Abstract
Aim
Attention is a goal‐directed cognitive process that facilitates the detection of task‐relevant sensory stimuli from dynamic environments. Anterior cingulate cortical area (ACA) is known to play a key role in attentional behavior, but the specific circuits mediating attention remain largely unknown. As ACA modulates sensory processing in the visual cortex (VIS), we aim to test a hypothesis that frontal top‐down neurons projecting from ACA to VIS (ACAVIS) contributes to visual attention behavior through chemogenetic approach.
Methods
Adult, male mice were trained to perform the 5‐choice serial reaction time task (5CSRTT) using a touchscreen system. An intersectional viral approach was used to selectively express inhibitory designer receptors exclusively activated by designer drugs (iDREADD) or a static fluorophore (mCherry) in ACAVIS neurons. Mice received counterbalanced injections (i.p.) of the iDREADD ligand (clozapine‐N‐oxide; CNO) or vehicle (saline) prior to 5CSRTT testing. Finally, mice underwent progressive ratio testing and open field testing following CNO or saline administration.
Results
Chemogenetic suppression of ACAVIS neuron activity decreased correct task performance during the 5CSRTT mainly driven by an increase in omission and a trending decrease in accuracy with no change in behavioral outcomes associated with motivation, impulsivity, or compulsivity. Breakpoint during the progressive ratio task and distance moved in the open field test were unaffected by ACAVIS neuron suppression. CNO administration itself had no effect on task performance in mCherry‐expressing mice.
Conclusion
These results identify long‐range frontal‐sensory ACAVIS projection neurons as a key enactor of top‐down attentional behavior and may serve as a beneficial therapeutic target.
Keywords: anterior cingulate cortex, attention, chemogenetics, top‐down projection, visual cortex
Circuit‐specific chemogenetic silencing demonstrated that frontal neurons projecting from anterior cingulate cortex to visual are essential for top‐down control of attention behavior in mice. This study suggests that long‐range frontal‐sensory projection neurons as a key enactor of top‐down attentional behavior and may serve as a beneficial therapeutic target.
1. INTRODUCTION
Top‐down attention is a cognitive process to facilitate the detection of relevant sensory stimulus from our complex and ever‐changing world. To date, neural signatures of attention have largely been investigated in the context of visual response modulation. 1 Previous functional connectivity studies in humans, 2 , 3 , 4 electrophysiology studies in human, monkey, and rodents, 5 , 6 , 7 , 8 rodent pharmacological studies, 9 , 10 , 11 and mouse optogenetic and chemogenetic studies 12 , 13 demonstrate that the frontal cortex—especially the anterior cingulate cortex area (ACA)—plays a key role in implementing a top‐down control of visual attention, although not restricted in this sensory modality. 8 However, due to the heterogeneous connectivity of ACA neurons with extensive cortical and subcortical regions, the precise neural circuits originating from the ACA that mediate attention remain largely unknown until recently. 12 , 14
A recent study in mice demonstrated that long‐range frontal cortico‐cortical projections from the ACA to the visual cortex (VIS: ACAVIS) modulate visual discrimination sensitivity. 15 We thus hypothesized that ACAVIS projection neurons are necessary for visual attentional behavior. Here, we aimed to test this hypothesis by using a chemogenetic approach to determine whether top‐down ACAVIS projections neurons play a critical role in regulating attentional behavior in freely moving mice. We chose to use a chemogenetic approach because it is not only useful to examine the causal role of neural circuits in behavior, but also is potentially amenable to clinical translation to treat psychiatric disorders. 16
2. METHODS
2.1. Animals
Adult, male C57BL/6 mice (Charles River Laboratories, Massachusetts) were group‐housed under a standard 12 hours light/dark cycle in a temperature‐ and humidity‐controlled vivarium. Male mice were included to directly compare the current results to our previous studies. 13 , 17 Training was initiated when mice were 9‐10 weeks old. Mice were allowed access to water for 2 hours each day and maintained approximately 85%‐90% of their ad libitum weight during behavioral training. Food was available ad libitum throughout the experiment. All animal protocols were approved by the Institutional Animal Care and Use Committee at Icahn School of Medicine at Mount Sinai.
2.2. Drugs
Clozapine‐N‐oxide (CNO; Tocris Bioscience) was fully dissolved in sterile saline. For behavior experiments, CNO was injected intraperitoneally (i.p.) at 10 mg/kg 30 minutes before testing.
2.3. Viral strategies and stereotaxic procedures
Mice were anesthetized with 2% isoflurane and head‐fixed in a mouse stereotaxic apparatus (Narishige). Bilateral ACA injections sites relative to bregma are as follows: AP +0.7, +0.2, −0.3 mm, ML ±0.2 mm, and DV −0.7 mm. Bilateral VIS injection sites relative to lambda are as follows: AP +0.0 mm, ML ±3.0 mm, DV −0.4 mm; AP +0.1 mm, ML ±2.85, 3.15 mm, and DV −0.4 mm. For circuit‐selective ACAVIS chemogenetic silencing, AAV8‐hSyn‐DIO‐hM4D(Gi)‐mCherry (Addgene, Titer: 1.8 × 1013 GC/mL) was injected in the ACA and canine adenovirus 2 carrying Cre‐recombinase (CAV‐Cre, Montpellier, Titer: 3.4 × 1012 GC/mL) or rAAV2‐CAG‐Cre‐WPRE (Boston Children's Hospital, Titer: 3.74 × 1013 GC/mL) was injected into VIS, bilaterally. In a separate cohort of mice, AAV8‐hSyn‐DIO‐mCherry (Addgene; Titer: 2.1 × 1013 GC/mL) was injected bilaterally into the ACA. Each infusion (500 nL) was made at 150 nL/min using a microinjector set (Nanoject III) and glass pulled syringe. The syringe was left in place for 1 minute following the injection to reduce backflow of virus. Behavioral testing occurred at least three weeks after viral injection to allow for maximal viral expression.
2.4. Behavior
2.4.1. 5‐Choice serial reaction time task
Apparatus: Testing was conducted following procedures previously described in 17 in eight Bussey‐Saksida operant chambers with a touchscreen system (Lafayette Instruments). Dimensions are as follows: a black plastic trapezoid (walls 20 cm high × 18 cm wide (at screen‐magazine) × 24 cm wide (at screen) × 6 cm wide (at magazine)). Stimuli were displayed on a touch‐sensitive screen (12.1 inch, screen resolution 600 × 800) divided into five response windows by a black plastic mask (4.0 × 4.0 cm, positioned centrally with windows spaced 1.0 cm apart, 1.5 cm above the floor) fitted in front of the touchscreen. Schedules were designed, and data were collected and analyzed using ABET II Touch software (Lafayette Instrument). The inputs and outputs of the multiple chambers were controlled by WhiskerServer software (Lafayette Instrument). Habituation: Before 5CSRTT training, mice were first acclimated to the operant chamber and milk reward. The food magazine was illuminated and diluted (30%) sweetened condensed milk (Eagle Brand) was dispensed every 40 seconds after mice entered the food magazine. Mice needed to enter reward tray 20 times during two consecutive sessions 30‐minute sessions before advancing to the next stage. Mice were then trained to touch the illuminated response window: during this phase, a white square stimulus was presented randomly at one response window until it was touched. If the mouse touched the stimulus, the milk reward was delivered in conjunction with a tone and magazine light. Touches to nonstimulus locations had no consequence. After reaching criterion on this phase (20 stimulus touches in 30 minutes for two consecutive days), mice advanced to the 5CSRTT training phase. Training: Mice were tested 5 days a week, 100 trials a day (or up to 30 minutes). Each trial began with the illumination of the magazine light. After mice exited the food magazine, there was an intertrial interval (ITI) period of 5 seconds before a stimulus was presented at one response window. If a mouse touched the screen during the ITI period, the response was recorded as premature and the mouse was punished with a 5 seconds time‐out (house light on). After the time‐out period, the magazine light illumination and house light switch off signaled onset of the next trial. After the ITI period, a stimulus appeared randomly in one of the five response windows for a set stimulus duration (this varied from 32 to 2 seconds, depending of stage of training). A limited‐hold period followed by the stimulus duration was 5 seconds, during which the stimulus was absent but the mouse was still able to respond to the location. Responses during stimulus presence and limited holding period could be recorded either as correct (touching the stimulus window) or incorrect (touching any other windows). A correct response was rewarded with a tone, and milk delivery, indicated by the illumination of the magazine light. Failure to respond to any window over the stimulus and limited‐hold period was counted as an omission. Incorrect responses and omissions were punished with a 5‐second time‐out. In addition, repeated screen touches after a correct or incorrect response were counted as perseverative responses. Animals started at stimulus duration of 32 seconds. With a goal to baseline mice at a stimulus duration of 2 seconds, the stimulus duration was sequentially reduced from 32, 16, 8, 4, to 2 seconds. Animals had to reach a criterion (≥50 trials, ≥80% accuracy, ≤20% omissions) over consecutive days to pass from one stage to the next. After reaching 5CSRTT baseline criterion with the 2 seconds stimulus duration, mice were injected with virus. 5CSRTT Testing: Following viral injections, mice were reestablished to baseline criterion and then challenged with an increased attentional demand by reducing and pseurandomly shuffling the stimulus duration to 2.0, 1.5, 1.0, and 0.8 seconds. Between experimental testing days, mice were subjected to 2 seconds stimulus duration training to confirm that the mice maintain stable baseline criterion. All injected mice passed the criteria and moved to testing phase. Attention and response control were assessed by measuring the following performance: correct percentage ((100 × (correct)/(correct + incorrect+omissions)), percentage accuracy (100 × correct responses/(correct responses + incorrect responses)), percentage omission (100 × omissions/(omissions + correct responses + incorrect responses)), percentage of premature responses, percentage of perseverative responses, latency to collect response (s), and latency to reward collection (s) after correct choices.
2.4.2. Progressive ratio task
Using the Bussey–Saksida chamber, mice were subjected to fixed ratio (FR) training in which the center response window was illuminated and needed to be touched for a fixed number of times, depending on schedule, in order for a milk reward to be dispensed. Mice were trained on FR1, followed by FR2, FR3, and FR5 schedules. Criterion was defined as completion of 30 trials in a single session. After passed the criterion on FR5 phase for three consecutive days, mice were subjected to the progressive ratio (PR) phase where the reward response requirement was incremented on a linear +4 basis (ie, 1, 5, 9, and 13). Once mice showed stable performances, defined as 10% breakpoint variability across two sessions, mice underwent four days of testing. Mice were administered CNO or saline 30 minutes prior to PR testing in a counterbalanced manner. The order of vehicle versus CNO was counterbalanced across mice.
2.4.3. Open field test
Locomotor activity was measured for 30 minutes in a square apparatus (43 × 43 × 33 cm) equipped with a panel of infrared beams (16 beams) located in the horizontal direction along the sides of each square apparatus. Data were collected with Fusion v4 software (Omnitech Electronics). Mice were given ad libitum access to water for at least one week prior to open field testing. For inhibitory DREADD experiments, activity was assessed twice for each mouse, once when vehicle was administered, and once when CNO was administered. The order of vehicle versus CNO administration was counterbalanced across mice. Activity sessions were separated by at least 7 drug‐free days.
2.5. Electrophysiological validation of iDREADD
Animals were decapitated under isoflurane anesthesia. Brains were quickly removed and transferred into ice‐cold artificial cerebrospinal fluid (ACSF) of the following composition (in mmol/L): 210.3 sucrose, 11 glucose, 2.5 KCl, 1 NaH2PO4, 26.2 NaHCO3, 0.5 CaCl2, and 4 MgCl2. Acute coronal slices of ACA (300 μm) contained both hemispheres. Slices were allowed to recover for 40 minutes at room temperature in the same solution, but with reduced sucrose (105.2 mmol/L) and addition of NaCl (59.5 mmol/L). Following recovery, slices were maintained at room temperature in standard ACSF composed of the following (in mmol/L): 119 NaCl, 2.5 KCl, 1 NaH2PO4, 26.2 NaHCO3, 11 glucose, 2 CaCl2, and 2 MgCl2. Slices were visualized under upright differential interference contrast microscope used for the identification of fluorescently labeled cells. Patch clamp recordings were made from fluorescently labeled ACAVIS projection neurons. Borosilicate glass electrodes (5‐7 MΩ) were filled with the internal solution containing (in mmol/L): 127.5 K‐methanesulfonate, 10 HEPES, 5 KCl, 5 Na‐phosphocreatine, 2 MgCl2, 2 Mg‐ATP, 0.6 EGTA, and 0.3 Na‐GTP (pH 7.25, 295 mOsm). Signals were low‐pass filtered at 3 kHz and sampled at 20 kHz. Neurons were included in the analysis if input resistance, series resistance and membrane potential did not change more than 10% during the course of recordings. For iDREADD validation, spontaneous firing rate was measured in a current clamp mode. Recordings were performed in normal ACSF with a depolarizing current of 1.6 ± 0.04 times rheobase injected to the cell. In order to establish steady‐state firing activity of recorded neurons, we slowly increased the depolarizing current over the course of 2‐3 minutes to elicit low level of sustained spontaneous firing. The recordings were initiated after the adjustment of the depolarizing current. Firing rate was quantified as the average instantaneous firing frequency measured over 3 minutes before and following CNO (10 μmol/L) application.
2.6. Immunohistochemistry
Anesthetized mice were transcardially perfused with cold phosphate‐buffered saline (PBS) followed by 4% paraformaldehyde (PFA) in PBS. The brains were postfixed in 4% PFA at 4°C for 3 hours and cryoprotected in 30% sucrose solution for 24 hours before embedding in Optimum Cutting Temperature (OCT, Tissue Tek). The frozen brains were sectioned into 35‐μm‐thick coronal sections using a cryostat (CM3050, Leica). Slices were collected at nine specific Bregma areas (2.10, 1.70, 1.18, 0.74, 0.14, −0.34, −0.82, −1.34, −1.82) to analyze the anterior‐posterior spread of virally infected cells. Every 6th 35‐μm‐thick coronal sections were washed in Tris‐buffered saline (TBS), pH 7.5 and incubated with NeuroTrace 435/455 blue fluorescent Nissl stain (Thermo Fisher Scientific Inc). NeuroTrace was used for focus adjustment. After washing, the sections were mounted on glass slides and coverslipped. Targeting and efficacy of iDREADD‐mCherry to visual cortex projecting ACA neurons was confirmed using anti‐mCherry Imaging was performed using LSM780 confocal microscopes (Zeiss). The first level (Min) represented the minimum number of mice showing signal in a given area, namely n = 1. The second level (Q1) represented the 25th percentile of mice with overlapping signal in a given area, the third level (Q2) represented the 50th percentile, and the fourth level (Q3) represented the 75th percentile. The fifth level (Q4) represented the maximum number of mice showing overlapping expression in a given area, namely the total number of mice in the group. All images were processed and analyzed on ImageJ software (NIH). According to the Allen Mouse Brain Atlas, regions of interest were defined.
2.7. Data analysis and statistics
Statistical analyses were performed using Prism (GraphPad). The 5CSRTT data (accuracy and omission) were analyzed using a 2‐way repeated measures of variance (ANOVA) with Drug (vehicle, CNO) and Stimulus duration (2.0, 1.5, 1.0, 0.8 seconds) as within‐subject factors. In the analysis of other 5CSRTT data and locomotion, a paired t test was used. Immunohistochemistry data without axon‐mapping was analyzed using Student's t test. All data are expressed as means ± SEM.
3. RESULTS
To determine whether ACAVIS projection neurons are required for visual attentional behavior, we performed chemogenetic suppression of top‐down ACAVIS neurons as mice performed freely moving attentional behavior during the 5CSRTT, an assay of visual attention. To selectively express inhibitory DREADD (iDREADD) into the top‐down ACAVIS neurons, we microinjected a Cre‐dependent AAV encoding iDREADD‐mCherry and a retrograde Cre encoding virus into the ACA and VIS, respectively (Figure 1A). We confirmed the viral expression location of iDREADD‐mCherry (33.18 cells/mm2) within the ACA and axonal terminal expression within the VIS (Figure 1B) and performed viral expression mapping of iDREADD‐mCherry‐expressing ACAVIS neurons (Figure 1C). Using whole‐cell patch clamp recordings in slice preparations, we validated that ACAVIS neurons expressing iDREADD‐mCherry demonstrated suppressed action potentials upon bath application of CNO, the ligand for DREADDs (Figure 1D).
FIGURE 1.
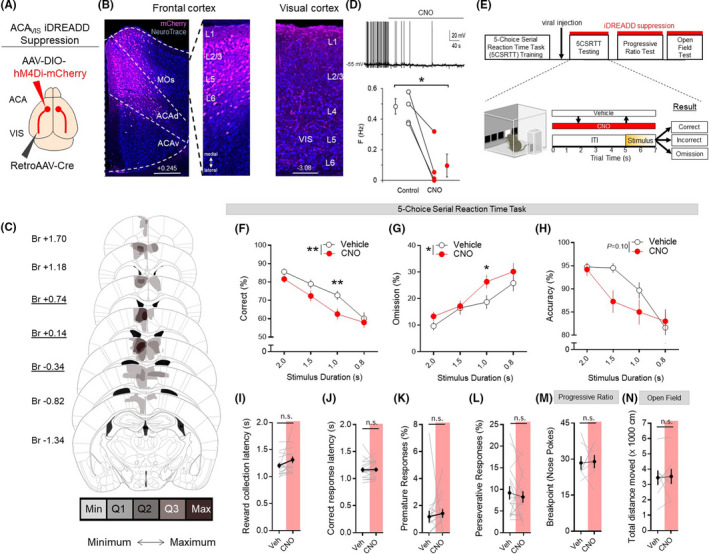
Chemogenetic suppression of ACAVIS neuron activity disrupts attentional behavior. A, Intersectional viral strategy: To selectively express iDREADD in ACAVIS neurons, Cre‐dependent iDREADD‐mCherry and retrograde Cre encoding AAVs were injected bilaterally into the ACA and VIS, respectively. B, Representative images of iDREADD (pink)‐expressing ACAVIS neurons and NeuroTrace (blue) in both dorsal ACA (ACAd) and secondary motor cortex (MOs) of frontal cortex (left, Scale bar = 200 μm) and axon terminals in the visual cortex (right, Scale bar = 100 μm). C, Histological verification of ACAVIS iDREADD viral expression location within the frontal cortex. Underlined distance from bregma (Br, mm) indicates injection location. Min: at least one mouse had viral expression in this area. Q1: >25% of mice had expression in this area. Q2: >50% of mice had expression in this area. Q3: >75% of mice had expression in this area. Max: 100% of mice had expression in this area. D, Electrophysiological validation of iDREADD in ACAVIS neurons. Top: Representative trace of whole‐cell patch recording from ACAVIS neuron in frontal cortex slice upon bath application of CNO. Bottom: CNO significantly decreased firing frequency of ACAVIS neurons expressing iDREADD during whole‐cell recording in slice (two‐tailed paired t test, t 3 = 5.09, *P = .0147, n = 3 mice, four cells). E, Experimental timeline: Mice were first trained on the 5CSRTT before viral injection. After allowing three weeks for maximal viral expression, mice underwent 5CSRTT testing. Mice were treated with saline (vehicle) or clozapine‐N‐oxide (CNO, 10 mg/kg) 30 min prior to testing in a counterbalanced manner with a fixed 5‐s intertrial interval (ITI) and pseudorandomized stimulus duration (2.0, 1.5, 1.0, or 0.8 s; n = 16 mice; 4944 total trials). F‐H, ACAVIS neuron activity suppression via acute CNO administration significantly decreased correct trials (%, two‐way repeated measures analysis of variance, (RM ANOVA), F 1,15 = 10.03, **P = .0064, Holm‐Sidak multiple comparisons at 2.0, 1.5, 1.0, and 0.8 s stimulus duration, P = .6028, .1575, **.008, .9443, n = 16 mice) and omissions (two‐way RM ANOVA, F 1,15 = 5.341, *P = .0344; Holm‐Sidak multiple comparisons at 2, 1.5, 1.0, and 0.8 s, P = .5797, .9980, *.0325, .4172, n = 16 mice), but had no significant yet trending effect on accuracy (two‐way RM ANOVA F 1,15 = 3.038, P = .10, n = 16 mice). I‐L, ACAVIS neuron suppression had no effect on reward collection latency (t 15 = 1.770, P = .0970), correct response latency (t 15 = 0.3928, P = .7000), premature responses (t 15 = 0.8159, P = .4273), and perseverative responses (t 15 = 0.6747, P = .5101) during 5CSRTT testing (n = 16 mice). M, Acute CNO administration had no effect on motivation as independently measured using a progressive ratio task (t 6 = 0.1448, P = .8896, n = 7 mice). N, Acute CNO administration had no effect on motor activity as independently measured during open field testing (t 7 = 0.1959, P = .8502, n = 8 mice). I‐L, Two‐tailed paired t test. Error bars indicate mean ± SEM, n.s. = nonsignificant, *P < .05, **P < .01. Data available in Table S1
Following 5CSRTT training, mice received viral injections and were rebaselined (4 out of 5 sessions with ≥50 trials, ≥80% accuracy, ≤20% omission at 2 seconds stimulus duration) before 5CSRTT testing. Mice underwent 5CSRTT testing in automated standardized Bussey‐Saksida touchscreen operant chambers 18 , 19 (Figure 1E). During the 5CSRTT, mice are required to maintain and divide their attention across five response windows located in their lower visual field on the touchscreen during a delay period in order to then identify the location where a brief stimulus is presented. If the mouse touches the correct location on the touchscreen, it suggests proper allocation of attention toward the touchscreen and the mouse receives a reward, however touching a nonstimulus response window (incorrect) or failing to respond to any response window (omission) were considered to reflect lapses in attentional performance. 13 , 20 , 21 Prior to daily test sessions, mice were administered counterbalanced systemic injections (i.p.) of either clozapine‐N‐oxide (CNO) (10 mg/kg) or vehicle (saline). During testing, the stimulus duration length was shuffled between 2.0, 1.5, 1.0, and 0.8 seconds in a pseudorandomized order in order to increase attentional load and each test session was separated by at least one day of re‐baseline training sessions with a 2 seconds stimulus duration to ensure stable baseline performance. Chemogenetic inhibition of ACAVIS neuron activity through CNO treatment significantly disrupted 5CSRTT performance, as indicated by a decreased overall correct %, mainly as a result of increased omissions and nonsignificant yet trending decrease in accuracy (Figure 1F‐H). These findings suggest that ACAVIS projection neurons play a critical role in performance outcome of the 5CSRTT.
Together with main measures of attentional capacity, the 5CSRTT provides a holistic evaluation of behavioral function by capturing additional actions including motivation (latency to collect the reward) and processing speed (correct response latency) as well as impulsivity (premature responses) and compulsivity (perseverative responses). 22 Neither iDREADD suppression of ACAVIS neurons nor mCherry control had an effect on correct response latency, reward collection latency, premature responses, or perseverative responses, suggesting that disrupted 5CSRTT performance was a result of reduced attention rather than changes in motivation or disrupted decision‐making (Figure 1I‐L). We also performed an independent progressive ratio task assay of motivation and corroborated that ACAVIS chemogenetic suppression has no impact on motivation as quantified by progressive ratio breakpoint (Figure 1M). CNO‐mediated suppression of ACAVIS neurons also had no effect on motor activity in the open field test (Figure 1N).
In a separate cohort of mice, the static fluorophore mCherry was expressed in the ACAVIS projection neurons to rule out effects of clozapine‐N‐oxide (CNO) or its parent compound clozapine in the absence of iDREADD because of CNO‐to‐clozapine back‐metabolism 23 (Figure 2A). CNO administration in the absence of iDREADD was shown to have no effect on performance on attentional behavior (Figure 2B‐D), reward collection latency (Figure 2E), correct response latency (Figure 2F), premature responses (Figure 2G), or perseverative responses (Figure 2H) during 5CSRTT testing. CNO also had no off‐target effects that resulted in changes in motivation during a progressive ratio task (Figure 2I) or motor activity in the open field test (Figure 2J). Collectively, these results demonstrate that ACAVIS projection neuron activity is causally important for visual attentional performance.
FIGURE 2.
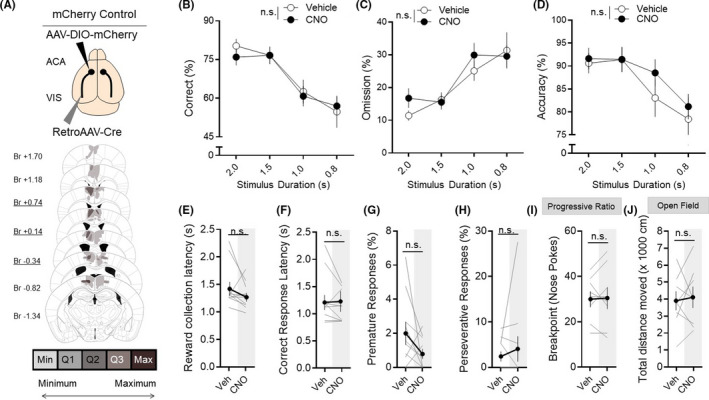
A, Top: Intersectional viral strategy, to selectively express static fluorophore mCherry into ACAVIS neurons, Cre‐dependent mCherry, and retrograde Cre encoding AAVs were injected bilaterally into the ACA and VIS, respectively. Bottom: Histological verification of ACAVIS mCherry viral expression location within the PFC. Underlined distance from bregma (Br, mm) indicates injection location. Min: at least one mouse had viral expression in this area. Q1: at least 25% of mice had expression in this area. Q2: >50% of mice had expression in this area. Q3: >75% of mice had expression in this area. Max: 100% of mice had expression in this area. B‐D, Acute CNO had no effect on correct % (two‐way RM ANOVA, F 1,9 = 0.1636, P = .6953), omissions % (two‐way RM ANOVA, F 1,9 = 0.9356, P = .3587), or accuracy % (two‐way RM ANOVA, F 1,9 = 1.546, P = .2452) during 5CSRTT testing (n = 10 mice; 2522 total trials). E‐H, Acute CNO had no effect on other measures during the 5CSRTT, including reward collection latency (t 9 = 1.379, P = .1963), correct response latency (t 9 = 1.396, P = .2013), premature responses (t 9 = 0.2023, P = .1341), or perseverative responses (t 9 = 0.2023, P = .4982) during 5CSRTT testing (n = 10 mice). I, Acute CNO had no effect on breakpoint during progressive ratio task (n = 8 mice, t 7 = 0.2023, P = .8454). J, Acute CNO had no effect on total distance moved during open field testing (n = 8 mice, t 7 = 0.3318, P = .7497). E‐J, Two‐tailed paired t test. Error bars indicate mean ± SEM, n.s. = nonsignificant. Data available in Table S2
4. DISCUSSION
In this study, we used circuit‐specific chemogenetic silencing to show that frontal‐sensory ACAVIS projection neurons are essential for top‐down control of attention behavior in male mice. As ACAVIS manipulation had no effect on reward collection latency during the 5CSRTT or breakpoint on the progressive ratio task, disrupted 5CSRTT performance is highly likely a direct result of altered attention rather than motivational state. 22 Furthermore, control studies with mice expressing mCherry ruled out off‐target effects of CNO administration. 23
A previous chemogenetic study in mice revealed a causal role of excitatory ACA neurons for visual attention during 5CSRTT, 13 but the specific ACA subpopulations contributing to this behavior remained largely unknown. Our study suggests that ACAVIS neurons at least in part contribute to the ACA‐dependent visual attentional performance together with other ACA neurons. 12 , 14 Another recent chemogenetic study indirectly linked ACAVIS neuron activity and attentional behavior by showing that transient chemogenetic suppression of ACAVIS neuron during adolescence leads to attention deficits in adulthood. 17 Our study provides more direct link ACAVIS neurons and attentional behavior by acutely suppressing neuronal activity during 5CSRTT. Our chemogenetic study in freely moving mice also complements previous findings associated with optogenetic manipulation of ACAVIS projection activity conducted in restrained head‐fixed condition 15 by demonstrating a causal role of ACAVIS neurons in a more unconstrained experimental condition. One limitation of the current study is that it was only conducted in male, but not female mice which limits the interpretation of the results for female mice. It is crucial that future studies directly compare baseline 5CSRTT attentional performance and the role of ACAVIS circuit between male and female mice.
Our study was conducted with translational Bussey‐Saksida touchscreen operant chambers with a high degree of automation and standardization, applying similar stimulus and response characteristics to those used by the analogous Cambridge Neuropsychological Test Automated Battery (CANTAB) in nonhuman primates and humans. 24 , 25 , 26 , 27 Our study establishes a proof‐of‐principle for future applications of chemogenetic targeting of frontal‐sensory attentional circuit in higher mammals such as monkeys, in which chemogenetics is increasingly becoming feasible. 28 , 29 A recent identification of FDA‐approved DREADD agonist is expected to further facilitate clinical translation of chemogenetics to treat psychiatric disorders. 30 Our study may ultimately inspire interventions specifically targeting top‐down frontal‐sensory circuits to improve attention in ADHD, 31 schizophrenia, 32 and autism 33 patients with improper frontal modulation of VIS activity.
CONFLICT OF INTEREST
Authors declare no competing financial interests or conflict of interest.
AUTHOR CONTRIBUTIONS
KJN, HK, and HM designed experiments. KJN, HK, and HM analyzed and wrote the manuscript with contributions from all co‐authors. KJN and HK performed chemogenetics experiments with assistance from SEM, JB, ENF, KC, and MD. YG performed slice electrophysiology. SEM, JB, and SI performed viral validation. HM supervised the research.
ANIMAL STUDIES
All animal protocols were approved by the Institutional Animal Care and Use Committee at Icahn School of Medicine at Mount Sinai.
Supporting information
Table S1‐S2
ACKNOWLEDGEMENT
This work was funded by NIH F31MH121010 to KJN and NIH R21NS105119, R21MH106919, and R01MH119523 to HM. We thank the members of the Morishita laboratory for helpful feedback.
Norman KJ, Koike H, McCraney SE, et al. Chemogenetic suppression of anterior cingulate cortical neurons projecting to the visual cortex disrupts attentional behavior in mice. Neuropsychopharmacol Rep. 2021;41:207–214. 10.1002/npr2.12176
Norman and Koike equal contribution.
DATA AVAILABILITY STATEMENT
All relevant data are included in Supporting Information.
REFERENCES
- 1. Clark K, Squire RF, Merrikhi Y, Noudoost B. Visual attention: Linking prefrontal sources to neuronal and behavioral correlates. Prog Neurogibol. 2015;132:59–80. [DOI] [PubMed] [Google Scholar]
- 2. Alexander MP, Stuss DT, Shallice T, Picton TW, Gillingham S. Impaired concentration due to frontal lobe damage from two distinct lesion sites. Neurology. 2005;65:572–9. [DOI] [PubMed] [Google Scholar]
- 3. Corbetta M, Miezin FM, Dobmeyer S, Shulman GL, Petersen SE. Selective and divided attention during visual discriminations of shape, color, and speed: functional anatomy by positron emission tomography. J Neurosci. 1991;11:2383–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weissman DH, Gopalakrishnan A, Hazlett CJ, Woldorff MG. Dorsal anterior cingulate cortex resolves conflict from distracting stimuli by boosting attention toward relevant events. Cereb Cortex. 2005;15:229–37. [DOI] [PubMed] [Google Scholar]
- 5. Johnston K, Levin HM, Koval MJ, Everling S. Top‐down control‐signal dynamics in anterior cingulate and prefrontal cortex neurons following task switching. Neuron. 2007;53:453–62. [DOI] [PubMed] [Google Scholar]
- 6. Nagai Y, Critchley HD, Featherstone E, Fenwick PB, Trimble MR, Dolan RJ. Brain activity relating to the contingent negative variation: an fMRI investigation. NeuroImage. 2004;21:1232–41. [DOI] [PubMed] [Google Scholar]
- 7. Totah NK, Kim YB, Homayoun H, Moghaddam B. Anterior cingulate neurons represent errors and preparatory attention within the same behavioral sequence. J Neurosci. 2009;29:6418–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu D, Deng H, Xiao X, Zuo Y, Sun J, Wang Z. Persistent neuronal activity in anterior cingulate cortex correlates with sustained attention in rats regardless of sensory modality. Sci Rep. 2017;7:43101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chudasama Y, Passetti F, Rhodes SE, Lopian D, Desai A, Robbins TW. Dissociable aspects of performance on the 5‐choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res. 2003;146:105–19. [DOI] [PubMed] [Google Scholar]
- 10. Passetti F, Chudasama Y, Robbins TW. The frontal cortex of the rat and visual attentional performance: dissociable functions of distinct medial prefrontal subregions. Cereb Cortex. 2002;12:1254–68. [DOI] [PubMed] [Google Scholar]
- 11. Pehrson AL, Bondi CO, Totah NK, Moghaddam B. The influence of NMDA and GABA(A) receptors and glutamic acid decarboxylase (GAD) activity on attention. Psychopharmacology. 2013;225:31–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim H, Ahrlund‐Richter S, Wang X, Deisseroth K, Carlen M. Prefrontal parvalbumin neurons in control of attention. Cell. 2016;164:208–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koike H, Demars MP, Short JA, Nabel EM, Akbarian S, Baxter MG, et al. Chemogenetic inactivation of dorsal anterior cingulate cortex neurons disrupts attentional behavior in mouse. Neuropsychopharmacology. 2016;41:1014–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. White MG, Panicker M, Mu C, Carter AM, Roberts BM, Dharmasri PA, et al. Anterior cingulate cortex input to the claustrum is required for top‐down action control. Cell Rep. 2018;22:84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang S, Xu M, Kamigaki T, Hoang Do JP, Chang WC, Jenvay S, et al. Selective attention. Long‐range and local circuits for top‐down modulation of visual cortex processing. Science. 2014;345:660–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. English JG, Roth BL. Chemogenetics – a transformational and translational platform. JAMA Neurol. 2015;72:1361–6. [DOI] [PubMed] [Google Scholar]
- 17. Nabel EM, Garkun Y, Koike H, Sadahiro M, Liang A, Norman KJ, et al. Adolescent frontal top‐down neurons receive heightened local drive to establish adult attentional behavior in mice. Nat Commun. 2020;11:3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bari A, Dalley JW, Robbins TW. The application of the 5‐choice serial reaction time task for the assessment of visual attentional processes and impulse control in rats. Nat Protoc. 2008;3:759–67. [DOI] [PubMed] [Google Scholar]
- 19. Carli M, Robbins TW, Evenden JL, Everitt BJ. Effects of lesions to ascending noradrenergic neurones on performance of a 5‐choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav Brain Res. 1983;9:361–80. [DOI] [PubMed] [Google Scholar]
- 20. Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–84. [DOI] [PubMed] [Google Scholar]
- 21. Lustig C, Kozak R, Sarter M, Young JW, Robbins TW. CNTRICS final animal model task selection: control of attention. Neurosci Biobehav Rev. 2013;37:2099–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Robbins TW. The 5‐choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology. 2002;163:362–80. [DOI] [PubMed] [Google Scholar]
- 23. Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa‐Shah P, Rodriguez LA, et al. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science. 2017;357:503–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bussey TJ, Holmes A, Lyon L, Mar AC, McAllister KA, Nithianantharajah J, et al. New translational assays for preclinical modelling of cognition in schizophrenia: the touchscreen testing method for mice and rats. Neuropharmacology. 2012;62:1191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mar AC, Horner AE, Nilsson SR, Alsio J, Kent BA, Kim CH, et al. The touchscreen operant platform for assessing executive function in rats and mice. Nat Protoc. 2013;8:1985–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Romberg C, Mattson MP, Mughal MR, Bussey TJ, Saksida LM. Impaired attention in the 3xTgAD mouse model of Alzheimer's disease: rescue by donepezil (Aricept). J Neurosci. 2011;31:3500–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Silverman JL, Gastrell PT, Karras MN, Solomon M, Crawley JN. Cognitive abilities on transitive inference using a novel touchscreen technology for mice. Cereb Cortex. 2013;25:1133–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nagai Y, Miyakawa N, Takuwa H, Hori Y, Oyama K, Ji B, et al. Deschloroclozapine, a potent and selective chemogenetic actuator enables rapid neuronal and behavioral modulations in mice and monkeys. Nat Neurosci. 2020;23:1157–67. [DOI] [PubMed] [Google Scholar]
- 29. Upright NA, Brookshire SW, Schnebelen W, Damatac CG, Hof PR, Browning PGF, et al. Behavioral effect of chemogenetic inhibition is directly related to receptor transduction levels in rhesus monkeys. J Neurosci. 2018;38:7969–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weston M, Kaserer T, Wu A, Mouravlev A, Carpenter JC, Snowball A, et al. Olanzapine: a potent agonist at the hM4D(Gi) DREADD amenable to clinical translation of chemogenetics. Sci Adv. 2019;5:eaaw1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mazaheri A, Coffey‐Corina S, Mangun GR, Bekker EM, Berry AS, Corbett BA. Functional disconnection of frontal cortex and visual cortex in attention‐deficit/hyperactivity disorder. Biol Psychiatry. 2010;67:617–23. [DOI] [PubMed] [Google Scholar]
- 32. Roiser JP, Wigton R, Kilner JM, Mendez MA, Hon N, Friston KJ, et al. Dysconnectivity in the frontoparietal attention network in schizophrenia. Front Psychiatry. 2013;4:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Minshew NJ, Keller TA. The nature of brain dysfunction in autism: functional brain imaging studies. Curr Opin Neurol. 2010;23:124–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S2
Data Availability Statement
All relevant data are included in Supporting Information.


