capable of fixing complement, and therefore could cause the direct death of nearby myocytes through the activation of apoptotic attack complexes.35 These findings suggest that COVID-19 may incite a form of myocarditis that is different from the typical lymphocytic myocarditis associated with other viral myocarditis presentations and may instead be associated with diffusely infiltrative cells of monocyte/macrophage lineage.35,41,44
Potential Mechanisms of COVID-19 Vaccine Myocarditis
SARS-CoV-2 mRNA vaccines contain nucleoside-modified mRNA, encoding the viral spike glycoprotein of SARS-CoV-2, but not live virus or DNA. They are encapsulated in lipid nanoparticles that act as delivery vehicles to transport mRNA into the cells and may include inactive ingredients such as buffer and salts. Once inside the host cells, the vaccine’s mRNA causes the cells to build the spike protein which then stimulates an adaptive immune response to identify and destroy a virus expressing spike protein. Vaccine-induced spike protein IgG antibodies prevent attachment of SARS-COV-2 to its host cell via spike protein binding to the angiotensin-converting enzyme 2 receptor, and thereby neutralizes the virus.
Selected RNA molecules can be immunogenic and stimulate the mammalian innate immune system, destroying the mRNA before it reaches target cells, preventing the spike protein and neutralizing antibody production. Nucleoside modifications of mRNA have been groundbreaking, shown to reduce innate immunogenicity, and result in less activation of cytokines, paving the path for mRNA vaccine development.45 COVID-19 mRNA vaccines have been shown to be highly effective and safe in large-scale trials.46,47 Systemic reactions to the vaccine, which are usually mild and transient, were reported more commonly among the younger population and more often after the second dose. Adverse cardiovascular effects in these trials were isolated, with incidences <0.05%, and did not include myocarditis.46,47
Although nucleoside modifications of mRNA have been shown to reduce their innate immunogenicity,45 in certain individuals with genetic predisposition,48 the immune response to mRNA may not be turned down and may drive the activation of an aberrant innate and acquired immune response. The dendritic cells or Toll-like receptor expressing cells exposed to RNA may still have the capacity to express cytokines and activation markers in certain individuals, although this may be markedly less when exposed to mRNA with nucleoside modifications than when treated with unmodified RNA.45 The immune system may therefore detect the mRNA in the vaccine as an antigen, resulting in activation of proinflammatory cascades and immunologic pathways that may play a role in the development of myocarditis as part of a systemic reaction in certain individuals.45,48 It will be important to monitor the possibility of such complications because the revolutionary use of mRNA is being considered for other vaccinations and therapies.
In published reports of myocarditis after COVID-19 vaccination, cardiac biopsy was reported in only 2 cases and did not demonstrate myocardial infiltrate11 or any evidence of myocarditis.9 This could be attributable to a sampling error in these few cases, or a different mechanism causing myocardial injury detected by cardiac biomarkers and MRI not manifest as traditional lymphocytic or eosinophilic myocarditis or myonecrosis on cardiac histopathology. SARS-COV-2 polymerase chain reaction and viral serology for other causes including hepatitis, Epstein-Barr virus, cytomegalovirus, parvovirus, mycoplasma, HIV, influenza A/B, respiratory syncytial virus, rhinovirus, enterovirus (Coxsackie A, Coxsackie B), adenovirus, and other causes were negative for acute or active infection, when tested, arguing against myocarditis caused by COVID-19 or other infections.10,14–18 Serology for autoimmune disorders with antinuclear antibodies and rheumatoid factor were negative, with no evidence of predilection to individuals with preexisting autoimmune disorders.10 There was also no evidence of leukocytosis, eosinophilia, anemia, thrombocytopenia, or transaminase elevation.19,12 d-Dimer was slightly elevated in 2 patients without evidence of pulmonary embolus or venous thromboembolic events,12,14 and erythrocyte sedimentation rate was mildly elevated in some cases.14 In 1 case report, a panel testing for variants in 121 genes potentially linked to cardiomyopathy was negative,17 arguing against an existing predisposition to cardiomyopathy attributable to known gene variants in that case.
By 1 case report, SARS-CoV-2 spike IgM and IgG neutralizing antibody levels were not significantly different in the patient with myocarditis than in individuals without myocarditis post–COVID-19 mRNA vaccination,17 arguing against a hyperimmune response.17 In the same report, the patient with myocarditis had elevated levels of IL-1 (interleukin 1) receptor antagonist, IL-5, IL-16, but not proinflammatory cytokines such as IL-6, tumor necrosis factor, IL-1B, IL-2, or interferon-γ levels. However, the patient had diminished levels of leukemia inhibitory factor, varying bidirectional profiles for IL-10, macrophage migration inhibitory factor, and vascular endothelial growth factor relative to an unvaccinated individual or a vaccinated individual without myocarditis.17 This patient also had higher levels of antibodies against some self-antigens such as aquaporin 4, endothelial cell antigen, and proteolipid protein 1.17 Historically, circulating heart-reactive autoantibodies have been reported at a higher frequency in patients with myocarditis and have been implicated in pathogenesis.49 These autoantibodies are usually directed against multiple antigens, some of which may have functional effects on cardiac myocytes.49 Thus, autoantibody generation could be one of the mechanisms whereby myocarditis may develop in susceptible individuals after vaccination. However, it should be noted that in the patient studied, autoantibody levels peaked on day 2 along with symptoms, but they did not recede as expected, as the clinical condition improved, although the follow-up was rather short. Autoantibodies are found more frequently in first-degree relatives of patients with cardiomyopathy than in the healthy population, raising the possibility that myocarditis may develop in a subgroup of patients with the appropriate genetic background. Also, the autoantibodies may not be pathogenic and could also be seen as a result of myocardial inflammation. In addition, this case patient had a 2-fold increase in the frequency of natural killer (NK) cells, which are the classical population of innate lymphoid cells, expressing a heterogeneous repertoire of germline-encoded receptors that allows them to destroy cells that are infected by viruses, cancer cells, or cells that are rejected. The surge in NK cells may have either contributed to the pathology or the disease resolution process. It is not clear whether the differences seen in this patient regarding relative increases in NK cells, autoantibodies, and a dysregulated cytokine profile reflect a causal pathological immune response or reactive adaptive responses to myocardial inflammation, and await validation by further studies.
Another important potential mechanism for myocarditis is molecular mimicry between the spike protein of SARS-CoV-2 and self-antigens.50 Antibodies against SARS-CoV-2 spike glycoproteins have been experimentally shown to cross-react with structurally similar human peptide protein sequences, including α-myosin.50 However, severe adverse events or autoimmune reactions have been very rare.46,47 Although COVID-19 vaccination does not appear to provoke de novo immune-mediated adverse events, it is possible that it may trigger preexisting dysregulated pathways in certain individuals with predisposition, resulting in a polyclonal B-cell expansion, immune complex formation, and inflammation.48
Earlier animal studies of vaccines for SARS-CoV-1 and Middle East respiratory syndrome coronavirus had raised concerns for enhanced disease with reexposure to wild-type virus after vaccination.51,52 These were triggered by different mechanisms, including neutrophilic and eosinophilic cellular infiltrates, possibly linked to Th17 responses, or nonneutralizing antibodies resulting in enhancement of antibody-induced cellular cytotoxicity, complement-dependent pathways, and aberrant activation of the innate and acquired immune system.53–56 Antibody-dependent enhancement of immunity was initially observed in the 1960s with respiratory syncytial virus and measles vaccines.57 It was characterized by nonneutralizing antibodies generated by past infection or vaccination failing to shut down the pathogen on reexposure and acting as a gateway by allowing the virus to gain entry, replicate, and lead to wider dissemination of illness and overreactive immune responses causing more severe illness. However, no evidence of either cellular immune enhancement or antibody-dependent enhancement of immunity was observed in non-human primate studies after SARS-CoV-2 virus challenge, either after vaccination or previous infection.58 These findings led an National Institutes of Health ACTIV study (Accelerating COVID-19 Therapeutic Interventions and Vaccines) panel to conclude that the risk of immune enhancement after COVID-19 immunizations was low, but required ongoing pharmacovigilance and monitoring.58 To date, neither COVID-19 disease nor the new COVID-19 vaccines have shown evidence of causing antibody-dependent enhancement of immunity or other forms of immune enhancement with reexposure. People infected with SARS-CoV-2 have not been reported to develop antibody-dependent enhancement of immunity on repeat exposure, and vaccine breakthrough COVID-19 cases are rare and mild. Furthermore, there is no evidence of acute COVID-19 infection during presentation with myocarditis cases after COVID-19 vaccination, arguing against a breakthrough infection as a cause (Table 4).
Reports to date also do not suggest a delayed hypersensitivity reaction, such as serum sickness–like reaction or eosinophilic myocarditis as a cause for myocarditis after mRNA COVID-19 vaccination.15 Although rare, delayed localized skin hypersensitivity reactions have been described with mRNA COVID-19 vaccination with a median latency of 7 days,59 unlike myocarditis emerging earlier within 3 to 4 days after vaccination. None of the case reports published to date had evidence of eosinophilia in peripheral blood or immune complex deposition or eosinophilic infiltrates in endomyocardial biopsy samples arguing against hypersensitivity, allergic or eosinophilic myocarditis.8–17 Lipid nanoparticles or adjuvants used in mRNA vaccines have not been shown to result in an immune or inflammatory response and have not been associated with myocarditis either.
Rare occurrences of vaccine-induced immune thrombotic thrombocytopenia have been reported after vaccination with the recombinant adenoviral vector encoding the spike protein antigen of SARS-COV-2.60 Although very rare thrombotic complications have been reported after mRNA COVID-19 vaccinations, these patients did not have thrombocytopenia or antiplatelet antibodies.61,62 None of the myocarditis cases reported after mRNA vaccination had evidence of thrombotic events, thrombocytopenia, or disseminated intravascular coagulation (Table 4). These patients also did not have persistent fever beyond the first few days, lymphadenopathy, hepatosplenomegaly, cytopenias (anemia, leukopenia, and thrombocytopenia), hypofibrinogenemia, transaminitis, extreme elevation in ferritin or multiorgan impairment to suggest a cytokine storm, hemophagocytic lymphohistiocytosis, or macrophage activation syndrome that results from overactivation of T lymphocytes and macrophages.63,64
Male predominance in myocarditis/pericarditis cases has been described in clinical and experimental studies before, and the reasons are unknown. An important possible explanation relates to sex hormone differences.3,65,66 Testosterone is thought to play a role, by a combined mechanism of inhibition of anti-inflammatory cells3,65–67 and commitment to a Th1-type immune response.68 Estrogen has inhibitory effects on proinflammatory T cells, resulting in a decrease in cell-mediated immune responses; and pericarditis incidence is higher in women during the postmenopausal period.69 Another contributing factor could be underdiagnosis in women. By our analysis of the VAERS database, as of June 6, 2021, there were 6235 reported cases of chest pain, 69% of which were in women, versus 30% in men.70 Despite a higher prevalence of chest pain in women, diagnostic evaluation, including ECG, laboratory biomarkers, echocardiography, and MRI, was performed and reported more often in male than in female patients presenting with chest pain after COVID vaccination (Bozkurt, unpublished data, 2021).
Assessing the risk
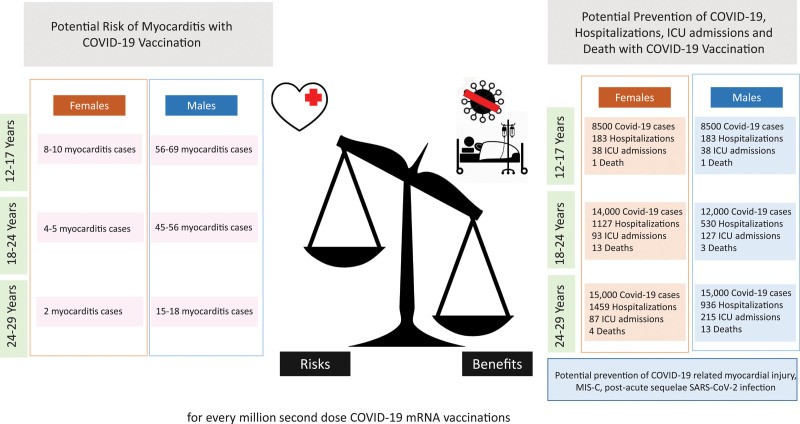
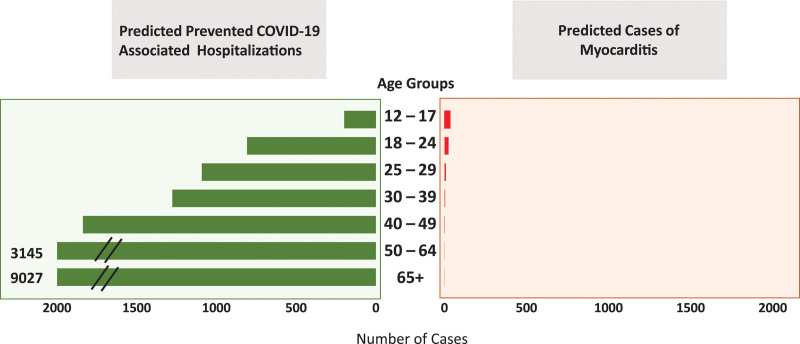
Despite these rare cases of myocarditis, the benefit-risk assessment for COVID-19 vaccination shows a favorable balance for all age and sex groups5 (Figures 2 and 3). Given the known potential risk of complications with COVID-19 infection, including hospitalizations and death even in younger adults (mortality remains 0.1–1 per 100 000 for persons 12–29 years of age), the risk-benefit decision remains overwhelmingly favorable for vaccination. Therefore, COVID-19 vaccination is currently recommended for everyone ≥12 years of age5 (Figure 3). COVID-19 vaccination not only prevents COVID-19–related hospitalizations and death, but also COVID-19–related complications such as myocarditis, multisystem inflammatory syndrome,33 and post–acute sequelae of SARS-CoV-2 infection or long COVID-19.74
Figure 2.
Predicted benefits of reduction in COVID-19–related hospitalizations and death and risks of myocarditis after second dose of mRNA COVID-19 vaccination by age group. Adapted from Centers for Disease Control and Prevention5 with permission. Copyright ©2021, Centers for Disease Control and Prevention (“COVID-19 mRNA vaccines in adolescents and young adults: Benefit-risk presentation”). Predictions for hospitalization and myocarditis rates were calculated for every million doses of mRNA vaccine based on hospitalization rates from Coronavirus Disease 2019 (COVID-19)–Associated Hospitalization Surveillance Network (COVID-NET) as of May 22.71 Benefit/risk were calculated over 120 days. To meet the ECG or rhythm-monitoring criterion, at least 1 of the following must be included: ST-segment or T-wave abnormalities, paroxysmal or sustained atrial, supraventricular, or ventricular arrhythmias, atrioventricular nodal conduction delays or intraventricular conduction defects. COVID-19 indicates coronavirus disease 2019; ICU, intensive care unit; MIS-C, multisystem inflammatory syndrome in children; and SARS-CoV-2, severe acute respiratory syndrome coronavirus-2. †Using either the original or revised Lake Louise criteria.72 ‡Using the Dallas criteria.73 §Autopsy cases may be classified as pericarditis on the basis of meeting histopathologic criteria of the pericardium.
Figure 3.
Potential risk of myocarditis with COVID-19 mRNA vaccination in the 120 days after vaccination and predicted prevention of COVID-19 cases, COVID-19–related hospitalizations, intensive care unit admissions, and deaths according to age groups and sex. Adapted from Centers for Disease Control and Prevention5 with permission. Copyright ©2021, Centers for Disease Control and Prevention (“COVID-19 mRNA vaccines in adolescents and young adults: Benefit-risk presentation”). Predictions for hospitalization and myocarditis rates were calculated for every million doses of mRNA vaccine based on hospitalization rates from Coronavirus Disease 2019 (COVID-19)–Associated Hospitalization Surveillance Network (COVID-NET) as of May 22, 2021. Benefit/risk was calculated over 120 days.
Management Strategies
Although rare, clinicians should be aware of the myocarditis and pericarditis risk, which should be considered in individuals presenting with chest pain within a week after vaccination, especially in the younger population. For initial evaluation, ECG and cardiac troponin level should be obtained, and inflammatory markers such as C-reactive protein and erythrocyte sedimentation rate can be helpful.5 For suspected cases, cardiology consultation and evaluation with echocardiography and cardiac MRI should be considered. An evaluation for acute COVID-19 infection (via polymerase chain reaction of respiratory tract sample) and past disease (via SARS-CoV-2 nucleocapsid and spike protein antibodies) would be helpful. Evaluation and management may vary depending on the patient’s age, clinical presentation, potential other causes and comorbidities, hemodynamic and rhythm stability, and clinical course. Patients with chest pain, evidence of myocardial injury, ECG changes, cardiac imaging abnormality, arrhythmia, hemodynamic instability after COVID-19 vaccination likely will require hospitalization and close follow-up.
In published case reports, in addition to supportive care, nonsteroidal anti-inflammatory drugs, steroids, and colchicine were used for management of some of the patients with myocarditis after COVID-19 vaccination. A few patients were treated with intravenous immunoglobulin and aspirin, and some were initiated on β-blocker and angiotensin-converting enzyme inhibitor therapy because of left ventricular systolic dysfunction. Although there are no prospective or randomized studies, it is reasonable to consider these therapies, especially in patients with significant symptoms and findings. Among patients with rapid resolution of symptoms, with preserved cardiac function and normal biomarkers or resolving cardiac biomarker abnormality, therapy may be deferred. In patients with persistent mild symptoms without hemodynamic instability, arrhythmia, significant left ventricular dysfunction or heart failure, colchicine, nonsteroidal anti-inflammatory drugs, and steroids may be considered. In patients with left ventricular dysfunction, heart failure, new-onset arrhythmia, or hemodynamic instability, intravenous steroids and intravenous immunoglobulin along with other cardiac or circulatory supportive measures can be considered. In patients with left ventricular systolic dysfunction, guideline-directed therapy including β-blockers and angiotensin-converting enzyme inhibitors should be initiated. Management should include a cardiologist for initial assessment, evaluation, treatment, and follow-up, and an infection disease specialist for guidance on subsequent immunization strategies.
Although the clinical course appears mild with likely resolution of symptoms and signs, it is reasonable to restrict or defer strenuous physical activity and competitive sports until after complete resolution of symptoms, signs, hemodynamic, rhythm, diagnostic, and biomarker abnormalities. If a person develops myocarditis or pericarditis after the first dose of an mRNA vaccine, CDC recommends that their second dose be delayed and that the second dose could be reconsidered on resolution of symptoms, signs, and findings, under certain circumstances.75 There is evolving evidence that a single-dose mRNA vaccine does not offer adequate protection in the general population against new SARS-COV-2 variants, and further studies are needed to determine efficacy of a single versus 2 doses of mRNA vaccination in different age groups.75 CDC recommends that all cases of myocarditis and pericarditis post–COVID-19 vaccination be reported to VAERS.5
Future Directions and Research Priorities
Studies are needed to elucidate the incidence, risk factors including genetic predisposition, prognosis, potential mechanisms, reasons for sex differences, clinical course, treatment strategies, and the long-term impact of myocarditis after COVID-19 vaccination.5
Future research studies should be designed and supported specifically: (1) to characterize the role of specific immune cell populations, their similarities and differences in the development of COVID-19, immunity post–COVID-19 vaccinations, myocardial injury and multisystem inflammatory syndrome in children related to COVID-19, and myocarditis related to COVID-19 vaccines; (2) to characterize histopathology, immunohistochemistry, ultrastructural, and functional changes of the myocardium in the setting of myocardial injury related to COVID-19, and myocarditis related to COVID-19 vaccines, and their correlation with cardiac imaging and cardiac biomarker findings; (3) to prospectively screen for the development of myocarditis and myocardial injury after COVID-19 vaccinations in different populations with specific emphasis on sex- and age-related differences; (4) to explore predisposing factors for the development of myocardial injury with COVID-19 or myocarditis with COVID-19 vaccines (eg, genetic factors, comorbidities, immunity or autoimmunity profile); (5) to explore the mechanisms for development of myocarditis related to COVID-19 mRNA vaccination, including but not limited to molecular mimicry, autoantibody formation, mRNA immune reactivity, trigger of preexisting dysregulated immune processes; it is also important to determine whether these factors are specific for spike delivery through the mRNA technology or possibly a rare event from mRNA vaccinations; (6) to prospectively characterize the clinical course and short- and long-term outcomes of myocardial injury related to COVID-19, and myocarditis related to COVID-19 vaccines; (7) to explore appropriate treatment and management strategies for myocardial injury related to COVID-19 and myocarditis related to COVID-19 vaccines; (8) to characterize cardiac biomarkers, cardiac function and structure in patients with prolonged symptoms after COVID-19, or myocarditis related to COVID-19 vaccine, if any; (9) to determine a risk-benefit ratio for different age and sex groups with different doses of COVID-19 vaccination; and (10) to provide guidance on return to play and return to activity for patients with evidence of myocardial injury related to COVID-19 and myocarditis related to COVID-19 vaccines.
A collaborative registry of myocarditis related to COVID-19 vaccination with data collected on patient demographics, clinical presentation, biomarkers including cardiac troponin, diagnostic findings of ECG, echocardiography and cardiac MRI, biomarkers, with a paired bioregistry with blood and cardiac tissue samples would be quite valuable and help answer some of these questions.
Conclusions
In summary, >177 million people have received at least 1 dose of COVID-19 vaccine (>300 million doses) in the United States, and CDC and other international organizations continue to monitor the safety of COVID-19 vaccines for any health problems including rare cases of myocarditis after vaccination.75 Despite rare cases of self-limited myocarditis, the benefit-risk assessment for COVID-19 vaccination shows a favorable balance for all age and sex groups; therefore, COVID-19 vaccination is currently recommended for everyone 12 years of age and older.
Sources of Funding
None.
Disclosures
Dr Bozkurt: Consultation for Bayer and scPharmaceuticals, Clinical Events Committee for Guide-HF Trial Abbott Pharmaceuticals, and Data Safety Monitoring Board for Anthem Trial by Liva Nova Pharmaceuticals. Dr Hotez: Inventor on a COVID-19 vaccine technology owned by Baylor College of Medicine that was licensed nonexclusively to vaccine companies in India (Biological E) and elsewhere. Dr Kamat reports no conflicts.
Nonstandard Abbreviations and Acronyms
- COVID-19
- coronavirus disease 2019
- CDC
- Centers for Disease Control and Prevention
- IL
- interleukin
- SARS
- severe acute respiratory syndrome
- SARS-CoV-2
- severe acute respiratory syndrome coronavirus 2
- VAERS
- Vaccine Adverse Event Reporting System
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
The podcast and transcript are available as a Data Supplement at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.121.056135.
For Sources of Funding and Disclosures, see page 482.
Contributor Information
Ishan Kamat, Email: ishan.kamat@bcm.edu.
Peter J. Hotez, Email: hotez@bcm.edu.
References
- 1.Su JR, McNeil MM, Welsh KJ, Marquez PL, Ng C, Yan M, Cano MV. Myopericarditis after vaccination, Vaccine Adverse Event Reporting System (VAERS), 1990–2018. Vaccine. 2021;39:839–845. doi: 10.1016/j.vaccine.2020.12.046 [DOI] [PubMed] [Google Scholar]
- 2.Tschöpe C, Ammirati E, Bozkurt B, Caforio ALP, Cooper LT, Felix SB, Hare JM, Heidecker B, Heymans S, Hübner N, et al. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol. 2021;18:169–193. doi: 10.1038/s41569-020-00435-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fairweather D, Cooper LT, Jr, Blauwet LA. Sex and gender differences in myocarditis and dilated cardiomyopathy. Curr Probl Cardiol. 2013;38:7–46. doi: 10.1016/j.cpcardiol.2012.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimabukuro TT, Nguyen M, Martin D, DeStefano F. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine. 2015;33:4398–4405. doi: 10.1016/j.vaccine.2015.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC). Advisory Committee on Immunization Practices (ACIP). Coronavirus disease 2019 (COVID-19) vaccines. Accessed July 6, 2021. https://www.cdc.gov/vaccines/acip/meetings/slides-2021-06.html
- 6.Gubernot D, Jazwa A, Niu M, Baumblatt J, Gee J, Moro P, Duffy J, Harrington T, McNeil MM, Broder K, et al. U.S. population-based background incidence rates of medical conditions for use in safety assessment of COVID-19 vaccines. Vaccine. 2021;39:3666–3677. doi: 10.1016/j.vaccine.2021.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marill MC. FDA to add myocarditis warning to mRNA COVID-19 vaccines. Medscape. June 23, 2021. Accessed July 6, 2021. https://www.medscape.com/viewarticle/953647
- 8.Marshall M, Ferguson ID, Lewis P, Jaggi P, Gagliardo C, Collins JS, Shaughnessya R, Carona R, Fuss C, Corbin KJE, et al. Symptomatic acute myocarditis in seven adolescents following Pfizer-BioNTech COVID-19 vaccination. Pediatrics. Published online June 4, 2021. doi: 10.1542/peds.2021-052478 [DOI] [PubMed] [Google Scholar]
- 9.Rosner CM, Genovese L, Tehrani BN, Atkins M, Bakhshi H, Chaudhri S, Damluji AA, de Lemos JA, Desai SS, Emaminia A, et al. Myocarditis temporally associated with COVID-19 vaccination. Circulation. 2021;144:503–506. doi: 10.1161/CIRCULATIONAHA.121.055891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abu Mouch S, Roguin A, Hellou E, Ishai A, Shoshan U, Mahamid L, Zoabi M, Aisman M, Goldschmid N, Berar Yanay N. Myocarditis following COVID-19 mRNA vaccination. Vaccine. 2021;39:3790–3793. doi: 10.1016/j.vaccine.2021.05.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larson KF, Ammirati E, Adler ED, Cooper LT, Jr, Hong KN, Saponara G, Couri D, Cereda A, Procopio A, Cavalotti C, et al. Myocarditis after BNT162b2 and mRNA-1273 vaccination. Circulation. 2021;144:507–509. doi: 10.1161/CIRCULATIONAHA.121.055913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ammirati E, Cavalotti C, Milazzo A, Pedrotti P, Soriano F, Schroeder JW, Morici N, Giannattasio C, Frigerio M, Metra M, et al. Temporal relation between second dose BNT162b2 mRNA Covid-19 vaccine and cardiac involvement in a patient with previous SARS-COV-2 infection. Int J Cardiol Heart Vasc. 2021;34:100774. doi: 10.1016/j.ijcha.2021.100774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bautista GJ, Pena OP, Bonilla Fernandez JA, Cardenes LA, Ramirez BL, Caballero DE. Acute myocarditis after administration of the BNT162b2 vaccine against COVID-19. Rev Esp Cardiol (Engl Ed). Published online April 27, 2021; S1885–5857(21)00133–X. doi: 10.1016/j.rec.2021.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mclean K, Johnson T. Myopericarditis in a previously healthy adolescent male following COVID-19 vaccination: a case report. Acad Emerg Med. Published online June 16, 2021. doi: 10.1111/acem.14322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Angelo T, Cattafi A, Carerj ML, Booz C, Ascenti G, Cicero G, Blandino A, Mazziotti S. Myocarditis after SARS-CoV-2 vaccination: a vaccine-induced reaction? Can J Cardiol. Published online June 9, 2021;S0828–282X(21)00286–5. doi: 10.1016/j.cjca.2021.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albert E, Aurigemma G, Saucedo J, Gerson DS. Myocarditis following COVID-19 vaccination. Radiol Case Rep. 2021;16:2142–2145. doi: 10.1016/j.radcr.2021.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muthukumar A, Narasimhan M, Li QZ, Mahimainathan L, Hitto I, Fuda F, Batra K, Jiang X, Zhu C, Schoggins J, et al. In-depth evaluation of a case of presumed myocarditis after the second dose of COVID-19 mRNA vaccine. Circulation. 2021;144:487–498. doi: 10.1161/CIRCULATIONAHA.121.056038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montgomery J, Ryan M, Engler R, Hoffman D, McClenathan B, Collins L, Loran D, Hrncir D, Herring K, Platzer M, et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol. Published online June 29, 2021. doi: 10.1001/jamacardio.2021.2833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim HW, Jenista ER, Wendell DC, Azevedo CF, Campbell MJ, Darty SN, Parker MA, Kim RJ. Patients with acute myocarditis following mRNA COVID-19 vaccination. JAMA Cardiol. Published online June 29, 2021. doi: 10.1001/jamacardio.2021.2828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Israel Ministry of Health. Surveillance of myocarditis (inflammation of the heart muscle) cases between December 2020 and May 2021 (including). June 2, 2021. Accessed July 6, 2021. https://www.gov.il/en/departments/news/01062021-03
- 21.World Health Organization. Severe acute respiratory syndrome (SARS). 2021. Accessed July 6, 2021. https://www.who.int/health-topics/severe-acute-respiratory-syndrome#tab=tab_1
- 22.Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A, Jain SS, Burkhoff D, Kumaraiah D, Rabbani L, et al. COVID-19 and cardiovascular disease. Circulation. 2020;141:1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941 [DOI] [PubMed] [Google Scholar]
- 23.Hendren NS, Drazner MH, Bozkurt B, Cooper LT, Jr. Description and proposed management of the acute COVID-19 cardiovascular syndrome. Circulation. 2020;141:1903–1914. doi: 10.1161/CIRCULATIONAHA.120.047349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oudit GY, Kassiri Z, Jiang C, Liu PP, Poutanen SM, Penninger JM, Butany J. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest. 2009;39:618–625. doi: 10.1111/j.1365-2362.2009.02153.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alhogbani T. Acute myocarditis associated with novel Middle East respiratory syndrome coronavirus. Ann Saudi Med. 2016;36:78–80. doi: 10.5144/0256-4947.2016.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitrani RD, Dabas N, Goldberger JJ. COVID-19 cardiac injury: implications for long-term surveillance and outcomes in survivors. Heart Rhythm. 2020;17:1984–1990. doi: 10.1016/j.hrthm.2020.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, Shchendrygina A, Escher F, Vasa-Nicotera M, Zeiher AM, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5:1265–1273. doi: 10.1001/jamacardio.2020.3557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rajpal S, Tong MS, Borchers J, Zareba KM, Obarski TP, Simonetti OP, Daniels CJ. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol. 2021;6:116–118. doi: 10.1001/jamacardio.2020.4916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark DE, Parikh A, Dendy JM, Diamond AB, George-Durrett K, Fish FA, Slaughter JC, Fitch W, Hughes SG, Soslow JH. COVID-19 myocardial pathology evaluation in athletes with cardiac magnetic resonance (COMPETE CMR). Circulation. 2021;143:609–612. doi: 10.1161/CIRCULATIONAHA.120.052573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martinez MW, Tucker AM, Bloom OJ, Green G, DiFiori JP, Solomon G, Phelan D, Kim JH, Meeuwisse W, Sills AK, et al. Prevalence of inflammatory heart disease among professional athletes with prior COVID-19 infection who received systematic return-to-play cardiac screening. JAMA Cardiol. 2021;6:745–752. doi: 10.1001/jamacardio.2021.0565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Starekova J, Bluemke DA, Bradham WS, Eckhardt LL, Grist TM, Kusmirek JE, Purtell CS, Schiebler ML, Reeder SB. Evaluation for myocarditis in competitive student athletes recovering from coronavirus disease 2019 with cardiac magnetic resonance imaging. JAMA Cardiol. Published online January 14, 2021; e207444. doi: 10.1001/jamacardio.2020.7444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daniels CJ, Rajpal S, Greenshields JT, Rosenthal GL, Chung EH, Terrin M, Jeudy J, Mattson SE, Law IH, Borchers J, et al. Prevalence of clinical and subclinical myocarditis in competitive athletes with recent SARS-CoV-2 infection: results from the Big Ten COVID-19 Cardiac Registry. JAMA Cardiol. Published online May 27, 2021; e212065. doi: 10.1001/jamacardio.2021.2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Most ZM, Hendren N, Drazner MH, Perl TM. Striking similarities of multisystem inflammatory syndrome in children and a myocarditis-like syndrome in adults: overlapping manifestations of COVID-19. Circulation. 2021;143:4–6. doi: 10.1161/CIRCULATIONAHA.120.050166 [DOI] [PubMed] [Google Scholar]
- 34.Feldstein LR, Tenforde MW, Friedman KG, Newhams M, Rose EB, Dapul H, Soma VL, Maddux AB, Mourani PM, Bowens C, et al. ; Overcoming COVID-19 Investigators. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA. 2021;325:1074–1087. doi: 10.1001/jama.2021.2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fox SE, Falgout L, Vander Heide RS. COVID-19 myocarditis: quantitative analysis of the inflammatory infiltrate and a proposed mechanism. Cardiovasc Pathol. Published online June 23, 2021; 107361. doi: 10.1016/j.carpath.2021.107361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skendros P, Mitsios A, Chrysanthopoulou A, Mastellos DC, Metallidis S, Rafailidis P, Ntinopoulou M, Sertaridou E, Tsironidou V, Tsigalou C, et al. Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J Clin Invest. 2020;130:6151–6157. doi: 10.1172/JCI141374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, Chilla S, Heinemann A, Wanner N, Liu S, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383:590–592. doi: 10.1056/NEJMc2011400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A, Sepe PA, Resasco T, Camporotondo R, Bruno R, et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22:911–915. doi: 10.1002/ejhf.1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lindner D, Fitzek A, Bräuninger H, Aleshcheva G, Edler C, Meissner K, Scherschel K, Kirchhof P, Escher F, Schultheiss HP, et al. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020;5:1281–1285. doi: 10.1001/jamacardio.2020.3551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kawakami R, Sakamoto A, Kawai K, Gianatti A, Pellegrini D, Nasr A, Kutys B, Guo L, Cornelissen A, Mori M, et al. Pathological evidence for SARS-CoV-2 as a cause of myocarditis: JACC Review Topic of the Week. J Am Coll Cardiol. 2021;77:314–325. doi: 10.1016/j.jacc.2020.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fox SE, Li G, Akmatbekov A, Harbert JL, Lameira FS, Brown JQ, Vander Heide RS. Unexpected features of cardiac pathology in COVID-19 infection. Circulation. 2020;142:1123–1125. doi: 10.1161/CIRCULATIONAHA.120.049465 [DOI] [PubMed] [Google Scholar]
- 43.Halushka MK, Vander Heide RS. Myocarditis is rare in COVID-19 autopsies: cardiovascular findings across 277 postmortem examinations. Cardiovasc Pathol. 2021;50:107300. doi: 10.1016/j.carpath.2020.107300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Basso C, Leone O, Rizzo S, De Gaspari M, van der Wal AC, Aubry MC, Bois MC, Lin PT, Maleszewski JJ, Stone JR. Pathological features of COVID-19-associated myocardial injury: a multicentre cardiovascular pathology study. Eur Heart J. 2020;41:3827–3835. doi: 10.1093/eurheartj/ehaa664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karikó K, Buckstein M, Ni H, Weissman D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23:165–175. doi: 10.1016/j.immuni.2005.06.008 [DOI] [PubMed] [Google Scholar]
- 46.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. ; C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, et al. ; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caso F, Costa L, Ruscitti P, Navarini L, Del Puente A, Giacomelli R, Scarpa R. Could Sars-coronavirus-2 trigger autoimmune and/or autoinflammatory mechanisms in genetically predisposed subjects? Autoimmun Rev. 2020;19:102524. doi: 10.1016/j.autrev.2020.102524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caforio AL, Mahon NJ, Tona F, McKenna WJ. Circulating cardiac autoantibodies in dilated cardiomyopathy and myocarditis: pathogenetic and clinical significance. Eur J Heart Fail. 2002;4:411–417. doi: 10.1016/s1388-9842(02)00010-7 [DOI] [PubMed] [Google Scholar]
- 50.Vojdani A, Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020;217:108480. doi: 10.1016/j.clim.2020.108480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Graham BS. Rapid COVID-19 vaccine development. Science. 2020;368:945–946. doi: 10.1126/science.abb8923 [DOI] [PubMed] [Google Scholar]
- 52.Jaume M, Yip MS, Cheung CY, Leung HL, Li PH, Kien F, Dutry I, Callendret B, Escriou N, Altmeyer R, et al. Anti-severe acute respiratory syndrome coronavirus spike antibodies trigger infection of human immune cells via a pH- and cysteine protease-independent FcγR pathway. J Virol. 2011;85:10582–10597. doi: 10.1128/JVI.00671-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sedova ES, Scherbinin DN, Lysenko AA, Alekseeva SV, Artemova EA, Shmarov MM. Non-neutralizing antibodies directed at conservative influenza antigens. Acta Naturae. 2019;11:22–32. doi: 10.32607/20758251-2019-11-4-22-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Halstead SB, Katzelnick L. COVID-19 vaccines: should we fear ADE? J Infect Dis. 2020;222:1946–1950. doi: 10.1093/infdis/jiaa518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hotez PJ, Bottazzi ME, Corry DB. The potential role of Th17 immune responses in coronavirus immunopathology and vaccine-induced immune enhancement. Microbes Infect. 2020;22:165–167. doi: 10.1016/j.micinf.2020.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hotez PJ, Corry DB, Bottazzi ME. COVID-19 vaccine design: the Janus face of immune enhancement. Nat Rev Immunol. 2020;20:347–348. doi: 10.1038/s41577-020-0323-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Swart RL, Kuiken T, Timmerman HH, van Amerongen G, Van Den Hoogen BG, Vos HW, Neijens HJ, Andeweg AC, Osterhaus AD. Immunization of macaques with formalin-inactivated respiratory syncytial virus (RSV) induces interleukin-13-associated hypersensitivity to subsequent RSV infection. J Virol. 2002;76:11561–11569. doi: 10.1128/jvi.76.22.11561-11569.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haynes BF, Corey L, Fernandes P, Gilbert PB, Hotez PJ, Rao S, Santos MR, Schuitemaker H, Watson M, Arvin A. Prospects for a safe COVID-19 vaccine. Sci Transl Med. 2020;12:eabe0948. doi: 10.1126/scitranslmed.abe0948 [DOI] [PubMed] [Google Scholar]
- 59.Johnston MS, Galan A, Watsky KL, Little AJ. Delayed localized hypersensitivity reactions to the Moderna COVID-19 vaccine: a case series. JAMA Dermatol. 2021;157:716–720. doi: 10.1001/jamadermatol.2021.1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384:2092–2101. doi: 10.1056/NEJMoa2104840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dias L, Soares-Dos-Reis R, Meira J, Ferrão D, Soares PR, Pastor A, Gama G, Fonseca L, Fagundes V, Carvalho M. Cerebral venous thrombosis after BNT162b2 mRNA SARS-CoV-2 vaccine. J Stroke Cerebrovasc Dis. 2021;30:105906. doi: 10.1016/j.jstrokecerebrovasdis.2021.105906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carli G, Nichele I, Ruggeri M, Barra S, Tosetto A. Deep vein thrombosis (DVT) occurring shortly after the second dose of mRNA SARS-CoV-2 vaccine. Intern Emerg Med. 2021;16:803–804. doi: 10.1007/s11739-021-02685-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schulert GS, Grom AA. Pathogenesis of macrophage activation syndrome and potential for cytokine- directed therapies. Annu Rev Med. 2015;66:145–159. doi: 10.1146/annurev-med-061813-012806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tang LV, Hu Y. Hemophagocytic lymphohistiocytosis after COVID-19 vaccination. J Hematol Oncol. 2021;14:87. doi: 10.1186/s13045-021-01100-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lyden DC, Olszewski J, Feran M, Job LP, Huber SA. Coxsackievirus B-3-induced myocarditis. Effect of sex steroids on viremia and infectivity of cardiocytes. Am J Pathol. 1987;126:432–438. [PMC free article] [PubMed] [Google Scholar]
- 66.Girón-González JA, Moral FJ, Elvira J, García-Gil D, Guerrero F, Gavilán I, Escobar L. Consistent production of a higher TH1:TH2 cytokine ratio by stimulated T cells in men compared with women. Eur J Endocrinol. 2000;143:31–36. doi: 10.1530/eje.0.1430031 [DOI] [PubMed] [Google Scholar]
- 67.Frisancho-Kiss S, Coronado MJ, Frisancho JA, Lau VM, Rose NR, Klein SL, Fairweather D. Gonadectomy of male BALB/c mice increases Tim-3(+) alternatively activated M2 macrophages, Tim-3(+) T cells, Th2 cells and Treg in the heart during acute coxsackievirus-induced myocarditis. Brain Behav Immun. 2009;23:649–657. doi: 10.1016/j.bbi.2008.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huber SA, Pfaeffle B. Differential Th1 and Th2 cell responses in male and female BALB/c mice infected with coxsackievirus group B type 3. J Virol. 1994;68:5126–5132. doi: 10.1128/JVI.68.8.5126-5132.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kytö V, Sipilä J, Rautava P. Clinical profile and influences on outcomes in patients hospitalized for acute pericarditis. Circulation. 2014;130:1601–1606. doi: 10.1161/CIRCULATIONAHA.114.010376 [DOI] [PubMed] [Google Scholar]
- 70.Centers for Disease Control and Prevention. The Vaccine Adverse Event Reporting System (VAERS) results. June 6, 2021. Accessed July 6, 2021. https://wonder.cdc.gov/vaers.html
- 71.Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19)-associated hospitalization surveillance network (COVID-NET). 2021. Accessed July 6, 2021. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covid-net/purpose-methods.html
- 72.Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, Kindermann I, Gutberlet M, Cooper LT, Liu P, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72:3158–3176. doi: 10.1016/j.jacc.2018.09.072 [DOI] [PubMed] [Google Scholar]
- 73.Aretz HT, Billingham ME, Edwards WD, Factor SM, Fallon JT, Fenoglio JJ, Jr, Olsen EG, Schoen FJ. Myocarditis. A histopathologic definition and classification. Am J Cardiovasc Pathol. 1987;1:3–14. [PubMed] [Google Scholar]
- 74.Hageman JR. Long COVID-19 or post-acute sequelae of SARS-CoV-2 infection in children, adolescents, and young adults. Pediatr Ann. 2021;50:e232–e233. doi: 10.3928/19382359-20210519-02 [DOI] [PubMed] [Google Scholar]
- 75.Centers for Disease Control and Prevention. Clinical considerations; myocarditis and pericarditis after receipt of mRNA COVID-19 vaccines among adolescents and young adults. May 28, 2021. Accessed July 6, 2021. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/myocarditis.html