Abstract
Otsuka Pharmaceutical Co., Ltd. successfully developed the first dopamine D2 receptor partial agonist approved for schizophrenia, the antipsychotic aripiprazole (Abilify®). The drug was approved for this indication in the United States in 2002 and has received approval in the United States, Europe, Japan, and many other countries for several indications including schizophrenia, acute mania, adjunctive treatment of major depressive disorder (MDD), irritability associated with autistic disorder, and Tourette's disorder. Otsuka next developed brexpiprazole (Rexulti®), another D2 receptor partial agonist, which was granted marketing approval in the United States in 2015 as adjunctive therapy in major depressive disorder and for the treatment of schizophrenia. In Japan, brexpiprazole also received approval as a treatment for schizophrenia in 2018. In this review, we describe Otsuka's research history and achievements over the preceding 40 years in the area of antipsychotic drug discovery for dopamine D2 receptor partial agonists.
Keywords: aripiprazole, brexpiprazole, dopamine D2 receptor partial agonist, dopamine‐system stabilizer, serotonin‐dopamine activity modulator
“Dopamine‐system stabilizer” activity of aripiprazole.
1. INTRODUCTION
Aripiprazole (Abilify®, Figure 1) is an antipsychotic agent that contains a carbostyril skeleton and acts as a partial agonist at dopamine D2 receptors. 1 , 2 , 3 , 4 At the time of its discovery, both its chemical structure and mechanism of action were novel and markedly different from those of existing conventional antipsychotics. 3 , 4 Aripiprazole was developed by Otsuka Pharmaceutical Co., Ltd. (hereinafter referred to as “Otsuka”) in collaboration with Bristol Myers Squibb and approved by the FDA in 2002 for the indication of schizophrenia. The drug was approved for the same indication in Europe in 2004, in Japan in 2006, and in more than 65 countries worldwide. Unlike conventional antipsychotics (dopamine D2 receptor antagonists), aripiprazole is characterized by its stabilizing effect on dopamine neurotransmission and is thus described as a dopamine‐system stabilizer (DSS). 5 , 6 , 7 A new formulation of aripiprazole, Abilify Maintena®, is a prolonged release aqueous suspension for IM injection that can exert a therapeutic effect for 4 weeks after a single dose. It was approved for the treatment of schizophrenia in the United States in 2013, in Europe in 2013 and in Japan in 2015. Altogether, it has been approved in more than 50 countries. 8 , 9 More recently, Abilify Maintena® was also approved for the indication of maintenance treatment of bipolar I disorder in adults. Aripiprazole has not only D2 receptor partial agonist activity but also has 5‐HT1A receptor partial agonist and 5‐HT2A receptor antagonist activities. However, the affinity of aripiprazole for D2 receptors is one order higher than the affinities for 5‐HT1A and 5‐HT2A receptors. Thus, aripiprazole is considered to act as a DSS at clinical doses.
FIGURE 1.
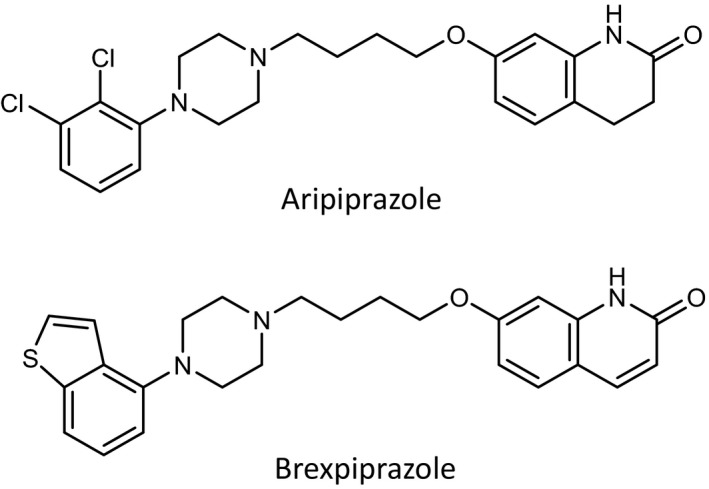
Chemical structures of aripiprazole and brexpiprazole
In 1999, Otsuka continued its drug discovery research efforts to find the next generation antipsychotic that combined the favorable characteristics of serotonin‐dopamine antagonists (SDAs), as epitomized by risperidone, and those of aripiprazole as a DSS. These efforts succeeded and led to the discovery and development of the novel antipsychotic brexpiprazole (Rexulti®, Figure 1). Brexpiprazole, which was developed with Lundbeck, was approved in the United States in 2015 both as adjunctive therapy in major depressive disorder (MDD) and as a treatment for schizophrenia. It was also approved for the indication of schizophrenia in Canada and Australia in 2017, in Japan in 2018, and in Europe in 2018. Recently, it was approved in Canada (2019) as adjunctive treatment in MDD. Brexpiprazole is an antipsychotic that belongs to a new category of drugs and is classified as a serotonin‐dopamine activity modulator (SDAM), which has almost equally high affinities for 5‐HT1A, 5‐HT2A, and D2 receptors and functions as a potent partial agonist at serotonin 5‐HT1A receptors, a potent antagonist at 5‐HT2A receptors, and a potent partial agonist at dopamine D2 receptors with lower intrinsic activity compared to aripiprazole. 10 , 11
In this review, we describe the research history and achievements of our CNS drug discovery division in the field of antipsychotic drugs.
2. ARIPIPRAZOLE
2.1. History of research and development
In the 1950s, chlorpromazine and haloperidol entered the market as antipsychotic drugs. These drugs were commonly referred to as typical antipsychotics or first‐generation antipsychotics; they possessed dopamine D2 receptor antagonist activity and were effective in treating the positive symptoms (eg, hallucinations and delusions) of schizophrenia. However, they had a number of drawbacks. Most notably, they were only minimally effective in the treatment of negative symptoms (eg, blunted affect, emotional withdrawal, and reduced motor activity) of schizophrenia, they induced extrapyramidal symptoms including akathisia, dystonia, parkinsonian syndrome, and tardive dyskinesia, and they elevated blood prolactin levels. In the 1980s, research on developing drugs that worked on both dopaminergic and serotonergic neurons was initiated. In 1990, clozapine was launched in the United States. Clozapine was in fact an old drug and although it was highly efficacious, it caused agranulocytosis and convulsions, significantly limiting its use. This was followed by the release of the first serotonin‐dopamine antagonist (SDA), risperidone, introducing the new class of atypical antipsychotics. Subsequently, other SDAs were approved, including olanzapine, quetiapine, and ziprasidone. These atypical antipsychotics are often referred to as second‐generation antipsychotics. Unlike their typical antipsychotic counterparts, atypical antipsychotics are less likely to induce extrapyramidal symptoms. However, each drug is different and each has its own shortcomings (ie, hyperprolactinemia with risperidone; weight gain, dyslipidemia, and sedation with olanzapine and quetiapine; and cardiac QTc prolongation with ziprasidone). Given these considerations, there existed an unmet medical need for new antipsychotics with better tolerability and an improved safety profile. 12 , 13
In 1972, Arvid Carlsson, who was awarded the 2000 Nobel Prize in Physiology or Medicine, along with his colleagues, hypothesized that receptors (autoreceptors) which negatively regulate dopamine synthesis/release and firing rate of dopamine neurons exist in the presynaptic region of dopaminergic neurons. 14 The existence of dopamine autoreceptors was subsequently proven. Based on the dopamine hyperactivity theory for schizophrenia, Otsuka began to work on agonists at presynaptic dopamine autoreceptors and eventually discovered OPC‐4392, a dopamine autoreceptor agonist, in 1980 on the basis of a hypothesis that a selective dopamine autoreceptor agonist would act as an antipsychotic and have fewer extrapyramidal symptoms. 15 While clinical trials conducted in Japan and Europe demonstrated that OPC‐4392 exhibited fewer extrapyramidal symptoms, as expected, and was effective in reducing negative symptoms, its effect on positive symptoms was inadequate. Otsuka then decided to discontinue the development of OPC‐4392 due to its lack of therapeutic effects on positive symptoms. The clinical results of OPC‐4392 prompted Otsuka to generate a working hypothesis that a compound that combines dopamine autoreceptor agonism, a property possessed by OPC‐4392, and potent postsynaptic dopamine D2 receptor antagonism, a property not possessed by OPC‐4392, may be effective in the treatment of both negative and positive symptoms of schizophrenia and less likely to induce extrapyramidal symptoms. Based on this hypothesis, in 1987, Otsuka finally discovered aripiprazole through in vivo/ex vivo screening using animal models. At the time, there were no reports available on a screening method capable of simultaneously detecting both presynaptic and postsynaptic pharmacological activities. Otsuka then decided to search for a new target compound by utilizing a range of conventional screening methods. Specifically, Otsuka studied compounds for their anti‐apomorphine activity (ie, postsynaptic dopamine D2 receptor antagonist activity) in mice and identified ones that were as equivalent as possible to haloperidol in terms of potency. Next, using as an index the inhibitory effect on a gamma‐butyrolactone‐ or reserpine‐induced increase in dopamine biosynthesis in the mouse forebrain, Otsuka selected compounds with dopamine autoreceptor agonist activity and potency equal to or greater than that of OPC‐4392. Other selection criteria included a weak α1 adrenergic receptor antagonist effect, which is related to cardiovascular side effects, and a low potential to induce catalepsy, which is predictive of extrapyramidal symptoms. These drug discovery efforts resulted in the synthesis of aripiprazole. Aripiprazole is pharmacologically characterized by its ability to act both as a presynaptic dopamine autoreceptor agonist and as a postsynaptic dopamine D2 receptor antagonist at almost the same dose, a characteristic not shared by conventional antipsychotics at the time. 3 , 4
2.2. Dopamine D2 receptor partial agonist activity
Analysis of the mechanism of aripiprazole's presynaptic dopamine autoreceptor agonist activity and postsynaptic dopamine D2 receptor antagonist activity revealed that aripiprazole was a dopamine D2 receptor partial agonist with a relatively low level of intrinsic activity. 1 In the presence of dopamine, aripiprazole decreased dopamine D2 receptor‐mediated transmission but did not result in full blockade. In the absence of dopamine, aripiprazole produced small increases in dopamine D2 receptor‐mediated transmission consistent with its intrinsic activity. Studies also demonstrated that aripiprazole exerted greater agonist activity at presynaptic dopamine D2 autoreceptors than at postsynaptic dopamine D2 receptors on the basis that the receptor reserve of presynaptic dopamine D2 autoreceptors was greater than that of postsynaptic dopamine D2 receptors. 1
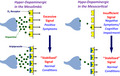
The concept of receptor partial agonism is not new. Rather, it is one that has long been used to explain receptor‐mediated responses. In short, a D2 receptor partial agonist is defined as a substance with affinity for D2 receptors, but one that possesses less intrinsic activity than that of the endogenous full agonist dopamine (ie, a substance that binds to a D2 receptor and produces the same qualitative response as dopamine; however, while this response is smaller than that produced by dopamine, it is not 0.). A D2 receptor partial agonist can reduce dopamine D2 receptor neurotransmission to the level of its own agonist activity (intrinsic activity), and this property was demonstrated for aripiprazole. In contrast, it can increase neurotransmission mediated by the D2 receptors to the level of its intrinsic activity by itself, and this property also was demonstrated for aripiprazole. Unlike conventional antipsychotics (ie, D2 receptor antagonists), aripiprazole acts as a DSS; it stabilizes the D2 receptor‐mediated neurotransmission to the level of its own intrinsic activity. 1 , 2 , 5 The mechanism of D2 receptor partial agonist action on dopaminergic neurotransmission is thus quite different from that of D2 receptor antagonists. The dopamine hyperactivity hypothesis of schizophrenia has identified dopamine as the culprit, but this substance is an important neurotransmitter closely linked to normal mental, motor, and hormonal regulation. It is no surprise that conventional antipsychotics (ie, D2 receptor antagonists) are riddled with side effects, as they completely block D2 receptor‐mediated physiological response to dopamine at higher‐than‐expected blood drug levels resulting from dose increase or due to an individual difference in drug absorption and metabolism. Therefore, conventional antipsychotics, which completely block the D2 receptor‐mediated physiological response to dopamine, may not be the optimal option for treating this condition. As a DSS, aripiprazole should possess a clinically preferable mechanism of action. For patients with schizophrenia, the onset of symptoms appears to be associated with increased activity of mesolimbic dopaminergic neurons (ie, onset of positive symptoms) as well as decreased mesocortical activity (ie, onset of negative symptoms and cognitive impairment) 16 and aripiprazole can work as a functional antagonist and as a functional agonist in areas of overactivity and underactivity, respectively 5 , 17 (Figure 2).
FIGURE 2.
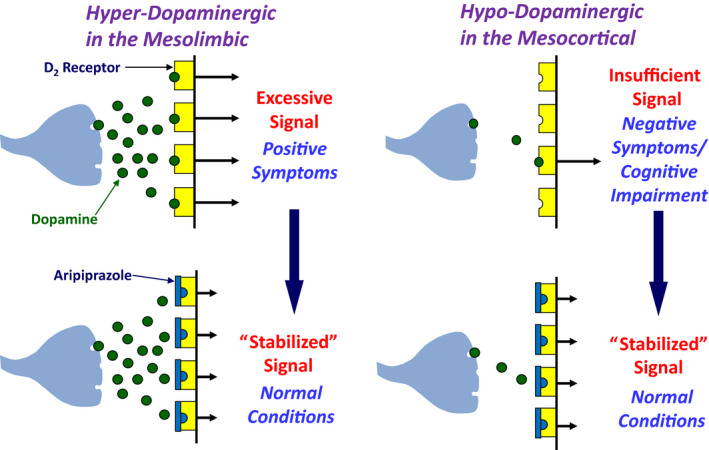
“Dopamine‐system stabilizer (DSS)” activity of aripiprazole
2.3. Clinical benefits based on dopamine D2 receptor partial agonist activity
This section summarizes how the D2 receptor partial agonist properties of aripiprazole are associated with its clinical utility. Conventional antipsychotics exert their therapeutic effects by blocking dopamine D2 receptors in the brain; however, they also cause side effects due to their potent antagonist action. These side effects include (a) extrapyramidal symptoms due to excessive blockade of nigrostriatal dopamine D2 receptors, 16 (b) depression and dysphoria due to excessive blockade of accumbal dopamine D2 receptors, 18 and (c) lactation, gynecomastia, menstrual abnormality, and sexual dysfunction associated with hyperprolactinemia due to excessive blockade of anterior pituitary dopamine D2 receptors. 16 , 19 These problems may contribute to poor tolerability and low drug adherence. 12 Aripiprazole causes extrapyramidal symptoms and hyperprolactinemia less frequently than conventional antipsychotics (ie, D2 receptor antagonists) 6 , 7 which may contribute to the perceived improvement in drug adherence. In fact, while it has often been observed that patients treated with antipsychotics associated with higher occupancy of brain dopamine D2/D3 receptors have lower levels of subjective well‐being, patients who switched their medication from risperidone or olanzapine to aripiprazole tablets (10‐30 mg/d) reported that their subjective sense of well‐being improved. 20 This was despite aripiprazole's high occupancy of brain dopamine D2/D3 receptors (82%‐92%) and this benefit lasted over a 6‐month treatment period with aripiprazole tablets. 20 In a 28‐week randomized open‐label trial to directly compare aripiprazole prolonged release injectable suspension (400 mg/4 weeks) and paliperidone palmitate, aripiprazole prolonged release injectable suspension was associated with a greater improvement in total scores for both subjective well‐being and the quality of life scale at Week 28 from baseline. 21 Aripiprazole's intrinsic activity, which does not cause excessive blockade of dopamine D2 receptor‐mediated signaling, may explain its favorable tolerability and safety profile as mentioned above.
Dopamine supersensitive psychosis (DSP) that occurs after chronic use of high‐dose antipsychotics is considered to result from a compensatory increase in dopamine D2 receptors (ie, upregulation) in response to excessive blockade of dopamine D2 receptors. 22 , 23 Since aripiprazole has an intrinsic activity and it does not cause excessive blockade of dopamine D2 receptors, it is unlikely to increase dopamine D2 receptors, suggesting that the risk of inducing DSP is low. Reports from animal experiments show that aripiprazole does not increase the number of dopamine D2 receptors 24 and therefore is less likely to enhance dopamine sensitivity. Interestingly, enhanced dopamine sensitivity induced by chronic treatment with haloperidol was reversed in rats after chronic treatment with aripiprazole. 24
Considering that blood concentrations of antipsychotics vary substantially among different individuals, a reasonable approach is to determine a dose range that accommodates individual variability. The aripiprazole tablet label specifies the range of effective doses (6‐30 mg/d in Japan, 10‐30 mg/d in the United States) to be used. The incidence of adverse events in schizophrenia, except somnolence, in patients receiving aripiprazole tablets did not increase dose‐dependently within the effective dose range, showing the drug's favorable tolerability and safety profile. 25 While conventional antipsychotics (ie, D2 receptor antagonists) usually have dose‐dependent side effects due to blockade of dopamine D2 receptors, aripiprazole may minimize such problems, probably because it does not cause excessive blockade of dopamine D2 receptor‐mediated signaling due to its intrinsic activity. In addition, aripiprazole also has relatively low affinities, compared to D2 receptors, for other receptor families (eg, adrenergic α1 receptors, histamine H1 receptors, and muscarinic M1 receptors) which may further contribute to reducing the incidence or severity of some side effects (eg, orthostatic hypotension, oversedation, weight gain, and anticholinergic side effects). 26
3. BREXPIPRAZOLE
3.1. History of research and development
In 1999, Otsuka's CNS drug discovery division, in conjunction with their clinical development colleagues in the United States and Japan, initiated an effort to determine what effects of aripiprazole could be improved in a second‐generation molecule. Although aripiprazole demonstrated a favorable tolerability and safety profile compared to other atypical antipsychotics such as risperidone, olanzapine and quetiapine, we were informed that in clinical practice aripiprazole was associated with higher incidences of adverse events such as akathisia, restlessness, and insomnia. By reviewing the clinical data and the feedback Otsuka received from practicing psychiatrists, a hypothesis emerged within Otsuka that adding a more potent effect on the serotonin system, a feature of SDAs, to a DSS could result in the development of an antipsychotic with a superior safety profile including reduced risks of the kinds of side effects (eg, akathisia, restlessness, and insomnia) that were frequently reported in the early period of aripiprazole therapy. Our drug discovery efforts focused on two points. First, it was postulated that increasing the potency of antagonism at serotonin 5‐HT2A receptors could reduce insomnia and other sleep disturbances, based on reports that serotonin 5‐HT2A receptor antagonists are able to ameliorate insomnia by enhancing slow‐wave sleep during the night. 27 , 28 Thus, we hypothesized that more potent antagonism at serotonin 5‐HT2A receptors may ameliorate insomnia induced by aripiprazole in clinical practice. In addition, it was hypothesized within Otsuka that the potent 5‐HT2A receptor antagonist activity might ameliorate aripiprazole‐induced akathisia, especially in adjunctive treatment of MDD. 29 , 30 Moreover, it was thought that increasing the agonist potency at serotonin 5‐HT1A receptors might further improve the symptoms of depression and anxiety. At that time, tandospirone, a selective 5‐HT1A receptor partial agonist, was approved in Japan for the indication of depressed mood in neurotic patients (ie, dysthymia, DSM‐5; persistent depressive disorder) and a cluster analysis of the Hamilton Anxiety Scale in the latter study demonstrated the superiority of tandospirone over diazepam in the treatment of depressed mood. 31 In addition, it was reported that adjunctive treatment with tandospirone improved memory function in patients with schizophrenia who were receiving ongoing treatment with typical antipsychotics. 32 Second, it was postulated that the observation of akathisia, insomnia and restlessness with aripiprazole could be ameliorated by reducing the intrinsic activity at dopamine D2 receptors. This hypothesis was supported by a report on patients with schizophrenia in whom akathisia, irritability, and excitement were observed when receiving L‐DOPA treatment and which disappeared with a reduction in the L‐DOPA dose or increase in the dose of antipsychotics. 33 With this in mind, research was initiated to uncover a new partial agonist. The results of the research based on both of these hypotheses led to the discovery of brexpiprazole.
Unlike aripiprazole, brexpiprazole has almost equally high affinities for 5‐HT1A, 5‐HT2A, and D2 receptors and exhibits potent partial agonist activity at 5‐HT1A receptors, potent antagonist activity at 5‐HT2A receptors, and potent partial agonist activity at D2 receptors with a reduced level of intrinsic activity. Based on this profile, brexpiprazole has been classified as an SDAM, a novel class of antipsychotics. 10 , 11
3.2. Pharmacology of brexpiprazole
3.2.1. Receptor binding affinity
Table 1 shows the binding affinities of brexpiprazole and other antipsychotics for human receptors in vitro. 10 , 34 , 35 Brexpiprazole is characterized by high affinity for 5‐HT1A and 5‐HT2A receptors (Ki = 0.12 and 0.47 nmol/L, respectively) as well as for D2L receptors (0.30 nmol/L). The affinity of brexpiprazole for D2L receptors is comparable to that of aripiprazole (0.34 nmol/L), and the affinities of brexpiprazole for 5‐HT1A and 5‐HT2A receptors are approximately 10‐fold higher than those of aripiprazole (1.7 and 3.4 nmol/L, respectively). Unlike aripiprazole, brexpiprazole has almost equally high affinities for not only D2L receptors but also 5‐HT1A and 5‐HT2A receptors. In addition, brexpiprazole shows moderate affinities for histamine H1 receptors (19 nmol/L) and adrenaline α1A receptors (3.8 nmol/L). 10 Although not shown in Table 1, brexpiprazole has higher affinity for α1B (Ki = 0.17 nmol/L) and α2C (Ki = 0.59 nmol/L) receptors 10 than aripiprazole (α1B: Ki = 35 nmol/L, α2C: Ki = 38 nmol/L). 26
TABLE 1.
Binding affinities of antipsychotics for human receptors (Ki , nmol/L)
| Receptors | Brexpiprazole | Aripiprazole | Olanzapine | Quetiapine | Risperidone | Haloperidol |
|---|---|---|---|---|---|---|
| D2 | 0.30 | 0.34 | 20 | 180 | 2.2 | 1.4 |
| D3 | 1.1 | 0.8 | 50 | 940 | 9.6 | 2.5 |
| 5‐HT1A | 0.12 | 1.7 | 2100 | 230 | 210 | 3600 |
| 5‐HT2A | 0.47 | 3.4 | 3.3 | 220 | 0.29 | 120 |
| 5‐HT2C | 34 | 96 | 10 | 1400 | 10 | 4700 |
| 5‐HT7 | 3.7 | 39 | 250 | 1800 | 3.0 | 1100 |
| α1 | 3.8 a | 52 a | 54 | 15 | 1.4 | 4.7 |
| H1 | 19 | 61 | 2.8 | 8.7 | 19 | 440 |
| M1 | >1000 | 6800 | 4.7 | 100 | 2800 | 1600 |
3.2.2. Partial agonism at 5‐HT1A receptors
The functional effects of brexpiprazole were investigated using guanosine 5′‐O‐(3‐[35S]thio)‐triphosphate ([35S] GTPγS) binding to recombinant human 5‐HT1A receptor‐expressing cells. Brexpiprazole partially increased GTPγS binding (EC50 = 0.49 nmol/L, E max = 60%) and was thus shown to be a 5‐HT1A receptor partial agonist with higher potency and slightly lower intrinsic activity than aripiprazole (EC50 = 2.1 nmol/L, E max = 73%). The potency of brexpiprazole is also higher than serotonin (EC50 = 5.1 nmol/L). The antianxiety drug buspirone also showed 5‐HT1A receptor partial agonist activity (EC50 = 24 nmol/L, E max = 78%). The potency and intrinsic activity of brexpiprazole are much higher and slightly lower than buspirone, respectively. 10
3.2.3. Antagonism at 5‐HT2A receptors
Antagonism at 5‐HT2A receptors, a major pharmacological action of atypical antipsychotics, is the central tenet of the serotonin hypothesis proposed by Meltzer et al. 36 The functional effects of brexpiprazole on receptors were explored using recombinant human 5‐HT2A receptor‐expressing cells, with production of inositol monophosphate as an index. Brexpiprazole inhibited the effect of serotonin in a concentration‐dependent manner (corrected IC50 = 6.5 nmol/L). 10 The effect of brexpiprazole on 5‐HT2A receptor agonist (±)‐2,5‐dimethoxy‐4‐iodoamphetamine‐induced head twitches in rats was also evaluated. This in vivo study demonstrated that brexpiprazole had a more potent antagonist effect on 5‐HT2A receptors than aripiprazole (Table 2). 10
TABLE 2.
Pharmacological effects of brexpiprazole and aripiprazole
| Drugs | ||||||||
|---|---|---|---|---|---|---|---|---|
| Items | ||||||||
| ED50 values (mg/kg, po) | Cataleptogenic liability | |||||||
| D2 antagonism/antipsychotic‐like effect | 5‐HT2A antagonism | EPS | ||||||
| APO‐induced hyperlocomotion (Rat)(a) | APO‐induced stereotyped behavior (Rat)(b) | Conditioned avoidance response (Rat)(c) | DOI‐induced head twitch (Rat) | Cataleptogenic effect (Rat)(d) | d/a ratio | d/b ratio | d/c ratio | |
| Brexpiprazole | 2.3 | 2.9 | 6.0 | 4.7 | 20 | 8.7 | 6.9 | 3.3 |
| Aripiprazole | 3.2 | 6.1 | 23 | 21 | 42 | 13.1 | 6.9 | 1.8 |
Cataleptogenic liability = (ED50 for induction of catalepsy)/(ED50 for inhibition of APO‐induced behavior) or (ED50 for inhibition of conditioned avoidance response). The data are quoted from Maeda et al. 10 , 11
Abbreviations: APO, apomorphine; DOI, (±)‐2,5‐dimethoxy‐4‐iodoamphetamine; EPS, extrapyramidal symptoms.
3.2.4. Partial agonism at D2/D3 receptors
The functional effects of brexpiprazole on receptors were explored using recombinant human D2L receptor‐expressing cells, with forskolin‐induced cyclic adenosine monophosphate (cAMP) accumulation as an index. Brexpiprazole partially inhibited cAMP accumulation (EC50 = 4.0 nmol/L, E max = 43%) and was thus shown to act as a D2 receptor partial agonist with almost the same potency and lower intrinsic activity compared to aripiprazole (EC50 = 5.6 nmol/L, E max = 61%) (Figure 3). 10 Brexpiprazole was also shown to act as a human D3 receptor partial agonist with similar potency and lower intrinsic activity (EC50 = 2.8 nmol/L, E max = 15%) than aripiprazole (EC50 = 5.9 nmol/L, E max = 28%). 10
FIGURE 3.
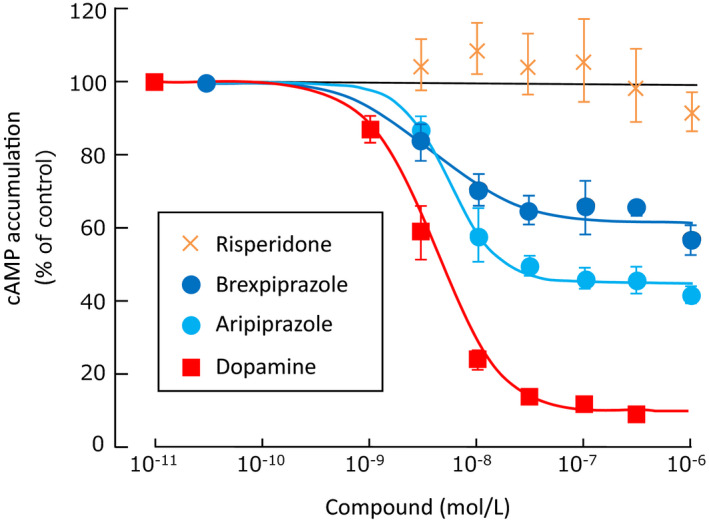
Dopamine D2L receptor partial agonist activity. Data are mean ± SD. Concentration‐response curves are shown for each compounds on forskolin‐induced cAMP accumulation in human D2L receptor‐expressing cells. Cyclic AMP accumulation was normalized to the percentage of forskolin‐induced cAMP accumulation (set at 100%). It is adapted from Maeda et al. 10
3.2.5. Anti‐apomorphine effect
In order to evaluate the functional D2 receptor antagonism of brexpiprazole in vivo, which may play a key role in improving positive symptoms, the effect of brexpiprazole on abnormal behavior induced by the D2 receptor agonist apomorphine was evaluated in rats. Brexpiprazole dose‐dependently and significantly inhibited hyperlocomotion and stereotyped behavior with ED50 values of 2.3 and 2.9 mg/kg, po, respectively; these inhibitory potencies were greater than those of aripiprazole (Table 2). Brexpiprazole also dose‐dependently reduced apomorphine‐induced blinking frequency in monkeys with an ED50 value of 0.03 mg/kg, po. 11
3.2.6. Inhibitory effect on conditioned avoidance response
The effect of brexpiprazole on the conditioned avoidance response in rats, a test model for predicting the effect of a drug on positive symptoms of schizophrenia, was evaluated. Brexpiprazole dose‐dependently and significantly inhibited an electric shock‐induced conditioned avoidance response with an ED50 value of 6.0 mg/kg, po; the inhibitory potency was greater than that of aripiprazole (Table 2). 11
3.2.7. Cataleptogenic effect
The cataleptogenic effect of brexpiprazole as an index of extrapyramidal symptoms was evaluated in rats. The cataleptogenic potency of brexpiprazole was greater than that of aripiprazole. However, the ED50 ratios for the cataleptogenic effect vs anti‐apomorphine effects or inhibitory effect on conditioned avoidance response of brexpiprazole were similar to those of aripiprazole (Table 2). 11
3.2.8. Effect on prolactin
It was reported that aripiprazole acts as a partial agonist on D2 receptors on the lactotroph cells in the isolated anterior pituitary gland of rats 37 and on both human D2S and D2L receptors expressed in GH4CL cells, which is a rat pituitary cell line. 38 In addition, aripiprazole decreased reserpine‐induced hyperprolactinemia in rats and the effect was antagonized by haloperidol, which suggests that aripiprazole acts as an agonist on D2 receptors on the lactotroph cells in the anterior pituitary gland when the inhibitory tone exerted by endogenous dopamine in the hypophyseal portal blood weakens. 37 The effect of brexpiprazole on reserpine‐induced hyperprolactinemia was evaluated in rats. Brexpiprazole at a dose of 3 mg/kg, po significantly decreased hyperprolactinemia, and doses of 10 and 30 mg/kg, po, which are approximately 3‐10 times higher than the doses that showed the anti‐apomorphine effects (ie, D2 receptor antagonist activity), did not significantly change prolactin levels, whereas the D2 receptor antagonist risperidone (3‐30 mg/kg, po) dose‐dependently and significantly increased hyperprolactinemia (Figure 4). 10
FIGURE 4.
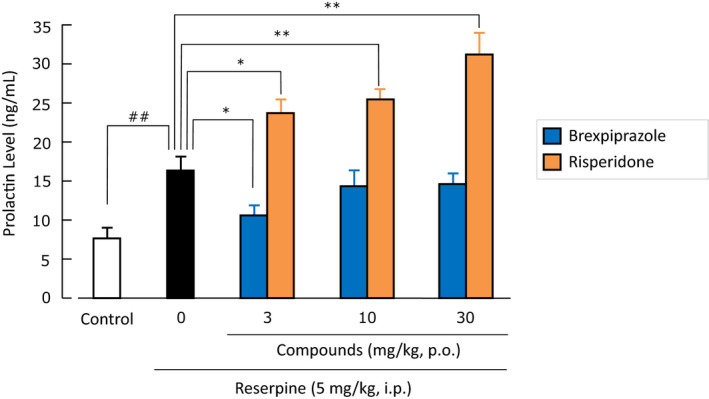
Effects of brexpiprazole and risperidone on reserpine‐induced hyperprolactinemia in rats. Data are mean ± SEM (n = 8). ## P < .01; *P < .05, **P < .01. It is quoted from Maeda et al. 10
3.2.9. Postsynaptic dopamine D2 receptor sensitizing effect
The postsynaptic dopamine D2 receptor sensitizing effect of brexpiprazole and haloperidol was investigated by evaluating low‐dose apomorphine‐induced stereotyped behavior in rats following 3 weeks of oral treatment with the drugs. Haloperidol (1 mg/kg, po) significantly augmented apomorphine‐induced stereotyped behavior 3 and 5 days after completion of 3‐week treatment, whereas the 3‐week treatment with brexpiprazole (6 mg/kg, po; equivalent potency to 1 mg/kg of haloperidol in rats) did not significantly augment stereotyped behavior. 39
3.2.10. Novel object recognition test
A novel object recognition test was conducted in rats subchronically treated with phencyclidine (subPCP), a psychedelic compound which induces a behavioral syndrome in rodents that bears close similarities to some of the core symptoms observed in schizophrenic patients, including cognitive deficit. Brexpiprazole completely reversed a subPCP‐induced decrease in the discrimination index at doses (1 and 3 mg/kg, po) that are almost equivalent to the doses at which brexpiprazole exerts its anti‐apomorphine effects. This effect of brexpiprazole was attenuated by the 5‐HT1A receptor antagonist WAY‐100635 and the 5‐HT2A receptor antagonist M100907 alone partially reversed subPCP‐induced impairment, thus suggesting that both 5‐HT1A receptor partial agonist and 5‐HT2A receptor antagonist activities may contribute to the beneficial effects of brexpiprazole. 11
4. CONCLUSION
This article provides a summary of the discovery research and development history, pharmacological properties, and clinical use of aripiprazole and brexpiprazole. Aripiprazole and brexpiprazole are D2 receptor partial agonists in contrast to conventional antipsychotics, which are D2 receptor antagonists. D2 receptor antagonists cause excessive blockade of dopamine D2 receptors and thereby induce side effects related to D2 receptor blockade. On the other hand, aripiprazole and brexpiprazole have fewer D2 receptor blockade‐related side effects because they have intrinsic activity and do not cause excessive blockade of D2 receptor‐mediated signaling. The D2 receptor partial agonists aripiprazole and brexpiprazole appear to have a physiologically sound mechanism of action in patients as they do not abolish the intrinsic physiological function of dopamine and can stabilize neurotransmission at D2 receptors.
Brexpiprazole has lower intrinsic activity at D2 receptors compared with aripiprazole. However, a study of cataleptogenic activity suggests that both brexpiprazole and aripiprazole have a wide safe dose range based on the therapeutic dose of D2 receptor antagonists and the dose that can cause the onset of extrapyramidal side effects. In addition, rat experiments demonstrate that brexpiprazole is associated with a low risk for hyperprolactinemia and no significant postsynaptic dopamine D2 receptor sensitizing effect after repeated administration, as has also been observed with aripiprazole. 24 , 37 , 38 These findings strongly suggest that even though the intrinsic activity of brexpiprazole at D2 receptors is reduced compared to aripiprazole, brexpiprazole still retains the benefit of a D2 receptor partial agonist (ie, does not cause excessive blockade of D2 receptor‐mediated signaling sufficient to induce adverse side effects). The lower intrinsic activity of brexpiprazole at D2 receptors relative to aripiprazole indicates a lower likelihood of inducing side effects that are considered to be associated with aripiprazole‐induced stimulating activity at D2 receptors. Indeed, clinical trials conducted both outside 40 , 41 , 42 , 43 and in Japan 44 , 45 demonstrated low incidences of adverse events especially akathisia, restlessness, and insomnia (activating adverse effects 43 ). Brexpiprazole had little or no significant effect on blood prolactin levels in schizophrenia 42 , 44 , 46 although aripiprazole was associated with decreases in blood prolactin levels. 6 , 7 , 25 This can possibly be attributed to the lower intrinsic activity of brexpiprazole. With all of the above taken into consideration, the antipsychotic brexpiprazole appears to be a D2 receptor partial agonist that is therapeutically more beneficial with fewer side effects compared to aripiprazole.
Compared to aripiprazole, brexpiprazole has a higher affinity for 5‐HT1A and 5‐HT2A receptors, and accordingly, it functions as a more potent partial agonist at 5‐HT1A receptors and as a more potent antagonist at 5‐HT2A receptors than aripiprazole. It has been hypothesized that partial agonism at 5‐HT1A receptors may play a role in reducing depression and anxiety 31 and improving cognitive function, 32 while also being associated with a more favorable side effect profile compared to other antipsychotics. 36 Brexpiprazole showed improvement in phencyclidine‐induced cognitive deficit in the novel objective recognition test in rats, and the effect was attenuated by the selective 5‐HT1A receptor antagonist WAY‐100635. In addition, brexpiprazole also had improving effects in other models of cognitive deficits, and the effects were almost completely antagonized by WAY‐100635 47 , 48 suggesting that the 5‐HT1A receptor partial agonist activity has an important role in improving cognitive deficits in animals. It was reported that aripiprazole improves phencyclidine‐induced impairment of recognition memory in mice, and the effect is antagonized by WAY‐100635. 49 However, in our study, which used rats instead of mice, aripiprazole did not improve phencyclidine‐induced cognitive deficit. 11 It has recently been reported that co‐treatment with buspirone, a 5‐HT1A receptor partial agonist, and atypical antipsychotic drugs outperformed atypical antipsychotic drugs alone in improving cognitive deficit and reducing the burden on the families of patients with schizophrenia. 50 It has been proposed that a potent antagonist effect on 5‐HT2A receptors is involved in improving insomnia and other sleep disturbances, 27 , 28 as well as akathisia, 29 , 30 psychotic symptoms, depression, anxiety, and reducing extrapyramidal symptoms in patients with schizophrenia. 36 The potent antagonism of brexpiprazole at the 5‐HT2A receptors may contribute to its favorable tolerability/safety profile in clinical practice.
Brexpiprazole also has high affinity for α1B and α2C receptors and acts as an antagonist at both receptors. 10 The implications of the high affinities of brexpiprazole for α1B and α2C receptors as antagonist effects are difficult to predict in clinical practice. A study using knockout mice suggests that α1B receptor antagonism might contribute to antipsychotic‐like activity and effects on psychostimulant‐ or opiate‐induced reward. 51 It was reported that prazosin, an α1 receptor antagonist, is effective in the treatment of post‐traumatic stress disorder (PTSD). 52 Although the degree to which α1B receptor antagonism is effective in the treatment of PTSD is not fully known, it has been reported that adjunctive treatment with brexpiprazole and escitalopram reduces behavioral stress responses in a rat model of PTSD‐like symptoms 53 and that brexpiprazole blocks PTSD‐like memory while promoting normal fear memory in a model that precisely recapitulates the two memory components of PTSD in mice. 54 Interestingly, it has been recently reported that adjunctive treatment with brexpiprazole improves treatment‐resistant complex PTSD in domestic family violence victims. 55 In addition, some studies have reported that α2C receptor antagonism might contribute to antipsychotic‐like activity, 56 , 57 , 58 , 59 antidepressant‐like activity, 56 , 59 and procognitive activity 57 , 59 , 60 in animals. Although brexpiprazole has high affinity for both α1B and α2C receptors, cardiovascular adverse events such as orthostatic hypotension do not appear to be a significant problem in clinical practice. 40 , 41 , 44 , 45
In conclusion, brexpiprazole is a promising novel antipsychotic indicated for the treatment of schizophrenia and other disorders with a unique mechanism of action associated with its pharmacological properties as an SDAM.
CONFLICT OF INTEREST
Tetsuro Kikuchi, Kenji Maeda, Mikio Suzuki, Tsuyoshi Hirose and Takashi Futamura are full‐time employees of Otsuka Pharmaceutical Co., Ltd., and Robert D. McQuade is a full‐time employee of Otsuka Pharmaceutical Development & Commercialization, Inc.
AUTHOR CONTRIBUTIONS
All authors contributed to the writing of this manuscript.
ACKNOWLEDGMENTS
The authors would like to express their deep gratitude to Catherine Weiss, PhD (Director, Global Medical Affairs, Otsuka Pharmaceutical Development & Commercialization, Inc) for the editorial support she gave to this manuscript.
Kikuchi T, Maeda K, Suzuki M, Hirose T, Futamura T, McQuade RD. Discovery research and development history of the dopamine D2 receptor partial agonists, aripiprazole and brexpiprazole. Neuropsychopharmacol Rep. 2021;41:134–143. 10.1002/npr2.12180
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Burris KD, Molski TF, Xu C, Ryan E, Tottori K, Kikuchi T, et al. Aripiprazole, a novel antipsychotic, is a high affinity partial agonist at human dopamine D2 receptors. J Pharmacol Exp Ther. 2002;302(1):381–9. [DOI] [PubMed] [Google Scholar]
- 2. Tadori Y, Miwa T, Tottori K, Burris KD, Stark A, Mori T, et al. Aripiprazole's low intrinsic activities at human dopamine D2L and D2S receptors render it a unique antipsychotic. Eur J Pharmacol. 2005;515(1–3):10–19. [DOI] [PubMed] [Google Scholar]
- 3. Kikuchi T, Tottori K, Uwahodo Y, Hirose T, Miwa T, Oshiro Y, et al. 7‐{4‐[4‐(2,3‐Dichlorophenyl)‐1‐piperazinyl]butyloxy}‐3,4‐dihydro‐2(1H)‐quinolinone (OPC‐14597), a new putative antipsychotic drug with both presynaptic dopamine autoreceptor agonistic activity and postsynaptic D2 receptor antagonistic activity. J Pharmacol Exp Ther. 1995;274(1):329–36. [PubMed] [Google Scholar]
- 4. Oshiro Y, Sato S, Kurahashi N, Tanaka T, Kikuchi T, Tottori K, et al. Novel antipsychotic agents with dopamine autoreceptor agonist properties: synthesis and pharmacology of 7‐[4‐(4‐phenyl‐1‐piperazinyl)butoxy]‐3,4‐dihydro‐2(1H)‐quinolinone derivatives. J Med Chem. 1998;41(5):658–67. [DOI] [PubMed] [Google Scholar]
- 5. Stahl SM. Dopamine system stabilizers, aripiprazole, and the next generation of antipsychotics, part 2: illustrating their mechanism of action. J Clin Psychiatry. 2001;62(12):923–4. [DOI] [PubMed] [Google Scholar]
- 6. Kane JM, Carson WH, Saha AR, McQuade RD, Ingenito GG, Zimbroff DL, et al. Efficacy and safety of aripiprazole and haloperidol versus placebo in patients with schizophrenia and schizoaffective disorder. J Clin Psychiatry. 2002;63(9):763–71. [DOI] [PubMed] [Google Scholar]
- 7. Potkin SG, Saha AR, Kujawa MJ, Carson WH, Ali M, Stock E, et al. Aripiprazole, an antipsychotic with a novel mechanism of action, and risperidone vs placebo in patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry. 2003;60(7):681–90. [DOI] [PubMed] [Google Scholar]
- 8. Kane JM, Sanchez R, Perry PP, Jin N, Johnson BR, Forbes RA, et al. Aripiprazole intramuscular depot as maintenance treatment in patients with schizophrenia: a 52‐week, multicenter, randomized, double‐blind, placebo‐controlled study. J Clin Psychiatry. 2012;73(5):617–24. [DOI] [PubMed] [Google Scholar]
- 9. Ishigooka J, Nakamura J, Fujii Y, Iwata N, Kishimoto T, Iyo M, et al. Efficacy and safety of aripiprazole once‐monthly in Asian patients with schizophrenia: a multicenter, randomized, double‐blind, non‐inferiority study versus oral aripiprazole. Schizophr Res. 2015;161(2–3):421–8. [DOI] [PubMed] [Google Scholar]
- 10. Maeda K, Sugino H, Akazawa H, Amada N, Shimada J, Futamura T, et al. Brexpiprazole I: in vitro and in vivo characterization of a novel serotonin‐dopamine activity modulator. J Pharmacol Exp Ther. 2014;350(3):589–604. [DOI] [PubMed] [Google Scholar]
- 11. Maeda K, Lerdrup L, Sugino H, Akazawa H, Amada N, McQuade RD, et al. Brexpiprazole II: antipsychotic‐like and procognitive effects of a novel serotonin‐dopamine activity modulator. J Pharmacol Exp Ther. 2014;350(3):605–14. [DOI] [PubMed] [Google Scholar]
- 12. Conley RR, Kelly DL. Current status of antipsychotic treatment. Curr Drug Targets CNS Neurol Disord. 2002;1(2):123–8. [DOI] [PubMed] [Google Scholar]
- 13. Newcomer JW. Second‐generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19(Suppl. 1):1–93. [DOI] [PubMed] [Google Scholar]
- 14. Carlsson A, Kehr W, Lindqvist M, Magnusson T, Atack CV. Regulation of monoamine metabolism in the central nervous system. Pharmacol Rev. 1972;24(2):371–84. [PubMed] [Google Scholar]
- 15. Yasuda Y, Kikuchi T, Suzuki S, Tsutsui M, Yamada K, Hiyama T. 7‐[3‐(4‐[2,3‐Dimethylphenyl]piperazinyl)‐propoxy]‐2(1H)‐quinolinone (OPC‐4392), a presynaptic dopamine autoreceptor agonist and postsynaptic D2 receptor antagonist. Life Sci. 1988;42(20):1941–54. [DOI] [PubMed] [Google Scholar]
- 16. Risch SC. Pathophysiology of schizophrenia and the role of newer antipsychotics. Pharmacotherapy. 1996;16(1 Pt 2):11–4. [PubMed] [Google Scholar]
- 17. Tadori Y, Forbes RA, McQuade RD, Kikuchi T. Receptor reserve‐dependent properties of antipsychotics at human dopamine D2 receptors. Eur J Pharmacol. 2009;607(1–3):35–40. [DOI] [PubMed] [Google Scholar]
- 18. Voruganti L, Slomka P, Zabel P, Costa G, So A, Mattar A, et al. Subjective effects of AMPT‐induced dopamine depletion in schizophrenia: correlation between dysphoric responses and striatal D2 binding ratios on SPECT imaging. Neuropsychopharmacology. 2001;25(5):642–50. [DOI] [PubMed] [Google Scholar]
- 19. Bostwick JR, Guthrie SK, Ellingrod VL. Antipsychotic‐induced hyperprolactinemia. Pharmacotherapy. 2009;29(1):64–73. [DOI] [PubMed] [Google Scholar]
- 20. Mizrahi R, Mamo D, Rusjan P, Graff A, Houle S, Kapur S. The relationship between subjective well‐being and dopamine D2 receptors in patients treated with a dopamine partial agonist and full antagonist antipsychotics. Int J Neuropsychopharmacol. 2009;12(5):715–21. [DOI] [PubMed] [Google Scholar]
- 21. Potkin SG, Loze JY, Forray C, Baker RA, Sapin C, Peters‐Strickland T, et al. Multidimensional assessment of functional outcomes in schizophrenia: results from qualify, a head‐to‐head trial of aripiprazole once‐monthly and paliperidone palmitate. Int J Neuropsychopharmacol. 2017;20(1):40–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Iyo M, Tadokoro S, Kanahara N, Hashimoto T, Niitsu T, Watanabe H, et al. Optimal extent of dopamine D2 receptor occupancy by antipsychotics for treatment of dopamine supersensitivity psychosis and late‐onset psychosis. J Clin Psychopharmacol. 2013;33(3):398–404. [DOI] [PubMed] [Google Scholar]
- 23. Nakata Y, Kanahara N, Iyo M. Dopamine supersensitivity psychosis in schizophrenia: concepts and implications in clinical practice. J Psychopharmacol. 2017;31(12):1511–8. [DOI] [PubMed] [Google Scholar]
- 24. Tadokoro S, Okamura N, Sekine Y, Kanahara N, Hashimoto K, Iyo M. Chronic treatment with aripiprazole prevents development of dopamine supersensitivity and potentially supersensitivity psychosis. Schizophr Bull. 2012;38(5):1012–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Marder SR, McQuade RD, Stock E, Kaplita S, Marcus R, Safferman AZ, et al. Aripiprazole in the treatment of schizophrenia: safety and tolerability in short‐term, placebo‐controlled trials. Schizophr Res. 2003;61(2–3):123–36. [DOI] [PubMed] [Google Scholar]
- 26. Shapiro DA, Renock S, Arrington E, Chiodo LA, Liu L‐X, Sibley DR, et al. Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology. 2003;28(8):1400–11. [DOI] [PubMed] [Google Scholar]
- 27. Lammers GJ, Arends J, Declerck AC, Kamphuisen HA, Schouwink G, Troost J. Ritanserin, a 5‐HT2 receptor blocker, as add‐on treatment in narcolepsy. Sleep. 1991;14(2):130–2. [PubMed] [Google Scholar]
- 28. Fish LR, Gilligan MT, Humphries AC, Ivarsson M, Ladduwahetty T, Merchant KJ, et al. 4‐Fluorosulfonylpiperidines: selective 5‐HT2A ligands for the treatment of insomnia. Bioorg Med Chem Lett. 2005;15(16):3665–9. [DOI] [PubMed] [Google Scholar]
- 29. Miller CH, Fleischhacker WW, Ehrmann H, Kane JM. Treatment of neuroleptic induced akathisia with the 5‐HT2 antagonist ritanserin. Psychopharmacol Bull. 1990;26(3):373–6. [PubMed] [Google Scholar]
- 30. Laoutidis ZG, Luckhaus C. 5‐HT2A receptor antagonists for the treatment of neuroleptic‐induced akathisia: a systematic review and meta‐analysis. Int J Neuropsychopharmacol. 2014;17(5):823–32. [DOI] [PubMed] [Google Scholar]
- 31. Yamawaki S. The use and development of anxiolytics in Japan. Eur Neuropsychopharmacol. 1999;9(Suppl. 6):S413–9. [DOI] [PubMed] [Google Scholar]
- 32. Sumiyoshi T, Matsui M, Yamashita I, Nohara S, Kurachi M, Uehara T, et al. The effect of tandospirone, a sertonin1A agonist, on memory function in schizophrenia. Biol Psychiatry. 2001;49(10):861–8. [DOI] [PubMed] [Google Scholar]
- 33. Inanaga K, Inoue K, Tachibana H, Oshima M, Kotorii T. Effect of L‐DOPA in schizophrenia. Folia Psychiatr Neurol Jpn. 1972;26(2):145–57. [DOI] [PubMed] [Google Scholar]
- 34. Citrome L, Stensbøl TB, Maeda K. The preclinical profile of brexpiprazole: what is its clinical relevance for the treatment of psychiatric disorders? Expert Rev Neurother. 2015;15(10):1219–29. [DOI] [PubMed] [Google Scholar]
- 35. Miyamoto S, Duncan GE, Mailman RB, Lieberman JA. Developing novel antipsychotic drugs: strategies and goals. Curr Opin CPNS Invest Drugs. 2000;2(1):25–39. [Google Scholar]
- 36. Meltzer HY. Serotonergic mechanisms as targets for existing and novel antipsychotics. Handb Exp Pharmacol. 2012;212:87–124. [DOI] [PubMed] [Google Scholar]
- 37. Inoue T, Domae M, Yamada K, Furukawa T. Effects of the novel antipsychotic agent 7‐{4‐[4‐(2,3‐dichlorophenyl)‐1‐piperazinyl]butyloxy}‐3,4‐dihydro‐2(1H)‐quinolinone (OPC‐14597) on prolactin release from the rat anterior pituitary gland. J Pharmacol Exp Ther. 1996;277(1):137–43. [PubMed] [Google Scholar]
- 38. Aihara K, Shimada J, Miwa T, Tottori K, Burris KD, Yocca FD, et al. The novel antipsychotic aripiprazole is a partial agonist at short and long isoforms of D2 receptors linked to the regulation of adenylyl cyclase activity and prolactin release. Brain Res. 2004;1003(1–2):9–17. [DOI] [PubMed] [Google Scholar]
- 39. Amada N, Akazawa H, Ohgi Y, Maeda K, Sugino H, Kurahashi N, et al. Brexpiprazole has a low risk of dopamine D 2 receptor sensitization and inhibits rebound phenomena related to D2 and serotonin 5‐HT 2A receptors in rats. Neuropsychopharmacol Rep. 2019;39(4):279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kane JM, Skuban A, Hobart M, Ouyang J, Weiller E, Weiss C, et al. Overview of short‐ and long‐term tolerability and safety of brexpiprazole in patients with schizophrenia. Schizophr Res. 2016;174(1–3):93–8. [DOI] [PubMed] [Google Scholar]
- 41. Citrome L, Ota A, Nagamizu K, Perry P, Weiller E, Baker RA. The effect of brexpiprazole (OPC‐34712) and aripiprazole in adult patients with acute schizophrenia: results from a randomized, exploratory study. Int Clin Psychopharmacol. 2016;31(4):192–201. [DOI] [PubMed] [Google Scholar]
- 42. McEvoy J, Citrome L. Brexpiprazole for the treatment of schizophrenia: a review of this novel serotonin‐dopamine activity modulator. Clin Schizophr Relat Psychoses. 2016;9(4):177–86. [PubMed] [Google Scholar]
- 43. Citrome L. Activating and sedating adverse effects of second‐generation antipsychotics in the treatment of schizophrenia and major depressive disorder: absolute risk increase and number needed to harm. J Clin Psychopharmacol. 2017;37(2):138–47. [DOI] [PubMed] [Google Scholar]
- 44. Ishigooka J, Iwashita S, Tadori Y. Efficacy and safety of brexpiprazole for the treatment of acute schizophrenia in Japan: a 6‐week, randomized, double‐blind, placebo‐controlled study. Psychiatry Clin Neurosci. 2018;72(9):692–700. [DOI] [PubMed] [Google Scholar]
- 45. Ishigooka J, Iwashita S, Tadori Y. Long‐term safety and effectiveness of brexpiprazole in Japanese patients with schizophrenia: a 52‐week, open‐label study. Psychiatry Clin Neurosci. 2018;72(6):445–53. [DOI] [PubMed] [Google Scholar]
- 46. Ivkovic J, Lindsten A, George V, Eriksson H, Hobart M. Effect of brexpiprazole on prolactin: an analysis of short‐ and long‐term studies in schizophrenia. J Clin Psychopharmacol. 2019;39(1):13–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yoshimi N, Futamura T, Hashimoto K. Improvement of dizocilpine‐induced social recognition deficits in mice by brexpiprazole, a novel serotonin–dopamine activity modulator. Eur Neuropsychopharmacol. 2015;25(3):356–64. [DOI] [PubMed] [Google Scholar]
- 48. Yoshimi N, Fujita Y, Ohgi Y, Futamura T, Kikuchi T, Hashimoto K. Effects of brexpiprazole, a novel serotonin‐dopamine activity modulator, on phencyclidine‐induced cognitive deficits in mice: a role for serotonin 5‐HT1A receptors. Pharmacol Biochem Behav. 2014;124:245–9. [DOI] [PubMed] [Google Scholar]
- 49. Nagai T, Murai R, Matsui K, Kamei H, Noda Y, Furukawa H, et al. Aripiprazole ameliorates phencyclidine‐induced impairment of recognition memory through dopamine D1 and serotonin 5‐HT1A receptors. Psychopharmacology(Berl). 2009;202(1–3):315–28. [DOI] [PubMed] [Google Scholar]
- 50. Wang Y, Yang X, Song X, Zhao L, Wei J, Wang J, et al. Co‐treatment of buspirone with atypical antipsychotic drugs (AAPDs) improved neurocognitive function in chronic schizophrenia. Schizophr Res. 2019;209:135–40. [DOI] [PubMed] [Google Scholar]
- 51. Drouin C, Darracq L, Trovero F, Blanc G, Glowinski J, Cotecchia S, et al. α1b‐Adrenergic receptors control locomotor and rewarding effects of psychostimulants and opiates. J Neurosci. 2002;22(7):2873–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Green B. Prazosin in the treatment of PTSD. J Psychiatr Pract. 2014;20(4):253–9. [DOI] [PubMed] [Google Scholar]
- 53. Cohen H, Zohar J, Kaplan Z, Arnt J. Adjunctive treatment with brexpiprazole and escitalopram reduces behavioral stress responses and increase hypothalamic NPY immunoreactivity in a rat model of PTSD‐like symptoms. Eur Neuropsychopharmacol. 2018;28(1):63–74. [DOI] [PubMed] [Google Scholar]
- 54. Ducourneau E‐G, Guette C, Perrot D, Mondesir M, Mombereau C, Arnt J, et al. Brexpiprazole blocks post‐traumatic stress disorder‐like memory while promoting normal fear memory. Mol Psychiatry. 2020. 10.1038/s41380-020-0852-z [DOI] [PubMed] [Google Scholar]
- 55. O'Connor M. Adjunctive therapy with brexpiprazole improves treatment resistant complex post traumatic stress disorder in domestic family violence victims. Australas Psychiatry. 2020;28(3):264–6. [DOI] [PubMed] [Google Scholar]
- 56. Quaglia W, Del Bello F, Giannella M, Piergentili A, Pigini M. α2C‐adrenoceptor modulators: a patent review. Expert Opin Ther Pat. 2011;21(4):455–81. [DOI] [PubMed] [Google Scholar]
- 57. Sallinen J, Holappa J, Koivisto A, Kuokkanen K, Chapman H, Lehtimäki J, et al. Pharmacological characterization of a structurally novel α2C‐adrenoseptor antagonist ORM‐10921 and its effects in neuropsychiatric models. Basic Clin Pharmacol Toxicol. 2013;113(4):239–49. [DOI] [PubMed] [Google Scholar]
- 58. Brosda J, Jantschak F, Pertz HH. α2‐Adrenoceptors are targets for antipsychotic drugs. Psychopharmacology(Berl). 2014;231(5):801–12. [DOI] [PubMed] [Google Scholar]
- 59. Uys MM, Shahid M, Harvey BH. Therapeutic potential of selectively targeting the α2C‐adrenoceptor in cognition, depression, and schizophrenia – new developments and future perspective. Front Psychiatry. 2017;8:144. 10.3389/fpsyt.2017.00144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kalkman HO, Loetscher E. α2C‐Adrenoceptor blockade by clozapine and other antipsychotic drugs. Eur J Pharmacol. 2003;462(1–3):33–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


