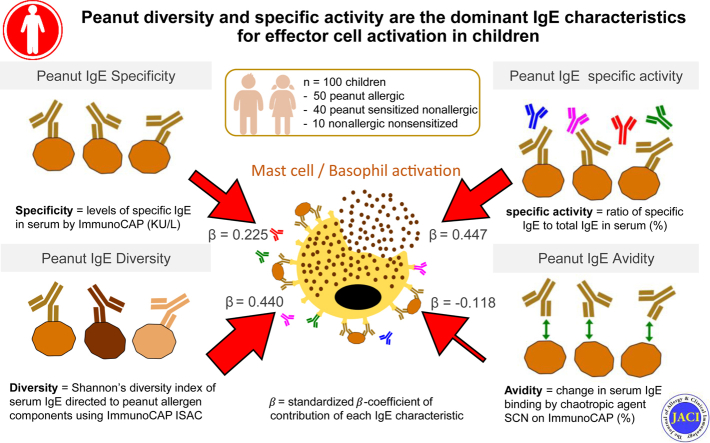
Abstract
Background
IgE mediates allergic reactions to peanut; however, peanut-specific IgE (sIgE) levels do not always equate to clinical peanut allergy. Qualitative differences between sIgE of peanut-sensitized but tolerant (PS) and peanut-allergic (PA) individuals may be important.
Objective
We sought to assess the influence of IgE characteristics on effector cell activation in peanut allergy.
Methods
A cohort of 100 children was studied. The levels of IgE to peanut and peanut components were measured. Specific activity (SA) was estimated as the ratio of allergen-sIgE to total IgE. Avidity was measured by ImmunoCAP with sodium thiocyanate. IgE diversity was calculated on the basis of ImmunoCAP-Immuno Solid-phase Allergen Chip assays for 112 allergens or for 6 peanut allergens. Whole-blood basophils and mast cell line Laboratory of Allergic Diseases 2 sensitized with patients’ plasma were stimulated with peanut or controls and assessed by flow cytometry.
Results
SA to peanut (P < .001), Ara h 1 (P = .004), Ara h 2 (P < .001), Ara h 3 (P = .02), and Ara h 6 (P < .001) and the avidity of peanut-sIgE (P < .001) were higher in PA than in PS individuals. Diversity for peanut allergens was greater in PA individuals (P < .001). All IgE characteristics were correlated with basophil and mast cell activation. Peanut SA (R = 0.447) and peanut diversity (R = 0.440) had the highest standardized β-coefficients in combined multivariable regression models (0.447 and 0.440, respectively).
Conclusions
IgE specificity, SA, avidity, and peanut diversity were greater in PA than in PS individuals. IgE peanut SA and peanut diversity had the greatest influence on effector cell activation and could be used clinically.
Key words: Avidity, diversity, specificity, specific activity, specific IgE, basophil activation test, mast cell activation test, peanut allergy, CD63
Abbreviations used: AUC, Area under the curve; ISAC, Immuno Solid-phase Allergen Chip; LAD2, Laboratory of Allergic Diseases 2; MAT, Mast cell activation test; NA, Nonsensitized nonallergic; PA, Peanut-allergic; PD, Peanut diversity; PS, Peanut-sensitized but tolerant; ROC, Receiver-operating characteristic; SA, Specific activity; sIgE, Specific IgE; SPT, Skin prick test; tIgE, Total IgE; WD, Whole diversity
Graphical abstract
Allergen-specific IgE (sIgE) detection as a diagnostic tool reports many false-positives.1,2 For some foods, including peanut, sIgE testing has improved dramatically over recent years through incorporation of allergen components.3,4 Alongside component testing, in vitro effector cell assays, such as the basophil activation test (BAT), have shown better diagnostic accuracy and discrimination between allergic and sensitized but tolerant individuals.5 A possible explanation for the superiority of BAT in reflecting individuals’ food-allergic phenotype is that it considers all characteristics of sIgE, rather than only levels, as well as possible blocking antibodies and intrinsic basophil sensitivity.6,7 The mast cell activation test (MAT) is also more specific than sIgE alone8 and has the advantage of not requiring fresh blood like the BAT. Because the mast cells are homogeneous and propagated from a single human mast cell,9 sensitized with plasma from different subjects, and washed before allergen stimulation, the MAT is well suited as an in vitro model to study the functional characteristics of IgE.
Examples of intrinsic functional characteristics of IgE likely to influence effector cell degranulation in response to allergen are specificity, specific activity (SA), diversity, and affinity.6 IgE specificity is known to correlate with allergic status, illustrated by the dominance of Ara h 2 sIgE as a diagnostic marker in peanut allergy.3,4,10 Conversely, levels of total IgE (tIgE) alone are considered useless as standalone in the diagnosis of specific allergies; however, the proportion of sIgE to tIgE, known as IgE SA, which reflects the proportion of IgE bound to effector cells that is specific for a given allergen, may be more informative.11, 12, 13 An individual’s reactivity to allergen is likely affected by the proportion of non-sIgE and its occupation of high affinity IgE receptors (FcεRI) on the surface of effector cells.11,12 Perhaps, an increased diversity in IgE repertoire is likely to dampen an allergen-specific response through the reduced chance of crosslinking on the cell surface.13 Conversely, IgE diversity to different epitopes within 1 allergen has been shown to increase basophil sensitivity to allergen and can possibly increase basophil reactivity.14, 15, 16 Both IgE diversity and basophil reactivity have been associated with severity of allergic reactions to peanut. IgE affinity for allergen is likely to influence effective IgE crosslinking able to cause effector cell activation.17 For polyclonal IgE responses to multivalent allergens, avidity, which refers to the collective effect of a combination of antigen-antibody interactions, reflects more accurately the strength with which IgE binds to allergen than affinity, which refers to molecular interactions between IgE and its epitopes.18
In this study, we aimed to determine the relative importance of IgE characteristics through the assessment of their diagnostic accuracy, influence on basophil and mast cell degranulation, and interplay with each other.
Methods
Study population
Peanut-allergic (PA), peanut-sensitized but tolerant (PS), and nonsensitized nonallergic (NA) individuals were evaluated as previously described.5 Peanut allergy was determined by positive response to oral food challenge, except for patients who had convincing clinical history of systemic allergic reactions within 1 year of sample collection, together with a skin prick test (SPT) wheal size greater than or equal to 8 mm and/or peanut-sIgE greater than or equal to 15 KUA/L.19 Peanut tolerance was defined by a negative oral food challenge or the ability to ingest greater than or equal to 4 g of peanut protein, twice weekly, without demonstrating an allergic response, as assessed by a validated peanut consumption questionnaire.20 Peanut sensitization was determined by SPT wheal size greater than or equal to 1 mm and/or a peanut-sIgE greater than or equal to 0.1 kUA/L. Children who were clinically unwell, had significant chronic illness, or were unwilling to participate were not included. One hundred patients were selected consecutively in chronological order of recruitment based on availability of plasma samples with sufficient volume. The study was approved by the South East London Research Ethics Committee 2, and written informed consent was obtained from parents of all children.
Allergen-sIgE measurements
ImmunoCAP ISAC (Immuno Solid-phase Allergen Chip) (Thermo Fisher, Uppsala, Sweden) was carried out according to manufacturer’s instructions. IgE binding to each allergen array was recorded using a LuxScan 10K scanner (Core Life Sciences, Irvine, Calif) and data analyzed using the Phadia Microarray Image Analysis Software (Thermo Fisher, Uppsala, Sweden).
IgE SA determination
IgE SA was determined by calculating the ratio of allergen-sIgE to tIgE levels, measured using the ImmunoCAP assays as detailed above, and expressed as percentage, using the following formula:
Avidity measurements
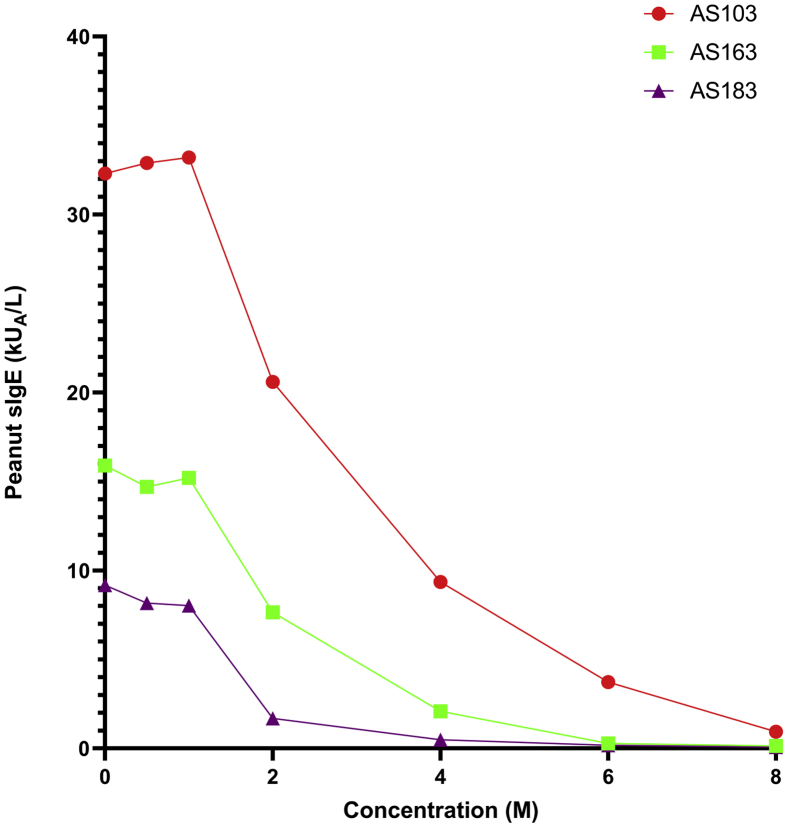
Avidity assays were performed to estimate the cumulative affinities of peanut-sIgE in plasma of both PA (n = 36) and PS (n = 24) patients (see Table E1 in this article's Online Repository at www.jacionline.org). The assay was developed on the basis of avidity ELISAs using thiocyanate solutions to interfere with antibody:antigen interactions.21,22 To determine the optimal concentration of sodium thiocyanate (NaSCN), plasma samples from 3 individuals, 1 PA individual (AS103) with peanut-sIgE levels closer to top of detection limit (100 kUA/L), 1 PA individual (AS163) with peanut-sIgE levels toward the lower detection limit of the assay, however with enough IgE to allow accurate inhibition (∼20 kUA/L), and 1 PS individual (AS183) with similar peanut IgE levels (∼20 kUA/L). We assayed plasma from each individual, in a 1:1 ratio, with NaSCN from 0 to 8 molar concentrations. Two molar NaSCN was selected as the optimal concentration, as it was the lowest concentration to cause measurable reduction in IgE binding, across both PA and PS individuals (see Fig E1 in this article’s Online Repository at www.jacionline.org). For avidity assays across the whole cohort, samples were selected on the basis of volume and peanut-sIgE level greater than or equal to 1 kUA/L. This minimum detection limit (≥1 kUA/L) was based on linearity experiments, in which serial dilutions spanning the detection limit of the whole assay were measured. For avidity estimations, patient plasma was incubated for 20 minutes at room temperature, with either PBS (negative control) or 2 molar NaSCN. Preincubated samples were then assayed for peanut-sIgE using ImmunoCAP. Avidity results were reported as “100 − %binding decrease” following NaSCN incubation and calculated using the formula below:
Fig E1.
Avidity ImmunoCAP development assay incorporating 2 PA individuals and 1 PS individual. Titration of sodium thiocyanate (NaSCN) using the following concentrations: 0 molar (PBS), 0.5 molar, 1 molar, 2 molar, 4 molar, 6 molar, and 8 molar.
Diversity measurements
The diversity of an individual’s IgE repertoire was generated from ImmunoCAP ISAC assays. Shannon’s diversity index23 was used to transform the data as follows:
where H is the diversity index and is the reported output; i = (1, ... S), S is the total number of allergens measured, and Pi is the proportion (relative abundance) of allergen, i, in total amount of allergen-sIgE levels.
Basophil and mast cell activation assays
Whole-blood BAT and MAT were performed as previously described,5,8,24 with BAT performed on 54 individuals (PA = 23, PS = 31) and MAT performed on 100 individuals (PA = 50, PS = 40, NA = 10). For the BAT, heparinized whole blood was stimulated for 30 minutes at 37°C with peanut extract diluted in RPMI media, resulting in serial 10-fold dilutions from 10 μg/mL to 0.1 ng/mL. Anti-IgE (1 μg/mL) and N-Formylmethionine-leucyl-phenylalanine (fMLP, 1 μM) were used as positive controls and RPMI alone as the negative control. For the MAT, Laboratory of Allergic Diseases 2 (LAD2) cells9 were cultured in rIL-4 5 days before overnight sensitization with patient plasma, as previously described. Serial dilutions of peanut extract (0.1-1000 ng/mL) or controls (anti-IgE 1 μg/mL, ionomycin 1 μg/mL, or 0.04% BSA RPMI) were added to presensitized cells and incubated for 1 hour at 37˚C. Basophils were identified as SSClow/CD203c+/CD123+/HLA-DR− and mast cells as forward scatter/viable cells. For both BAT and MAT, surface expression of CD63 was measured by flow cytometry using FACS Canto II with FACSDiva software (BD Biosciences, San Diego, Calif) and data were analyzed using FlowJo software version 7.6.1 (TreeStar, Ashland, Ore).
Statistical analyses
Comparisons of groups were done using Mann-Whitney U test and Kruskal-Wallis P tests. The diagnostic performance of all measurements was examined against allergic status to peanut using receiver-operating characteristic (ROC)-curve analyses. Associations between individuals’ characteristics and mast cell activation were analyzed using Spearman rank correlation, with mast cell activation distributed in a non-normal fashion. Linear regression models were generated for each characteristic of IgE investigated in this study, with their regression (β) coefficients from univariable analyses reported in Table II. Multivariable linear regression models were designed including peanut-sIgE levels, peanut SA, peanut avidity, peanut diversity (PD), and whole diversity (WD), in a number of combinations as outlined in Table III. For standardized beta (β) coefficients, input data were standardized by generating Z scores, using the following formula, before performing linear regression analyses:
where Z is standardized data point, x is observed data point, μ is mean of sample, and σ is SD of the sample. Statistical analyses were performed using GraphPad Prism 7.0 (GraphPad Software, San Diego, Calif), SPSS 25.0 (IBM SPSS Statistics, New York, NY), and MedCalC 19.4.1 (MedCalc Software, Ostend, Belgium).
Table II.
Summary table reporting the discriminative ability and association of each characteristic with mast cell activation (%CD63+ LAD2 cells)
| Characteristic | PA vs PS t test P value (P value adjusted for multiple comparisons∗) | Correlation |
Linear regression (n = 90) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R value (n = 90) | P value (n = 90) | R value (PA, n = 50) | P value (PA, n = 50) | R value (PS, n = 40) | P value (PS, n = 40) | Β-coefficient | Coefficient SE | Standardized β-coefficient | P value | R2 value | ||
| Peanut-sIgE (kUA/L) | .0019 (.030) | 0.787 | <.0001 | 0.716 | <.0001 | 0.702 | <.0001 | 0.094 | 0.016 | 0.521 | <.0001 | 0.271 |
| Ara h 1 sIgE (kUA/L) | .0838 (1) | 0.621 | <.0001 | 0.637 | <.0001 | 0.475 | .0019 | 0.307 | 0.050 | 0.551 | <.0001 | 0.304 |
| Ara h 2 sIgE (kUA/L) | <.0001 (<.0001) | 0.666 | <.0001 | 0.641 | <.0001 | 0.208 | .1977 | 0.212 | 0.037 | 0.526 | <.0001 | 0.268 |
| Ara h 3 sIgE (kUA/L) | .0456 (.7296) | 0.613 | <.0001 | 0.587 | <.0001 | 0.556 | .0002 | 0.490 | 0.118 | 0.405 | <.0001 | 0.164 |
| Ara h 6 sIgE (kUA/L) | <.0001 (<.0001) | 0.658 | <.0001 | 0.625 | <.0001 | 0.205 | .2042 | 0.384 | 0.074 | 0.484 | <.0001 | 0.234 |
| Ara h 8 sIgE (kUA/L) | .4022 (1) | 0.385 | .0001 | 0.311 | .0280 | 0.444 | .0041 | −0.065 | 0.091 | −0.078 | .463 | 0.006 |
| Ara h 9 sIgE (kUA/L) | .0834 (1) | 0.219 | .0385 | 0.237 | .0975 | 0.445 | .0036 | 0.146 | 0.410 | 0.038 | .722 | 0.001 |
| Peanut SA | <.0001 (<.0001) | 0.659 | <.0001 | 0.604 | <.0001 | 0.485 | .0015 | 0.980 | 0.129 | 0.628 | <.0001 | 0.395 |
| Ara h 1 SA | .0038 (.0608) | 0.502 | <.0001 | 0.580 | <.0001 | 0.158 | .3316 | 2.722 | 0.361 | 0.626 | <.0001 | 0.386 |
| Ara h 2 SA | <.0001 (<.0001) | 0.515 | <.0001 | 0.523 | .0001 | −0.209 | .1950 | 1.494 | 0.245 | 0.545 | <.0001 | 0.297 |
| Ara h 3 SA | .0002 (.0032) | 0.418 | <.0001 | 0.457 | .0008 | 0.024 | .8847 | 4.231 | 1.224 | 0.346 | <.0001 | 0.120 |
| Ara h 6 SA | <.0001 (.0001) | 0.513 | <.0001 | 0.411 | .0030 | −0.031 | .8533 | 2.194 | 0.455 | 0.455 | <.0001 | 0.209 |
| Ara h 8 SA | .2862 (1) | 0.146 | .169 | 0.012 | .2957 | 0.194 | .2302 | 0.045 | 1.055 | 0.005 | .966 | 0.000 |
| Ara h 9 SA | .3529 (1) | −0.008 | .942 | −0.081 | .6262 | 0.248 | .1219 | −2.850 | 3.569 | −0.085 | .427 | 0.007 |
| PD | <.0001 (<.0001) | 0.649 | <.0001 | 0.565 | <.0001 | 0.233 | .1477 | 23.763 | 3.085 | 0.635 | <.0001 | 0.396 |
| WD | .4945 (1) | 0.494 | <.0001 | 0.442 | .0006 | 0.545 | .0002 | 8.362 | 2.525 | 0.333 | .001 | 0.111 |
| Avidity (%inhibition)† | <.0001 | 0.236 | .069 | −0.207 | .1168 | −0.344 | .0999 | 0.267 | 0.177 | 0.174 | .183 | 0.036 |
Discriminative ability was assessed using Mann-Whitney t test and associations with mast cell activation are reported as both Spearman rank (R value) correlation and logistic regression (β-coefficient). P value refers to the comparison between PA and PS patients.
For avidity analyses n = 60, with PA (n = 36) and PS (n = 24).
Adjustment for multiple comparisons was done with Bonferroni-Dunn.
Table III.
Summary of mechanistic multivariable linear regression models (n = 60), in which combinations of functional IgE characteristics were used to predict %CD63+ LAD2 cell activation
| Model no. | Characteristic | Unstandardized β- coefficient | Coefficient SE | Standardized β-coefficient | P value | R2 value | VIF |
|---|---|---|---|---|---|---|---|
| 1 | Peanut-sIgE | 0.037 | 0.020 | 0.225 | .066 | 0.415 | 1.384 |
| PD | 20.528 | 5.203 | 0.534 | <.0001 | 1.750 | ||
| Avidity | −0.141 | 0.180 | −0.092 | .436 | 1.322 | ||
| 2 | Peanut SA | 0.664 | 0.156 | 0.447 | <.0001 | 0.530 | 1.314 |
| PD | 16.940 | 4.470 | 0.440 | <.0001 | 1.608 | ||
| Avidity | −0.181 | 0.158 | −0.118 | .258 | 1.275 | ||
| 3 | Peanut-sIgE | 0.077 | 0.019 | 0.472 | <.0001 | 0.255 | 1.008 |
| WD | 2.171 | 4.306 | 0.057 | .628 | 1.035 | ||
| Avidity | 0.223 | 0.180 | 0.145 | .224 | 1.043 | ||
| 4 | Peanut SA | 0.993 | 0.154 | 0.668 | <.0001 | 0.447 | 1.084 |
| WD | 7.383 | 3.786 | 0.201 | .056 | 1.078 | ||
| Avidity | 0.121 | 0.157 | 0.079 | .444 | 1.063 |
Unstandardized and standardized β-coefficients are reported alongside significance and variance inflation factor (VIF). VIF >10 was used to indicate any collinearity.
Results
Study population
One hundred children (50 PA, 40 PS, 10 NA), aged 5 months to 17 years, 69% male, were studied. PA individuals were significantly older than PS individuals (P = .003). All demographic, clinical, and serological features of the study population are presented in Table I.
Table I.
Demographic and clinical features and serum sIgE levels of the study population (n = 100)
| Demographic, clinical, and immunologic features | PA (n = 50) | PS (n = 40) | NA (n = 10) | P value |
|---|---|---|---|---|
| Age (y) | 8.31 (1.68-17.91) | 5.93 (0.52-15.81) | 5.96 (0.77-12.58) | .003 |
| Sex: male | 36 (72) | 25 (62.5) | 8 (80) | .371 |
| Atopic eczema | 17 (34) | 18 (45) | 2 (20) | .287 |
| Asthma/wheezing | 29 (58) | 13 (33) | 1 (10) | .016 |
| Allergic rhinitis | 33 (66) | 14 (35) | 1 (10) | .003 |
| Other food allergy | 38 (76) | 33 (83) | 2 (20) | .453 |
| SPT wheal size to peanut (mm) | 9 (1-34)† | 3 (0-12)† | 0 (0-0) | <.0001 |
| tIgE (kUA/L) | 297.5 (4-3,550) | 469 (7-10,714) | 29.5 (6-397) | .5757 |
| sIgE to peanut (kUA/L) | 12 (0.2-568) | 2.41 (0.04-128) | 0.01 (0-0.9) | .0019 |
| sIgE to Ara h 1 (kUA/L) | 0.80 (0-199) | 0.11 (0-88.3) | 0 (0-0.01) | .0838 |
| sIgE to Ara h 2 (kUA/L) | 2.54 (0.01-278) | 0.07 (0-82.3) | 0.04 (0.01-0.07) | <.0001 |
| sIgE to Ara h 3 (kUA/L) | 0.36 (0-89.6) | 0.06 (0-7.28) | 0.01 (0-0.04) | .0456 |
| sIgE to Ara h 6 (kUA/L) | 3.835 (0-155) | 0.03 (0-86) | 0 (0-0.29) | <.0001 |
| sIgE to Ara h 8 (kUA/L) | 0.09 (0-185) | 0.04 (0-88.6) | 0 (0-0.02) | .4022 |
| sIgE to Ara h 9 (kUA/L) | 0.02 (0-871) | 0.05 (0-43.9) | 0.01 (0-0.02) | .0834 |
| Peanut SA (%) | 5.03 (0.06-81.71) | 0.82 (0.02-7.68) | 0.03 (0-0.18) | <.0001 |
| Ara h 1 SA (%) | 0.37 (0-24.34) | 0.03 (0-2.12) | 0 (0-0.04) | .0038 |
| Ara h 2 SA (%) | 2.48 (0.01-55.26) | 0.012 (0-1.86) | 0.09 (0.01-0.83) | <.0001 |
| Ara h 3 SA (%) | 0.14 (0-9.98) | 0.019 (0-0.90) | 0.01 (0-0.67) | .0002 |
| Ara h 6 SA (%) | 1.91 (0-25.63) | 0.01 (0-1.06) | 0 (0-0.41) | <.0001 |
| Ara h 8 SA (%) | 0.05 (0-11.90) | 0.01 (0-7.77) | 0 (0-0.04) | .2862 |
| Ara h 9 SA (%) | 0.01 (0-1.5) | 0.01 (0-4.72) | 0.01 (0-0.17) | .3529 |
| PD | 0.97 (0-1.41) | 0 (0-1.35) | 0 (0-0) | <.0001 |
| WD | 1.985 (0-3.39) | 1.82 (0-3.1) | 0 (0-1.32) | .4945 |
| Avidity (%inhibition)‡ | 41.07 (15.94-72.5) | 28.63 (7.43-50) | — | <.0001 |
| MAT to peanut (%CD63+ LAD2 cells) | 24.6 (0-79.9) | 4.18 (0-31.65) | 1.09 (0-3.9) | <.0001 |
| BAT to peanut† (%CD63+ basophils)∗ | 37.55 (1.75-90.79) | 1.57 (0-16.6) | 0.36 (0-0.99) | <.0001 |
Values are expressed as n (%) or median (range). P value refers to the comparison between PA and PS patients.
BAT = PA (n = 23), PS (n = 54), NA (n = 7).
SPT = PA (n = 49), PS (n = 38), PA + PS (n = 87).
For avidity analyses, n = 60, with PA (n = 36) and PS (n = 24).
Peanut-sIgE is strongly correlated with mast cell activation
We previously reported that sIgE levels to peanut (P < .01), Ara h 2 (P < .0001), Ara h 3 (P = .046), and Ara h 6 (P < .0001) were significantly higher in PA than in PS individuals, with Ara h 2 and Ara h 6 showing the greatest diagnostic utility.10 The other components tested did not discriminate between PA and PS individuals (see Fig E2 in this article’s Online Repository at www.jacionline.org).
Fig E2.
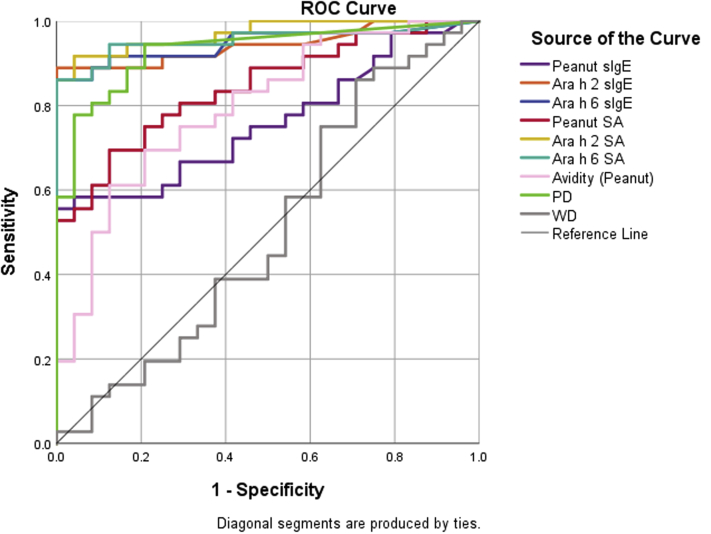
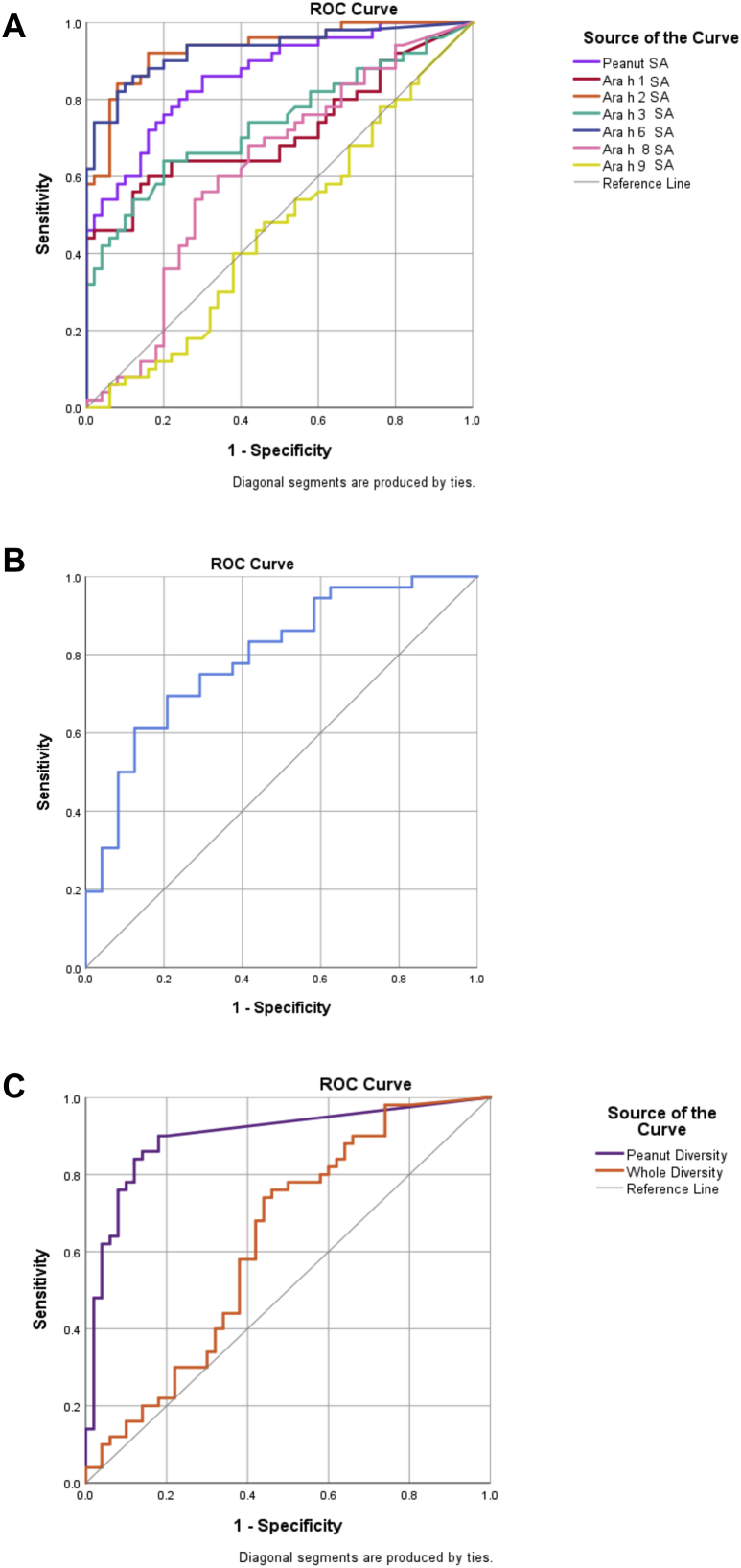
ROC curves for the most discriminative characteristics, allowing for comparison, in the subpopulation of n = 60, initially selected for avidity analyses. Displayed are ROC curves for peanut-sIgE (purple), Ara h 2 sIgE (orange), Ara h 6 sIgE (blue), peanut SA (red), Ara h 2 SA (yellow), Ara h 6 SA (turquoise), avidity (pink), PD (light green), and WD (gray). For corresponding areas under the curves, see Table E4.
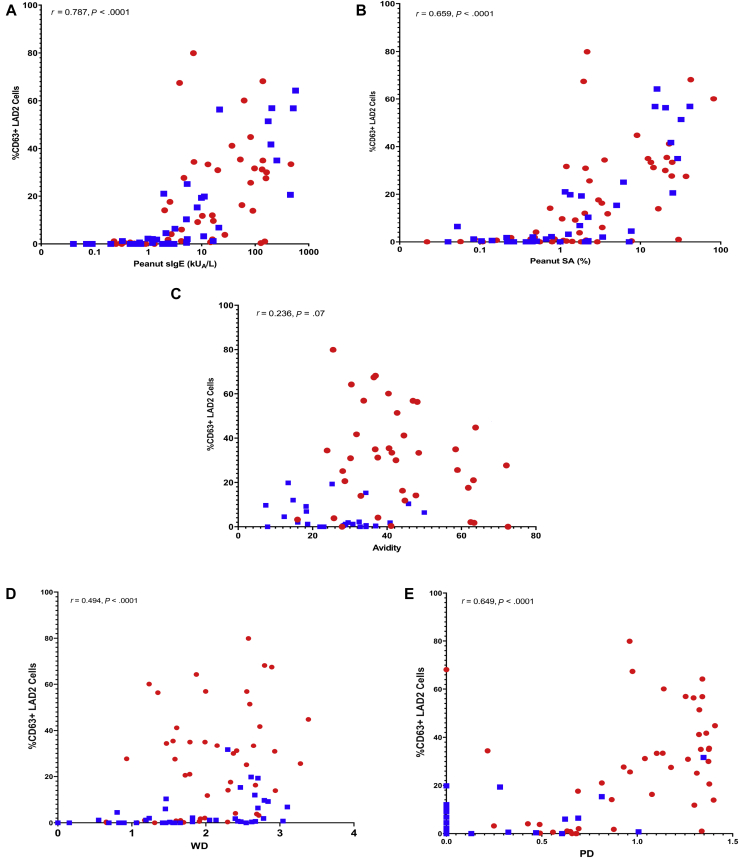
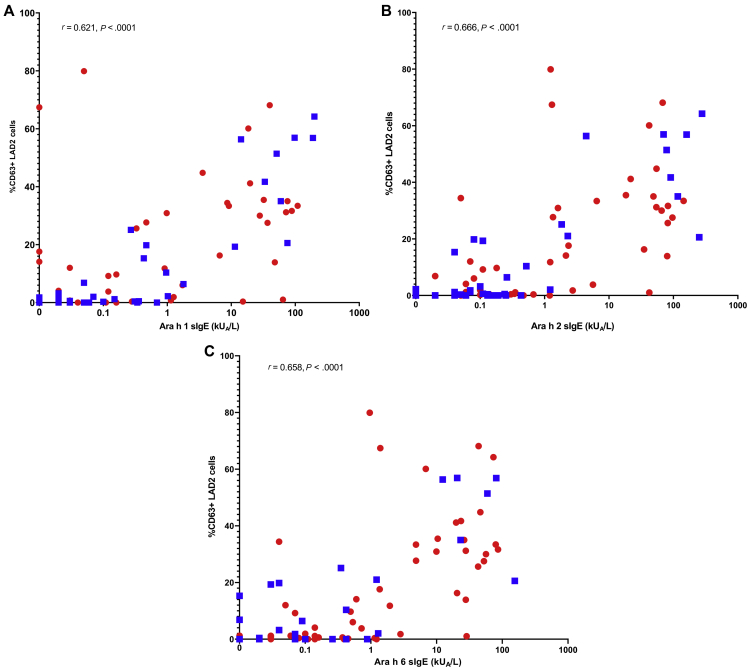
Here, we assessed to what degree the levels of sIgE correlated with peanut-induced mast cell activation. Peanut-sIgE was strongly correlated with peanut-induced mast cell activation, reporting an R value of 0.787 (P < .0001), with Ara h 2 reporting 0.666 (P < .0001), Ara h 6 0.658 (P < .0001), and Ara h 1 0.621 (P < .0001) (Fig 3, A; see Fig E3 in this article’s Online Repository at www.jacionline.org; Table II). A full characteristic correlation matrix, with corresponding P values, has been reported in Tables E2 and E3 in this article’s Online Repository at www.jacionline.org.
Fig 3.
Spearman’s correlation between (A) peanut-sIgE levels, (B) peanut SA, (C) peanut avidity, (D) WD, and (E) PD, and mast cell activation. %CD63+ LAD 2 cells represent cell activation following the MAT. Correlation reported across peanut-sensitized population (n = 90), with PA individuals (red) and PS individuals (blue). R values have been displayed on the figure and in Table II.
Fig E3.
Spearman correlation between (A) Ara h 1, (B) Ara h 2, (C) Ara h 6 sIgE levels and mast cell activation. %CD63+ LAD 2 cells represent cell activation following the MAT. Correlation reported across peanut-sensitized population (n = 90), with PA individuals (red) and PS individuals (blue). R values have been displayed on the figure and in Table II.
IgE SA improves the discrimination between PA and PS
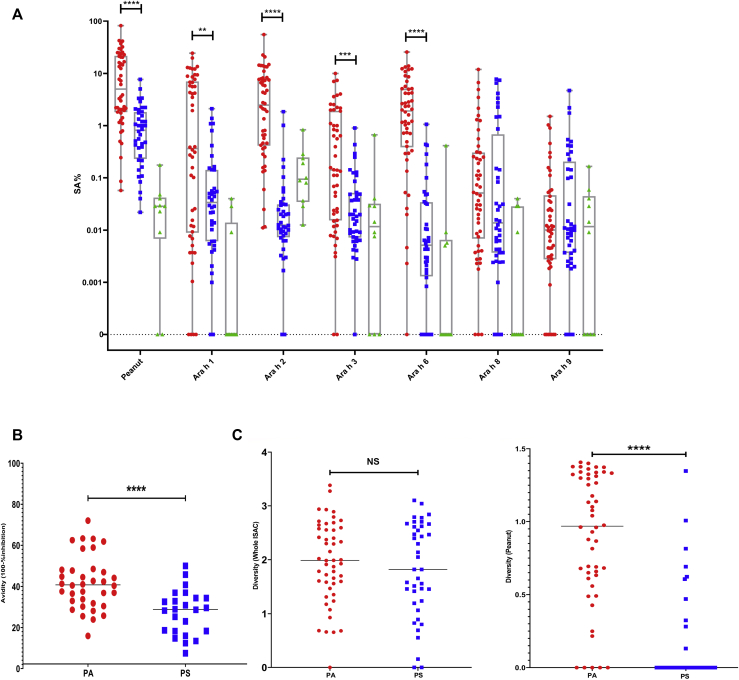
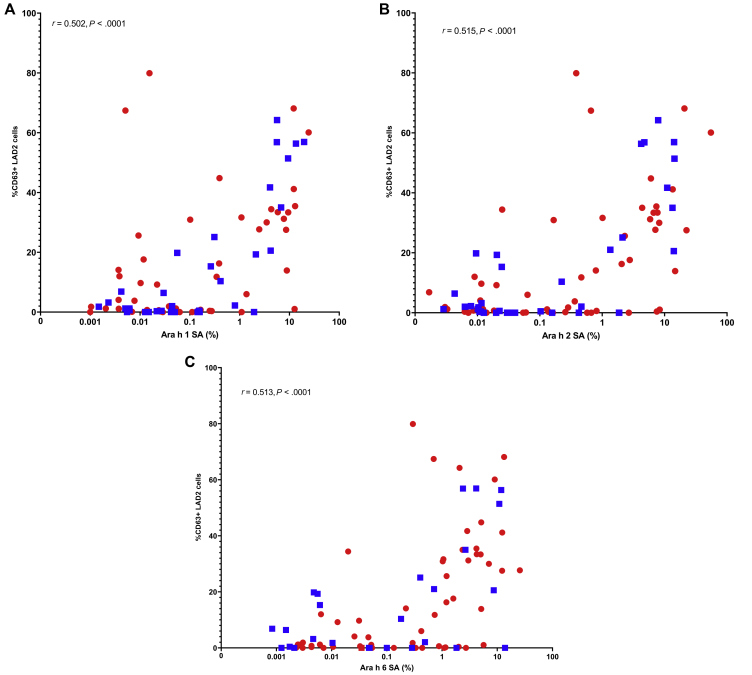
IgE SA values to peanut (P < .0001), Ara h 1 (P = .0038), Ara h 2 (P < .0001), Ara h 3 (P = .02), and Ara h 6 (P < .0001) were higher in PA than in PS individuals (Fig 1, A; Table II). In ROC analyses, peanut and allergen components showed a larger area under the ROC curve for SA than for sIgE alone—for instance, Ara h 2 SA had an area under the curve (AUC) of 0.933, followed by Ara h 6 (0.930), whole peanut (0.862), Ara h 3 (0.738), and Ara h 1 (0.716), (Fig 2, A; see Table E4 in this article’s Online Repository at www.jacionline.org), with peanut SA showing a significantly higher AUC (P = .0038) than peanut-sIgE, in the same population (see Table E5 in this article’s Online Repository at www.jacionline.org). SA correlated with mast cell activation across both PA and PS individuals (n = 90), with peanut SA reporting the highest R value of 0.659 (P < .0001), followed by Ara h 2 (0.515, P < .0001) and Ara h 6 (0.513, P < .0001) (Fig 3, B; see Fig E3 in this article’s Online Repository at www.jacionline.org; Table II).
Fig 1.
Distribution of IgE (A) SA, (B) avidity for peanut, and (C) diversity in PA individuals (in red, n = 50), PS individuals (in blue, n = 40), and NA individuals (in green, n = 10). The Mann-Whitney U test was used for the comparison between PA individuals and PS individuals. NS, Not significant; ISAC, Immuno Solid-phase Allergen Chip. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Fig 2.
ROC curves for IgE (A) SA, (B) peanut avidity, and (C) diversity across the whole study population (n = 100). For corresponding AUCs, see Table II. Labels have been included in the figure, where necessary.
Avidity of IgE for peanut is higher in patients with peanut allergy and correlates with mast cell activation
PA individuals (n = 36) showed a significantly higher avidity score (mean ± SD, 41.82 ± 13.00 %inhibition of IgE binding) than PS (n = 24) individuals (mean ± SD, 27.41 ± 10.95) (P < .0001, Mann-Whitney U test); however, there was an overlap between the groups (Fig 1, B). The avidity ROC analyses exhibited an AUC of 0.795 (Fig 2, B; see Tables E4 and E6 in this article’s Online Repository at www.jacionline.org). There was a very weak, but insignificant, correlation between the avidity of peanut-sIgE and mast cell activation in response to peanut (R = 0.236; P = .069; Fig 3, C).
IgE diversity for peanut allergens strongly correlates with mast cell activation
To measure IgE diversity, we transformed the ISAC data using Shannon index to create 2 variables: WD, incorporating all 112 allergens, and PD, considering the peanut allergens Ara h 1, 2, 3, 6, 8, and 9. Diversity within the peanut allergens was significantly greater (P < .0001) in PA (mean, 0.89) than in PS (mean, 0.16) individuals; however, WD did not discriminate (P = .495) between PA (mean, 1.96) and PS (mean, 1.81) individuals (Fig 1, C and D). ROC analyses were performed to see how these variables were distinct in allergic versus nonallergic subjects (Fig 2, C; Table E4). The AUC was 0.898 for PD and 0.628 for WD. PD also reported greater correlation with mast cell activation (R = 0.649; P < .0001) compared with WD (R = 0.494; P < .0001) (Fig 3, D and E).
Peanut IgE diversity and SA are the major determinants of mast cell and basophil activation
Having studied each characteristic of IgE separately, we assessed their relative importance and influence on effector cell activation. We generated logistic regression models incorporating the most important IgE characteristics based on their ability to discriminate between the PA and PS populations, their correlation with LAD2 cell activation, and significant (P < .2) standardized β-coefficients in simple logistic regression (Table III).
Four multivariable mechanistic logistic regression models were designed, incorporating 3 characteristics dissimilar in nature and acquisition, to reduce the probability of collinearity. Model 1 incorporated peanut-sIgE, PD, and avidity, generating standardized β-coefficients of 0.225 (P = .066), 0.534 (P < .0001), and −0.092 (P = .436), respectively. This suggested peanut IgE diversity to peanut allergens was the most influential characteristic of IgE in mast cell activation. This effect was mirrored when assessing basophil activation (see supplementary model s5), albeit with a greater relative influence reported by PD. In model 2, peanut-sIgE was replaced with peanut SA, and the influence that PD had on effector cell activation was lower compared with model 1 but still statistically significant. Peanut SA, PD, and avidity displayed coefficients of 0.447 (P < .0001), 0.440 (P < .0001), and 0.118 (P = .2580), respectively, with both PD and peanut SA reporting a significant impact on effector cell activation.
Models 3 and 4 mirrored models 1 and 2; however, WD was incorporated to replace PD. In model 3, peanut-sIgE was the only characteristic to influence effector cell activation with any significance, exhibiting a standardized β-coefficient of 0.472 (P < .0001). A similar effect was seen in model 4, with peanut SA replacing peanut-sIgE. Similar trends were seen considering basophil activation in models S6 and S7. Peanut SA influenced activation to a greater extent, reporting a standardized β-coefficient of 0.668 (P < .0001). Models 3 and 4 showed the positive impact peanut avidity measurements may have in the prediction of effector cell activation, albeit with poor significance. Supplementary models were designed to directly compare the influence of similar characteristics (see Table E7 in this article’s Online Repository at www.jacionline.org). Of note, supplementary model S2, containing only peanut-sIgE and SA, reports SA with a greater standardized β-coefficient and significance.
Given that the biology of primary basophils can differ from LAD2 cells, namely in terms of IgE receptor density and intrinsic cellular sensitivity, which can vary between patients, we assessed the relative importance of the same IgE characteristics on basophil activation. Univariable and multivariable logistic regression models were fitted using the available whole-blood BAT results in the same patient population (n = 54) (Table I).5 Both peanut SA and PD reported the highest standardized β-coefficients across both univariable, 0.762 (P < .0001) and 0.843 (P < .0001), respectively, and multiple multivariable regression models, such as supplementary model 4, reporting standardized β-coefficients of 0.335 and 0.690, respectively (see Tables E8 and E9 in this article’s Online Repository at www.jacionline.org).
Discussion
Levels of allergen-sIgE do not always reflect clinical reactivity to peanut, which raises the hypothesis that, beyond levels, the qualitative characteristics of sIgE may influence effector cell response to peanut allergens. In this study, we tested samples from a well-characterized cohort of 100 children being assessed for peanut allergy on the BAT and MAT to peanut, which reflect in vitro the clinical reactivity to peanut,8 and quantified SA, diversity, and avidity of IgE for peanut allergens in each subject. PA children had higher proportion of allergen-sIgE, higher diversity, and higher avidity of IgE for peanut allergens. These variables, as well as peanut and peanut component sIgE, correlated with effector cell activation to a greater or lesser extent. Using multivariable logistic regression models, the IgE characteristics that appeared to be most determinant of effector cell activation following peanut stimulation were SA and PD, suggesting that the IgE repertoire bound to receptors on the surface of mast cells is key in influencing degranulation. The greater proportion of allergen-sIgE and the broader array of peanut allergens that IgE recognizes, the greater the mast cell activation in response to peanut and, consequently, the greater the likelihood and severity of peanut allergy.8 To our knowledge, this is the first study assessing the functional characteristics of polyclonal-sIgE in a patient population, as well as the first study to quantify avidity using ImmunoCAP and to introduce the concept of diversity as a quantitative variable, which showed unprecedented influence on effector cell activation.
Quantifying allergen-sIgE has shown diagnostic utility in that elevated levels are associated with a higher likelihood of food allergy.25 Component-sIgE and epitope-sIgE detection added further resolution in peanut allergy diagnosis.3,4,26, 27, 28 We recently showed the importance of IgE specificity to peanut 2S albumins, Ara h 2 and Ara h 6, with Ara h 2 being the most dominant IgE-binding allergen and inducer of effector cell activation.10 Unsurprisingly, in this study, we made similar observations. However, because whole peanut extract was used as the stimulant in the MAT and whole peanut extract represents better the peanut ingested by patients, peanut-sIgE levels, rather than sIgE to peanut components, were used to represent specificity across multivariable models. The peanut components included Ara h 8 and also Ara h 9, which are less likely to cause systemic reactions, but we opted to include them in the analyses, because, from a mechanistic perspective, low-affinity interactions can contribute to effector cell degranulation.29
We used 2 in vitro systems to test the functionality of IgE and use as end points for the univariate and multivariate models: the BAT and the MAT. The use of the 2 cell types is an important validation of our findings. We used the LAD2 cells as the mast cells in the MAT.8 The uniformity of using human mast cell line removes the heterogeneity that others have seen when using primary mast cells,30 and allows us an excellent functional effector cell platform for exploring isolated IgE characteristics, with the least possible “noise” from mast cell–intrinsic features.30 Moreover, the MAT assay isolates receptor-bound antibodies, through initial wash steps, removing unbound antibodies from assay. This ablates the effect of blocking antibodies in the periphery, such as allergen-specific IgG4, and encourages the assessment of receptor-bound IgE.24
We report that peanut-sIgE significantly influences both basophil and mast cell activation, as exemplified in the univariable logistic regression models and multivariable models 1, 3, and S6. Impressively, SA improved discrimination between PA and PS individuals and had greater influence on both basophil and mast cell activation. It can be observed for some components, such as Ara h 2, that SA in the NA group reports higher medians than the PS group. This is due to the difference in tIgE levels between the 2 groups, in which tIgE is typically within the normal range in the NA group, and largely elevated in the PS group due to their atopic nature. The importance of SA has been highlighted by Christensen et al,6 showing that higher relative concentrations of allergen-sIgE to nonallergen sIgE increased both basophil sensitivity and maximum basophil degranulation. Clinically, IgE SA has been shown as a strong predictor in the outcome of oral food challenges11 and the efficacy of anti-IgE treatment in allergic individuals.31 However, other studies have failed to demonstrate improved diagnostic performance and showed comparable AUCs for both sIgE levels and SA to peanut, and peanut components.32 In our study, we report a significant increase in AUCs with peanut SA compared with standalone peanut-sIgE, suggesting that determining sIgE:tIgE ratios may have diagnostic benefits. Moreover, the increased influence on effector cell activation shown by incorporating peanut SA, over peanut-sIgE, in multivariable regression models also supports the utility of SA as a better predictor of allergic reactivity. The direct comparison of peanut-sIgE and SA in single models, S2 and S9 (Tables E5 and E6), further substantiates SA’s dominance as an influential characteristic over standalone sIgE titers. Conceptually, an increase in IgE SA or ratio of relevant sIgE to irrelevant sIgE acts to increase the probability that 2 IgE antibodies attached to FcεRI will become complexed with an allergenic molecule, thus inducing degranulation.
It is well established that high-affinity binding of IgE to allergen is a characteristic that influences the mechanisms of allergic reactions, and has been demonstrated across both experimental and clinical studies.17,33 It has recently been reported that production of high-affinity IgE is regulated by a distinct subset of previously unidentified T follicular helper cells, characterized by their high IL-13 expression.34 High-affinity allergen-sIgE is required to outcompete lower affinity allergen-specific-IgG and initiate the allergic response.35 Despite this, a role for low-affinity interactions has been previously demonstrated using both high- and low-affinity IgE of same specificity and showing marked reduction in effector cell degranulation in response to inhibition of only low-affinity sIgE.31,36 This implies that summative avidity of allergen-sIgE is a potentially more important consideration than the affinity of individual IgE molecules.
The development of avidity ELISAs has enabled the estimation of avidity in polyclonal samples, by using different chaotropic agents.21,37, 38, 39, 40 We applied these principles using the well-established, validated, and fully quantitative ImmunoCAP technology. We showed a high degree of significant discrimination between PA and PS individuals, in which peanut-sIgE from PA individuals was far more tolerant of NaSCN addition than peanut-sIgE from PS individuals, with a greater proportion remaining bound to peanut ImmunoCAP. A recently published study suggested that this difference in avidity between clinically distinct sensitized populations could be a consequence of allergen exposure, with chronic allergen exposure leading to the accumulation of long-lived plasma cells, generating higher affinity IgE.41 We expected that our data for peanut-sIgE avidity would have higher impact on effector cell activation. This may be a consequence of limitations within the variable itself. We proposed a fully quantitative method for determining IgE avidity in patient samples, in which removal of low-avidity interactions in the presence of NaSCN was not influenced by the levels of sIgE in the sample. However, unlike the other variables tested, the avidity score is presented as relative inhibition, and therefore relies heavily on the original peanut-sIgE level, determined in the absence of NaSCN. We believe that IgE avidity can become more influential in cases in which IgE levels/concentrations and diversity are comparable between allergic and nonallergic individuals.
The diversity of one’s IgE repertoire as an influential characteristic in the mechanisms of allergy is lesser established. Allergen-sIgE levels, from both the whole allergen and the components perspectives, are exceptionally informative for the diagnosis and mechanistic understanding of peanut allergy; however, we do not normally consider the specificity of remaining IgE. Over recent years, the phenomena of “epitope/molecular spreading” has been described in allergy, whereby IgE specificity and concentration, to components/epitopes within clinically relevant allergens, diversifies over time and creates a more heterogeneous pool of allergen-sIgE.14,42,43 We used ImmunoCAP ISAC technology and designed variables, based on Shannon diversity, to reflect the diversity and concentration of IgE to both a wide array of allergens (WD) and allergen components within peanut (PD).44,45 When applying Shannon’s diversity principles to our population, WD did not discriminate between PA and PS individuals. This was not an unexpected finding, because most children in our cohort were highly atopic presenting with multiple allergic comorbidities. However, because both PA and PS individuals had detectable IgE to peanut and peanut major allergens, we could have expected a similar outcome with PD, but this was not the case because PD was able to discriminate between these cohorts in a highly significant manner. Moreover, PD showed unusually high diagnostic value and correlated well with mast cell activation. Perhaps most impressive was the influence that PD had on effector cell activation, where in simple logistic regression models, PD reported the outright highest standardized β-coefficients (P < .0001), reflecting influence on both mast cell and basophil activation. In multivariable mechanistic models, PD and peanut SA outperformed avidity in model 2, reporting comparably significant influence on mast cell activation, but with PD reporting slightly more significant impact on basophil activation. When plotted in isolation with peanut SA, PD reported a slightly greater standardized β-coefficient, considering both mast cell and basophil activation. The molecular spreading phenomenon is the most viable rationale for higher PD, in PA compared with PS individuals. This phenomenon has been described in children with hay fever, whereby Phleum pratense sensitization across different allergenic molecules increases/diversifies temporally, following the initial onset of disease.43 Under the assumption that some PS children eventually develop clinical peanut allergy, we could predict an increase in their IgE diversity to peanut allergens over time with progression of their allergy. Similar observations have been reported in the Learning Early About Peanut allergy (LEAP) study.46
Overall, for each of the IgE characteristics measured, there was a significant difference between PA and PS individuals, all showing improved diagnostic utility over peanut-sIgE levels and influenced effector cell activation. Between many of these variables, significant correlation was reported, reflecting the fact that allergic individuals tend to report high levels/scores across different functional IgE characteristics, namely higher levels of peanut-sIgE, higher peanut SA, higher PD, and higher avidity for peanut allergens. Similarly, as discussed, many PA individuals also report polysensitization to the different peanut allergens. This can often introduce concerns of multicollinearity when building multivariable models. In our study, IgE characteristics were included in the same model only if dissimilar in nature and acquisition, with collinearity presumed minimal as demonstrated by the low variance inflation factor scores. Our conclusions require, nevertheless, verification in future studies. In our cohort of 90 subjects with detectable peanut-sIgE, PD and peanut SA held the most influence on effector cell activation, with SA offering improved diagnostic accuracy over peanut-sIgE levels. It is likely that mast cell reactivity, and thus clinical peanut allergy, is a consequence of multiple IgE characteristics and none should be discounted in further investigations into the mechanisms of food allergy. Other IgE characteristics, such as glycosylation/posttranslational modifications, should be investigated, with the inhibitory effect of desialyation of glycans on IgE from allergic individuals potentially leading to therapeutic benefits.47 The clinical implications of IgE characteristics may extend to the effect of immunomodulatory treatments, such as allergen-specific immunotherapy48 and biologics, and could be used as biomarkers of response to treatment.
Key messages.
-
•
IgE specificity, SA, avidity, and diversity are significantly different between PA and PS individuals and are positively correlated with effector cell activation.
-
•
Multivariable models indicate that IgE diversity to peanut allergens and peanut IgE SA have the greatest influence on effector cell activation.
Acknowledgments
We thank Professor George Du Toit, Dr Suzana Radulovic, and the Paediatric Allergy Team at the Evelina London Children’s Hospital for their effort in recruiting participants and collecting samples to the study, and acknowledge support by the UK National Institute for Health Research comprehensive Biomedical Research Centre award to Guy’s and St Thomas’ National Health Service (NHS) Foundation Trust, in partnership with King’s College London and King’s College Hospital NHS Foundation Trust.
Footnotes
This work was supported by the Medical Research Council (MRC Clinician Scientist FellowshipMR/M008517/1, MRC Centenary Early Career Award, and MRC Clinical Research Training Fellowship no. G0902018 awarded to A.F.S.), Asthma UK (grant no. AUK-BC-2015-01), and the UK National Institute for Health Research comprehensive Biomedical Research Centre award to Guy’s and St Thomas’ National Health Service (NHS) Foundation Trust, in partnership with King’s College London and King’s College Hospital NHS Foundation Trust.
Disclosure of potential conflict of interest: A. F. Santos reports grants and personal fees from Medical Research Council (grants nos. MR/T032081/1, MR/M008517/1, G090218); grants from Asthma UK (G1000758) and the National Institute for Health Research through the Biomedical Research Centre award to Guy’s and St Thomas’ NHS Foundation Trust, during the conduct of the study; grants from Immune Tolerance Network/National Institute of Allergy and Infectious Diseases, National Institutes of Health, Asthma UK and Food Allergy Research and Education (FARE); personal fees from Thermo Scientific, Nutricia, Infomed, Novartis, Allergy Therapeutics, and Buhlmann, as well as research support from Buhlmann and Thermo Fisher Scientific through a collaboration agreement with King’s College London. The rest of the authors declare that they have no relevant conflicts of interest.
Appendix
Fig E4.
Spearman correlation between (A) Ara h 1, (B) Ara h 2, (C) Ara h 6 IgE SA and mast cell activation. %CD63+ LAD 2 cells represent cell activation following the MAT. Correlation reported across peanut-sensitized population (n = 90), with PA individuals (red) and PS individuals (blue). R values have been displayed on the figure and in Table II.
Table E1.
Demographic/clinical features and serum sIgE levels of the study population, used explicitly in avidity assays (n = 60)
| Feature | PA (n = 36) | PS (n = 24) | P value∗ |
|---|---|---|---|
| Age (y) | 8.37 (2.06-16.27) | 6.05 (0.52-13.71) | .0155 |
| Sex: male | 24 (66.67) | 15 (62.5) | — |
| SPT to peanut (mm) | 10 (1-34) | 3 (0-9)∗ | <.0001 |
| tIgE (kUA/L) | 518 (19-3550) | 547 (28-6036) | .5845 |
| sIgE to peanut (kUA/L) | 31.95 (1.2-568) | 5.195 (0.99-20.9) | .0005 |
| sIgE to Ara h 1 (kUA/L) | 8.92 (0-199) | 0.16 (0-11.4) | .0099 |
| sIgE to Ara h 2 (kUA/L) | 12.3375 (0.05-278) | 0.07 (0-0.52) | <.0001 |
| sIgE to Ara h 3 (kUA/L) | 1.475 (0-89.6) | 0.07 (0.02-7.28) | .0115 |
| sIgE to Ara h 6 (kUA/L) | 10.17 (0-155) | 0.03 (0-0.49) | <.0001 |
| sIgE to Ara h 8 (kUA/L) | 0.215 (0-185) | 0.11 (0-88.6) | .7787 |
| sIgE to Ara h 9 (kUA/L) | 0.04 (0-8.71) | 0.13 (0-13.9) | .0354 |
Values are expressed as n (%) or median (range). P value refers to the comparison between PA and PS patients and was generated using Mann-Whitney t tests.
SPT in PS individuals (n = 23).
Table E2.
Correlation matrix for each characteristic assessed in this study across PA and PS individuals (n = 90), including peanut avidity (n = 60)
| Characteristic | Age | MAT (%CD63+ LAD2 cells) | Peanut-sIgE | Ara h 1 sIgE | Ara h 2 sIgE | Ara h 3 sIgE | Ara h 6 sIgE | Ara h 8 sIgE | Ara h 9 sIgE | Peanut SA | Ara h 1 SA | Ara h 2 SA | Ara h 3 SA | Ara h 6 SA | Ara h 8 SA | Ara h 9 SA | WD | PD | Peanut avidity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 1.000 | 0.357 | 0.288 | 0.116 | 0.431 | 0.192 | 0.345 | 0.425 | −0.038 | 0.069 | −0.024 | 0.311 | −0.053 | 0.239 | 0.265 | −0.269 | 0.370 | 0.363 | 0.249 |
| MAT (%CD63+ LAD2 cells) | 0.357 | 1.000 | 0.787 | 0.621 | 0.666 | 0.613 | 0.658 | 0.383 | 0.219 | 0.659 | 0.502 | 0.515 | 0.418 | 0.513 | 0.146 | −0.008 | 0.494 | 0.649 | 0.236 |
| Peanut-sIgE | 0.288 | 0.787 | 1.000 | 0.820 | 0.692 | 0.865 | 0.670 | 0.438 | 0.416 | 0.707 | 0.584 | 0.448 | 0.547 | 0.444 | 0.046 | 0.006 | 0.580 | 0.645 | −0.020 |
| Ara h 1 sIgE | 0.116 | 0.621 | 0.820 | 1.000 | 0.567 | 0.874 | 0.535 | 0.126 | 0.386 | 0.623 | 0.859 | 0.418 | 0.677 | 0.384 | −0.214 | 0.068 | 0.348 | 0.551 | 0.159 |
| Ara h 2 sIgE | 0.431 | 0.666 | 0.692 | 0.567 | 1.000 | 0.582 | 0.880 | 0.268 | 0.066 | 0.599 | 0.473 | 0.841 | 0.462 | 0.736 | −0.002 | −0.189 | 0.351 | 0.809 | 0.490 |
| Ara h 3 sIgE | 0.192 | 0.613 | 0.865 | 0.874 | 0.582 | 1.000 | 0.527 | 0.270 | 0.426 | 0.589 | 0.666 | 0.353 | 0.718 | 0.319 | −0.118 | −0.001 | 0.430 | 0.547 | 0.021 |
| Ara h 6 sIgE | 0.345 | 0.658 | 0.670 | 0.535 | 0.880 | 0.527 | 1.000 | 0.242 | 0.020 | 0.616 | 0.479 | 0.790 | 0.474 | 0.891 | 0.021 | −0.147 | 0.296 | 0.832 | 0.470 |
| Ara h 8 sIgE | 0.425 | 0.383 | 0.438 | 0.126 | 0.268 | 0.270 | 0.242 | 1.000 | 0.442 | −0.001 | −0.164 | −0.036 | −0.160 | −0.017 | 0.779 | 0.101 | 0.842 | 0.216 | −0.106 |
| Ara h 9 sIgE | −0.038 | 0.219 | 0.416 | 0.386 | 0.066 | 0.426 | 0.020 | 0.442 | 1.000 | −0.033 | 0.089 | −0.235 | 0.030 | −0.231 | 0.138 | 0.692 | 0.490 | 0.075 | −0.213 |
| Peanut SA | 0.069 | 0.659 | 0.707 | 0.623 | 0.599 | 0.589 | 0.616 | −0.001 | −0.033 | 1.000 | 0.749 | 0.723 | 0.780 | 0.687 | 0.006 | 0.025 | 0.135 | 0.628 | 0.166 |
| Ara h 1 SA | −0.024 | 0.502 | 0.584 | 0.859 | 0.473 | 0.666 | 0.479 | −0.164 | 0.089 | 0.749 | 1.000 | 0.579 | 0.834 | 0.539 | −0.211 | 0.131 | 0.047 | 0.512 | 0.210 |
| Ara h 2 SA | 0.311 | 0.515 | 0.448 | 0.418 | 0.841 | 0.353 | 0.790 | −0.036 | −0.235 | 0.723 | 0.579 | 1.000 | 0.586 | 0.871 | −0.012 | −0.141 | 0.017 | 0.736 | 0.491 |
| Ara h 3 SA | −0.053 | 0.418 | 0.547 | 0.677 | 0.462 | 0.718 | 0.474 | −0.160 | 0.030 | 0.780 | 0.834 | 0.586 | 1.000 | 0.545 | −0.154 | 0.088 | −0.031 | 0.517 | 0.067 |
| Ara h 6 SA | 0.239 | 0.513 | 0.444 | 0.384 | 0.736 | 0.319 | 0.891 | −0.017 | −0.231 | 0.687 | 0.539 | 0.871 | 0.545 | 1.000 | 0.007 | −0.117 | 0.014 | 0.744 | 0.493 |
| Ara h 8 SA | 0.265 | 0.146 | 0.046 | −0.214 | −0.002 | −0.118 | 0.021 | 0.779 | 0.138 | 0.006 | −0.211 | −0.012 | −0.154 | 0.007 | 1.000 | 0.237 | 0.537 | 0.015 | −0.061 |
| Ara h 9 SA | −0.269 | −0.008 | 0.006 | 0.068 | −0.189 | −0.001 | −0.147 | 0.101 | 0.692 | 0.025 | 0.131 | −0.141 | 0.088 | −0.117 | 0.237 | 1.000 | 0.123 | −0.083 | −0.094 |
| WD | 0.370 | 0.494 | 0.580 | 0.348 | 0.351 | 0.430 | 0.296 | 0.842 | 0.490 | 0.135 | 0.047 | 0.017 | −0.031 | 0.014 | 0.537 | 0.123 | 1.000 | 0.285 | −0.239 |
| PD | 0.363 | 0.649 | 0.645 | 0.551 | 0.809 | 0.547 | 0.832 | 0.216 | 0.075 | 0.628 | 0.512 | 0.736 | 0.517 | 0.744 | 0.015 | −0.083 | 0.285 | 1.000 | 0.414 |
| Peanut avidity | 0.249 | 0.236 | −0.020 | 0.159 | 0.490 | 0.021 | 0.470 | −0.106 | −0.213 | 0.166 | 0.210 | 0.491 | 0.067 | 0.493 | −0.061 | −0.094 | −0.239 | 0.414 | 1.000 |
R values from Spearman correlation have been reported here; for corresponding P values, see Table E3.
Table E3.
Correlation matrix for each characteristic assessed in this study across PA and PS individuals (n = 90), including peanut avidity (n = 60)
| Characteristic | Age | MAT (%CD63+ LAD2 cells) | Peanut-sIgE | Ara h 1 sIgE | Ara h 2 sIgE | Ara h 3 sIgE | Ara h 6 sIgE | Ara h 8 sIgE | Ara h 9 sIgE | Peanut SA | Ara h 1 SA | Ara h 2 SA | Ara h 3 SA | Ara h 6 SA | Ara h 8 SA | Ara h 9 SA | WD | PD | Peanut avidity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | .00056 | .00596 | .27444 | .00002 | .06955 | .00086 | .00003 | .72318 | .51760 | .82589 | .00283 | .61999 | .02349 | .01169 | .01021 | .00034 | .00044 | .05503 | |
| MAT (%CD63+ LAD2 cells) | .00056 | .00000 | .00000 | .00000 | .00000 | .00000 | .00019 | .03853 | .00000 | .00000 | .00000 | .00004 | .00000 | .16917 | .94174 | .00000 | .00000 | .06897 | |
| Peanut-sIgE | .00596 | .00000 | .00000 | .00000 | .00000 | .00000 | .00002 | .00004 | .00000 | .00000 | .00001 | .00000 | .00001 | .66737 | .95223 | .00000 | .00000 | .87807 | |
| Ara h 1 sIgE | .27444 | .00000 | .00000 | .00000 | .00000 | .00000 | .23674 | .00017 | .00000 | .00000 | .00004 | .00000 | .00019 | .04291 | .52120 | .00078 | .00000 | .22635 | |
| Ara h 2 sIgE | .00002 | .00000 | .00000 | .00000 | .00000 | .00000 | .01059 | .53394 | .00000 | .00000 | .00000 | .00000 | .00000 | .98654 | .07482 | .00069 | .00000 | .00007 | |
| Ara h 3 sIgE | .06955 | .00000 | .00000 | .00000 | .00000 | .00000 | .01007 | .00003 | .00000 | .00000 | .00064 | .00000 | .00220 | .26768 | .99553 | .00002 | .00000 | .87582 | |
| Ara h 6 sIgE | .00086 | .00000 | .00000 | .00000 | .00000 | .00000 | .02166 | .84925 | .00000 | .00000 | .00000 | .00000 | .00000 | .84575 | .16804 | .00469 | .00000 | .00015 | |
| Ara h 8 sIgE | .00003 | .00019 | .00002 | .23674 | .01059 | .01007 | .02166 | .00001 | .98997 | .12195 | .73753 | .13151 | .87723 | .00000 | .34142 | .00000 | .04055 | .42142 | |
| Ara h 9 sIgE | .72318 | .03853 | .00004 | .00017 | .53394 | .00003 | .84925 | .00001 | .75874 | .40462 | .02567 | .78122 | .02819 | .19307 | .00000 | .00000 | .47942 | .10218 | |
| Peanut SA | .51760 | .00000 | .00000 | .00000 | .00000 | .00000 | .00000 | .98997 | .75874 | .00000 | .00000 | .00000 | .00000 | .95836 | .81483 | .20348 | .00000 | .20589 | |
| Ara h 1 SA | .82589 | .00000 | .00000 | .00000 | .00000 | .00000 | .00000 | .12195 | .40462 | .00000 | .00000 | .00000 | .00000 | .04589 | .21951 | .66325 | .00000 | .10709 | |
| Ara h 2 SA | .00283 | .00000 | .00001 | .00004 | .00000 | .00064 | .00000 | .73753 | .02567 | .00000 | .00000 | .00000 | .00000 | .90742 | .18384 | .87080 | .00000 | .00007 | |
| Ara h 3 SA | .61999 | .00004 | .00000 | .00000 | .00000 | .00000 | .00000 | .13151 | .78122 | .00000 | .00000 | .00000 | .00000 | .14827 | .41057 | .77012 | .00000 | .60884 | |
| Ara h 6 SA | .02349 | .00000 | .00001 | .00019 | .00000 | .00220 | .00000 | .87723 | .02819 | .00000 | .00000 | .00000 | .00000 | .94563 | .27125 | .89866 | .00000 | .00006 | |
| Ara h 8 SA | .01169 | .16917 | .66737 | .04291 | .98654 | .26768 | .84575 | .00000 | .19307 | .95836 | .04589 | .90742 | .14827 | .94563 | .02439 | .00000 | .88657 | .64115 | |
| Ara h 9 SA | .01021 | .94174 | .95223 | .52120 | .07482 | .99553 | .16804 | .34142 | .00000 | .81483 | .21951 | .18384 | .41057 | .27125 | .02439 | .24687 | .43530 | .47294 | |
| WD | .00034 | .00000 | .00000 | .00078 | .00069 | .00002 | .00469 | .00000 | .00000 | .20348 | .66325 | .87080 | .77012 | .89866 | .00000 | .24687 | .00638 | .06595 | |
| PD | .00044 | .00000 | .00000 | .00000 | .00000 | .00000 | .00000 | .04055 | .47942 | .00000 | .00000 | .00000 | .00000 | .00000 | .88657 | .43530 | .00638 | .00100 | |
| Avidity | .05503 | .06897 | .87807 | .22635 | .00007 | .87582 | .00015 | .42142 | .10218 | .20589 | .10709 | .00007 | .60884 | .00006 | .64115 | .47294 | .06595 | .00100 |
P values from Spearman correlation have been reported here; for corresponding R values, see Table E2.
Table E4.
Diagnostic performance of each characteristic in the determination of peanut allergy
| Characteristic | AUC | Lower bound (95% CI) | Upper bound (95% CI) | Optimal Cutoff | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|
| Peanut-sIgE (kUA/L) | 0.752 | 0.658 | 0.845 | 21.25 | 32.29 | 93.10 |
| Ara h 1 sIgE (kUA/L) | 0.668 | 0.559 | 0.777 | 2.68 | 32.86 | 90.00 |
| Ara h 2 sIgE (kUA/L) | 0.916 | 0.857 | 0.975 | 0.28 | 82.00 | 94.00 |
| Ara h 3 sIgE (kUA/L) | 0.672 | 0.563 | 0.780 | 1.10 | 33.33 | 87.10 |
| Ara h 6 sIgE (kUA/L) | 0.908 | 0.846 | 0.970 | 0.32 | 82.00 | 90.00 |
| Ara h 8 sIgE (kUA/L) | 0.624 | 0.511 | 0.737 | 0.02 | 60.00 | 31.43 |
| Ara h 9 sIgE (kUA/L) | 0.468 | 0.354 | 0.581 | 0.86 | 14.58 | 82.69 |
| Peanut S A (%) | 0.862 | 0.793 | 0.932 | 1.79 | 76.00 | 80.00 |
| Ara h 1 S A (%) | 0.716 | 0.613 | 0.820 | 2.29 | 44.00 | 100.00 |
| Ara h 2 S A (%) | 0.933 | 0.885 | 0.981 | 0.12 | 92.00 | 84.00 |
| Ara h 3 S A (%) | 0.738 | 0.639 | 0.837 | 0.05 | 64.00 | 80.00 |
| Ara h 6 S A (%) | 0.930 | 0.878 | 0.981 | 0.29 | 82.00 | 92.00 |
| Ara h 8 S A (%) | 0.618 | 0.507 | 0.730 | 0.043 | 54.00 | 72.00 |
| Ara h 9 S A (%) | 0.465 | 0.351 | 0.578 | 0.015 | 40.00 | 62.00 |
| PD | 0.898 | 0.831 | 0.964 | 0.48 | 84.00 | 88.00 |
| WD | 0.628 | 0.517 | 0.739 | 1.58 | 74.00 | 56.00 |
| Avidity (%inhibition)∗ | 0.795 | 0.681 | 0.910 | 37.23 | 61.10 | 87.50 |
The AUCs, respective 95% CIs, optimal cutoffs, sensitivity, and specificity have been reported alongside each characteristic.
Avidity cohort, n = 60.
Table E5.
Pairwise comparison of ROC analyses in the same sample cohort (n = 100)
| Characteristic comparisons | Diagnostic performance (ROC analyses) |
Pairwise comparison 8of AUCs (P value) | |||||
|---|---|---|---|---|---|---|---|
| AUC | Lower bound (95% CI) | Upper bound (95% CI) | AUC | Lower bound (95% CI) | Upper bound (95% CI) | ||
| Peanut-sIgE (kUA/L)-Peanut SA (%) | 0.752 | 0.658 | 0.845 | 0.862 | 0.793 | 0.932 | .0038 |
| Ara h 1 sIgE (kUA/L)-Ara h 1 SA (%) | 0.668 | 0.559 | 0.777 | 0.716 | 0.613 | 0.820 | .0898 |
| Ara h 2 sIgE (kUA/L)-Ara h 2 SA (%) | 0.916 | 0.857 | 0.975 | 0.933 | 0.885 | 0.981 | .5415 |
| Ara h 3 sIgE (kUA/L)-Ara h 3 SA (%) | 0.672 | 0.563 | 0.780 | 0.738 | 0.639 | 0.837 | .0664 |
| Ara h 6 sIgE (kUA/L)-Ara h 6 SA (%) | 0.908 | 0.846 | 0.970 | 0.930 | 0.878 | 0.981 | .2420 |
| Ara h 8 sIgE (kUA/L)-Ara h 8 SA (%) | 0.624 | 0.511 | 0.737 | 0.618 | 0.507 | 0.730 | .8743 |
| Ara h 9 sIgE (kUA/L)-Ara h 9 SA (%) | 0.468 | 0.354 | 0.581 | 0.465 | 0.351 | 0.578 | .9552 |
| WD-PD | 0.628 | 0.517 | 0.739 | 0.898 | 0.831 | 0.964 | <.0001 |
Comparison of AUC paired data ROC curves as described by deLong et al.E1
Table E6.
Diagnostic performance of selected characteristics in the determination of peanut allergy, in reduced study cohort used for avidity assays, as governed by the areas under the ROC curve
| Characteristic | AUC | Lower bound (95% CI) | Upper bound (95% CI) |
|---|---|---|---|
| sIgE to peanut (kUA/L) | 0.760 | 0.641 | 0.880 |
| sIgE to Ara h 2 (kUA/L) | 0.944 | 0.885 | 1.000 |
| sIgE to Ara h 6 (kUA/L) | 0.947 | 0.889 | 1.000 |
| Peanut SA (%) | 0.843 | 0.746 | 0.939 |
| Ara h 2 SA (%) | 0.970 | 0.933 | 1.000 |
| Ara h 6 SA (%) | 0.954 | 0.899 | 1.000 |
| PD | 0.931 | 0.866 | 0.995 |
| WD | 0.505 | 0.348 | 0.661 |
| Avidity (%inhibition) | 0.795 | 0.681 | 0.910 |
The AUCs and their respective 95% CIs were generated in the cohort described in Table E1 and allows for comparison of diagnostic performance in the reduced study cohort.
Table E7.
Simple multivariable logistic regression models (n = 90), in which combinations of functional IgE characteristics were used to predict %CD63+ LAD2 cell activation
| Supplementary model no. | Characteristic | Unstandardized β-coefficient | Coefficient SE | Standardized β-coefficient | P value | R2 value | VIF |
|---|---|---|---|---|---|---|---|
| S1 | PD | 22.005 | 3.196 | 0.588 | <.0001 | 0.411 | 1.101 |
| WD | 3.894 | 2.143 | 0.155 | .073 | 1.101 | ||
| S2 | Peanut-sIgE | 0.050 | 0.016 | 0.279 | .003 | 0.454 | 1.322 |
| Peanut SA | 0.765 | 0.142 | 0.491 | <.0001 | 1.322 | ||
| S3 | PD | 15.752 | 3.260 | 0.421 | <.0001 | 0.523 | 1.381 |
| Peanut SA | 0.635 | 0.136 | 0.407 | <.0001 | 1.381 |
Unstandardized and standardized β-coefficients are reported alongside significance and variance inflation factor (VIF). VIF >10 was used to indicate any collinearity.
Table E8.
Simple linear regression models, in which functional IgE characteristics were used to predict %CD63+ basophil activation
| Characteristic | Unstandardized β-coefficient | Coefficient SE | Standardized β- coefficient | P value | R2 value |
|---|---|---|---|---|---|
| Peanut-sIgE | 0.151 | 0.019 | 0.737 | <.0001 | 0.543 |
| Ara h 1 sIgE | 0.392 | 0.067 | 0.632 | <.0001 | 0.399 |
| Ara h 2 sIgE | 0.338 | 0.041 | 0.756 | <.0001 | 0.564 |
| Ara h 3 sIgE | 0.875 | 0.130 | 0.682 | <.0001 | 0.465 |
| Ara h 6 sIgE | 0.674 | 0.091 | 0.717 | <.0001 | 0.514 |
| Ara h 8 sIgE | −0.232 | 0.280 | −0.114 | .410 | 0.130 |
| Ara h 9 sIgE | −0.011 | 1.783 | −0.001 | .995 | 0.000 |
| Peanut SA | 1.434 | 0.169 | 0.762 | <.0001 | 0.581 |
| Ara h 1 SA | 3.772 | 0.584 | 0.584 | <.0001 | 0.445 |
| Ara h 2 SA | 2.119 | 0.322 | 0.674 | <.0001 | 0.455 |
| Ara h 3 SA | 11.606 | 1.555 | 0.719 | <.0001 | 0.517 |
| Ara h 6 SA | 5.681 | 0.908 | 0.655 | <.0001 | 0.429 |
| Ara h 8 SA | −1.692 | 2.109 | −0.111 | .426 | 0.012 |
| Ara h 9 SA | −2.567 | 5.088 | −0.070 | .616 | 0.005 |
| WD | 8.378 | 4.315 | 0.260 | .058 | 0.068 |
| PD | 40.432 | 3.578 | 0.843 | <.0001 | 0.711 |
| Avidity | 0.635 | 0.378 | 0.281 | .102 | 0.079 |
Unstandardized and standardized β-coefficients are reported alongside significance (n = 54).
Table E9.
Simple multivariable linear regression models, in which combinations of functional IgE characteristics were used to predict %CD63+ basophil activation (n = 54)
| Model no. | Characteristic | Unstandardized β-coefficient | Coefficient SE | Standardized β-coefficient | P value | R2 value | VIF |
|---|---|---|---|---|---|---|---|
| S4∗ | Peanut-sIgE | 0.043 | 0.025 | 0.213 | .098 | 0.765 | 2.045 |
| PD | 38.851 | 7.119 | 0.751 | <.0001 | 2.50 | ||
| Avidity | −0.230 | 0.227 | −0.102 | .317 | 1.326 | ||
| S5∗ | Peanut SA | 0.616 | 0.187 | 0.335 | .002 | 0.809 | 1.690 |
| PD | 35.673 | 5.787 | 0.690 | <.0001 | 2.037 | ||
| Avidity | −0.243 | 0.200 | −0.108 | .234 | 1.280 | ||
| S6∗ | Peanut-sIgE | 0.138 | 0.025 | 0.688 | <.0001 | 0.540 | 1.041 |
| WD | 1.372 | 6.740 | 0.025 | .840 | 1.007 | ||
| Avidity | 0.352 | 0.280 | 0.156 | .219 | 1.037 | ||
| S7∗ | Peanut SA | 1.374 | 0.214 | 0.748 | <.0001 | 0.607 | 1.071 |
| WD | 9.911 | 6.270 | 0.180 | .124 | 1.021 | ||
| Avidity | 0.280 | 0.260 | 0.124 | .290 | 1.049 | ||
| S8 | PD | 41.936 | 3.885 | 0.874 | <.0001 | 0.716 | 1.179 |
| WD | −2.594 | 2.610 | −0.81 | .325 | 1.179 | ||
| S9 | Peanut-sIgE | 0.098 | 0.016 | 0.482 | <.0001 | 0.760 | 1.299 |
| Peanut SA | 0.998 | 0.147 | 0.531 | <.0001 | 1.299 | ||
| S10 | PD | 28.841 | 3.927 | 0.601 | <.0001 | 0.796 | 1.680 |
| Peanut SA | 0.714 | 0.154 | 0.380 | <.0001 | 1.680 |
Unstandardized and standardized β-coefficients are reported alongside significance and variance inflation factor (VIF). VIF >10 was used to indicate any collinearity. For models containing avidity as an input characteristic, n = 35 is observed.
For models that include avidity analyses, n = 35.
References
- 1.Klemans R.J.B., van Os-Medendorp H., Blankestijn M., Bruijnzeel-Koomen C.A.F.M., Knol E.F., Knulst A.C. Diagnostic accuracy of specific IgE to components in diagnosing peanut allergy: a systematic review. Clin Exp Allergy. 2015;45:720–730. doi: 10.1111/cea.12412. [DOI] [PubMed] [Google Scholar]
- 2.Soares-Weiser K., Takwoingi Y., Panesar S.S., Muraro A., Werfel T., Hoffmann-Sommergruber K. The diagnosis of food allergy: a systematic review and meta-analysis. Allergy. 2014;69:76–86. doi: 10.1111/all.12333. [DOI] [PubMed] [Google Scholar]
- 3.Klemans R.J.B., Broekman H.C.H.P., Knol E.F., Bruijnzeel-Koomen C.A.F.M., Otten H.G., Pasmans S.G.M.A. Ara h 2 is the best predictor for peanut allergy in adults. J Allergy Clin Immunol Pract. 2013;1:632–638.e1. doi: 10.1016/j.jaip.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 4.Dang T.D., Tang M., Choo S., Licciardi P.V., Koplin J.J., Martin P.E. Increasing the accuracy of peanut allergy diagnosis by using Ara h 2. J Allergy Clin Immunol. 2012;129:1056–1063. doi: 10.1016/j.jaci.2012.01.056. [DOI] [PubMed] [Google Scholar]
- 5.Santos A.F., Douiri A., Bécares N., Wu S.-Y., Stephens A., Radulovic S. Basophil activation test discriminates between allergy and tolerance in peanut-sensitized children. J Allergy Clin Immunol. 2014;134:645–652. doi: 10.1016/j.jaci.2014.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christensen L.H., Holm J., Lund G., Riise E., Lund K. Several distinct properties of the IgE repertoire determine effector cell degranulation in response to allergen challenge. J Allergy Clin Immunol. 2008;122:298–304. doi: 10.1016/j.jaci.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 7.MacGlashan D.W., Saini S.S. Omalizumab increases the intrinsic sensitivity of human basophils to IgE-mediated stimulation. J Allergy Clin Immunol. 2013;132:906–911.e4. doi: 10.1016/j.jaci.2013.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santos A.F., Couto-Francisco N., Bécares N., Kwok M., Bahnson H.T., Lack G. A novel human mast cell activation test for peanut allergy. J Allergy Clin Immunol. 2018;142:689–691.e9. doi: 10.1016/j.jaci.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirshenbaum A.S., Akin C., Wu Y., Rottem M., Goff J.P., Beaven M.A. Characterization of novel stem cell factor responsive human mast cell lines LAD 1 and 2 established from a patient with mast cell sarcoma/leukemia: activation following aggregation of FcepsilonRI or FcgammaRI. Leuk Res. 2003;27:677–682. doi: 10.1016/s0145-2126(02)00343-0. [DOI] [PubMed] [Google Scholar]
- 10.Hemmings O., Du Toit G., Radulovic S., Lack G., Santos A.F. Ara h 2 is the dominant peanut allergen despite similarities with Ara h 6. J Allergy Clin Immunol. 2020;146:621–630.e5. doi: 10.1016/j.jaci.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta R.S., Lau C.H., Hamilton R.G., Donnell A., Newhall K.K. Predicting outcomes of oral food challenges by using the allergen-specific IgE–total IgE ratio. J Allergy Clin Immunol Pract. 2014;2:300–305. doi: 10.1016/j.jaip.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Horimukai K., Hayashi K., Tsumura Y., Nomura I., Narita M., Ohya Y. Total serum IgE level influences oral food challenge tests for IgE-mediated food allergies. Allergy. 2015;70:334–337. doi: 10.1111/all.12562. [DOI] [PubMed] [Google Scholar]
- 13.Dehlink E., Baker A.H., Yen E., Nurko S., Fiebiger E. Relationships between levels of serum IgE, cell-bound IgE, and IgE-receptors on peripheral blood cells in a pediatric population. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flinterman A.E., Knol E.F., Lencer D.A., Bardina L., den Hartog Jager C.F., Lin J. Peanut epitopes for IgE and IgG4 in peanut-sensitized children in relation to severity of peanut allergy. J Allergy Clin Immunol. 2008;121:737–743.e10. doi: 10.1016/j.jaci.2007.11.039. [DOI] [PubMed] [Google Scholar]
- 15.Shreffler W.G., Beyer K., Chu T.-H.T., Burks A.W., Sampson H.A. Microarray immunoassay: association of clinical history, in vitro IgE function, and heterogeneity of allergenic peanut epitopes. J Allergy Clin Immunol. 2004;113:776–782. doi: 10.1016/j.jaci.2003.12.588. [DOI] [PubMed] [Google Scholar]
- 16.Patil S.U., Shreffler W.G. Immunology in the Clinic Review Series; focus on allergies: basophils as biomarkers for assessing immune modulation. Clin Exp Immunol. 2012;167:59–66. doi: 10.1111/j.1365-2249.2011.04503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mita H., Yasueda H., Akiyama K. Affinity of IgE antibody to antigen influences allergen-induced histamine release. Clin Exp Allergy. 2000;30:1583–1589. doi: 10.1046/j.1365-2222.2000.00921.x. [DOI] [PubMed] [Google Scholar]
- 18.Chardin H., Mercier K., Frydman C., Vollmer N. Surface plasmon resonance imaging: a method to measure the affinity of the antibodies in allergy diagnosis. J Immunol Methods. 2014;405:23–28. doi: 10.1016/j.jim.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 19.Roberts G., Lack G. Diagnosing peanut allergy with skin prick and specific IgE testing. J Allergy Clin Immunol. 2005;115:1291–1296. doi: 10.1016/j.jaci.2005.02.038. [DOI] [PubMed] [Google Scholar]
- 20.Fox A.T., Sasieni P., du Toit G., Syed H., Lack G. Household peanut consumption as a risk factor for the development of peanut allergy. J Allergy Clin Immunol. 2009;123:417–423. doi: 10.1016/j.jaci.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 21.Poirier M.P., Ahlstedt S., Ford J., Dolen W.K. Use of thiocyanate elution to estimate relative avidity of allergen specific IgE antibodies. Allergy Asthma Proc. 1997;18:359–362. doi: 10.2500/108854197778558025. [DOI] [PubMed] [Google Scholar]
- 22.Almanzar G., Ottensmeier B., Liese J., Prelog M. Assessment of IgG avidity against pertussis toxin and filamentous hemagglutinin via an adapted enzyme-linked immunosorbent assay (ELISA) using ammonium thiocyanate. J Immunol Methods. 2013;387:36–42. doi: 10.1016/j.jim.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Morris E.K., Caruso T., Buscot F., Fischer M., Hancock C., Maier T.S. Choosing and using diversity indices: insights for ecological applications from the German Biodiversity Exploratories. Ecol Evol. 2014;4:3514–3524. doi: 10.1002/ece3.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santos A.F., James L.K., Bahnson H.T., Shamji M.H., Couto-Francisco N.C., Islam S. IgG4 inhibits peanut-induced basophil and mast cell activation in peanut-tolerant children sensitized to peanut major allergens. J Allergy Clin Immunol. 2015;135:1249–1256. doi: 10.1016/j.jaci.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sampson H.A. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol. 2001;107:891–896. doi: 10.1067/mai.2001.114708. [DOI] [PubMed] [Google Scholar]
- 26.Santos A.F., Barbosa-Morais N.L., Hurlburt B.K., Ramaswamy S., Hemmings O., Kwok M. IgE to epitopes of Ara h 2 enhance the diagnostic accuracy of Ara h 2-specific IgE. Allergy. 2020;75:2309–2318. doi: 10.1111/all.14301. [DOI] [PubMed] [Google Scholar]
- 27.Nicolaou N., Murray C., Belgrave D., Poorafshar M., Simpson A., Custovic A. Quantification of specific IgE to whole peanut extract and peanut components in prediction of peanut allergy. J Allergy Clin Immunol. 2011;127:684–685. doi: 10.1016/j.jaci.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 28.Nicolaou N., Poorafshar M., Murray C., Simpson A., Winell H., Kerry G. Allergy or tolerance in children sensitized to peanut: prevalence and differentiation using component-resolved diagnostics. J Allergy Clin Immunol. 2010;125:191–197.e13. doi: 10.1016/j.jaci.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 29.Handlogten M.W., Kiziltepe T., Serezani A.P., Kaplan M.H., Bilgicer B. Inhibition of weak-affinity epitope-IgE interactions prevents mast cell degranulation. Nat Chem Biol. 2013;9:789–795. doi: 10.1038/nchembio.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tam I.Y.S., Lau H.Y.A., Tam S.Y., Lee T.H. Mast cell activation test using patient-derived mast cells exhibits distinct combinatorial phenotypic profiles among allergic patients. Allergy. 2020;75:1796–1799. doi: 10.1111/all.14225. [DOI] [PubMed] [Google Scholar]
- 31.Johansson S.G., Nopp A., Oman H., Ankerst J., Cardell L.O., Gronneberg R. The size of the disease relevant IgE antibody fraction in relation to ‘total-IgE’ predicts the efficacy of anti-IgE (Xolair) treatment. Allergy. 2009;64:1472–1477. doi: 10.1111/j.1398-9995.2009.02051.x. [DOI] [PubMed] [Google Scholar]
- 32.Grabenhenrich L., Lange L., Härtl M., Kalb B., Ziegert M., Finger A. The component-specific to total IgE ratios do not improve peanut and hazelnut allergy diagnoses. J Allergy Clin Immunol. 2016;137:1751–1760.e8. doi: 10.1016/j.jaci.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 33.Pierson-Mullany L.K., Jackola D.R., Blumenthal M.N., Rosenberg A. Evidence of an affinity threshold for IgE-allergen binding in the percutaneous skin test reaction. Clin Exp Allergy. 2002;32:107–116. doi: 10.1046/j.0022-0477.2001.01244.x. [DOI] [PubMed] [Google Scholar]
- 34.Gowthaman U., Chen J.S., Zhang B., Flynn W.F., Lu Y., Song W. Identification of a T follicular helper cell subset that drives anaphylactic IgE. Science. 2019;365 doi: 10.1126/science.aaw6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim K.E., Rosenberg A., Roberts S., Blumenthal M.N. The affinity of allergen specific IgE and the competition between IgE and IgG for the allergen in Amb a V sensitive individuals. Mol Immunol. 1996;33:873–880. doi: 10.1016/0161-5890(96)84613-x. [DOI] [PubMed] [Google Scholar]
- 36.Collins A.M., Basil M., Nguyen K., Thelian D. Rat basophil leukaemia (RBL) cells sensitized with low affinity IgE respond to high valency antigen. Clin Exp Allergy. 1996;26:964–970. [PubMed] [Google Scholar]
- 37.Blackburn N.K., Besselaar T.G., Schoub B.D., O’Connell K.F. Differentiation of primary cytomegalovirus infection from reactivation using the urea denaturation test for measuring antibody avidity. J Med Virol. 1991;33:6–9. doi: 10.1002/jmv.1890330103. [DOI] [PubMed] [Google Scholar]
- 38.Thomas H.I.J., Morgan-Capner P., Enders G., O’Shea S., Caldicott D., Best J.M. Persistence of specific IgM and low avidity specific IgG1 following primary rubella. J Virol Methods. 1992;39:149–155. doi: 10.1016/0166-0934(92)90133-x. [DOI] [PubMed] [Google Scholar]
- 39.Pullen G.R., Fitzgerald M.G., Hosking C.S. Antibody avidity determination by ELISA using thiocyanate elution. J Immunol Methods. 1986;86:83–87. doi: 10.1016/0022-1759(86)90268-1. [DOI] [PubMed] [Google Scholar]
- 40.El-Khouly F., Lewis S.A., Pons L., Burks A.W., Hourihane J.O.B. IgG and IgE avidity characteristics of peanut allergic individuals. Pediatr Allergy Immunol. 2007;18:607–613. doi: 10.1111/j.1399-3038.2007.00542.x. [DOI] [PubMed] [Google Scholar]
- 41.Asrat S., Kaur N., Liu X., Ben L.-H., Kajimura D., Murphy A.J. Chronic allergen exposure drives accumulation of long-lived IgE plasma cells in the bone marrow, giving rise to serological memory. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.aav8402. [DOI] [PubMed] [Google Scholar]
- 42.Custovic A., Sonntag H.-J., Buchan I.E., Belgrave D., Simpson A., Prosperi M.C.F. Evolution pathways of IgE responses to grass and mite allergens throughout childhood. J Allergy Clin Immunol. 2015;136:1645–1652.e8. doi: 10.1016/j.jaci.2015.03.041. [DOI] [PubMed] [Google Scholar]
- 43.Hatzler L., Panetta V., Lau S., Wagner P., Bergmann R.L., Illi S. Molecular spreading and predictive value of preclinical IgE response to Phleum pratense in children with hay fever. J Allergy Clin Immunol. 2012;130:894–901.e5. doi: 10.1016/j.jaci.2012.05.053. [DOI] [PubMed] [Google Scholar]
- 44.Shannon C.E. A mathematical theory of communication. Bell System Techn J. 1948;27:379–423. [Google Scholar]
- 45.Nagendra H. Opposite trends in response for the Shannon and Simpson indices of landscape diversity. Appl Geogr. 2002;22:175–186. [Google Scholar]
- 46.Ylescupidez A., Du Toit G., Salavoura K., Brough H., Radulovic S., Bahnson H.T. Longitudinal trajectories of eczema severity, duration, and affected body-region predict risk of food allergy in combination with filaggrin gene mutations. Allergy. 2020;75:63–64. [Google Scholar]
- 47.Shade K.-T.C., Conroy M.E., Washburn N., Kitaoka M., Huynh D.J., Laprise E. Sialylation of immunoglobulin E is a determinant of allergic pathogenicity. Nature. 2020;582:265–270. doi: 10.1038/s41586-020-2311-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Di Lorenzo G., Mansueto P., Pacor M.L., Rizzo M., Castello F., Martinelli N. Evaluation of serum s-IgE/total IgE ratio in predicting clinical response to allergen-specific immunotherapy. J Allergy Clin Immunol. 2009;123:1103–1110.e4. doi: 10.1016/j.jaci.2009.02.012. [DOI] [PubMed] [Google Scholar]
Reference
- DeLong E.R., DeLong D.M., Clarke-Pearson D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]