Abstract
Aim
Approximately one‐third of patients with major depressive disorder develop treatment‐resistant depression. One‐third of patients with treatment‐resistant depression demonstrate resistance to ketamine, which is a novel antidepressant effective for this disorder. The objective of this study was to examine the utility of resting‐state functional magnetic resonance imaging for the prediction of treatment response to ketamine in treatment‐resistant depression.
Methods
An exploratory seed‐based resting‐state functional magnetic resonance imaging analysis was performed to examine baseline resting‐state functional connectivity differences between ketamine responders and nonresponders before treatment with multiple intravenous ketamine infusions.
Results
Fifteen patients with treatment‐resistant depression received multiple intravenous subanesthetic (0.5 mg/kg/40 minutes) ketamine infusions, and nine were identified as responders. The exploratory resting‐state functional magnetic resonance imaging analysis identified a cluster of significant baseline resting‐state functional connectivity differences associating ketamine response between the amygdala and subgenual anterior cingulate gyrus in the right hemisphere. Using anatomical region of interest analysis of the resting‐state functional connectivity, ketamine response was predicted with 88.9% sensitivity and 100% specificity. The resting‐state functional connectivity of significant group differences between responders and nonresponders retained throughout the treatment were considered a trait‐like feature of heterogeneity in treatment‐resistant depression.
Conclusion
This study suggests the possible clinical utility of resting‐state functional magnetic resonance imaging for predicting the antidepressant effects of ketamine in treatment‐resistant depression patients and implicated resting‐state functional connectivity alterations to determine the trait‐like pathophysiology underlying treatment response heterogeneity in treatment‐resistant depression.
Keywords: functional connectivity, ketamine, resting‐state functional MRI, treatment response prediction, treatment‐resistant depression
This study illustrates that the alteration in the RSFC within the right AN in TRD patients reflects the antidepressant response to ketamine at baseline. The alteration remained throughout the 2‐week treatment with multiple ketamine infusions and seemed to reflect the trait‐like features underlying treatment heterogeneity in TRD. By employing an anatomical ROI of the sc/sgACC, the present study also suggests the possible clinical utility of the rsfMRI to predict the treatment response to ketamine in TRD patients.

1. INTRODUCTION
Major depressive disorder (MDD) is a highly heterogeneous disorder in terms of treatment response. Several patients show improvement with placebo; however, one‐fourth of patients show insufficient treatment response despite application of multiple adequate pharmacological treatment options. 1 MDD showing insufficient response to treatment following two different types of antidepressants is known as treatment‐resistant depression (TRD). 2 This treatment response heterogeneity makes treatment optimization an obstacle for patients. Predicting treatment response at an early stage of MDD and choosing an optimized treatment for each individual are critical issues. Despite numerous efforts to identify treatment response predictors in multiple domains, 3 to date, no single pretreatment variable has been distinguished as a robust predictor.
Ketamine is a conventional dissociative anesthetic agent acting on the N‐methyl‐D‐aspartate (NMDA) receptor. In this decade, ketamine has gathered considerable attention as a novel antidepressant agent applicable to TRD. The single application of a subanesthetic intravenous or intranasal dose of ketamine shows a remarkable antidepressant effect in TRD patients 4 ; however, nearly 40% of TRD patients exhibit resistance to ketamine. 5 Heterogeneity in treatment response to ketamine has been shown, which has been used to divide TRD into ketamine responders and nonresponders. Previous studies have investigated the neural substrates of ketamine response using clinical variables, biochemistry, or neuroimaging. Rong et al 6 concluded that a high body mass index (BMI) and family history of alcohol use in a first‐degree relative (family history positive [FHP]) are the most consistent predictors of ketamine response reported to date. However, their biological roles in the antidepressant effects of ketamine are uncertain.
Neuroimaging has greatly contributed to the progress of brain science in recent decades. 3 Resting‐state functional magnetic resonance imaging (rsfMRI) has been developed as a noninvasive, task burden‐free approach, which allows for the identification of spontaneous neural activities. Among the properties of spontaneous neural activities identified using rsfMRI, examining the resting‐state functional connectivity (RSFC) between two or more brain regions would help understand the construction of large‐scale brain networks and their pathophysiology. Several studies have reported different RSFC alterations in large‐scale remote neural networks underlying the pathophysiology 7 or treatment response heterogeneity 8 of MDD. Sheline et al 9 demonstrated large‐scale RSFC disruptions of specific brain networks, including the cognitive control network, default mode network (DMN), and affective network (AN) in MDD patients. These remote networks compose neural subsystems associated with different domains of human cognition. The cognitive control network plays an important role in attention and/or working memory demanding cognitive tasks. In contrast, the DMN is associated with task negative cognitive domains such as self‐referential processing. The AN affects vigilance, autonomic function, visceral function, and behavioral selection driven by emotional stimuli. 9 Since MDD widely affects the brain functions associated with these remote subnetworks, its roles in these remote networks in the pathophysiology of MDD have been highlighted. Among these networks, converging evidence from previous imaging studies has suggested that the activity of the AN and RSFC of the DMN play critical roles in the treatment response heterogeneity of MDD. 10 , 11 Long et al recently highlighted with their meta‐analysis that the baseline RSFC within the DMN predicts treatment response in MDD regardless of treatment type. 12
Regarding ketamine, studies have demonstrated that subanesthetic doses alter brain activities including RSFC. A single application of ketamine changes the RSFC in the DMN of healthy individuals. 13 Moreover, ketamine treatment changes the reactivity of the neural components in the AN, 14 , 15 regional cerebral blood flow, 16 specific RSFCs, 17 , 18 and global brain functional connectivity 19 in TRD. However, the details of RSFC alteration in terms of treatment response heterogeneity in TRD patients are largely unknown. Furthermore, the clinical utilization of rsfMRI for prediction of treatment response in TRD has not been verified.
In the present study, based on our hypothesis that RSFC alterations within the AN or DMN represent the neural basis for treatment response heterogeneity in TRD, we assessed a new potential predictor of antidepressant effects of ketamine using rsfMRI, which would contribute to treatment optimization in TRD patients.
2. METHODS
2.1. Ethics statement
The study was approved by the Ethics Committee of the Kurume University School of Medicine, Kurume, Japan and performed in accordance with the Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects (https://www.mhlw.go.jp/file/06-Seisakujouhou-10600000-Daijinkanboukouseikagakuka/0000153339.pdf). All patients provided written informed consent before screening.
2.2. Patients
Participants were patients with TRD aged between 20 and 69 years, recruited from the outpatient clinic of Kurume University Hospital and related clinics from August 2014 to March 2019. To be eligible for the present study, patients diagnosed with MDD or bipolar disorder (BD) had to fulfill the criteria specified by the International Classification of Diseases, 10th revision and the following TRD criteria: 1. Patients diagnosed with MDD who had failed to respond to at least two suitable antidepressant agents with adequate dosage and duration and at least one adequate treatment course with a mood stabilizer or antipsychotics (monotherapy or augmentation) associated with the current episode; 2. Patients who met the BD criteria who had failed to respond to adequate treatments with a mood stabilizer or to an atypical‐antipsychotic in the current episode; 3. Patients with a previous history of any mood episode with mood‐incongruent psychotic features and suicidal events, with severe medical conditions, and with specific physical risks relating to ketamine use were excluded from the study. For the original purposes of the UMIN‐CTR No. UMIN000017529 study, additional exclusion criteria were applied regarding the safety of lithium treatment. The full inclusion and exclusion criteria are presented in Appendix S1.
Data including demographics and clinical characteristics, sex, age, illness duration, length of current episodes, family history of psychiatric disorders, educational history, and medication history were collected from all participants.
2.3. Treatment and psychometric measures
Ketamine treatment and rsfMRI assessment were performed on admission. On the day of admission, participants were randomly assigned to the placebo or the lithium groups via double‐blind randomization to examine the primary aim of the study—to assess the efficacy and safety of adjunctive lithium pretreatment before ketamine treatment. The participants were administered oral placebo or lithium carbonate (600‐800 mg) daily throughout the study. After the randomization, participants received up to four open‐label infusions of racemic ketamine hydrochloride (0.5 mg/kg/40 min) twice weekly over 2 weeks, following discontinuation of their antidepressant medications tapered over a 7‐ to 10‐day period. Ketamine was administered by a designated anesthesiologist under physical monitoring. Participants could withdraw from the study at any time. Discontinuation was considered according to each participant's physical and psychiatric risks (see Appendix S1). Treatment response was measured as an improvement of the Montgomery‐Asberg Depression Rating Scale (MADRS) 20 4 hours after each participant's last administration of ketamine. MADRS scoring was performed using the SIGMA structured interview. 21 Mood elevation was measured using the Young Mania Rating Scale (YMRS) 22 throughout the study. Full and partial responders were defined as patients who had a greater than 50% decrease or 40% to 50% decrease in the MADRS score after treatment, respectively. The schematic overview of the study design is shown in Figure 1.
FIGURE 1.
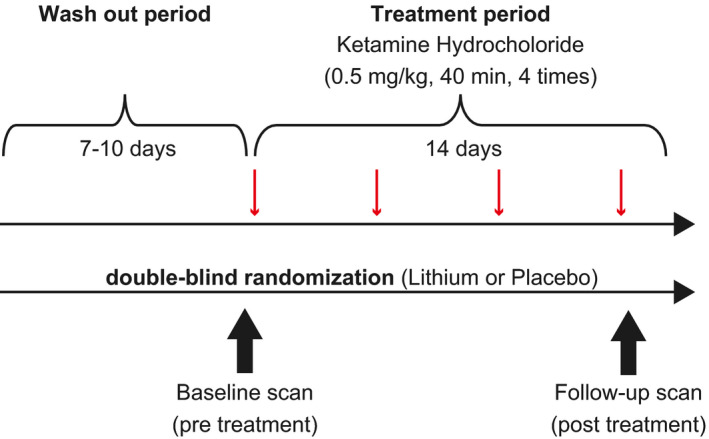
Schematic overview of the study design. Intravenous racemic ketamine infusion was scheduled four times in 2 weeks, following the wash out period, under the double‐blind random assignment of pretreatment with lithium carbonate (600‐800 mg/day) or placebo. Resting‐state fMRI scans were performed before treatment (baseline; 16 hours before the first ketamine administration) and after the last infusion (follow‐up; 6‐24 hours after the last infusion) of ketamine.fMRI, functional magnetic resonance imaging
2.4. Resting‐state fMRI procedure
2.4.1. Image acquisition
RsfMRI scans were scheduled 16 hours before the first ketamine administration (baseline) and 6‐24 hours after the last ketamine treatment (follow‐up). All imaging data were acquired using a GE Discovery 3.0 Tesla MRI scanner (Discovery MR750; General Electric, Milwaukee, WI). Structural images for spatial registration were obtained using T1 weighted high‐resolution three‐dimensional spoiled gradient‐recalled (SPGR) acquisition (repetition time = 1100 ms, echo time = 5 ms, inversion time = 450 ms, field of view = 220 mm × 220 mm, matrix = 420 × 224, flip angle = 12°, voxel size = 0.5 × 0.5 × 1 mm3). A gradient‐echo planer imaging (EPI) sequence sensitive to blood oxygenation level‐dependent contrast (T2* weighting, repetition time/echo time = 3000/35 ms, field of view = 240 mm × 240 mm, data matrix = 128 × 128, flip angle = 60°, in‐place resolution = 1.875 mm × 1.875 mm, slice thickness = 4 mm, and 40 axial slices covering the whole brain) was used for rsfMRI data (200 volumes) acquisition.
2.4.2. fMRI imaging preprocessing
Primary Digital Imaging and Communications in Medicine images of each participant were converted into Neuroimaging Informatics Technology Initiative format using dcm2niigui software (http://www.cabiatl.com/mricro/mricron/dcm2nii.html). All images were analyzed using the FMRIB Software Library (FSL) 23 5.0.6 software package (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSL) including components called MELODIC (Multivariate Exploratory Linear Optimized Decomposition into Independent Components) and Randomise (FSL's tool for nonparametric permutation inference on neuroimaging data). First, to minimize physiological noise and scanner‐related artifacts, each of the four‐dimensional EPI images was processed using MELODIC. MELODIC decomposes obtained EPI time series data into different spatial and temporal components according to its algorithm of probabilistic independent component analysis. 24 Neuroimaging experts independently assessed each attribute obtained from each independent component manually by referring frequency power and spatial extent. Based on the a priori agreement whereby two or more evaluators considered that if a particular component reflected noise or artifact, the component was removed from the original data. The kappa statistics for these discrimination coincidences showed a kappa value of 0.885 (95% confidence interval: 0.8592‐0.9107). Second, using the FMRI Expert Analysis Tool (FEAT), after the removal of the first 10 volumes, the reconstructed “denoized” data were filtered by a high‐pass filter (cutoff 0.01 Hz). Further, images were also corrected for motion and slice acquisition timing, and brain volume masking was applied and, spatially smoothed using a 6‐mm full‐width‐half‐maximum Gaussian kernel. The obtained structural SPGR images of each participant were used for accurate spatial registration into the Montreal Neurological Institute (MNI) template of 2 × 2 × 2 mm spatial resolution.
2.4.3. Obtaining RSFC maps and analysis
Whole‐brain RSFC maps were obtained in a seed‐based manner. The a priori seed regions were determined for each hemispheric amygdala and bilateral ventral precuneus in the MNI space, referring to the Harvard‐Oxford Atlas. 25 The masks of these regions were transformed into individual EPI spaces, and the mean of individual time series signals of each specific region (seeds, white matter and cerebral ventricle) was extracted. The RSFC between the seed region and each voxel of the whole brain were computed as contrast of parameter estimates of the general linear modeling (GLM), which employed the extracted seed time series data as explanatory variables and FEAT was used for each patient and were scanned separately. Averaged signals of white matter and cerebral ventricle were included in the design matrices of the GLM as regressions for no interest.
To examine regions with significant differences in RSFC of ketamine responders and nonresponders within the AN or DMN at baseline, the two‐sample unpaired t‐test was applied for the exploratory group analysis using permutation inference via Randomise. The 5000 times repeated permutation was employed to obtain an empirical null distribution of values. The Threshold‐Fee‐Cluster‐Enhancement (TFCE) transformation 26 was later applied to find significant clusters [family wise errors (FEW) corrected, P <.05] within the masked standard MNI space. Corresponding masked brain regions comprising the AN and the DMN respectively, were based on previous imaging studies. Boundaries of bilateral regions of each network excluded seed regions defined based on the Harvard‐Oxford Atlas (Figure S1).
Based on the results of the exploratory group analysis, the cluster showing significant RSFC differences between responders and nonresponders at baseline was determined. Overlap of the cluster and the anatomical parcellation of the cingulate cortex was performed in accordance with Beckmann et al, 27 and the anatomical region including the cluster was determined (the target region). Using the transformed anatomical mask of the target region sectioned into individual areas, the averaged GLM and contrasts of parameter estimates were extracted using the fslmeants command‐line program at each scan (RSFC between the seed and the target; RSFCs‐t). Prediction of the antidepressant activity of ketamine was tested using extracted RSFCs‐t demographics and clinical characteristics at baseline using receiver operating characteristic (ROC) curves analysis. The relationship between the baseline RSFCs‐t and treatment response was verified using Spearman's Rank‐Order Correlation and the Mann‐Whitney U‐test. RSFCs‐t changes according to the treatment were examined by comparing the follow‐up rsfMRI scans between responders and nonresponders using the Mann‐Whitney U‐test.
Furthermore, RSFC changes according to symptomatic improvement via ketamine treatment in the AN and the DMN was examined using nonparametric permutation paired t‐test (5000 times permutation followed by TFCE, FEW‐corrected P <.05, using Randomise) comparing RSFC between the baseline scan and the follow‐up scan of the responders group.
2.5. Statistical analysis
Baseline demographics were compared between ketamine responders and nonresponders using chi‐squared or two‐sample unpaired t‐tests. Since lithium pretreatment showed a tendency to influence responses to ketamine, the impact of lithium pretreatment on extracted RSFCs‐t was examined by multiple regression analysis adjusting for previously reported demographic factors 6 associated with ketamine response. The relationship between demographics, RSFCs‐t at baseline, and antidepressant effects of ketamine throughout the study is summarized using sensitivity, specificity, positive, and negative predictive values.
All statistical analyses, other than computing three‐dimensional RSFC maps, were performed using JMP Pro 12 (SAS Institute). P <.05 was considered statistically significant.
3. RESULTS
3.1. Demographics, clinical characteristics, and treatment results
Enrolled participants included 17 patients with TRD, 16 diagnosed with MDD, and one with BD. Fifteen participants (all right‐handed, average baseline MADRS score: 27.6 ± 5.5) received ketamine infusion and 11 participants completed all four scheduled ketamine infusions. There were nine ketamine responders (60%), of whom eight were classified as full responders. The mean MADRS score decreased to 6.8 ± 2.5 for the responders group, while the nonresponders group maintained an average symptom score of 25.2 ± 6.4. Details of participant outcomes are shown in Figure S2.
Ketamine infusion produced mild mood elevation during infusion, but none of the patients developed manic symptoms as measured by the YMRS (average 2.1 ± 2.1) throughout the study. Adverse events occurred in 14 participants (Table S1), and the most common adverse event throughout the study was dissociation, during ketamine infusion (n = 12, 80%). Ketamine responders and nonresponders were not significantly different in age, sex, BMI, or clinical characteristics, as listed in Table 1. The lithium pretreatment group (n = 7) showed a tendency of increased response to ketamine treatment relative to the placebo group (n = 8) (P =.057, chi‐squared test).
TABLE 1.
Demographics, clinical characteristics, and ketamine treatment response of the participants
| Total (n = 15) | Responders (n = 9, 60%) | Nonresponders (n = 6, 40%) | Statistics | P‐value | |
|---|---|---|---|---|---|
| Sex (female), n (%) | 9 (60) | 4 (44.4) | 5 (83.3) | Χ2 = 2.268 | 0.132 |
| Age (years ± SD) | 45.9 ± 12.5 | 42.9 ± 9.8 | 50.3 ± 14.6 | t=−1.099 | 0.291 |
| BMI (kg/m2 ± SD) | 22.9 ± 4.9 | 22.9 ± 4.7 | 23.0 ± 5.3 | t=−0.037 | 0.970 |
| Years since first episode | 8.3 ± 5.81 | 8.0 ± 6.0 | 8.8 ± 5.5 | t=−0.245 | 0.810 |
| Length of current episodes (years ± SD) | 1.3 ± 2.1 | 0.8 ± 0.6 | 2.1 ± 3.1 | t=−0.939 | 0.390 |
| Family history of mood disorders n, (%) | 7 (46.6) | 4 (44.4) | 3 (50) | Χ2 = 0.044 | 0.832 |
| Education (years ± SD) | 14.1 ± 2.4 | 14.1 ± 2.2 | 14 ± 2.5 | t = 0.083 | 0.934 |
| Baseline MADRS score | 27.6 ± 5.5 | 27 ± 4.2 | 28.5 ± 6.8 | t=−0.489 | 0.632 |
| Lithium n, (%) | 7 (46.7) | 6 (75) | 1 (16.7) | Χ2 = 3.616 | 0.057 |
Results from the chi‐square test (Χ2 ) and unpaired t‐test (t).
BMI, body mass index; MADRS, Montgomery‐Asberg Depression Rating Scale; SD, standard deviation.
3.2. Imaging results
3.2.1. Seed‐based exploratory analysis
The seed‐based exploratory analysis of the main effect of a group difference resulted in a significant cluster defining ketamine responders and nonresponders between the amygdala seed region and the subgenual anterior cingulate cortex (sgACC) in the AN in the right hemisphere at baseline (FEW‐corrected, P corr < 0.05, peak at x: 4, y: 40, z: −4, t = 8.13, Figure 2A). The seed‐based exploratory analysis in the AN with left amygdala seed and the analysis in the DMN with precuneus seed illustrated no significant difference between ketamine responders and nonresponders at baseline (FEW‐corrected, P corr < 0.05).
FIGURE 2.
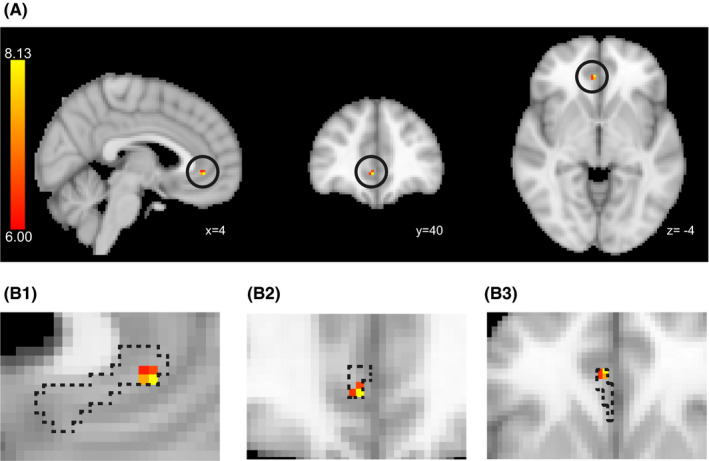
Significant cluster showing the significant RSFC differences between ketamine responders and nonresponders at baseline. A, Thresholded t map depicting the regions of significant RSFC difference between ketamine responders and nonresponders at baseline. Circles indicate the location of significant clusters (Right amygdala seed. Peak at x = 4, y = 38, z = −4; in MNI coordinates. The color bar indicates the t‐value). B, Overlap of the thresholded region and subdivision of the ACC. The dotted area indicates sc/sgACC defined by Beckmann et al 27 ). (B‐1) Sagittal, (B‐2) Coronal, (B‐3) Axial. The RSFCs‐t is defined as the RSFC between the dotted area and the right amygdala. ACC, anterior cingulate cortex; MNI, Montreal Neurological Institute; RSFC, resting‐state functional connectivity; scACC, subcallosal anterior cingulate cortex; sgACC, subgenual anterior cingulate cortex
3.2.2. Predictability of antidepressant effect of ketamine using the anatomical region of interest
Based on localization of the significant cluster revealed by the seed‐based exploratory analysis, we focused on the subdivision of the ACC defined by the preceding cortical parcellation study using diffusion tractography. 27 This subdivision (the dotted area in Figure 2B), lying across the sgACC to the subcallosal ACC (scACC), is characterized by rich neural connectivity to the amygdala. Employing this correct subdivision of the ACC as the target region of interest (ROI), we extracted the RSFCs‐t at each scan. We tested the predictability of antidepressant of ketamine using RSFCs‐t, demographics, or clinical characteristics at baseline. RSFCs‐t at baseline showed a significant negative correlation with improvement of depressive symptoms in terms of % MADRS reduction (Spearman's rank correlation coefficient, ρ = −0.56, P =.02, Figure 3A). When the 40% MADRS change was applied to the cutoff, the RSFCs‐t at baseline indicated a significant group difference between responders and nonresponders (Mann‐Whitney U‐test, P =.004, Figure 3B). The area under the ROC curve was 0.92 for RSFCs‐t (Figure 3C). When the RSFCs‐t cut‐off value of 0.041 was applied, the predicted antidepressant effect of ketamine had a sensitivity of 88.9%, specificity of 100%, positive predictive value of 100%, and negative predictive value of 85.7%. No demographics and clinical factors resulted in a meaningful prediction of the antidepressant effect of ketamine. The multiple regression adjustments for demographic factors showed no significant effect of lithium pretreatment outcome on RSFCs‐t.
FIGURE 3.
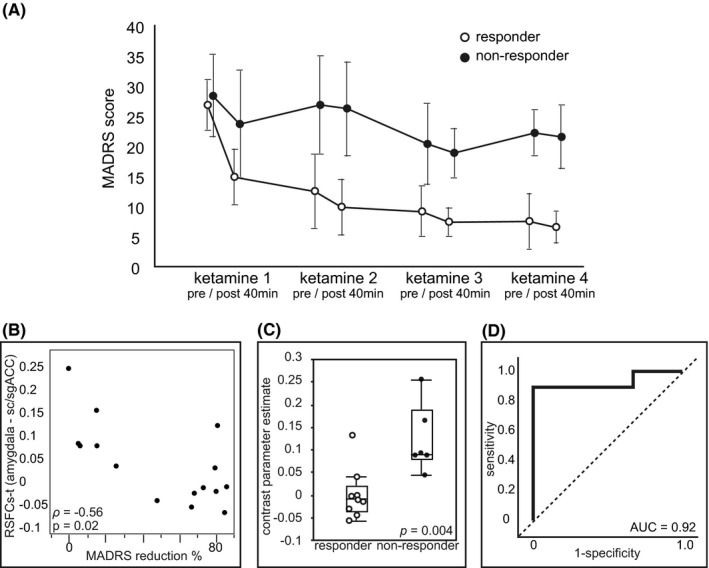
RSFC between the amygdala and the sc/sgACC in the right hemisphere (RSFCs‐t) at baseline predicts treatment response to ketamine. A, Time course change in depression severity as measured using the MADRS score (mean ± SD) throughout the study. Trajectories of depression severity are plotted for responder and nonresponder subgroups, defined using the final MADRS score. Each measurement corresponds to before and 40 minutes after each scheduled infusion of racemic ketamine (0.5 mg/kg/40 minutes). B, Distribution of baseline RSFCs‐t and %change in the MADRS score after the treatment. Correlation is significant with Spearman's rank‐order correlation (ρ = −0.56, P =.02). C, The box plot represents the RSFCs‐t indicating a significant difference between responders and nonresponders at baseline. Responders are shown in white dots and nonresponders are shown in black dots (P =.004, Mann‐Whitney U‐test. Box = 25th and 75th percentiles; bars = minutes and max values.). D, Nonparametric ROC curve analysis for RSFCs‐t (AUC = 0.92). AUC, area under the curve; MADRS, Montgomery‐Asberg Depression Rating Scale; ROC, receiver operating characteristics; RSFC, resting‐state functional connectivity; scACC, subcallosal anterior cingulate cortex; SD, standard deviation; sgACC, subgenual anterior cingulate cortex
3.2.3. Change in RSFCs‐t postketamine treatment
Change in RSFCs‐t postketamine treatment is illustrated in Figure 4.
FIGURE 4.
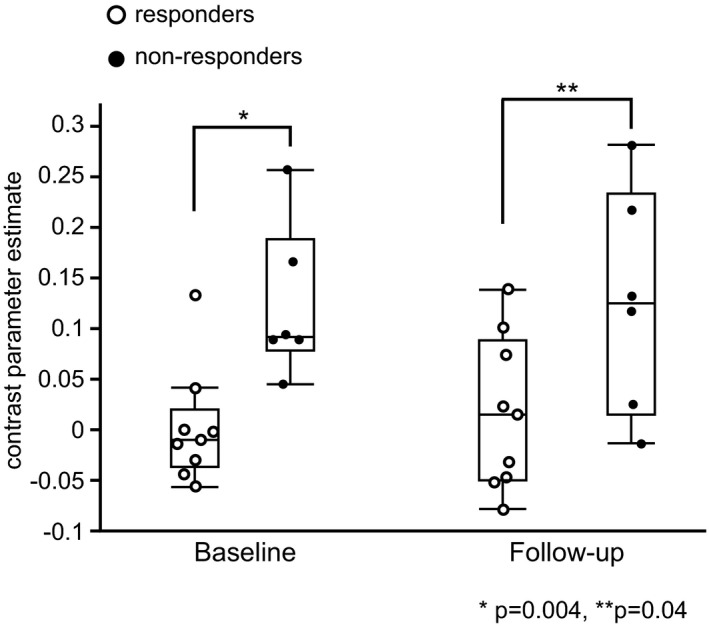
Change in RSFCs‐t postketamine treatment. The box plot represents the group differences in RSFCs‐t between the responders and nonresponders at baseline and at the follow‐up scan. The RSFCs‐t retained significant baseline difference between the responders and nonresponders at the follow‐up scan (P =.04, Mann‐Whitney U‐test). RSFCs‐t, resting‐state functional connectivity between the seed and the target
Same as the baseline result, the group differences in RSFCs‐t between the responders and nonresponders remained significant at the follow‐up scan (P =.04, Mann‐Whitney U‐test).
3.2.4. RSFC changes according to symptomatic improvement
All three seed‐based exploratory analyses (left and right amygdala seeds within the AN and precuneus seed within the DMN) failed to find clusters of significant difference according to symptomatic improvement in the responders (nonparametric paired t‐test, FEW‐corrected, P corr < 0.05).
4. DISCUSSION
This study was the first to demonstrate the ability of rsfMRI to predict the antidepressant effects of standard subanesthetic (0.5 mg/kg/40 min) ketamine treatment in TRD patients. The changes in RSFC between the amygdala and sc/sgACC in the right hemisphere at baseline, which predicted the antidepressant effect of ketamine with high sensitivity and specificity, irrespective of lithium or placebo pretreatment, may represent a potential biomarker for treatment response in TRD patients.
In the present study, we employed both patients with MDD and BD suffering from TRD. Since ketamine has demonstrated equivalent antidepressant effects in treatment‐resistant MDD and BD, the biological mechanisms underpinning response to ketamine are believed to be similar in both disorders. 28 We focused on the biological basis of the treatment response to ketamine, regardless of different operational diagnoses, and included both patients with MDD as well as BD in the present study.
The main pharmacological activity of ketamine is NMDA receptor antagonism. How a subanesthetic dose of ketamine produces a rapid antidepressant effect is still uncertain; however, the proposed mechanisms involve multiple neural pathways that dependent on or independent of NMDA receptor antagonism. 29 Complexity in the mechanisms of action of ketamine and the complex pathophysiology of TRD would limit the antidepressant response ratio of ketamine to approximately 60%. The search for biomarkers in predicting antidepressant response to ketamine is meaningful for unraveling the pathophysiology of TRD as well as for individualized treatment optimization. High BMI and FHP have been reported to be effective predictors. 6 However, in the present study, baseline BMI did not predict the antidepressant effect of ketamine. In an original article reporting an association between baseline BMI and ketamine mood response, 30 the average BMI of the study population was over 30. This BMI occurs in less than 2.5% of the Japanese population. The average BMI of 22.5 in the participants of the present study population matched the average Japanese BMI reported in 2017 (http://www.nibiohn.go.jp/eiken/). This suggests that the worldwide applicability of BMI as a predictor of the antidepressant effect of ketamine is limited. FHP was absent in our study population. Phelps et al 31 reported that an FHP for alcohol dependence could predict the ketamine mood response with a sensitivity and specificity of 80% and 69%, respectively. The higher sensitivity and specificity of the RSFCs‐t biomarker in the present study suggest that it could provide more accurate prediction. Other biological markers 6 that predict the antidepressant effects of ketamine have been reported and include low adiponectin, high vitamin B12 levels, a low delta sleep ratio, low glutamine/glutamate ratio, and brain‐derived neurotrophic factor gene polymorphism; however, these factors require further validation.
We employed the anatomical ROIs of the ACC as defined previously 27 to extract RSFCs‐t in the prediction analysis instead of functionally defined clusters on the assumption of clinical application. Beckmann et al divided the cingulate cortex into eight parcels based on anatomical connectivity. 27 Their cluster 1 (the dotted area in Figure 2B) is characterized by rich direct neural projection to amygdala. Since the boundary between the sgACC and the scACC exhibits anatomical variations, 32 we employed the entire cluster 1 for the ROI analysis and retrieved the result of sufficient prediction of antidepressant effect of ketamine. Employing cluster 1 as the ROI would simplify the procedure of the prediction analysis given its simple definition along the rostrum of the corpus callosum. This would aid the clinical utility of rsfMRI for treatment response prediction in TRD. Salvadore et al 33 showed that functional connectivity between these regions in the left hemisphere predicts an antidepressive effect of ketamine in TRD patients using magnetoencephalography under a cognitive task. Nevertheless, despite the differences in the imaging techniques used, their findings are consistent with those of the present study, except for potential effects due to hemispheric opposition. According to the functional laterality of the amygdala, while the left side is likely to participate in more overt emotional processing, the right side is likely to participate in more covert processing. 34 The hemispheric dissociation between the present study and that reported by Sarvadore et al 33 might be a reflection of differences in data acquisition, that is under total resting conditions or when subjected to a cognitive task. Chen et al 18 recently reported that RSFC between the right superior frontal gyrus and striatum at baseline predicted treatment response to very low dose (0.2 mg/kg) ketamine infusion but not to ordinary subanesthetic dose (0.5 mg/kg) in TRD. We did not assess the dose difference of subanesthetic ketamine. Since the clinical effect of very low dose ketamine infusion was not established, further study is needed to investigate the difference of the present result and the preceding result by Chen et al. 18
Numerous studies investigating RSFC have provided insights regarding pathophysiology 7 , 35 , 36 and prediction of treatment response in MDD. 3 , 12 , 37 Herein, we examined changes in RSFC in two brain networks: the AN and the DMN. RSFC within the AN is altered in MDD. 7 , 38 Regarding treatment response heterogeneity or prediction of treatment outcomes, associations between local structure, anatomical connectivity, local activity, and reactivity of the AN and treatment response of MDD have been reported previously. 39 In particular, the amygdala and ACC are the key regions reported to be associated with treatment response to antidepressants. 39 , 40 , 41 , 42 Recently, the role of the DMN in the pathophysiology of MDD has gathered increasing attention. Previous imaging studies have consistently reported alterations in RSFC within the DMN in patients with MDD. 7 , 35 , 43 These findings have been extended to the pathophysiology of TRD. 10 , 44 , 45 , 46 However, fewer studies have reported a relationship between changes in RSFC and treatment response heterogeneity in TRD patients. 18 , 47 , 48
In this study, the seed‐based exploratory analysis at baseline revealed a cluster that was associated with treatment response to ketamine within the AN, but not the DMN. This suggests that the AN plays a key role in treatment response in TRD with respect to the DMN in treatment‐sensitive depression (TSD). Previous imaging studies that investigated local brain structure, activities, or RSFC in the AN of TRD patients also support this idea. The scACC, thalamic, and amygdala volumes are associated with treatment response to deep brain stimulation. 49 Successful treatment with electroconvulsive therapy modified the RSFC between the amygdala and fusiform face area, 50 and patients who responded to repetitive transcranial stimulation treatment also showed higher N‐acetylaspartate levels in the left ACC. 51 Further, blunted amygdala reactivity during emotional face recognition tasks has been correlated with treatment response to mindfulness‐based cognitive therapy. 52 These observations support the role of the AN as a common neural basis of treatment response in TRD patients.
Our exploratory analysis illustrated that increased RSFC between the amygdala and sgACC in the right hemisphere is associated with ketamine resistance. Increased RSFC in the AN has been observed in several psychiatric disorders, 17 , 53 and in an animal model 54 of early life stress, known as a major risk factor of adult MDD. Increased RSFC of remote networks has been reported as the neurological basis of the depressive state of MDD and is an indicator of symptom severity. Furthermore, the increased RSFC maintained in patients who are symptom‐free could be considered as a trait‐like feature of the disease. 17 This study illustrated how the baseline RSFC alteration continued to maintain differences between treatment responders and nonresponders regardless of the current depressive symptoms. This suggested that there may be a trait‐like feature associated with ketamine resistance represented in the increased RSFC rather than state, symptom related feature. Similar modifications in RSFC observed in TSD patients supports this idea. 55 , 56 Further study is needed to reveal whether these trait‐like features are present in individuals before onset of the disease, or formed as a result of different chronic disease processes occurring in TRD.
Infusion of a subanesthetic dose of ketamine influences both rapid and prolonged brain activities in healthy participants and patients with MDD. Ionescu et al 57 summarized 47 neuroimaging studies and concluded that ketamine affects different areas of the brain in various ways to contribute to the symptomatic improvement of MDD. Regarding the effects on neural circuitry, the authors proposed that ketamine causes a “disconnection” of several brain networks showing hyper‐connectivity during a mood episode in MDD. However, the detailed mechanisms involved in ketamine's “disconnection” effect are largely unknown.
Studies that examined RSFC in patients with TRD reported RSFC changes associated with symptomatic improvement via ketamine treatment. Abdallah et al 19 reported that a single ketamine infusion increased global brain connectivity in the frontal lobe 24 hours after treatment. Simultaneously, when compared with nonresponders, responders showed increased RSFC between the caudate and visual cortex and, between the lateral prefrontal cortex and sensory cortex, respectively. Chen et al 58 demonstrated a significant decrease in RSFC between the left and right ACC, and between the right dorsolateral prefrontal and right frontal cortex 48 hours after a single ketamine infusion. Our exploratory analysis failed to find state‐dependent RSFC modifications associated with symptomatic improvement in the responders. Chen et al 58 reported an association between RSFC and the MADRS subscales indicating suicide ideation, but failed to demonstrate RSFC’s association with overall MADRS. Morris et al 59 reported that patients with MDD showed hyper‐activity of sgACC and increased RSFC between the hippocampus and the sgACC. Ketamine normalized the hyper‐activity of the sgACC that was associated with anticipatory anhedonia and anxiety, but not with the MADRS score. Changes in the RSFC might not be completely reflective of overall symptomatic improvement, measured by scales similar to the MADRS. Only one study has reported RSFC alterations between the sgACC and prefrontal cortex reflecting both the baseline difference between responders and nonresponders and overall MADRS reduction after a single ketamine infusion. 60 Since this study reported a lower response rate compared to other studies, it is largely unclear whether changes in single RSFC can be reflective of overall symptomatic improvement as measured using scales similar to the MADRS. Scheele et al 61 reported the ketamine response of a patient with TRD having bilateral amygdala damage. This important case report suggested that the activity of the entire amygdala is unnecessary for the antidepressant activity of ketamine. Combined with previous findings, 41 , 52 our results suggest that the baseline amygdala “hyper‐connectivity” to the sgACC in the right hemisphere might obstruct the antidepressant effect of ketamine. This speculation is consistent with the clinical course of the case reported by Scheele et al 61 However, the case also suggested that the amygdala was not vital to the development of treatment resistance in MDD. These findings suggest the involvement of a multi‐level pathophysiology in the neural networks underlying the formation of depressive symptoms, development of treatment resistance, and treatment response to agents including ketamine. The present results might represent a part of a multiple level pathophysiology.
Certain limitations of this study must be noted. The sample size was small because of the strict inclusion criteria regarding previous treatment response. However, the detected main difference of responders and nonresponders in RSFC via nonparametric statistical analysis was sufficiently robust to remain significant after multiple comparison correction. The open‐label administration of ketamine could have introduced biases regarding the reporting of responses, but it is unclear whether this would affect group differences. Lack of controls including healthy or TSD patients, makes it difficult to generalize the results of this study to all MDD patients. A longitudinal study recruiting patients at disease onset is needed. Lastly, the present study did not investigate differences between transient and sustained responders to ketamine.
5. CONCLUSIONS
This study illustrates that the alteration in the RSFC within the right AN in TRD patients reflects the antidepressant response to ketamine at baseline. The alteration remained throughout the 2‐week treatment with multiple ketamine infusions and seemed to reflect the trait‐like features underlying treatment heterogeneity in TRD. By employing an anatomical ROI of the sc/sgACC, the present study also suggests the possible clinical utility of rsfMRI in predicting the treatment response to ketamine in TRD patients.
Since there may be a multi‐level pathophysiology underlying the formation of depressive symptoms, treatment resistance, and treatment response, a multivariate model able to predict treatment response 62 , 63 is of increasing importance in clinical practice. Gathering reliable variables, including findings from rsfMRI, corresponding to each treatment are needed in future studies to improve stratification.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
TN, NH, and MT contributed to the concept and design of the study. TN, MT, NH, MI, KU, TH, TA, and NU performed the acquisition, analysis, and interpretation of data. The manuscript was drafted by TN and MT All authors approved the final version.
APPROVAL AND INFORMED CONSENT
The protocol for this research project has been approved by a suitably constituted Ethics Committee of the institution and it conforms to the provisions of the Declaration of Helsinki. The Ethics Committee of the Kurume University School of Medicine, Kurume, Japan, Approval No. 14 097. All informed consent was obtained from the subject(s) and/or guardian(s).
REGISTRY AND THE REGISTRATION NO. OF THE STUDY/TRIAL
The study was conducted as part of the study registered as UMIN‐CTR No. UMIN000017529. All patients provided written informed consent before screening.
ANIMAL STUDIES
N/A.
Supporting information
Fig S1
Fig S2
Supplementary Material
Appendix S1
ACKNOWLEDGEMENTS
The authors would like to thank all volunteers for their contribution. The authors also thank members of the Psychopharmacology section, Department of Neuropsychiatry, Kurume University School of Medicine; staff from the Department of Biostatistics center, Kurume University; and anesthesiologists of the Department of Anesthesiology, Kurume University School of Medicine.
Nakamura T, Tomita M, Horikawa N, et al. Functional connectivity between the amygdala and subgenual cingulate gyrus predicts the antidepressant effects of ketamine in patients with treatment‐resistant depression. Neuropsychopharmacol Rep. 2021;41:168–178. 10.1002/npr2.12165
Funding information
The authors declare that this research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. The IRB did not grant the deposit of raw data in a publicly accessible data archive or repository at the time of approval since the procedure was not included in the study protocol or informed consent document.
REFERENCES
- 1. Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer‐term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–17. [DOI] [PubMed] [Google Scholar]
- 2. Fava M, Davidson KG. Definition and epidemiology of treatment‐resistant depression. Psychiatr Clin North Am. 1996;19:179–200. [DOI] [PubMed] [Google Scholar]
- 3. Perlman K, Benrimoh D, Israel S, Rollins C, Brown E, Tunteng JF, et al. A systematic meta‐review of predictors of antidepressant treatment outcome in major depressive disorder. J Affect Disord. 2019;15:503–15. [DOI] [PubMed] [Google Scholar]
- 4. Papadimitropoulou K, Vossen C, Karabis A, Donatti C, Kubitz N. Comparative efficacy and tolerability of pharmacological and somatic interventions in adult patients with treatment‐resistant depression: a systematic review and network meta‐analysis. Curr Med Res Opin. 2017;33:701–11. [DOI] [PubMed] [Google Scholar]
- 5. Molero P, Ramos‐Quiroga JA, Martin‐Santos R, Calvo‐Sánchez E, Gutiérrez‐Rojas L, Meana JJ. Antidepressant efficacy and tolerability of ketamine and esketamine: a critical review. CNS Drugs. 2018;32:411–20. [DOI] [PubMed] [Google Scholar]
- 6. Rong C, Park C, Rosenblat JD, Subramaniapillai M, Zuckerman H, Fus D, et al. Predictors of response to ketamine in treatment resistant major depressive disorder and bipolar disorder. Int J Environ Res Public Health. 2018;15:771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Helm K, Viol K, Weiger TM, Tass PA, Grefkes C, Del Monte D, et al. Neuronal connectivity in major depressive disorder: a systematic review. Neuropsychiatr Dis Treat. 2018;17:2715–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dichter GS, Gibbs D, Smoski MJ. A systematic review of relations between resting‐state functional‐MRI and treatment response in major depressive disorder. J Affect Disord. 2015;1:8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sheline YI, Price JL, Yan Z, Mintun MA. Resting‐state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci USA. 2010;107:11020–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Kwaasteniet BP, Rive MM, Ruhé HG, Schene AH, Veltman DJ, Fellinger L, et al. Decreased resting‐state connectivity between neurocognitive networks in treatment resistant depression. Front Psychiatry. 2015;2:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Posner J, Hellerstein DJ, Gat I, Mechling A, Klahr K, Wang Z, et al. Antidepressants normalize the default mode network in patients with dysthymia. JAMA Psychiatry. 2013;70:373–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Long Z, Du L, Zhao J, Wu S, Zheng Q, Lei X. Prediction on treatment improvement in depression with resting state connectivity: a coordinate‐based meta‐analysis. J Affect Disord. 2020;1:62–8. [DOI] [PubMed] [Google Scholar]
- 13. Scheidegger M, Walter M, Lehmann M, Metzger C, Grimm S, Boeker H, et al. Ketamine decreases resting state functional network connectivity in healthy subjects: implications for antidepressant drug action. PLoS One. 2012;7:e44799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reed JL, Nugent AC, Furey ML, Szczepanik JE, Evans JW, Zarate CA. Ketamine normalizes brain activity during emotionally valenced attentional processing in depression. Neuroimage Clin. 2018;5:92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Loureiro JR, Leaver A, Vasavada M, Sahib AK, Kubicki A, Joshi S, et al. Modulation of amygdala reactivity following rapidly acting interventions for major depression. Hum Brain Mapp. 2020;41:1699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sahib AK, Loureiro JR, Vasavada MM, Kubicki A, Joshi SH, Wang K, et al. Single and repeated ketamine treatment induces perfusion changes in sensory and limbic networks in major depressive disorder. Eur Neuropsychopharmacol. 2020;33:89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anticevic A, Brumbaugh MS, Winkler AM, Lombardo LE, Barrett J, Corlett PR, et al. Global prefrontal and fronto‐amygdala dysconnectivity in bipolar I disorder with psychosis history. Biol Psychiatry. 2013;73:565–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen MH, Chang WC, Lin WC, Tu PC, Li CT, Bai YM, et al. Functional dysconnectivity of frontal cortex to striatum predicts ketamine infusion response in treatment‐resistant depression. Int J Neuropsychopharmacol. 2020;23:791–798. 10.1093/ijnp/pyaa056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abdallah CG, Averill LA, Collins KA, Geha P, Schwartz J, Averill C, et al. Ketamine treatment and global brain connectivity in major depression. Neuropsychopharmacology. 2017;42:1210–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9. [DOI] [PubMed] [Google Scholar]
- 21. Williams JB, Kobak KA. Development and reliability of a structured interview guide for the Montgomery Asberg Depression Rating Scale (SIGMA). Br J Psychiatry. 2008;192:52–8. [DOI] [PubMed] [Google Scholar]
- 22. Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–35. [DOI] [PubMed] [Google Scholar]
- 23. Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T. Bayesian analysis of neuroimaging data in FSL. NeuroImage. 2009;45(1 Suppl 1):S173–86. [DOI] [PubMed] [Google Scholar]
- 24. Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting‐state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360:1001–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968–80. [DOI] [PubMed] [Google Scholar]
- 26. Smith SM, Nichols TE. Threshold‐free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage. 2009;44:83–98. [DOI] [PubMed] [Google Scholar]
- 27. Beckmann M, Johansen‐Berg H, Rushworth MF. Connectivity‐based parcellation of human cingulate cortex and its relation to functional specialization. J Neurosci. 2009;29:1175–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Park LT, Falodun TB, Zarate CA. Ketamine for treatment‐resistant mood disorders. Focus (Am Psychiatr Publ). 2019;17:8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zanos P, Gould TD. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. 2018;23:801–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Niciu MJ, Ionescu DA, Guevara S, Machado‐Vieira R, Richards EM, Brutsche NE, et al. Clinical predictors of ketamine response in treatment‐resistant major depression. J Clin Psychiatry. 2014;75:e417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Phelps LE, Brutsche N, Moral JR, Luckenbaugh DA, Manji HK, Zarate CA. Family history of alcohol dependence and initial antidepressant response to an N‐methyl‐D‐aspartate antagonist. Biol Psychiatry. 2009;65:181–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Palomero‐Gallagher N, Mohlberg H, Zilles K, Vogt B. Cytology and receptor architecture of human anterior cingulate cortex. J Comp Neurol. 2008;508:906–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Salvadore G, Cornwell BR, Sambataro F, Latov D, Colon‐Rosario V, Carver F, et al. Anterior cingulate desynchronization and functional connectivity with the amygdala during a working memory task predict rapid antidepressant response to ketamine. Neuropsychopharmacology. 2010;35:1415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gainotti G. Emotions and the right hemisphere: can new data clarify old models? Neuroscientist. 2019;25:258–70. [DOI] [PubMed] [Google Scholar]
- 35. Dutta A, McKie S, Deakin JF. Resting state networks in major depressive disorder. Psychiatry Res. 2014;224:139–51. [DOI] [PubMed] [Google Scholar]
- 36. Brakowski J, Spinelli S, Dörig N, Bosch OG, Manoliu A, Holtforth MG, et al. Resting state brain network function in major depression ‐ depression symptomatology, antidepressant treatment effects, future research. J Psychiatr Res. 2017;92:147–59. [DOI] [PubMed] [Google Scholar]
- 37. Fonseka TM, MacQueen GM, Kennedy SH. Neuroimaging biomarkers as predictors of treatment outcome in major depressive disorder. J Affect Disord. 2018;233:21–35. [DOI] [PubMed] [Google Scholar]
- 38. Tang S, Lu L, Zhang L, Hu X, Bu X, Li H, et al. Abnormal amygdala resting‐state functional connectivity in adults and adolescents with major depressive disorder: a comparative meta‐analysis. EBioMedicine. 2018;36:436–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Saxena S, Brody AL, Ho ML, Zohrabi N, Maidment KM, Baxter LR. Differential brain metabolic predictors of response to paroxetine in obsessive‐compulsive disorder versus major depression. Am J Psychiatry. 2003;160:522–32. [DOI] [PubMed] [Google Scholar]
- 41. Williams LM, Korgaonkar MS, Song YC, Paton R, Eagles S, Goldstein‐Piekarski A, et al. Amygdala reactivity to emotional faces in the prediction of general and medication‐specific responses to antidepressant treatment in the randomized iSPOT‐D trial. Neuropsychopharmacology. 2015;40:2398–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pizzagalli D, Pascual‐Marqui RD, Nitschke JB, Oakes TR, Larson CL, Abercrombie HC, et al. Anterior cingulate activity as a predictor of degree of treatment response in major depression: evidence from brain electrical tomography analysis. Am J Psychiatry. 2001;158:405–15. [DOI] [PubMed] [Google Scholar]
- 43. Korgaonkar MS, Rekshan W, Gordon E, Rush AJ, Williams LM, Blasey C, et al. Magnetic resonance imaging measures of brain structure to predict antidepressant treatment outcome in major depressive disorder. EBioMedicine. 2014;2:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lui S, Wu Q, Qiu L, Yang X, Kuang W, Chan RC, et al. Resting‐state functional connectivity in treatment‐resistant depression. Am J Psychiatry. 2011;168:642–8. [DOI] [PubMed] [Google Scholar]
- 45. Luan SX, Zhang L, Wang R, Zhao H, Liu C. A resting‐state study of volumetric and functional connectivity of the habenular nucleus in treatment‐resistant depression patients. Brain Behav. 2019;9:e01229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. He Z, Cui Q, Zheng J, Duan X, Pang Y, Gao Q, et al. Frequency‐specific alterations in functional connectivity in treatment‐resistant and ‐sensitive major depressive disorder. J Psychiatr Res. 2016;82:30–9. [DOI] [PubMed] [Google Scholar]
- 47. Moreno‐Ortega M, Prudic J, Rowny S, Patel GH, Kangarlu A, Lee S, et al. Resting state functional connectivity predictors of treatment response to electroconvulsive therapy in depression. Sci Rep. 2019;25:5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ge R, Downar J, Blumberger DM, Daskalakis ZJ, Vila‐Rodriguez F. Functional connectivity of the anterior cingulate cortex predicts treatment outcome for rTMS in treatment‐resistant depression at 3‐month follow‐up. Brain Stimul. 2020;13:206–14. [DOI] [PubMed] [Google Scholar]
- 49. Sankar T, Chakravarty MM, Jawa N, Li SX, Giacobbe P, Kennedy SH, et al. Neuroanatomical predictors of response to subcallosal cingulate deep brain stimulation for treatment‐resistant depression. J Psychiatry Neurosci. 2020;45:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang J, Wei Q, Wang L, Zhang H, Bai T, Cheng L, et al. Functional reorganization of intra‐ and internetwork connectivity in major depressive disorder after electroconvulsive therapy. Hum Brain Mapp. 2018;39:1403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zheng H, Jia F, Guo G, Quan D, Li G, Wu H, et al. Abnormal anterior cingulate N‐acetylaspartate and executive functioning in treatment‐resistant depression after rTMS therapy. Int J Neuropsychopharmacol. 2015;18:pyv059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ferri J, Eisendrath SJ, Fryer SL, Gillung E, Roach BJ, Mathalon DH. Blunted amygdala activity is associated with depression severity in treatment‐resistant depression. Cogn Affect Behav Neurosci. 2017;17:1221–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kleinhans NM, Reiter MA, Neuhaus E, Pauley G, Martin N, Dager S, et al. Subregional differences in intrinsic amygdala hyperconnectivity and hypoconnectivity in autism spectrum disorder. Autism Res. 2016;9:760–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Johnson FK, Delpech JC, Thompson GJ, Wei L, Hao J, Herman P, et al. Amygdala hyper‐connectivity in a mouse model of unpredictable early life stress. Transl Psychiatry. 2018;21:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Goldstein‐Piekarski AN, Staveland BR, Ball TM, Yesavage J, Korgaonkar MS, Williams LM. Intrinsic functional connectivity predicts remission on antidepressants: a randomized controlled trial to identify clinically applicable imaging biomarkers. Transl Psychiatry. 2018;6:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Li L, Li B, Bai Y, Liu W, Wang H, Leung HC, et al. Abnormal resting state effective connectivity within the default mode network in major depressive disorder: a spectral dynamic causal modeling study. Brain Behav. 2017;7:e00732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ionescu DF, Felicione JM, Gosai A, Cusin C, Shin P, Shapero BG, et al. Ketamine‐associated brain changes: a review of the neuroimaging literature. Harv Rev Psychiatry. 2018;26:320–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chen MH, Lin WC, Tu PC, Li CT, Bai YM, Tsai SJ, et al. Antidepressant and antisuicidal effects of ketamine on the functional connectivity of prefrontal cortex‐related circuits in treatment‐resistant depression: a double‐blind, placebo‐controlled, randomized, longitudinal resting fMRI study. J Affect Disord. 2019;1:15–20. [DOI] [PubMed] [Google Scholar]
- 59. Morris LS, Costi S, Tan A, Stern ER, Charney DS, Murrough JW. Ketamine normalizes subgenual cingulate cortex hyper‐activity in depression. Neuropsychopharmacology. 2020;45:975–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gärtner M, Aust S, Bajbouj M, Fan Y, Wingenfeld K, Otte C, et al. Functional connectivity between prefrontal cortex and subgenual cingulate predicts antidepressant effects of ketamine. Eur Neuropsychopharmacol. 2019;29:501–8. [DOI] [PubMed] [Google Scholar]
- 61. Scheele D, Zimbal S, Feinstein JS, Delis A, Neumann C, Mielacher C, et al. Treatment‐resistant depression and ketamine response in a patient with bilateral amygdala damage. Am J Psychiatry. 2019;176(12):982–6. [DOI] [PubMed] [Google Scholar]
- 62. Osuch E, Gao S, Wammes M, Théberge J, Williamson P, Neufeld RJ, et al. Complexity in mood disorder diagnosis: fMRI connectivity networks predicted medication‐class of response in complex patients. Acta Psychiatr Scand. 2018;138:472–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chin Fatt CR, Jha MK, Cooper CM, Fonzo G, South C, Grannemann B, et al. Effect of intrinsic patterns of functional brain connectivity in moderating antidepressant treatment response in major depression. Am J Psychiatry. 2020;177:143–54. [DOI] [PubMed] [Google Scholar]
- 64. LeDoux JE, Brown R. A higher‐order theory of emotional consciousness. Proc Natl Acad Sci USA. 2017;114:E2016–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Price RB, Lane S, Gates K, Kraynak TE, Horner MS, Thase ME, et al. Parsing heterogeneity in the brain connectivity of depressed and healthy adults during positive mood. Biol Psychiatry. 2017;81:347–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. McCormick EM, Telzer EH. Contributions of default mode network stability and deactivation to adolescent task engagement. Sci Rep. 2018;21:18049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Supplementary Material
Appendix S1
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. The IRB did not grant the deposit of raw data in a publicly accessible data archive or repository at the time of approval since the procedure was not included in the study protocol or informed consent document.


