Abstract
The childhood obesity epidemic is expected to increase cardiovascular disease risk, but the impact of obesity on vascular function in children is not fully understood. The purpose of this study was to determine the effect of obesity and maturation on vascular function in normal weight (BMI: 25–75 percentile) and obese (BMI: ≥95 percentile) children ages 8–18 years old. Large and small artery elasticity (LAEI and SAEI, respectively), measured by diastolic radial pulsewave contour analysis, and reactive hyperemia index (RHI), measured by peripheral arterial tonometry, were obtained, along with anthropometric and biochemical outcomes, in 61 normal weight and 62 obese children. SAEI and LAEI increased with age and were 30% and 18% higher, respectively, in obese children (P < 0.01). In contrast, reactive hyperemia increased with age in the normal weight group but did not differ between groups. Multivariate modeling was used to select variables that explained differences in vascular outcomes. The best model for LAEI in normal weight children was height alone (r2 = 0.49), whereas for obese children the best model included height + fat mass (r2 = 0.40). For SAEI, there were no significant models for normal weight children, but for obese children the best model included lean mass + fat mass (r2 = 0.36). Obese children had greater lean and fat mass, and more advanced Tanner stages than their normal weight peers. The increased elasticity observed in obese children appears to reflect accelerated growth and maturation without affecting vascular reactivity measured by reactive hyperemia. Longitudinal follow up will be essential in determining effects on future vascular disease risk.
INTRODUCTION
The prevalence of obesity has rapidly increased in the last century, and now ranks as one of the major causes of morbidity and mortality in the industrialized world (1). Obesity among children rose in the last 20 years to its current level of ~18% in the United States (1). Childhood obesity significantly increases morbidity and mortality from cardiovascular disease. In the Bogalusa Heart Study 58% of obese children 5–10 years old had one risk factor for cardiovascular disease and 25% had two or more (2). The Bogalusa study and similar longitudinal studies in Iowa and Finland have shown that childhood obesity and lipid profiles predict the degree of thickening of the carotid intima-media in early adulthood (20–40 years old (3–5)). It is less clear when and how childhood obesity adversely affects vascular function.
In adults, impaired vascular endothelial function, measured by reactive hyperemia or flow-mediated dilation of the brachial artery, is one of the earliest signs of vascular damage and is associated with abnormal coronary angiographic findings (6). Low arterial compliance, another index of vascular function, has also been associated with microvascular disease, which contributes to end-organ damage, stroke, and renal dysfunction (7). Both arterial compliance and endothelial function decline with age throughout adulthood, although these changes may be attenuated in people who exercise frequently (8–11). Risk factors such as hypertension, elevated low-density lipoprotein cholesterol, low high-density lipoprotein cholesterol, and obesity also predict the development of increased arterial stiffness (low compliance) in adults (12).
The pattern of vascular function changes during childhood and the impact of obesity are not as well established. A recent cross-sectional study showed that both large and small arterial compliance increased from ages ~9–30 years old and then declined from 30–80 years in healthy people (13). However, another study (14) reported that the decline in peripheral large arterial compliance may begin during adolescence, as shown by an increase in brachial-ankle pulse wave velocity in healthy children 9–17 years old. Vascular endothelial reactivity, thought to reflect healthier arteries, was reported to be either the same or greater in healthy children (10 years old) compared to young adults (27 years), but the difference depended on the method used to normalize data (15).
Similar to these inconsistent changes with age, the reported effects of obesity on vascular function in children are mixed. Endothelial function and central arterial compliance may be the same or lower in obese vs. normal weight children (6,16–19). In contrast, Dangart and colleagues (20) reported that carotid-radial pulse wave velocity was lower (indicating that arterial compliance was increased) in obese adolescent girls than in girls within the normal range for BMI. Those authors proposed that hyperinsulinemia associated with obesity might stimulate chronic vasorelaxation and higher vascular compliance, although that hypothesis has not been proven. Subsequent corroboration of the results of Dangardt et al. (20) was recently provided by Chalmers et al. (21), who demonstrated that obese children had higher small and large arterial elasticity (compliance) than age-matched normal weight children. The difference in small artery elasticity persisted even after controlling for height, race, sex, and Tanner maturation stage (21). The studies by Dangardt and Chalmers had relatively small sample sizes (51 and 67 total children, subdivided into normal weight and obese groups, respectively) and questions remain with regard to how much of the reported increased arterial compliance in obese children could be ascribed to differences in body composition, maturation, or biochemical variables like insulin and blood lipids that are expected to vary with obesity status.
The purpose of the present investigation was to identify differences in vascular function (arterial compliance and vascular reactive hyperemic response) in obese and normal weight children, and to assess whether these differences are attributable to variations in age, maturation (Tanner stage and height), body composition, or clinical biochemical variables.
METHODS
Subjects
One hundred twenty-three boys and girls aged 8–18 years old were recruited to participate in the study. Sixty-two healthy, obese children (30 males, 32 females), defined as having a BMI greater than the 95th percentile for age and sex on the Centers for Disease Control and Prevention growth charts, were recruited from the Diabetes and Endocrinology clinic at the University of Oklahoma Health Sciences Center and using local advertisements. Sixty-one normal weight children (31 males, 30 females), with a BMI between the 25–75th percentile, were recruited in a similar fashion.
Informed written consent and assent were obtained in accordance with the guidelines of the University of Oklahoma Health Sciences Center Institutional Review Board for Human Subjects. Each svubject completed a questionnaire that addressed family medical history and recent patterns of the child’s exercise and diet. Each subject also completed the “Modifiable Activities Questionnaire for Adolescents” to quantify physical activity over the past 12 months (22). Volume of physical activity was calculated as metabolic equivalents of task and expressed as metabolic equivalent hours/week.
Anthropometric measures
A pediatrician completed a routine medical history and physical examination and determined Tanner staging for pubertal status. Height was measured to the nearest 0.5 cm using a standard stadiometer. Weight was measured to the nearest 0.5 kg using a digital scale. BMI was calculated from these measures and expressed as a percentile using Centers for Disease Control and Prevention norms for the child’s age and sex. Blood pressure was measured using an appropriate sized cuff, based on arm circumference and corresponding American Heart Association cuff size guidelines. Body composition was quantified using dual energy X-ray absorptiometry (DEXA, GE iDXA, Fairfield, CT).
Arterial elasticity
The elasticity (compliance) of the large and small arteries was measured using diastolic pulse-wave analysis (HDI/ Pulsewave CR-2000, Hypertension Diagnostics, Eagan, MN). After 10 min of supine rest, an upper arm blood pressure cuff was placed on one arm. The opposite wrist was placed in a stabilizer to gently immobilize the wrist and minimize movement of the tonometric sensor placed over the radial artery. Once optimal waveforms and a stable baseline were achieved, arterial waveforms were recorded for 30 s and then digitized at 200 samples per second and stored by the device. Calculations of small and large artery arterial elasticity (SAEI and LAEI respectively) were derived from analysis of the pulse wave contour by the CR-2000 software. The mean of three replicates for each participant was used for data analyses.
Reactive hyperemia peripheral arterial tonometry
Reactive hyperemia peripheral arterial tonometry was performed to assess vascular endothelial function. During the test, the change in vascular tone in the fingertips was recorded before and after arterial occlusion (Endo-PAT2000; Itamar, Franklin, MA). Plethysmographic sensors were placed on the index finger of each hand. After a 5-min baseline measurement, an upper arm blood pressure cuff was inflated to elicit brachial artery occlusion in one limb for 5 min. Once the cuff is released, the resumption of blood flow stimulates vasodilation, and a downstream hyperemic response, which is detected as increased pulse pressure by the finger tip sensor. The reactive hyperemia index (RHI) was calculated as the ratio of the average pulse wave amplitude measured over 60 s starting 1 min after cuff deflation, relative to the average pulse wave amplitude measured at baseline. Concurrent measurements from the contra-lateral arm were used to normalize for changes in systemic vascular tone during the measurement period.
Serum analysis
A fasting blood sample was collected for measurement of serum glucose, insulin, C-reactive protein (CRP) and lipids. Glucose was measured by the glucose oxidase method (YSI 2300 STAT plus, Yellow Springs, OH). Insulin was measured using Human Insulin ELISA kit from Millipore (St Charles, MO). Glucose and insulin values were used to calculate fasting insulin resistance with the HOMAIR model (23). CRP and lipids (total cholesterol, low-density lipoprotien cholesterol, and high-density lipoprotien cholesterol, and triglycerides) were measured by the Veteran’s Administration Hospital Clinical Laboratory in Oklahoma City, OK.
Statistical analysis
All outcomes were inspected for normality of distribution. Because the outcomes measured appeared to be normally distributed, t-tests and regression models were used to identify differences between the normal weight and obese groups in anthropometric, serum, and arterial function measurements. Univariate modeling established correlations between each arterial function measure and the anthropometric and serum data. Variables that were significantly correlated with the outcomes were then included in multivariate regression models. Multiple models were developed to identify the model that best accounted for between-group differences in the measures of arterial function. SAS version 9.1 (Cary, NC) was used to calculate the regression models. Because the study explored novel hypotheses, we did not adjust for multiple comparisons, but considered any association significant that yielded a P value <0.05.
RESULTS
Anthropometric measures
The normal weight and obese groups were both comprised of similar numbers of males and females. Habitual physical activity as assessed by the questionnaire was not different between the two groups (normal weight = 32.6 ± 32.1 metabolic equivalent hour/week; obese = 37.2 ± 45.6). As shown in Table 1, the obese subjects were heavier (P < 0.01) as expected from the study design and had greater fat mass (P < 0.01) and lean mass (P < 0.01) compared to the normal weight subjects. The obese group was also taller (P < 0.01) and had more advanced Tanner staging (P < 0.05). Systolic and diastolic blood pressures were higher in the obese group compared to the normal weight group (P < 0.01 and P < 0.05 respectively).
Table 1.
Participant characteristics
| Normal weight (N = 61) | Obese (N = 63) | |
|---|---|---|
| Age (years) | 13.3 ± 3.0 | 13.9 ± 2.5 |
| Weight (kg) | 46.7 ± 13.6 | 85.7 ± 22.4 |
| Height (cm) | 154.4 ± 17.2 | 162.1 ± 12.6 |
| Tanner stage | 2.9 ± 1.3 | 3.4 ± 1.1 |
| Fat mass (kg) | 11.6 ± 4.6 | 37.0 ± 13.7 |
| Lean mass (kg) | 30.6 ± 10.3 | 43.3 ± 11.4 |
| Systolic blood pressure (mm Hg) | 108 ± 8 | 117 ± 8 |
| Diastolic blood pressure (mmHg) | 58 ± 5 | 60 ± 7 |
The two groups differed on all measures (P < 0.05) with the exception of age.
Serum measures
Fasting glucose concentration (Table 2) did not differ between groups but insulin was higher in the obese group (P < 0.01) resulting in an increased HOMAIR (P < 0.01). The total cholesterol, triglycerides, and low-density lipoprotien cholesterol were all higher in the obese group (P < 0.01), but high-density lipoprotein cholesterol did not differ between groups. CRP was also elevated in the obese group compared to the normal weight group (P < 0.01).
Table 2.
Serum biochemical outcomes
| Normal weight | Obese | |
|---|---|---|
| Glucose (mmol/l) | 4.61 ± 0.33 | 4.66 ± 0.39 |
| Insulin (pmol/l) | 47.71 ± 40.63 | 162.58 ± 338.99 |
| HOMAIR | 1.41 ± 0.09 | 4.99 ± 4.29 |
| Triglycerides (mmol/l) | 0.81 ± 0.35 | 1.27 ± 0.67 |
| Cholesterol (mmol/l) | 4.12 ± 0.70 | 4.95 ± 1.86 |
| HDL (mmol/l) | 1.25 ± 0.27 | 1.23 ± 0.35 |
| LDL (mmol/l) | 2.38 ± 0.64 | 3.23 ± 1.68 |
| CRP (nmol/l) | 7.05 ± 12.57 | 30.86 ± 46.57 |
All measures obtained after overnight fast. All outcomes except glucose and HDL differed (P < 0.05) between the two groups.CRP, C-reactive protein; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Vascular measures
As shown in Figure 1 the RHI did not differ between normal weight and overweight groups overall although the RHI values tended to be lower in obese children at the older end of the age range studied. In the normal weight group, RHI was positively correlated with age (r = 0.42, P = 0.001), with an average increase of 0.07 arbitrary units per year (95% confidence interval (CI): 0.03, 0.11; P = 0.01). However this relationship with age was not evident in the obese group. Additionally, RHI was not significantly correlated with any other anthropometric, family history, blood pressure, or serum biochemical outcome in either the normal weight or obese groups. Finally, RHI was not significantly correlated with either SAEI or LAEI in either the normal weight or obese groups.
Figure 1.
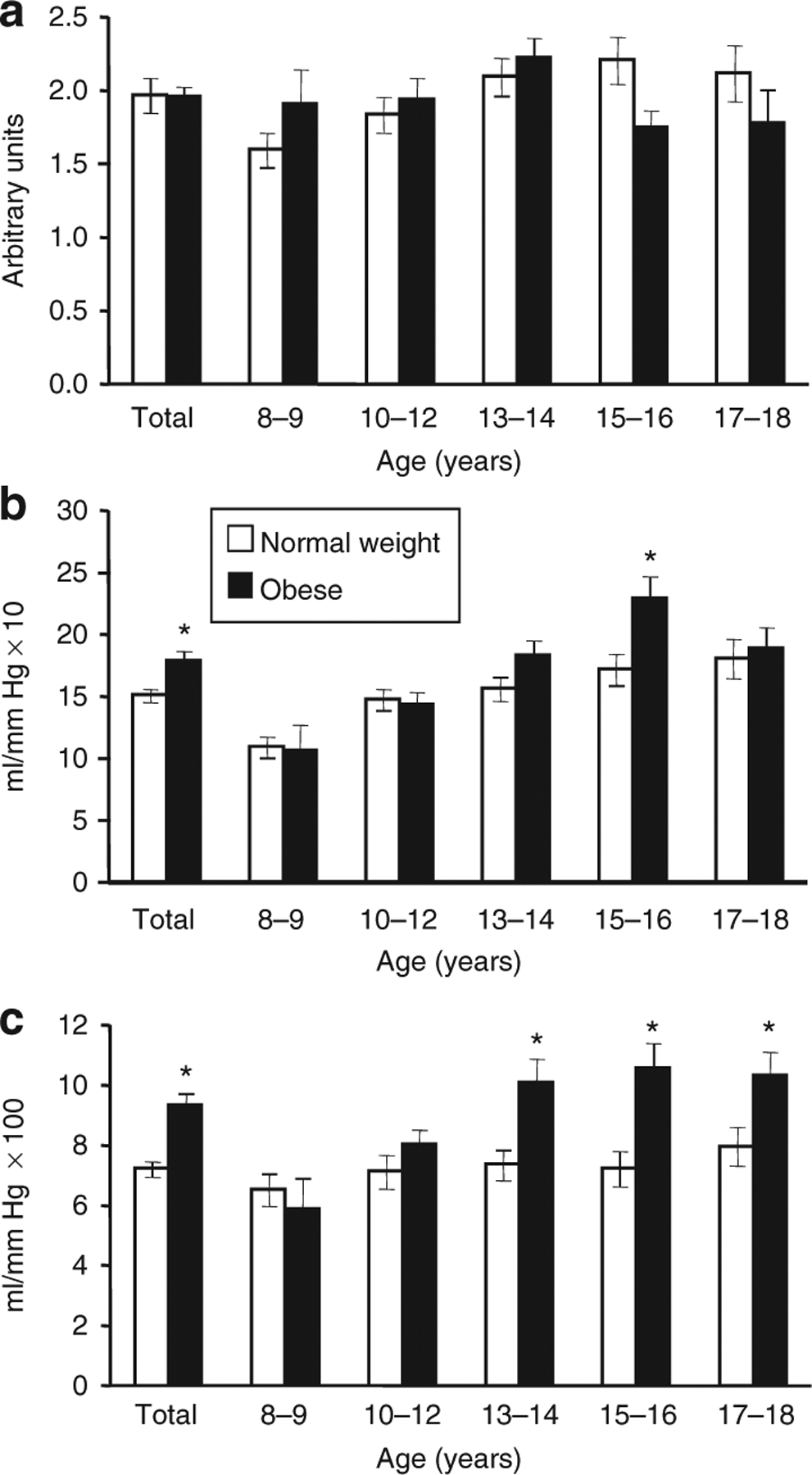
(a) Reactive hyperemia index (RHI) and (b) large arterial elasticity index (LAEI) and (c) small arterial elasticity index (SAEI) in normal weight and obese children. Values are for mean ± s.e.m. of the entire normal weight and obese groups (Total) and subgroups defined by age in 2-year increments. RHI increased with age in the normal weight group. LAEI increased with age in both normal weight and obese groups. SAEI increased significantly with age in obese, but not in normal weight children. *Obese > normal weight within age group (P < 0.05). Numbers of subjects per age group were 11 and 5, 16 and 17, 16 and 18, 8 and 16, and 9 and 7 for normal weight and obese respectively.
SAEI and LAEI (Figure 1) were higher in the obese group compared to the normal weight group (P < 0.01 and P = 0.006, respectively). Univariate regression was performed to identify variables that were significantly correlated with SAEI and LAEI and could be considered in multivariate models. As described below, age, body size, and composition outcomes were significant correlates but none of the serum biochemistry variables, including fasting insulin and HOMAIR, or blood pressure, were significantly correlated with LAEI or SAEI in either the normal weight or obese group. Likewise, family history of hypertension, diabetes, stroke, heart disease, and/or elevated cholesterol was not correlated with LAEI or SAEI.
Three to four repeated measurements of SAEI and LAEI were performed for each child, and intraclass correlation coefficients were calculated for both measures using separate mixed models that estimated within-subject and between-subject variances. The intraclass correlation coefficients, which is a ratio of between-subject variance to total variance, and therefore reflects a measure’s ability to distinguish between subjects, was 0.70 for SAEI and 0.78 for LAEI.
Figure 1 depicts the increase in SAEI with advancing age. SAEI increased 0.51 ml/mm Hg × 100 for each year of age among obese children (95% CI: 0.21, 0.81; P = 0.001) but was not associated with age in normal weight children (P = 0.106). Among children of normal weight, SAEI did not correlate with any single anthropometric or serum measure; the strongest correlation variable for SAEI was lean mass, but this was a nonsignificant relationship (r = 0.24, P = 0.66). In the obese group, age, Tanner Stage, height, lean mass, and fat mass were all significantly and positively correlated with SAEI. When these variables were included in a multivariate model, fat mass was the most significant single predictor of SAEI (r2 = 0.31). SAEI increased 0.13 ml/mm Hg × 100 for every 1 kg increase in fat mass (95% CI: 0.08, 0.18; P ≤ 0.01). Adding lean mass (r2 = 0.29), which independently increased SAEI 0.15 ml/mm Hg × 100 for every 1 kg increase in lean mass (95% CI: 0.09, 0.21; P ≤ 0.01), further raised the model’s predictive power (r2 = 0.36). Holding lean mass constant, SAEI increased 0.09 ml/mm Hg × 100 for every 1 kg increase in fat mass (95% CI: 0.03, 0.15; P ≤ 0.01). No other variables added significantly to the model.
Variables that individually predicted LAEI in both normal weight and obese groups were age, height, Tanner Stage, and lean mass. Figure 1 shows that LAEI increased with age for both groups. A regression model that treated age as a continuous variable demonstrated no age × group interaction (P = 0.138), and estimated that LAEI increased in either group by 0.95 ml/mm Hg × 10 (95% CI: 0.64, 1.26; P < 0.001) for each year of age. The regression model also estimated LAEI to be 2.05 ml/mm Hg × 10 higher in the obese group (95% CI: 0.32, 3.79; P = 0.021) than in the children of normal weight. In the normal weight children alone, height was the strongest predictor of LAEI (r2 = 0.49). LAEI increased 0.17 ml/mm Hg × 10 for every 1 cm increased in height (95% CI: 0.13, 0.22; P ≤ 0.01). Lean mass was nearly as strongly related (r2 = 0.44). For every 1 kg increase in lean mass, LAEI increased 0.27 ml/mm Hg × 10 (95% CI: 0.19, 0.35; P ≤ 0.01). In the normal weight group lean mass and height were highly correlated (r = 0.93). In the obese group, height was the strongest single predictor of LAEI (r2 = 0.33). For each 1 cm increase in height, LAEI increased 0.30 ml/mm Hg × 10 (95% CI: 0.19, 0.41; P ≤ 0.01). Adding fat mass to the model increased its predictive power (r2 = 0.40). Holding fat mass constant, LAEI increased 0.21 ml/mm Hg × 10 (95% CI: 0.09, 0.34; P ≤ 0.01) for every 1 cm increase in height. Substituting lean mass for height in the LAEI model for the obese group resulted in only slightly lower predictive power, alone (r2 = 0.30) or when combined with fat mass (r2 = 0.36). For each 1 kg increase in lean mass, LAEI increased 0.32 ml/mm Hg × 10 (95% CI: 0.19, 0.44; P ≤ 0.01), and holding fat mass constant, LAEI increased 0.21 ml/mm Hg × 10 for every 1 kg increased in lean mass (95% CI: 0.06, 0.36; P ≤ 0.01). As with the normal weight group, height, and lean mass were also highly correlated (r = 0.85). Since lean mass was a common predictor variable for SAEI and LAEI those relationships are shown in Figure 2.
Figure 2.
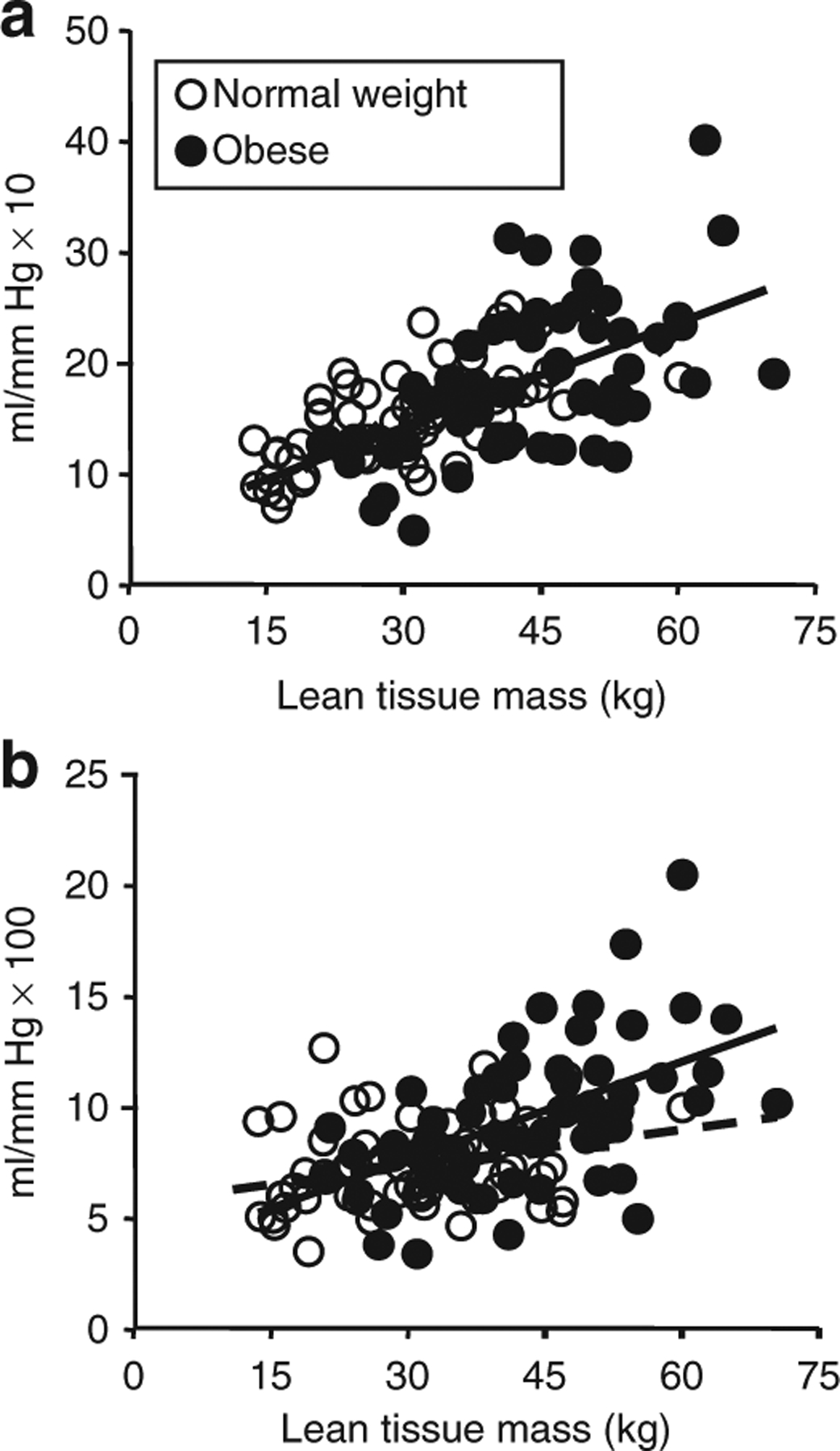
Association between total body lean tissue mass and (a) large arterial elasticity index (LAEI) and (b) small arterial elasticity index (SAEI). SAEI and LAEI were positively correlated with lean mass in both the normal weight (r = 0.24 and 0.67 respectively) and obese (r = 0.54 and 0.55 respectively) groups. Regression lines shown are for obese (solid) and normal weight (dashed), respectively.
DISCUSSION
The goal of the current study was to determine how vascular endothelial function and arterial compliance in children are affected by the presence of obesity. The main new findings were that large and small artery elasticity increased with age and were higher in obese children compared to their normal weight peers, particularly in children ≥13 years old. Multivariate regression models revealed that LAEI was most strongly associated with height and lean mass and SAEI with lean mass in both normal weight and obese children. Since obese children were taller, had greater lean and fat mass, and more advanced Tanner scores, our results point to advanced growth and maturation as an underlying explanation for the increase in arterial compliance in obese vs. normal weight children. After adjusting for height and lean mass, differences in arterial compliance between normal weight and obese groups were no longer evident.
No difference in RHI was noted overall between the obese and normal weight groups. Age was a modestly strong predictor of RHI in the normal weight group, which has not to our knowledge previously been reported in youth. This suggests that vascular reactivity may continue to develop in childhood and early adolescence for normal weight youth, although further work is needed to clarify this pattern and the underlying mechanisms. In contrast, no significant predictors of RHI were identified in the obese group. This could mean that the microvasculature response to sheer stress is not as readily impaired by obesity as it appears to be in adults. In the Framingham cohort study RHI in adults (mean ± s.d. age = 40 ± 9 years) was shown to be inversely related to BMI (24). The results of the current study suggest that RHI may begin to decline in obese children after age ~15 years as there was a trend in that direction, which if confirmed in young adults, would suggest that exposure to the effects of obesity for several years may be required before microvascular reactive hyperemia is adversely affected. In comparison, studies of flow mediated dilatation in children, a measure of the effect of endothelial reactivity on brachial artery diameter, have shown that it may be reduced as much as 50% in obese children (6,18). The flow-mediated dilatation methods used in those reports reflect the macrovascular response to occlusion, whereas the peripheral tonometry approach used in the present study likely reflects more of a microvasculature response (25). This raises the possibility that the effect of obesity on vascular endothelial function in children may appear or be manifest differently in large and small vessels. RHI has been shown to be reduced in children with type 1 diabetes (26), although to our knowledge RHI results in obese children have not been previously reported. RHI was also not correlated with the other vascular measures, SAEI, or LAEI. RHI and SAEI are both thought to reflect aspects of microcirculatory function, although their control mechanisms most likely differ. SAEI is a measure of the resting tone of the vessels and is correlated with the constitutive release of nitric oxide for resulting from constitutive nitric oxide synthase activity (27). The RHI measures vasculature response to ischemia, which results from reperfusion shear stress and stimulation of nitrogen oxide production through endothelial nitric oxide synthase (eNOS) (28,29). Thus, nitrogen oxide is thought to contribute to the regulation of both resting vascular tone and the reactive hyperemic response, though different mechanisms for its release appear to be involved. It is possible that variations in specific cytokines may impede the release of nitrogen oxide after brief ischemia in obese children thereby resulting in minimal difference in RHI between the normal weight and obese children.
An important novel result was that arterial elasticity was positively related to lean body mass and that differences in lean mass appear to explain why SAEI and LAEI is higher in obese children. Lean mass increases in childhood with growth, although as expected, obese children in the present study had greater lean mass than their normal weight peers. This increase in lean mass in obese children may be due in part to the higher mechanical stress resulting from the impact of carrying extra weight, possibly compounded by effects of adipocyte-related hormones. Lean mass is comprised of skeletal muscle and vital organs that are metabolically active and have higher vascular density than adipose tissue, so that the total body vascular volume is expected to increase with lean mass accretion (30). A larger vascular network could explain the greater arterial compliance in obese children, as pressure waves are distributed over a greater surface area.
In contrast to our findings in children, arterial compliance is not increased in obese adults despite the fact that obese adults also have greater lean mass than their normal weight peers (12). Obese adults have more atherosclerosis compared to obese children (3–5), which attenuates vascular smooth muscle relaxation. The increased luminal pressure in athero-sclerotic vessels in older obese people due to atherosclerosis and decreased elastin is expected to result in decreased arterial compliance. Arterial compliance declines during adulthood in healthy people, and declines even more rapidly in late adulthood (13). Collectively, the present study, along with two recent reports (20,21), suggest that obesity may increase the rate of maturation of the vascular system in children as a result of accelerated growth and pubertal maturation. Such a premature change in vascular function is consistent with the earlier development of cardiovascular disease risk in people who were obese during childhood (2). We speculate that childhood obesity causes an earlier peak in vascular compliance, followed by a subsequent hastening of the decline in LAEI and SAEI expected in adulthood (i.e., “shifting the curve” to the left). Since this study was performed cross-sectionally, at a single point in time, testing of this hypothesis likely will require longitudinal measures of vascular compliance in obese youth followed into adulthood.
In the present study, fat mass was a positively-associated predictor of both LAEI and SAEI in obese children, even after adjusting for lean mass. However, this relationship was not evident in the normal weight group, who had, as expected, lower fat mass. This finding suggests that adipocyte-related hormones or cytokines may contribute to increased arterial compliance (elasticity) in children. One potential mechanism that could support this premise is an increase in vascular eNOS, leading to increased nitric oxide, greater smooth muscle relaxation, and increased arterial compliance (31). At least two adiposity-related hormones, insulin and visfatin, are elevated in obesity, and are known to increase eNOS (32–34). In adults, both eNOS protein and mRNA content are higher in the subcutaneous fat of obese individuals relative to that of normal weight controls (35,36). Although direct infusions of insulin are associated with eNOS release and vasodilatation (37), insulin resistance is associated with lower eNOS mRNA expression and activity in adults (38). In the current study, we observed no relationship between fasting insulin or HOMA scores and vascular compliance, despite the fact that the obese children had higher fasting insulin levels and HOMA scores, and higher vascular compliance. These observations suggest that insulin per se is unlikely to be the primary mediator of arterial compliance differences in this population.
Some limitations of the present study are acknowledged. We limited the normal weight group to a BMI range between the 25–75th percentiles based on age and sex according to the Centers for Disease Control and Prevention growth charts, so that we could examine distinct (normal weight vs. obese) cohorts, thereby increasing the likelihood of detecting differences between groups. Normal weight children between the 5–25th percentiles and 75–85th percentiles were not studied. However, in our regression analyses we considered body composition and other outcomes as continuous variables independent of BMI-based categorization. Thus, the BMI inclusion criteria did not limit the ability to identify height, and lean and fat mass as the leading predictors of arterial compliance. In this study, we did not specifically analyze visceral adiposity, a known predictor of cardiovascular risk in adults (39). Although visceral fat in adults is related to many cardiovascular outcomes, its relationship to small and large arterial compliance has not been reported in children. Finally, while the methods used to quantify arterial elasticity and endothelial function have been validated in other studies of children (13,26,40), other approaches that measure similar or different aspects of vascular health in children might have been examined and compared. Further work using multiple techniques is needed to fully characterize how growth, maturation, and obesity affect the vascular health of children.
In conclusion, the results of the current study demonstrate that compliance (elasticity) of the large and small arteries is positively associated with body size (height and lean body mass) in children 8–18 years old. The accrual of lean mass during growth may account for the previous observations that small and large arterial compliance increase with age during childhood and early adulthood. We also found that obese children had increased arterial compliance compared to their normal weight peers, but this can be accounted for by their greater lean and fat mass along with a more advanced maturation stage relative to their normal weight peers. Additionally, in obese children arterial compliance also was positively correlated with fat mass suggesting that adipose tissue, and not insulin per se, may have an underappreciated influence on arterial compliance. The current study demonstrates that differences in body composition exert strong influences on vascular compliance in children. Further studies are needed to determine long-term effects on vascular health and disease risk in adulthood.
ACKNOWLEDGMENTS
Funding for this study was provided by the Endocrine Fellows Foundation Marilyn Fishman Grant for Diabetes Research, the Lawson Wilkins Pediatric Endocrine Society Clinical Scholars Award, the University of Oklahoma Health Sciences Center Department of Pediatric Diabetes and Endocrinology and NIH Grant Number P20 RR 024215 from the COBRE Program of the National Center for Research Resources.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1.Skilton MR, Celermajer DS. Endothelial dysfunction and arterial abnormalities in childhood obesity. Int J Obes (Lond) 2006;30:1041–1049. [DOI] [PubMed] [Google Scholar]
- 2.Zalesin KC, Franklin BA, Miller WM, Peterson ED, McCullough PA. Impact of obesity on cardiovascular disease. Endocrinol Metab Clin North Am 2008;37:663–684. [DOI] [PubMed] [Google Scholar]
- 3.Davis PH, Dawson JD, Riley WA, Lauer RM. Carotid intimal-medial thickness is related to cardiovascular risk factors measured from childhood through middle age: The Muscatine Study. Circulation 2001;104:2815–2819. [DOI] [PubMed] [Google Scholar]
- 4.Freedman DS, Dietz WH, Tang R et al. The relation of obesity throughout life to carotid intima-media thickness in adulthood: the Bogalusa Heart Study. Int J Obes Relat Metab Disord 2004;28:159–166. [DOI] [PubMed] [Google Scholar]
- 5.Juonala M, Viikari JS, Kähönen M et al. Childhood levels of serum apolipoproteins B and A-I predict carotid intima-media thickness and brachial endothelial function in adulthood: the cardiovascular risk in young Finns study. J Am Coll Cardiol 2008;52:293–299. [DOI] [PubMed] [Google Scholar]
- 6.Aggoun Y, Farpour-Lambert NJ, Marchand LM et al. Impaired endothelial and smooth muscle functions and arterial stiffness appear before puberty in obese children and are associated with elevated ambulatory blood pressure. Eur Heart J 2008;29:792–799. [DOI] [PubMed] [Google Scholar]
- 7.Short KR, Blackett PR, Gardner AW, Copeland KC. Vascular health in children and adolescents: effects of obesity and diabetes. Vasc Health Risk Manag 2009;5:973–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeSouza CA, Shapiro LF, Clevenger CM et al. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 2000;102:1351–1357. [DOI] [PubMed] [Google Scholar]
- 9.Clarkson P, Montgomery HE, Mullen MJ et al. Exercise training enhances endothelial function in young men. J Am Coll Cardiol 1999;33:1379–1385. [DOI] [PubMed] [Google Scholar]
- 10.McVeigh GE, Bratteli CW, Morgan DJ et al. Age-related abnormalities in arterial compliance identified by pressure pulse contour analysis: aging and arterial compliance. Hypertension 1999;33:1392–1398. [DOI] [PubMed] [Google Scholar]
- 11.Cohn JN, Quyyumi AA, Hollenberg NK, Jamerson KA. Surrogate markers for cardiovascular disease: functional markers. Circulation 2004;109:IV31–IV46. [DOI] [PubMed] [Google Scholar]
- 12.Arnett DK, Evans GW, Riley WA. Arterial stiffness: a new cardiovascular risk factor? Am J Epidemiol 1994;140:669–682. [DOI] [PubMed] [Google Scholar]
- 13.Gardner AW, Parker DE. Association between arterial compliance and age in participants 9 to 77 years old. Angiology 2010;61:37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niboshi A, Hamaoka K, Sakata K, Inoue F. Characteristics of brachial-ankle pulse wave velocity in Japanese children. Eur J Pediatr 2006;165:625–629. [DOI] [PubMed] [Google Scholar]
- 15.Thijssen DH, Bullens LM, van Bemmel MM et al. Does arterial shear explain the magnitude of flow-mediated dilation?: a comparison between young and older humans. Am J Physiol Heart Circ Physiol 2009;296:H57–H64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernhall B, Agiovlasitis S. Arterial function in youth: window into cardiovascular risk. J Appl Physiol 2008;105:325–333. [DOI] [PubMed] [Google Scholar]
- 17.Sakuragi S, Abhayaratna K, Gravenmaker KJ et al. Influence of adiposity and physical activity on arterial stiffness in healthy children: the lifestyle of our kids study. Hypertension 2009;53:611–616. [DOI] [PubMed] [Google Scholar]
- 18.Tounian P, Aggoun Y, Dubern B et al. Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: a prospective study. Lancet 2001;358:1400–1404. [DOI] [PubMed] [Google Scholar]
- 19.Urbina EM, Kimball TR, McCoy CE et al. Youth with obesity and obesity-related type 2 diabetes mellitus demonstrate abnormalities in carotid structure and function. Circulation 2009;119:2913–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dangardt F, Osika W, Volkmann R, Gan LM, Friberg P. Obese children show increased intimal wall thickness and decreased pulse wave velocity. Clin Physiol Funct Imaging 2008;28:287–293. [DOI] [PubMed] [Google Scholar]
- 21.Pereira MA, FitzerGerald SJ, Gregg EW et al. A collection of Physical Activity Questionnaires for health-related research. Med Sci Sports Exerc 1997;29:S1–205. [PubMed] [Google Scholar]
- 22.Pereira MA, FitzerGerald SJ, Gregg EW et al. A collection of Physical Activity Questionnaires for health-related research. Med Sci Sports Exerc 1997;29:S1–205. [PubMed] [Google Scholar]
- 23.Matthews DR, Hosker JP, Rudenski AS et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 24.Hamburg NM, Keyes MJ, Larson MG et al. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation 2008;117:2467–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dhindsa M, Sommerlad SM, DeVan AE et al. Interrelationships among noninvasive measures of postischemic macro- and microvascular reactivity. J Appl Physiol 2008;105:427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haller MJ, Stein J, Shuster J et al. Peripheral artery tonometry demonstrates altered endothelial function in children with type 1 diabetes. Pediatr Diabetes 2007;8:193–198. [DOI] [PubMed] [Google Scholar]
- 27.Persichini T, Cantoni O, Suzuki H, Colasanti M. Cross-talk between constitutive and inducible NO synthase: an update. Antioxid Redox Signal 2006;8:949–954. [DOI] [PubMed] [Google Scholar]
- 28.Li H, Förstermann U. Nitric oxide in the pathogenesis of vascular disease. J Pathol 2000;190:244–254. [DOI] [PubMed] [Google Scholar]
- 29.Cheng C, van Haperen R, de Waard M et al. Shear stress affects the intracellular distribution of eNOS: direct demonstration by a novel in vivo technique. Blood 2005;106:3691–3698. [DOI] [PubMed] [Google Scholar]
- 30.Ripoll E, Sillau AH, Banchero N. Changes in the capillarity of skeletal muscle in the growing rat. Pflugers Arch 1979;380:153–158. [DOI] [PubMed] [Google Scholar]
- 31.Wilkinson IB, Qasem A, McEniery CM et al. Nitric oxide regulates local arterial distensibility in vivo. Circulation 2002;105:213–217. [DOI] [PubMed] [Google Scholar]
- 32.Lovren F, Pan Y, Shukla PC et al. Visfatin activates eNOS via Akt and MAP kinases and improves endothelial cell function and angiogenesis in vitro and in vivo: translational implications for atherosclerosis. Am J Physiol Endocrinol Metab 2009;296:E1440–E1449. [DOI] [PubMed] [Google Scholar]
- 33.Ritchie SA, Kohlhaas CF, Boyd AR et al. Insulin-stimulated phosphorylation of endothelial nitric oxide synthase at serine-615 contributes to nitric oxide synthesis. Biochem J 2010;426:85–90. [DOI] [PubMed] [Google Scholar]
- 34.Yamawaki H, Hara N, Okada M, Hara Y. Visfatin causes endothelium-dependent relaxation in isolated blood vessels. Biochem Biophys Res Commun 2009;383:503–508. [DOI] [PubMed] [Google Scholar]
- 35.Elizalde M, Rydén M, van Harmelen V et al. Expression of nitric oxide synthases in subcutaneous adipose tissue of nonobese and obese humans. J Lipid Res 2000;41:1244–1251. [PubMed] [Google Scholar]
- 36.Galvin VB, Barakat H, Kemeny G et al. Endothelial nitric oxide synthase content in adipose tissue from obese and lean African American and white American women. Metab Clin Exp 2005;54:1368–1373. [DOI] [PubMed] [Google Scholar]
- 37.Westerbacka J, Vehkavaara S, Bergholm R et al. Marked resistance of the ability of insulin to decrease arterial stiffness characterizes human obesity. Diabetes 1999;48:821–827. [DOI] [PubMed] [Google Scholar]
- 38.Kearney MT, Duncan ER, Kahn M, Wheatcroft SB. Insulin resistance and endothelial cell dysfunction: studies in mammalian models. Exp Physiol 2008;93:158–163. [DOI] [PubMed] [Google Scholar]
- 39.Ohman MK, Wright AP, Wickenheiser KJ, Luo W, Eitzman DT. Visceral adipose tissue and atherosclerosis. Curr Vasc Pharmacol 2009;7:169–179. [DOI] [PubMed] [Google Scholar]
- 40.Dickinson KM, Keogh JB, Clifton PM. Effects of a low-salt diet on flow-mediated dilatation in humans. Am J Clin Nutr 2009;89:485–490. [DOI] [PubMed] [Google Scholar]


