Two orthorhombic polymorphs of the anti-inflammatory corticosteroid tixocortol pivalate have been identified. The two structures are characterized by layers of molecules connected by strong O—H⋯O hydrogen bonds.
Keywords: tixocortol, pivalone, crystal structure, polymorphs
Abstract
Two polymorphs, (I) and (II), of (S)-{2-[(8S,9S,10R,11S,13S,14S,17R)-11,17-dihydroxy-10,13-dimethyl-3-oxo-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-17-yl]-2-oxoethyl} 2,2-dimethylpropanethioate, C26H38O5S, have been identified. They are orthorhombic, non-centrosymmetric (P212121). The structures display layers of molecules conected via O—H⋯O hydrogen bonds along the b-axis direction in polymorph (I) and along the c-axis direction in polymorph (II). The structure of (II) exhibits disorder of the main molecule.
Chemical context
Tixocortol pivalate, also named Pivalone®, is a corticosteroid with local and topical anti-inflammatory activity (Davies et al., 1981 ▸; Jezequel et al., 1979 ▸; Liddle et al., 1960 ▸; Mazauric & Alligier, 1978 ▸; Nugent et al., 1963 ▸; Uphill, 1981 ▸) equal to that of hydrocortisone. As a corticosteroid, Tixocortol pivalate is used topically to relieve contact allergies and is also frequently recommended as a screening test for class A corticosteroids (Bircher et al., 1995 ▸; Burden & Beck, 1992 ▸; Lauerma, 1991 ▸; Bouley, 2013 ▸). Surprisingly, the structure of tixocortol pivalate has never been determined. It was therefore of interest to obtain two polymorphs, (I) and (II), of the title compound prepared by total enantio-selective synthesis.

Structural commentary
The presence of two polymorphs was confirmed by powder X-ray diffraction (PXRD) and the structures were determined by single crystal X-ray diffraction (SCXRD). The absolute configuration of its seven asymmetric carbons was established. Both polymorphs of the title compound consist of a (S)-{2-[(8S,9S,10R,11S,13S,14S,17R)-11,17-dihydroxy-10,13-dimethyl-3-oxo-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-17-yl]-2-oxoethyl} 2,2-dimethylpropanethioate molecule in the asymmetric unit (Figs. 1 ▸ and 2 ▸). The general shape of the molecule is strongly influenced by the conformation of one five-membered ring and three six-membered rings. In both polymorphs (Table 1 ▸), the five-membered ring (C8–C12) adopts an envelope form, both central six-membered rings (C9/C14–C17/C10 and C16/C18–C21/C17) adopt chair conformations and the six-membered ring with the double bond (C18/C19/C23–C26) adopts a half-chair conformation (Cremer & Pople, 1975 ▸). The superposition of the molecules, with the Automatic Molecule Overlay feature of Mercury (Macrae et al., 2020 ▸), results in an r.m.s.d. of 0.829 and a maximum deviation of 2.545 Å if no flexibility is allowed and in values of 0.336 and 0.856, respectively, if flexibility is allowed. The main difference is on the dimethyl-sulfanyl-propanone group whose position is imposed by crystal packing.
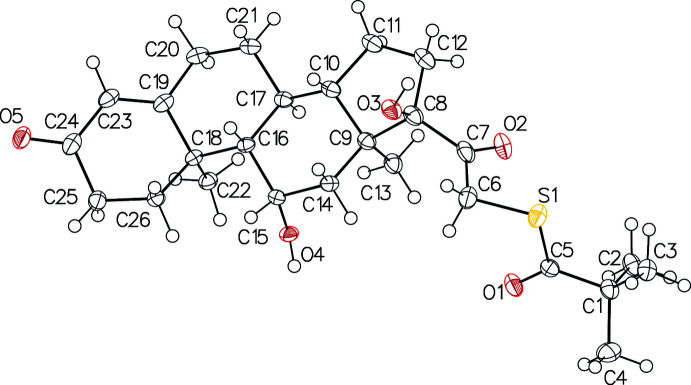
Figure 1.
ORTEP view of polymorph (I). Displacement ellipsoids are drawn at the 50% probability level.
Figure 2.
ORTEP view of polymorph (II). Displacement ellipsoids are drawn at the 30% probability level. The minor component of the disorder is omitted for clarity.
Table 1. Ring puckering parameters.
| Compound | PL358 (I) | SY20C174 (II) |
|---|---|---|
| C8–C12 | Q2 = 0.4847 (18) Å | Q2 = 0.441 (5) Å |
| Envelope conformation | φ2 = 39.4 (2)° | φ2 = 41.4 (6)° |
| C9/C14–C17/C10 | Q = 0.5519 (17) Å | Θ = 9.45 (18) ° | φ2 = 53.2 (11)° | Q = 0.556 (4) Å | Θ = 13.4 (4) ° | φ2 = 37 (2)° |
| Chair conformation | Q2 = 0.0908 (17) Å | Q3 = 54.4444 (17) Å | φ2 = 53.2 (11)° | Q2 = 0.128 (4) Å | Q3 = 0.541 (4) Å | φ2 = 37 (2)° |
| C16/C18–C21/C17 | Q = 0.5450 (17) Å | Θ = 175.11 (18) ° | φ2 = 170 (2)° | Q = 0.538 (4) Å | Θ = 173.2 (4) ° | φ2 = 196 (4)° |
| Chair conformation | Q2 = 0.0457 (17) Å | Q3 = −0.5431 (17) Å | φ2 = 170 (2)° | Q2 = 0.0065 (4) Å | Q3 = −0.534 (4) Å | φ2 = 196 (4)° |
| C18/C19/C23–C26 | Q = 0.4724 (18) Å | Θ = 52.7 (2) ° | φ2 = 266.8 (3)° | Q = 0.454 (4) Å | Θ = 55.6 (5) ° | φ2 = 281.9 (7)° |
| Half-chair conformation | Q2 = 0.3756 (18) Å | Q3 = 0.2865 (18) Å | φ2 = 266.8 (3)° | Q2 = 0.375 (4) Å | Q3 = 0.256 (4) Å | φ2 = 281.9 (7)° |
Supramolecular features
The crystal packing in both structures is stabilized by one O—H⋯O hydrogen bond (Figs. 3 ▸ and 4 ▸, Tables 2 ▸ and 3 ▸) producing layers along (010) for polymorph (I) (PL358) and along (001) for polymorph (II) (SY20C174). The geometry of these interactions indicates that these are strong hydrogen bonds.
Figure 3.
View of the hydrogen bond-network in polymorph (I).
Figure 4.
View of the hydrogen-bond network in polymorph (II).
Table 2. Hydrogen-bond geometry (Å, °) for PL358 (I) .
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O3—H3⋯O5i | 0.84 | 2.07 | 2.9021 (17) | 169 |
Symmetry code: (i) -x+1, y+{\script{1\over 2}}, -z+{\script{1\over 2}}.
Table 3. Hydrogen-bond geometry (Å, °) for SY20C174 (II) .
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O3—H3⋯O5i | 0.84 | 1.96 | 2.802 (4) | 175 |
Symmetry code: (i) -x+{\script{1\over 2}}, -y+1, z+{\script{1\over 2}}.
Morphology prediction
In both polymorphs, it was observed that the same type of hydrogen bonds plays a dominant role in the formation of hydrogen-bonded networks. However, the arrangements of molecules in the crystal packing of polymorphs (I) and (II) are different. The different arrangements can also be seen in the external shape and size of the crystals. The theoretical crystal habits of polymorphs (I) and (II) were predicted based on the BFDH model with Mercury (Fig. 5 ▸). The morphologies of Pivalone polymorphs (I) and (II) display significant differences in their main crystal dimension.
Figure 5.
View of the crystal morphology of polymorph (I) (top) and (II) (bottom).
Synthesis and crystallization
Tixocortol pivalate (Fig. 6 ▸) has been produced as follows (Bouley, 2013 ▸): in a dry inerted flask, cesium thiopivalate (620 g, 2.48 mol) and tetrahydrofuran (1460 mL) are stirred at room temperature. A hydrocortisone mesylate (995 g, 2.26 mol) solution in THF (4600 mL) is added in 1 h below 293 K. After 16 h of stirring, the reaction mixture is cooled below 283 K and water (12320 mL) is added. After addition, the reaction mixture is stirred for approximately 2 h. The precipitate is filtered and washed with water (10 × 820 mL). After drying under vacuum at 323 K for one night, the product is isolated as a white powder (yield 93%, purity by HPLC 98.5%).
Figure 6.
Reaction scheme for the synthesis of tixocortol pivalate.
Powder X-ray diffraction (PXRD)
Analyses were performed at room temperature from 2θ = 3 to 50° with an increasing step size of 0.02° and a count time of 120 s. The X-ray powder diffraction patterns were registered in transmission mode unless mentioned otherwise. The samples (few milligrams) are introduced without being crushed in 1 mm diameter glass capillaries to avoid preferential orientation. The capillaries are sealed to avoid contact with air. The analysis is performed in transmission mode by using a focusing X-ray mirror with divergence slits and anti-scatter slits (aperture 0.5°), on an Empyrean diffractometer from PANalytical Company (PANalytical, 2011 ▸) equipped with a copper anticathode tube (wavelength λ Kα1 = 1.54060 Å/Kα2 = 1.54443 Å) and with a PIXcel 1D detector with anti-scatter slits of 7.5 mm. The calibration of the analytical instrument is checked before each analytical batch according to quality systems.
Unit-cell parameters were obtained using indexing methods included in ITO (Visser, 1969 ▸) or DICVOL (Boultif & Louër, 2004 ▸). Le Bail (Le Bail, 1988 ▸) refinement was performed by using JANA2006 (Petříček et al., 2014 ▸) with the most plausible unit cell. The cell parameters found at room temperature were compared to those found from single crystal at different temperatures (Table 4 ▸). The cell parameters at low temperature and at ambient temperature found from single crystal and from powder diffraction are similar, confirming that no phase change occurs with different temperatures. The simulated PXRD patterns were calculated (Palmer, 2015 ▸) from SCXRD with cell parameters obtained at room temperature (Fig. 7 ▸).
Table 4. Cell parameters determined from SCXRD and PXRD at different temperatures.
| Compound | PL358 | PL358 | PL358 | SY20C174 | SY20C174 | SY20C174 |
|---|---|---|---|---|---|---|
| XRD measurement | SCXRD | SCXRD | PXRD | SCXRD | SCXRD | PXRD |
| Temperature | 110 K | 295 K | 295 K | 100 K | 298 K | 295 K |
| Space group | P212121 | P212121 | P212121 | P212121 | P212121 | P212121 |
| a | 6.4201 (2) | 6.467 (5) | 6.4775 (2) | 6.0146 (2) | 6.157 (9) | 6.1573 (2) |
| b | 17.6239 (7) | 17.887 (12) | 17.9583 (7) | 19.2817 (7) | 19.46 (3) | 19.4684 (7) |
| c | 20.8997 (8) | 20.897 (15) | 20.9335 (7) | 20.9887 (7) | 20.92 (3) | 20.8859 (9) |
| Volume | 2364.7 (1) | 2417 (5) | 2435.1 (1) | 2434.1 (1) | 2508 (11) | 2503.7 (2) |
Figure 7.
PXRD patterns of polymorphs (I) and (II) and their simulated patterns from the SCXRD study at room temperature.
Structure solution and refinement
Crystal data, data collection and structure refinement details are summarized in Table 5 ▸. The dimethyl-sulfanyl-propanone group was found to be disordered over two positions 77 (1)%/23 (1)% in polymotph (II). The SAME (Sheldrick, 2015b ▸) restraint was employed for the minor disordered part to maintain a reasonable model. All non-hydrogen atoms were refined anisotropically, except the minor disorder component. Hydrogen-atom positions were calculated geometrically and refined using the riding model. All H atoms, on carbon atoms, were placed at calculated positions using a riding model with C—H = 0.95 Å (aromatic), 0.99 Å (methylene) or 1 Å (methine) with U iso(H) = 1.2U eq(C). H atoms on oxygen atoms were located in difference-Fourier maps. Their positional parameters were refined as an idealized OH group (AFIX 147), (Sheldrick, 2015b ▸) with U iso(H) = 1.5U eq(O). The TWIN/BASF instruction was used to refine the Flack parameter.
Table 5. Experimental details.
| PL358 (I) | SY20C174 (II) | |
|---|---|---|
| Crystal data | ||
| Chemical formula | C26H38O5S | C26H38O5S |
| M r | 462.62 | 462.62 |
| Crystal system, space group | Orthorhombic, P212121 | Orthorhombic, P212121 |
| Temperature (K) | 110 | 100 |
| a, b, c (Å) | 6.4201 (2), 17.6239 (7), 20.8997 (8) | 6.0146 (2), 19.2817 (7), 20.9887 (7) |
| V (Å3) | 2364.74 (15) | 2434.10 (14) |
| Z | 4 | 4 |
| Radiation type | Mo Kα | Cu Kα |
| μ (mm−1) | 0.17 | 1.46 |
| Crystal size (mm) | 0.46 × 0.25 × 0.24 | 0.18 × 0.06 × 0.05 |
| Data collection | ||
| Diffractometer | Nonius Kappa APEXII | Bruker D8 Venture |
| Absorption correction | Multi-scan (SADABS; Krause et al., 2015 ▸) | Multi-scan (SADABS; Krause et al., 2015 ▸) |
| T min, T max | 0.912, 0.958 | 0.707, 0.862 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 74424, 5424, 5180 | 30900, 4303, 3803 |
| R int | 0.034 | 0.102 |
| Refinement | ||
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.028, 0.072, 1.04 | 0.055, 0.130, 1.07 |
| No. of reflections | 5424 | 4303 |
| No. of parameters | 296 | 329 |
| No. of restraints | 0 | 16 |
| H-atom treatment | H-atom parameters constrained | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.26, −0.23 | 0.26, −0.39 |
| Absolute structure | Flack x determined using 2176 quotients [(I +)−(I −)]/[(I +)+(I −)] (Parsons et al., 2013 ▸) | Flack x obtained from refinement |
| Absolute structure parameter | 0.027 (13) | 0.11 (4) |
Supplementary Material
Crystal structure: contains datablock(s) PL358, SY20C174, New_Global_Publ_Block. DOI: 10.1107/S2056989021007167/dj2019sup1.cif
Structure factors: contains datablock(s) PL358. DOI: 10.1107/S2056989021007167/dj2019PL358sup2.hkl
Supporting information file. DOI: 10.1107/S2056989021007167/dj2019PL358sup4.cdx
Structure factors: contains datablock(s) SY20C174. DOI: 10.1107/S2056989021007167/dj2019SY20C174sup3.hkl
Supporting information file. DOI: 10.1107/S2056989021007167/dj2019SY20C174sup5.cdx
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
The Ministère de l’Enseignement Supérieur et de la Recherche, the Centre National de la Recherche Scientifique (CNRS) and the Conseil Régional de Bourgogne Franche-Comté are gratefully acknowledged. This work is supported by the Université de Bourgogne and the Conseil Régional de Bourgogne Franche-Comté through the Plan d’Actions Régional pour l’Innovation (PARI) and the European Union through the PO FEDER-FSE Bourgogne 2014/2020 programs. The X-ray analyses were recorded in the ‘Pôle Chimie Moléculaire’, the technological platform for chemical analysis and molecular synthesis (http://www.wpcm.fr), which relies on the Institute of the Molecular Chemistry of the University of Burgundy (ICMUB) and SATT Sayens™, a Burgundy University private subsidiary.
supplementary crystallographic information
(S)-{2-[(8S,9S,10R,11S,13S,14S,17R)-11,17-Dihydroxy-10,13-dimethyl-3-oxo-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-17-yl]-2-oxoethyl} 2,2-dimethylpropanethioate (PL358) . Crystal data
| C26H38O5S | Dx = 1.299 Mg m−3 |
| Mr = 462.62 | Mo Kα radiation, λ = 0.71073 Å |
| Orthorhombic, P212121 | Cell parameters from 9539 reflections |
| a = 6.4201 (2) Å | θ = 2.3–27.4° |
| b = 17.6239 (7) Å | µ = 0.17 mm−1 |
| c = 20.8997 (8) Å | T = 110 K |
| V = 2364.74 (15) Å3 | Prism, clear light colourless |
| Z = 4 | 0.46 × 0.25 × 0.24 mm |
| F(000) = 1000 |
(S)-{2-[(8S,9S,10R,11S,13S,14S,17R)-11,17-Dihydroxy-10,13-dimethyl-3-oxo-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-17-yl]-2-oxoethyl} 2,2-dimethylpropanethioate (PL358) . Data collection
| Nonius Kappa APEXII diffractometer | 5424 independent reflections |
| Radiation source: X-ray tube, Siemens KFF Mo 2K-180 | 5180 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.034 |
| Detector resolution: 512 x 512 pixels mm-1 | θmax = 27.5°, θmin = 3.0° |
| φ and ω scans' | h = −8→8 |
| Absorption correction: multi-scan (SADABS; Krause et al., 2015) | k = −22→22 |
| Tmin = 0.912, Tmax = 0.958 | l = −27→27 |
| 74424 measured reflections |
(S)-{2-[(8S,9S,10R,11S,13S,14S,17R)-11,17-Dihydroxy-10,13-dimethyl-3-oxo-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-17-yl]-2-oxoethyl} 2,2-dimethylpropanethioate (PL358) . Refinement
| Refinement on F2 | Hydrogen site location: inferred from neighbouring sites |
| Least-squares matrix: full | H-atom parameters constrained |
| R[F2 > 2σ(F2)] = 0.028 | w = 1/[σ2(Fo2) + (0.0409P)2 + 0.6195P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.072 | (Δ/σ)max < 0.001 |
| S = 1.04 | Δρmax = 0.26 e Å−3 |
| 5424 reflections | Δρmin = −0.22 e Å−3 |
| 296 parameters | Absolute structure: Flack x determined using 2176 quotients [(I+)-(I-)]/[(I+)+(I-)] (Parsons et al., 2013) |
| 0 restraints | Absolute structure parameter: 0.027 (13) |
| Primary atom site location: dual |
(S)-{2-[(8S,9S,10R,11S,13S,14S,17R)-11,17-Dihydroxy-10,13-dimethyl-3-oxo-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-17-yl]-2-oxoethyl} 2,2-dimethylpropanethioate (PL358) . Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
(S)-{2-[(8S,9S,10R,11S,13S,14S,17R)-11,17-Dihydroxy-10,13-dimethyl-3-oxo-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-17-yl]-2-oxoethyl} 2,2-dimethylpropanethioate (PL358) . Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| S1 | 0.57981 (8) | 0.59132 (2) | 0.30170 (2) | 0.02260 (11) | |
| O3 | 0.6410 (2) | 0.35175 (7) | 0.23101 (6) | 0.0154 (2) | |
| H3 | 0.671037 | 0.360570 | 0.192609 | 0.023* | |
| O5 | 0.2936 (2) | −0.10124 (7) | 0.40054 (6) | 0.0186 (3) | |
| O2 | 0.9568 (2) | 0.49062 (7) | 0.30543 (6) | 0.0219 (3) | |
| O4 | 0.6656 (3) | 0.25785 (7) | 0.46752 (6) | 0.0243 (3) | |
| H4 | 0.576170 | 0.276912 | 0.492065 | 0.036* | |
| O1 | 0.4852 (3) | 0.52261 (8) | 0.40864 (7) | 0.0352 (4) | |
| C10 | 0.8689 (2) | 0.24334 (9) | 0.30097 (7) | 0.0105 (3) | |
| H10 | 0.757227 | 0.229873 | 0.269614 | 0.013* | |
| C16 | 0.6649 (2) | 0.16686 (9) | 0.37858 (7) | 0.0101 (3) | |
| H16 | 0.572073 | 0.153973 | 0.341722 | 0.012* | |
| C24 | 0.4122 (3) | −0.04919 (9) | 0.41447 (8) | 0.0133 (3) | |
| C22 | 0.7966 (3) | 0.09961 (10) | 0.48265 (8) | 0.0139 (3) | |
| H22A | 0.939136 | 0.110305 | 0.468479 | 0.021* | |
| H22B | 0.793474 | 0.050904 | 0.505250 | 0.021* | |
| H22C | 0.749690 | 0.139990 | 0.511490 | 0.021* | |
| C7 | 0.8049 (3) | 0.45608 (10) | 0.28770 (8) | 0.0152 (3) | |
| C18 | 0.6505 (2) | 0.09589 (9) | 0.42376 (7) | 0.0101 (3) | |
| C17 | 0.8818 (2) | 0.17768 (9) | 0.34806 (7) | 0.0108 (3) | |
| H17 | 0.986748 | 0.189289 | 0.382019 | 0.013* | |
| C23 | 0.6075 (3) | −0.03662 (9) | 0.37971 (8) | 0.0133 (3) | |
| H23 | 0.657156 | −0.075699 | 0.352367 | 0.016* | |
| C26 | 0.4233 (3) | 0.08633 (9) | 0.44563 (7) | 0.0116 (3) | |
| H26A | 0.395372 | 0.122300 | 0.480995 | 0.014* | |
| H26B | 0.329792 | 0.099703 | 0.409691 | 0.014* | |
| C21 | 0.9454 (3) | 0.10574 (9) | 0.31190 (8) | 0.0140 (3) | |
| H21A | 0.855784 | 0.100246 | 0.273627 | 0.017* | |
| H21B | 1.091004 | 0.111287 | 0.297053 | 0.017* | |
| C20 | 0.9280 (3) | 0.03384 (9) | 0.35248 (8) | 0.0138 (3) | |
| H20A | 0.950163 | −0.011075 | 0.324867 | 0.017* | |
| H20B | 1.038671 | 0.034177 | 0.385458 | 0.017* | |
| C9 | 0.8031 (3) | 0.31896 (9) | 0.33168 (7) | 0.0110 (3) | |
| C11 | 1.0623 (3) | 0.26354 (9) | 0.26139 (8) | 0.0142 (3) | |
| H11A | 1.076384 | 0.229298 | 0.224088 | 0.017* | |
| H11B | 1.190147 | 0.260105 | 0.287716 | 0.017* | |
| C1 | 0.4236 (3) | 0.65833 (11) | 0.41260 (8) | 0.0219 (4) | |
| C19 | 0.7196 (2) | 0.02742 (9) | 0.38462 (8) | 0.0112 (3) | |
| C15 | 0.5701 (3) | 0.24038 (9) | 0.40657 (8) | 0.0142 (3) | |
| H15 | 0.418893 | 0.230653 | 0.414522 | 0.017* | |
| C5 | 0.4924 (3) | 0.58328 (10) | 0.38238 (9) | 0.0184 (3) | |
| C14 | 0.5861 (3) | 0.30893 (9) | 0.36064 (8) | 0.0134 (3) | |
| H14A | 0.483971 | 0.302354 | 0.325584 | 0.016* | |
| H14B | 0.548391 | 0.355646 | 0.384264 | 0.016* | |
| C25 | 0.3715 (3) | 0.00567 (9) | 0.46830 (8) | 0.0138 (3) | |
| H25A | 0.458872 | −0.007601 | 0.505705 | 0.017* | |
| H25B | 0.223523 | 0.002816 | 0.481369 | 0.017* | |
| C8 | 0.8139 (3) | 0.37093 (9) | 0.27071 (8) | 0.0126 (3) | |
| C13 | 0.9608 (3) | 0.34721 (9) | 0.38172 (8) | 0.0155 (3) | |
| H13A | 0.975665 | 0.309141 | 0.415590 | 0.023* | |
| H13B | 0.911232 | 0.394965 | 0.400299 | 0.023* | |
| H13C | 1.096090 | 0.355534 | 0.361145 | 0.023* | |
| C12 | 1.0227 (3) | 0.34651 (10) | 0.23951 (8) | 0.0155 (3) | |
| H12A | 1.137374 | 0.379980 | 0.253852 | 0.019* | |
| H12B | 1.012823 | 0.349257 | 0.192295 | 0.019* | |
| C6 | 0.5917 (3) | 0.49264 (10) | 0.28061 (9) | 0.0205 (4) | |
| H6A | 0.491554 | 0.464500 | 0.307701 | 0.025* | |
| H6B | 0.546071 | 0.487033 | 0.235619 | 0.025* | |
| C4 | 0.5645 (4) | 0.72401 (12) | 0.39145 (14) | 0.0431 (6) | |
| H4A | 0.708534 | 0.713275 | 0.404017 | 0.065* | |
| H4B | 0.518021 | 0.771070 | 0.411956 | 0.065* | |
| H4C | 0.556793 | 0.729660 | 0.344859 | 0.065* | |
| C3 | 0.2026 (4) | 0.67460 (16) | 0.38883 (13) | 0.0430 (6) | |
| H3A | 0.203839 | 0.679901 | 0.342158 | 0.065* | |
| H3B | 0.151797 | 0.721734 | 0.408214 | 0.065* | |
| H3C | 0.110650 | 0.632594 | 0.400915 | 0.065* | |
| C2 | 0.4239 (6) | 0.64969 (16) | 0.48501 (10) | 0.0564 (9) | |
| H2A | 0.332983 | 0.607392 | 0.497155 | 0.085* | |
| H2B | 0.372756 | 0.696584 | 0.504713 | 0.085* | |
| H2C | 0.565977 | 0.639511 | 0.499814 | 0.085* |
(S)-{2-[(8S,9S,10R,11S,13S,14S,17R)-11,17-Dihydroxy-10,13-dimethyl-3-oxo-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-17-yl]-2-oxoethyl} 2,2-dimethylpropanethioate (PL358) . Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S1 | 0.0352 (3) | 0.01312 (19) | 0.0195 (2) | 0.00396 (19) | 0.01024 (19) | 0.00171 (16) |
| O3 | 0.0179 (6) | 0.0176 (6) | 0.0106 (5) | −0.0006 (5) | −0.0030 (5) | 0.0007 (4) |
| O5 | 0.0195 (6) | 0.0148 (6) | 0.0216 (6) | −0.0049 (5) | 0.0009 (5) | −0.0018 (5) |
| O2 | 0.0258 (7) | 0.0141 (6) | 0.0257 (7) | −0.0061 (5) | −0.0009 (6) | 0.0018 (5) |
| O4 | 0.0479 (9) | 0.0151 (6) | 0.0100 (6) | 0.0015 (6) | 0.0044 (6) | −0.0025 (5) |
| O1 | 0.0617 (11) | 0.0218 (7) | 0.0221 (7) | 0.0018 (7) | 0.0063 (7) | 0.0068 (6) |
| C10 | 0.0096 (7) | 0.0121 (7) | 0.0099 (7) | 0.0001 (6) | 0.0002 (6) | −0.0007 (6) |
| C16 | 0.0111 (7) | 0.0097 (7) | 0.0093 (6) | 0.0006 (6) | 0.0011 (6) | −0.0002 (6) |
| C24 | 0.0172 (8) | 0.0099 (7) | 0.0128 (7) | 0.0012 (6) | −0.0020 (7) | 0.0024 (6) |
| C22 | 0.0157 (8) | 0.0138 (8) | 0.0122 (7) | 0.0002 (6) | −0.0027 (6) | −0.0015 (6) |
| C7 | 0.0225 (9) | 0.0147 (8) | 0.0082 (7) | 0.0000 (7) | 0.0026 (7) | 0.0028 (6) |
| C18 | 0.0114 (7) | 0.0095 (7) | 0.0094 (7) | −0.0004 (6) | −0.0006 (5) | −0.0010 (6) |
| C17 | 0.0107 (7) | 0.0106 (7) | 0.0111 (7) | 0.0006 (6) | 0.0007 (6) | −0.0006 (6) |
| C23 | 0.0156 (8) | 0.0113 (7) | 0.0132 (7) | 0.0012 (6) | 0.0003 (6) | −0.0023 (6) |
| C26 | 0.0128 (7) | 0.0108 (7) | 0.0111 (7) | −0.0001 (6) | 0.0012 (6) | −0.0009 (6) |
| C21 | 0.0142 (7) | 0.0125 (7) | 0.0154 (7) | 0.0012 (6) | 0.0045 (6) | −0.0003 (6) |
| C20 | 0.0127 (7) | 0.0111 (7) | 0.0177 (8) | 0.0018 (6) | 0.0032 (7) | −0.0018 (6) |
| C9 | 0.0125 (7) | 0.0104 (7) | 0.0102 (7) | −0.0005 (6) | −0.0004 (6) | 0.0007 (6) |
| C11 | 0.0128 (7) | 0.0149 (7) | 0.0149 (7) | −0.0006 (7) | 0.0030 (6) | 0.0006 (6) |
| C1 | 0.0255 (9) | 0.0243 (9) | 0.0158 (8) | 0.0083 (8) | −0.0004 (8) | −0.0027 (7) |
| C19 | 0.0124 (7) | 0.0119 (7) | 0.0092 (7) | 0.0028 (6) | −0.0014 (6) | 0.0004 (6) |
| C15 | 0.0167 (8) | 0.0116 (7) | 0.0142 (7) | 0.0013 (6) | 0.0045 (7) | −0.0001 (6) |
| C5 | 0.0209 (8) | 0.0195 (8) | 0.0146 (7) | 0.0021 (7) | −0.0015 (7) | 0.0016 (7) |
| C14 | 0.0141 (7) | 0.0111 (7) | 0.0151 (7) | 0.0019 (6) | 0.0024 (6) | 0.0010 (6) |
| C25 | 0.0154 (8) | 0.0140 (8) | 0.0119 (7) | −0.0013 (6) | 0.0028 (6) | 0.0000 (6) |
| C8 | 0.0140 (8) | 0.0126 (7) | 0.0110 (7) | −0.0012 (6) | −0.0016 (6) | 0.0007 (6) |
| C13 | 0.0195 (8) | 0.0138 (7) | 0.0133 (7) | −0.0007 (7) | −0.0046 (7) | −0.0013 (6) |
| C12 | 0.0170 (8) | 0.0150 (8) | 0.0143 (8) | −0.0013 (7) | 0.0035 (6) | 0.0012 (6) |
| C6 | 0.0276 (9) | 0.0137 (8) | 0.0203 (8) | 0.0030 (7) | −0.0007 (8) | −0.0039 (6) |
| C4 | 0.0458 (14) | 0.0216 (10) | 0.0621 (16) | 0.0019 (10) | 0.0085 (14) | −0.0157 (10) |
| C3 | 0.0298 (12) | 0.0568 (16) | 0.0424 (13) | 0.0196 (11) | −0.0046 (11) | −0.0140 (12) |
| C2 | 0.105 (3) | 0.0496 (15) | 0.0150 (9) | 0.0370 (18) | −0.0069 (13) | −0.0052 (9) |
(S)-{2-[(8S,9S,10R,11S,13S,14S,17R)-11,17-Dihydroxy-10,13-dimethyl-3-oxo-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-17-yl]-2-oxoethyl} 2,2-dimethylpropanethioate (PL358) . Geometric parameters (Å, º)
| S1—C5 | 1.7827 (18) | C20—H20A | 0.9900 |
| S1—C6 | 1.7957 (18) | C20—H20B | 0.9900 |
| O3—H3 | 0.8400 | C20—C19 | 1.501 (2) |
| O3—C8 | 1.426 (2) | C9—C14 | 1.529 (2) |
| O5—C24 | 1.227 (2) | C9—C8 | 1.571 (2) |
| O2—C7 | 1.208 (2) | C9—C13 | 1.538 (2) |
| O4—H4 | 0.8400 | C11—H11A | 0.9900 |
| O4—C15 | 1.447 (2) | C11—H11B | 0.9900 |
| O1—C5 | 1.203 (2) | C11—C12 | 1.553 (2) |
| C10—H10 | 1.0000 | C1—C5 | 1.531 (3) |
| C10—C17 | 1.521 (2) | C1—C4 | 1.534 (3) |
| C10—C9 | 1.538 (2) | C1—C3 | 1.531 (3) |
| C10—C11 | 1.534 (2) | C1—C2 | 1.521 (3) |
| C16—H16 | 1.0000 | C15—H15 | 1.0000 |
| C16—C18 | 1.570 (2) | C15—C14 | 1.546 (2) |
| C16—C17 | 1.543 (2) | C14—H14A | 0.9900 |
| C16—C15 | 1.546 (2) | C14—H14B | 0.9900 |
| C24—C23 | 1.466 (2) | C25—H25A | 0.9900 |
| C24—C25 | 1.506 (2) | C25—H25B | 0.9900 |
| C22—H22A | 0.9800 | C8—C12 | 1.552 (2) |
| C22—H22B | 0.9800 | C13—H13A | 0.9800 |
| C22—H22C | 0.9800 | C13—H13B | 0.9800 |
| C22—C18 | 1.549 (2) | C13—H13C | 0.9800 |
| C7—C8 | 1.543 (2) | C12—H12A | 0.9900 |
| C7—C6 | 1.520 (3) | C12—H12B | 0.9900 |
| C18—C26 | 1.538 (2) | C6—H6A | 0.9900 |
| C18—C19 | 1.524 (2) | C6—H6B | 0.9900 |
| C17—H17 | 1.0000 | C4—H4A | 0.9800 |
| C17—C21 | 1.531 (2) | C4—H4B | 0.9800 |
| C23—H23 | 0.9500 | C4—H4C | 0.9800 |
| C23—C19 | 1.342 (2) | C3—H3A | 0.9800 |
| C26—H26A | 0.9900 | C3—H3B | 0.9800 |
| C26—H26B | 0.9900 | C3—H3C | 0.9800 |
| C26—C25 | 1.535 (2) | C2—H2A | 0.9800 |
| C21—H21A | 0.9900 | C2—H2B | 0.9800 |
| C21—H21B | 0.9900 | C2—H2C | 0.9800 |
| C21—C20 | 1.529 (2) | ||
| C5—S1—C6 | 99.71 (9) | C5—C1—C4 | 111.30 (16) |
| C8—O3—H3 | 109.5 | C3—C1—C5 | 107.18 (17) |
| C15—O4—H4 | 109.5 | C3—C1—C4 | 108.15 (19) |
| C17—C10—H10 | 106.4 | C2—C1—C5 | 108.89 (16) |
| C17—C10—C9 | 113.83 (13) | C2—C1—C4 | 111.2 (2) |
| C17—C10—C11 | 118.79 (13) | C2—C1—C3 | 110.0 (2) |
| C9—C10—H10 | 106.4 | C23—C19—C18 | 123.42 (15) |
| C11—C10—H10 | 106.4 | C23—C19—C20 | 120.46 (15) |
| C11—C10—C9 | 104.26 (13) | C20—C19—C18 | 116.09 (13) |
| C18—C16—H16 | 104.3 | O4—C15—C16 | 110.17 (13) |
| C17—C16—H16 | 104.3 | O4—C15—H15 | 107.5 |
| C17—C16—C18 | 113.58 (13) | O4—C15—C14 | 110.62 (13) |
| C17—C16—C15 | 114.07 (13) | C16—C15—H15 | 107.5 |
| C15—C16—H16 | 104.3 | C16—C15—C14 | 113.19 (13) |
| C15—C16—C18 | 114.64 (12) | C14—C15—H15 | 107.5 |
| O5—C24—C23 | 121.75 (15) | O1—C5—S1 | 120.96 (15) |
| O5—C24—C25 | 123.32 (16) | O1—C5—C1 | 124.65 (17) |
| C23—C24—C25 | 114.93 (14) | C1—C5—S1 | 114.36 (13) |
| H22A—C22—H22B | 109.5 | C9—C14—C15 | 113.34 (13) |
| H22A—C22—H22C | 109.5 | C9—C14—H14A | 108.9 |
| H22B—C22—H22C | 109.5 | C9—C14—H14B | 108.9 |
| C18—C22—H22A | 109.5 | C15—C14—H14A | 108.9 |
| C18—C22—H22B | 109.5 | C15—C14—H14B | 108.9 |
| C18—C22—H22C | 109.5 | H14A—C14—H14B | 107.7 |
| O2—C7—C8 | 122.04 (17) | C24—C25—C26 | 109.05 (13) |
| O2—C7—C6 | 122.91 (16) | C24—C25—H25A | 109.9 |
| C6—C7—C8 | 115.05 (15) | C24—C25—H25B | 109.9 |
| C22—C18—C16 | 114.12 (13) | C26—C25—H25A | 109.9 |
| C26—C18—C16 | 108.77 (12) | C26—C25—H25B | 109.9 |
| C26—C18—C22 | 110.05 (12) | H25A—C25—H25B | 108.3 |
| C19—C18—C16 | 106.91 (12) | O3—C8—C7 | 109.59 (14) |
| C19—C18—C22 | 106.50 (13) | O3—C8—C9 | 107.43 (13) |
| C19—C18—C26 | 110.42 (13) | O3—C8—C12 | 111.23 (13) |
| C10—C17—C16 | 108.20 (12) | C7—C8—C9 | 112.25 (13) |
| C10—C17—H17 | 109.9 | C7—C8—C12 | 113.50 (14) |
| C10—C17—C21 | 108.97 (13) | C12—C8—C9 | 102.54 (13) |
| C16—C17—H17 | 109.9 | C9—C13—H13A | 109.5 |
| C21—C17—C16 | 110.02 (13) | C9—C13—H13B | 109.5 |
| C21—C17—H17 | 109.9 | C9—C13—H13C | 109.5 |
| C24—C23—H23 | 118.4 | H13A—C13—H13B | 109.5 |
| C19—C23—C24 | 123.21 (15) | H13A—C13—H13C | 109.5 |
| C19—C23—H23 | 118.4 | H13B—C13—H13C | 109.5 |
| C18—C26—H26A | 108.9 | C11—C12—H12A | 110.5 |
| C18—C26—H26B | 108.9 | C11—C12—H12B | 110.5 |
| H26A—C26—H26B | 107.7 | C8—C12—C11 | 106.20 (13) |
| C25—C26—C18 | 113.48 (13) | C8—C12—H12A | 110.5 |
| C25—C26—H26A | 108.9 | C8—C12—H12B | 110.5 |
| C25—C26—H26B | 108.9 | H12A—C12—H12B | 108.7 |
| C17—C21—H21A | 109.0 | S1—C6—H6A | 108.5 |
| C17—C21—H21B | 109.0 | S1—C6—H6B | 108.5 |
| H21A—C21—H21B | 107.8 | C7—C6—S1 | 115.15 (13) |
| C20—C21—C17 | 113.14 (13) | C7—C6—H6A | 108.5 |
| C20—C21—H21A | 109.0 | C7—C6—H6B | 108.5 |
| C20—C21—H21B | 109.0 | H6A—C6—H6B | 107.5 |
| C21—C20—H20A | 109.2 | C1—C4—H4A | 109.5 |
| C21—C20—H20B | 109.2 | C1—C4—H4B | 109.5 |
| H20A—C20—H20B | 107.9 | C1—C4—H4C | 109.5 |
| C19—C20—C21 | 112.07 (14) | H4A—C4—H4B | 109.5 |
| C19—C20—H20A | 109.2 | H4A—C4—H4C | 109.5 |
| C19—C20—H20B | 109.2 | H4B—C4—H4C | 109.5 |
| C10—C9—C8 | 98.89 (12) | C1—C3—H3A | 109.5 |
| C10—C9—C13 | 112.53 (13) | C1—C3—H3B | 109.5 |
| C14—C9—C10 | 108.37 (13) | C1—C3—H3C | 109.5 |
| C14—C9—C8 | 115.37 (13) | H3A—C3—H3B | 109.5 |
| C14—C9—C13 | 111.59 (13) | H3A—C3—H3C | 109.5 |
| C13—C9—C8 | 109.50 (13) | H3B—C3—H3C | 109.5 |
| C10—C11—H11A | 110.9 | C1—C2—H2A | 109.5 |
| C10—C11—H11B | 110.9 | C1—C2—H2B | 109.5 |
| C10—C11—C12 | 104.17 (13) | C1—C2—H2C | 109.5 |
| H11A—C11—H11B | 108.9 | H2A—C2—H2B | 109.5 |
| C12—C11—H11A | 110.9 | H2A—C2—H2C | 109.5 |
| C12—C11—H11B | 110.9 | H2B—C2—H2C | 109.5 |
| O3—C8—C12—C11 | −88.38 (16) | C26—C18—C19—C23 | −10.2 (2) |
| O5—C24—C23—C19 | 166.22 (16) | C26—C18—C19—C20 | 171.80 (13) |
| O5—C24—C25—C26 | −136.63 (16) | C21—C20—C19—C18 | −52.86 (18) |
| O2—C7—C8—O3 | −159.55 (15) | C21—C20—C19—C23 | 129.03 (16) |
| O2—C7—C8—C9 | 81.2 (2) | C9—C10—C17—C16 | 59.62 (17) |
| O2—C7—C8—C12 | −34.5 (2) | C9—C10—C17—C21 | 179.23 (13) |
| O2—C7—C6—S1 | −0.9 (2) | C9—C10—C11—C12 | −31.87 (16) |
| O4—C15—C14—C9 | 76.44 (17) | C9—C8—C12—C11 | 26.18 (16) |
| C10—C17—C21—C20 | −170.47 (13) | C11—C10—C17—C16 | −177.02 (13) |
| C10—C9—C14—C15 | 52.98 (17) | C11—C10—C17—C21 | −57.42 (18) |
| C10—C9—C8—O3 | 72.69 (15) | C11—C10—C9—C14 | 168.13 (13) |
| C10—C9—C8—C7 | −166.78 (14) | C11—C10—C9—C8 | 47.53 (15) |
| C10—C9—C8—C12 | −44.62 (14) | C11—C10—C9—C13 | −67.98 (16) |
| C10—C11—C12—C8 | 2.91 (17) | C19—C18—C26—C25 | 41.50 (17) |
| C16—C18—C26—C25 | 158.52 (13) | C15—C16—C18—C22 | −71.13 (17) |
| C16—C18—C19—C23 | −128.32 (16) | C15—C16—C18—C26 | 52.16 (17) |
| C16—C18—C19—C20 | 53.64 (17) | C15—C16—C18—C19 | 171.40 (13) |
| C16—C17—C21—C20 | −51.99 (18) | C15—C16—C17—C10 | −51.38 (17) |
| C16—C15—C14—C9 | −47.76 (19) | C15—C16—C17—C21 | −170.32 (13) |
| C24—C23—C19—C18 | −3.6 (3) | C5—S1—C6—C7 | −95.53 (14) |
| C24—C23—C19—C20 | 174.33 (15) | C14—C9—C8—O3 | −42.61 (18) |
| C22—C18—C26—C25 | −75.79 (17) | C14—C9—C8—C7 | 77.93 (18) |
| C22—C18—C19—C23 | 109.31 (17) | C14—C9—C8—C12 | −159.91 (14) |
| C22—C18—C19—C20 | −68.74 (17) | C25—C24—C23—C19 | −14.5 (2) |
| C7—C8—C12—C11 | 147.50 (14) | C8—C7—C6—S1 | 178.95 (12) |
| C18—C16—C17—C10 | 174.74 (12) | C8—C9—C14—C15 | 162.72 (13) |
| C18—C16—C17—C21 | 55.80 (16) | C13—C9—C14—C15 | −71.47 (17) |
| C18—C16—C15—O4 | 55.85 (18) | C13—C9—C8—O3 | −169.48 (13) |
| C18—C16—C15—C14 | −179.71 (13) | C13—C9—C8—C7 | −48.95 (18) |
| C18—C26—C25—C24 | −59.02 (17) | C13—C9—C8—C12 | 73.22 (15) |
| C17—C10—C9—C14 | −60.92 (16) | C6—S1—C5—O1 | 9.35 (19) |
| C17—C10—C9—C8 | 178.48 (13) | C6—S1—C5—C1 | −168.80 (14) |
| C17—C10—C9—C13 | 62.97 (17) | C6—C7—C8—O3 | 20.62 (19) |
| C17—C10—C11—C12 | −159.84 (14) | C6—C7—C8—C9 | −98.66 (17) |
| C17—C16—C18—C22 | 62.48 (17) | C6—C7—C8—C12 | 145.63 (15) |
| C17—C16—C18—C26 | −174.24 (12) | C4—C1—C5—S1 | −38.1 (2) |
| C17—C16—C18—C19 | −55.00 (16) | C4—C1—C5—O1 | 143.8 (2) |
| C17—C16—C15—O4 | −77.53 (16) | C3—C1—C5—S1 | 79.96 (19) |
| C17—C16—C15—C14 | 46.91 (19) | C3—C1—C5—O1 | −98.1 (2) |
| C17—C21—C20—C19 | 50.36 (19) | C2—C1—C5—S1 | −161.1 (2) |
| C23—C24—C25—C26 | 44.08 (19) | C2—C1—C5—O1 | 20.9 (3) |
(S)-{2-[(8S,9S,10R,11S,13S,14S,17R)-11,17-Dihydroxy-10,13-dimethyl-3-oxo-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-17-yl]-2-oxoethyl} 2,2-dimethylpropanethioate (PL358) . Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O3—H3···O5i | 0.84 | 2.07 | 2.9021 (17) | 169 |
Symmetry code: (i) −x+1, y+1/2, −z+1/2.
(S)-{2-[(8S,9S,10R,11S,13S,14S,17R)-11,17-Dihydroxy-10,13-dimethyl-3-oxo-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-17-yl]-2-oxoethyl} 2,2-dimethylpropanethioate (SY20C174) . Crystal data
| C26H38O5S | Dx = 1.262 Mg m−3 |
| Mr = 462.62 | Cu Kα radiation, λ = 1.54178 Å |
| Orthorhombic, P212121 | Cell parameters from 6178 reflections |
| a = 6.0146 (2) Å | θ = 3.1–66.5° |
| b = 19.2817 (7) Å | µ = 1.46 mm−1 |
| c = 20.9887 (7) Å | T = 100 K |
| V = 2434.10 (14) Å3 | Plate, clear light colourless |
| Z = 4 | 0.18 × 0.06 × 0.05 mm |
| F(000) = 1000 |
(S)-{2-[(8S,9S,10R,11S,13S,14S,17R)-11,17-Dihydroxy-10,13-dimethyl-3-oxo-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-17-yl]-2-oxoethyl} 2,2-dimethylpropanethioate (SY20C174) . Data collection
| Bruker D8 Venture diffractometer | 4303 independent reflections |
| Radiation source: sealed X-ray tube, high brilliance microfocus sealed tube, Cu | 3803 reflections with I > 2σ(I) |
| QUAZAR MX multilayer optics monochromator | Rint = 0.102 |
| Detector resolution: 1024 x 1024 pixels mm-1 | θmax = 66.7°, θmin = 3.1° |
| φ and ω scans' | h = −7→6 |
| Absorption correction: multi-scan (SADABS; Krause et al., 2015) | k = −22→22 |
| Tmin = 0.707, Tmax = 0.862 | l = −24→25 |
| 30900 measured reflections |
(S)-{2-[(8S,9S,10R,11S,13S,14S,17R)-11,17-Dihydroxy-10,13-dimethyl-3-oxo-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-17-yl]-2-oxoethyl} 2,2-dimethylpropanethioate (SY20C174) . Refinement
| Refinement on F2 | Hydrogen site location: inferred from neighbouring sites |
| Least-squares matrix: full | H-atom parameters constrained |
| R[F2 > 2σ(F2)] = 0.055 | w = 1/[σ2(Fo2) + (0.052P)2 + 1.3292P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.130 | (Δ/σ)max < 0.001 |
| S = 1.07 | Δρmax = 0.26 e Å−3 |
| 4303 reflections | Δρmin = −0.39 e Å−3 |
| 329 parameters | Absolute structure: Flack x obtained from refinement |
| 16 restraints | Absolute structure parameter: 0.11 (4) |
| Primary atom site location: dual |
(S)-{2-[(8S,9S,10R,11S,13S,14S,17R)-11,17-Dihydroxy-10,13-dimethyl-3-oxo-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-17-yl]-2-oxoethyl} 2,2-dimethylpropanethioate (SY20C174) . Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refined as a 2-component inversion twin. |
(S)-{2-[(8S,9S,10R,11S,13S,14S,17R)-11,17-Dihydroxy-10,13-dimethyl-3-oxo-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-17-yl]-2-oxoethyl} 2,2-dimethylpropanethioate (SY20C174) . Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| O2 | 0.5899 (6) | 0.4360 (2) | 0.72379 (16) | 0.0542 (10) | |
| O3 | 0.2156 (5) | 0.50617 (16) | 0.61339 (15) | 0.0390 (7) | |
| H3 | 0.209393 | 0.545661 | 0.630189 | 0.059* | |
| O4 | 0.4523 (5) | 0.28363 (14) | 0.49153 (14) | 0.0367 (7) | |
| H4 | 0.369010 | 0.253012 | 0.507049 | 0.055* | |
| O5 | 0.2955 (7) | 0.36601 (17) | 0.17678 (14) | 0.0487 (9) | |
| C19 | 0.5824 (7) | 0.4099 (2) | 0.3207 (2) | 0.0332 (9) | |
| C23 | 0.5075 (8) | 0.4126 (2) | 0.2605 (2) | 0.0384 (10) | |
| H23 | 0.550048 | 0.451088 | 0.235126 | 0.046* | |
| C10 | 0.5508 (7) | 0.4744 (2) | 0.5179 (2) | 0.0324 (9) | |
| H10 | 0.415291 | 0.501122 | 0.505311 | 0.039* | |
| C26 | 0.2974 (7) | 0.3182 (2) | 0.34159 (18) | 0.0312 (9) | |
| H26A | 0.270014 | 0.275824 | 0.366990 | 0.037* | |
| H26B | 0.167597 | 0.349225 | 0.347184 | 0.037* | |
| C21 | 0.7010 (7) | 0.4915 (2) | 0.4084 (2) | 0.0346 (9) | |
| H21A | 0.832545 | 0.517167 | 0.424124 | 0.042* | |
| H21B | 0.578497 | 0.525229 | 0.402795 | 0.042* | |
| C18 | 0.5052 (7) | 0.3548 (2) | 0.36772 (19) | 0.0288 (9) | |
| C24 | 0.3664 (8) | 0.3607 (2) | 0.2319 (2) | 0.0406 (11) | |
| C15 | 0.3269 (7) | 0.34602 (19) | 0.48133 (19) | 0.0296 (9) | |
| H15 | 0.180184 | 0.332125 | 0.462922 | 0.036* | |
| C8 | 0.4270 (8) | 0.4760 (2) | 0.6258 (2) | 0.0386 (10) | |
| C17 | 0.6327 (7) | 0.4380 (2) | 0.4582 (2) | 0.0292 (9) | |
| H17 | 0.763081 | 0.408045 | 0.468950 | 0.035* | |
| C9 | 0.4772 (7) | 0.4237 (2) | 0.5707 (2) | 0.0322 (10) | |
| C22 | 0.6971 (8) | 0.3019 (2) | 0.3744 (2) | 0.0345 (9) | |
| H22A | 0.751711 | 0.289182 | 0.331957 | 0.052* | |
| H22B | 0.642942 | 0.260357 | 0.396306 | 0.052* | |
| H22C | 0.818350 | 0.322694 | 0.399048 | 0.052* | |
| C11 | 0.7052 (8) | 0.5257 (2) | 0.5512 (2) | 0.0387 (10) | |
| H11A | 0.698529 | 0.571722 | 0.530401 | 0.046* | |
| H11B | 0.860739 | 0.508887 | 0.550330 | 0.046* | |
| C20 | 0.7564 (7) | 0.4589 (2) | 0.3438 (2) | 0.0378 (11) | |
| H20A | 0.775716 | 0.496321 | 0.311979 | 0.045* | |
| H20B | 0.899444 | 0.433782 | 0.347301 | 0.045* | |
| C12 | 0.6174 (8) | 0.5295 (3) | 0.6202 (2) | 0.0448 (12) | |
| H12A | 0.561469 | 0.576745 | 0.629549 | 0.054* | |
| H12B | 0.737654 | 0.518446 | 0.650724 | 0.054* | |
| C6 | 0.2076 (9) | 0.4088 (2) | 0.7138 (2) | 0.0441 (11) | |
| H6AA | 0.196918 | 0.361138 | 0.696528 | 0.053* | 0.770 (4) |
| H6AB | 0.082226 | 0.435978 | 0.696182 | 0.053* | 0.770 (4) |
| H6BC | 0.120508 | 0.394023 | 0.676151 | 0.053* | 0.230 (4) |
| H6BD | 0.119404 | 0.443918 | 0.737153 | 0.053* | 0.230 (4) |
| C13 | 0.6669 (8) | 0.3752 (2) | 0.5918 (2) | 0.0381 (10) | |
| H13A | 0.721108 | 0.348936 | 0.554993 | 0.057* | |
| H13B | 0.611557 | 0.343021 | 0.624265 | 0.057* | |
| H13C | 0.788640 | 0.402879 | 0.609613 | 0.057* | |
| C25 | 0.3151 (8) | 0.2983 (2) | 0.27161 (19) | 0.0373 (10) | |
| H25A | 0.433983 | 0.263261 | 0.266140 | 0.045* | |
| H25B | 0.173190 | 0.277449 | 0.257299 | 0.045* | |
| C14 | 0.2796 (7) | 0.3837 (2) | 0.54478 (19) | 0.0303 (9) | |
| H14A | 0.154299 | 0.416247 | 0.538403 | 0.036* | |
| H14B | 0.232769 | 0.349021 | 0.576884 | 0.036* | |
| C7 | 0.4242 (9) | 0.4410 (2) | 0.6919 (2) | 0.0421 (11) | |
| C16 | 0.4439 (7) | 0.39282 (19) | 0.43159 (19) | 0.0270 (9) | |
| H16 | 0.326901 | 0.426958 | 0.418849 | 0.032* | |
| S1 | 0.1815 (3) | 0.40537 (8) | 0.79935 (7) | 0.0450 (5) | 0.770 (4) |
| O1 | 0.3483 (7) | 0.2850 (2) | 0.76836 (19) | 0.0451 (11) | 0.770 (4) |
| C1 | 0.3521 (12) | 0.3028 (4) | 0.8813 (3) | 0.0361 (18) | 0.770 (4) |
| C2 | 0.6090 (12) | 0.3076 (8) | 0.8878 (8) | 0.039 (3) | 0.770 (4) |
| H2A | 0.652836 | 0.294448 | 0.931111 | 0.058* | 0.770 (4) |
| H2B | 0.657117 | 0.355274 | 0.879249 | 0.058* | 0.770 (4) |
| H2C | 0.679074 | 0.276143 | 0.857122 | 0.058* | 0.770 (4) |
| C4 | 0.272 (2) | 0.2291 (5) | 0.8928 (5) | 0.058 (3) | 0.770 (4) |
| H4A | 0.339000 | 0.198135 | 0.861101 | 0.087* | 0.770 (4) |
| H4B | 0.110117 | 0.227401 | 0.889187 | 0.087* | 0.770 (4) |
| H4C | 0.316890 | 0.214251 | 0.935628 | 0.087* | 0.770 (4) |
| C3 | 0.2438 (11) | 0.3521 (3) | 0.9302 (3) | 0.0404 (15) | 0.770 (4) |
| H3A | 0.278848 | 0.336261 | 0.973456 | 0.061* | 0.770 (4) |
| H3B | 0.082257 | 0.352166 | 0.924197 | 0.061* | 0.770 (4) |
| H3C | 0.301585 | 0.399154 | 0.924132 | 0.061* | 0.770 (4) |
| C5 | 0.3022 (10) | 0.3230 (3) | 0.8119 (3) | 0.0332 (12) | 0.770 (4) |
| S1A | 0.2553 (10) | 0.3349 (3) | 0.7649 (3) | 0.055 (2)* | 0.230 (4) |
| O1A | 0.297 (2) | 0.4314 (7) | 0.8496 (6) | 0.042 (4)* | 0.230 (4) |
| C1A | 0.355 (4) | 0.3187 (10) | 0.8972 (9) | 0.023 (7)* | 0.230 (4) |
| C2A | 0.615 (5) | 0.309 (3) | 0.899 (3) | 0.06 (2)* | 0.230 (4) |
| H2AA | 0.683993 | 0.351239 | 0.915726 | 0.089* | 0.230 (4) |
| H2AB | 0.669427 | 0.300462 | 0.855557 | 0.089* | 0.230 (4) |
| H2AC | 0.652259 | 0.269623 | 0.926175 | 0.089* | 0.230 (4) |
| C3A | 0.283 (5) | 0.3463 (15) | 0.9609 (11) | 0.056 (8)* | 0.230 (4) |
| H3AA | 0.340463 | 0.316272 | 0.994771 | 0.084* | 0.230 (4) |
| H3AB | 0.120195 | 0.347280 | 0.962866 | 0.084* | 0.230 (4) |
| H3AC | 0.341046 | 0.393392 | 0.966587 | 0.084* | 0.230 (4) |
| C4A | 0.260 (6) | 0.2455 (13) | 0.8820 (15) | 0.038 (8)* | 0.230 (4) |
| H4AA | 0.330574 | 0.227462 | 0.843296 | 0.058* | 0.230 (4) |
| H4AB | 0.099349 | 0.248796 | 0.875531 | 0.058* | 0.230 (4) |
| H4AC | 0.291241 | 0.214194 | 0.917714 | 0.058* | 0.230 (4) |
| C5A | 0.310 (3) | 0.3695 (9) | 0.8412 (8) | 0.041 (5)* | 0.230 (4) |
(S)-{2-[(8S,9S,10R,11S,13S,14S,17R)-11,17-Dihydroxy-10,13-dimethyl-3-oxo-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-17-yl]-2-oxoethyl} 2,2-dimethylpropanethioate (SY20C174) . Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O2 | 0.057 (2) | 0.066 (2) | 0.0398 (19) | −0.0032 (19) | −0.0148 (17) | −0.0134 (17) |
| O3 | 0.0435 (18) | 0.0303 (16) | 0.0434 (18) | 0.0078 (14) | −0.0008 (14) | −0.0107 (13) |
| O4 | 0.056 (2) | 0.0208 (14) | 0.0328 (16) | −0.0010 (14) | 0.0056 (15) | 0.0014 (12) |
| O5 | 0.077 (2) | 0.0398 (18) | 0.0297 (17) | 0.0135 (18) | −0.0042 (17) | 0.0032 (13) |
| C19 | 0.034 (2) | 0.031 (2) | 0.035 (2) | 0.0098 (18) | 0.0098 (18) | 0.0075 (18) |
| C23 | 0.049 (3) | 0.030 (2) | 0.036 (2) | 0.009 (2) | 0.011 (2) | 0.008 (2) |
| C10 | 0.029 (2) | 0.025 (2) | 0.043 (2) | 0.0000 (17) | −0.0052 (19) | −0.0017 (19) |
| C26 | 0.033 (2) | 0.030 (2) | 0.031 (2) | −0.0031 (18) | 0.0027 (19) | 0.0025 (17) |
| C21 | 0.031 (2) | 0.026 (2) | 0.047 (3) | −0.0033 (18) | 0.000 (2) | 0.0037 (18) |
| C18 | 0.032 (2) | 0.026 (2) | 0.029 (2) | 0.0006 (17) | 0.0017 (17) | 0.0055 (17) |
| C24 | 0.053 (3) | 0.038 (2) | 0.031 (2) | 0.017 (2) | 0.007 (2) | 0.0035 (19) |
| C15 | 0.036 (2) | 0.022 (2) | 0.030 (2) | −0.0032 (18) | −0.0003 (18) | −0.0013 (16) |
| C8 | 0.040 (2) | 0.032 (2) | 0.044 (3) | 0.005 (2) | −0.005 (2) | −0.009 (2) |
| C17 | 0.026 (2) | 0.0237 (19) | 0.038 (2) | 0.0022 (16) | −0.0028 (17) | 0.0020 (17) |
| C9 | 0.033 (2) | 0.027 (2) | 0.037 (2) | 0.0015 (17) | −0.0020 (18) | −0.0033 (18) |
| C22 | 0.042 (2) | 0.028 (2) | 0.033 (2) | 0.003 (2) | 0.004 (2) | 0.0018 (17) |
| C11 | 0.036 (2) | 0.030 (2) | 0.050 (3) | −0.0019 (19) | −0.007 (2) | −0.006 (2) |
| C20 | 0.036 (2) | 0.031 (2) | 0.047 (3) | −0.0004 (18) | 0.008 (2) | 0.010 (2) |
| C12 | 0.046 (3) | 0.037 (2) | 0.051 (3) | −0.001 (2) | −0.007 (2) | −0.015 (2) |
| C6 | 0.058 (3) | 0.041 (3) | 0.033 (2) | 0.012 (2) | −0.002 (2) | 0.000 (2) |
| C13 | 0.043 (3) | 0.034 (2) | 0.037 (2) | 0.005 (2) | −0.006 (2) | −0.0056 (19) |
| C25 | 0.044 (2) | 0.036 (2) | 0.032 (2) | 0.004 (2) | −0.002 (2) | 0.0002 (18) |
| C14 | 0.032 (2) | 0.025 (2) | 0.034 (2) | −0.0018 (17) | 0.0041 (17) | 0.0008 (17) |
| C7 | 0.052 (3) | 0.038 (2) | 0.037 (3) | 0.004 (2) | −0.004 (2) | −0.016 (2) |
| C16 | 0.026 (2) | 0.0223 (19) | 0.033 (2) | 0.0016 (16) | −0.0008 (17) | 0.0049 (16) |
| S1 | 0.0628 (10) | 0.0413 (9) | 0.0309 (8) | 0.0206 (8) | 0.0025 (7) | −0.0008 (6) |
| O1 | 0.052 (3) | 0.042 (2) | 0.042 (2) | 0.007 (2) | −0.015 (2) | −0.013 (2) |
| C1 | 0.041 (4) | 0.031 (4) | 0.036 (4) | 0.008 (3) | −0.001 (3) | −0.008 (3) |
| C2 | 0.033 (5) | 0.044 (6) | 0.039 (5) | 0.005 (3) | −0.007 (3) | −0.007 (4) |
| C4 | 0.071 (6) | 0.037 (4) | 0.066 (6) | 0.001 (4) | −0.013 (5) | 0.012 (4) |
| C3 | 0.041 (4) | 0.043 (4) | 0.037 (4) | 0.006 (3) | −0.001 (3) | −0.001 (3) |
| C5 | 0.032 (3) | 0.030 (3) | 0.037 (3) | 0.001 (2) | 0.000 (2) | −0.004 (3) |
(S)-{2-[(8S,9S,10R,11S,13S,14S,17R)-11,17-Dihydroxy-10,13-dimethyl-3-oxo-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-17-yl]-2-oxoethyl} 2,2-dimethylpropanethioate (SY20C174) . Geometric parameters (Å, º)
| O2—C7 | 1.204 (6) | C6—H6AA | 0.9900 |
| O3—H3 | 0.8400 | C6—H6AB | 0.9900 |
| O3—C8 | 1.422 (6) | C6—H6BC | 0.9900 |
| O4—H4 | 0.8400 | C6—H6BD | 0.9900 |
| O4—C15 | 1.436 (5) | C6—C7 | 1.514 (7) |
| O5—C24 | 1.238 (5) | C6—S1 | 1.804 (4) |
| C19—C23 | 1.341 (6) | C6—S1A | 1.807 (5) |
| C19—C18 | 1.523 (6) | C13—H13A | 0.9800 |
| C19—C20 | 1.491 (6) | C13—H13B | 0.9800 |
| C23—H23 | 0.9500 | C13—H13C | 0.9800 |
| C23—C24 | 1.443 (7) | C25—H25A | 0.9900 |
| C10—H10 | 1.0000 | C25—H25B | 0.9900 |
| C10—C17 | 1.519 (6) | C14—H14A | 0.9900 |
| C10—C9 | 1.542 (6) | C14—H14B | 0.9900 |
| C10—C11 | 1.525 (6) | C16—H16 | 1.0000 |
| C26—H26A | 0.9900 | S1—C5 | 1.767 (6) |
| C26—H26B | 0.9900 | O1—C5 | 1.203 (6) |
| C26—C18 | 1.536 (6) | C1—C2 | 1.554 (9) |
| C26—C25 | 1.522 (5) | C1—C4 | 1.519 (11) |
| C21—H21A | 0.9900 | C1—C3 | 1.543 (9) |
| C21—H21B | 0.9900 | C1—C5 | 1.538 (9) |
| C21—C17 | 1.524 (6) | C2—H2A | 0.9800 |
| C21—C20 | 1.531 (6) | C2—H2B | 0.9800 |
| C18—C22 | 1.547 (6) | C2—H2C | 0.9800 |
| C18—C16 | 1.572 (6) | C4—H4A | 0.9800 |
| C24—C25 | 1.495 (6) | C4—H4B | 0.9800 |
| C15—H15 | 1.0000 | C4—H4C | 0.9800 |
| C15—C14 | 1.543 (6) | C3—H3A | 0.9800 |
| C15—C16 | 1.549 (5) | C3—H3B | 0.9800 |
| C8—C9 | 1.565 (6) | C3—H3C | 0.9800 |
| C8—C12 | 1.547 (7) | S1A—C5A | 1.767 (16) |
| C8—C7 | 1.543 (7) | O1A—C5A | 1.209 (17) |
| C17—H17 | 1.0000 | C1A—C2A | 1.57 (2) |
| C17—C16 | 1.536 (6) | C1A—C3A | 1.50 (2) |
| C9—C13 | 1.540 (6) | C1A—C4A | 1.55 (2) |
| C9—C14 | 1.518 (6) | C1A—C5A | 1.553 (19) |
| C22—H22A | 0.9800 | C2A—H2AA | 0.9800 |
| C22—H22B | 0.9800 | C2A—H2AB | 0.9800 |
| C22—H22C | 0.9800 | C2A—H2AC | 0.9800 |
| C11—H11A | 0.9900 | C3A—H3AA | 0.9800 |
| C11—H11B | 0.9900 | C3A—H3AB | 0.9800 |
| C11—C12 | 1.544 (7) | C3A—H3AC | 0.9800 |
| C20—H20A | 0.9900 | C4A—H4AA | 0.9800 |
| C20—H20B | 0.9900 | C4A—H4AB | 0.9800 |
| C12—H12A | 0.9900 | C4A—H4AC | 0.9800 |
| C12—H12B | 0.9900 | ||
| C8—O3—H3 | 109.5 | C7—C6—S1 | 113.1 (4) |
| C15—O4—H4 | 109.5 | C7—C6—S1A | 111.5 (4) |
| C23—C19—C18 | 122.2 (4) | S1—C6—H6AA | 109.0 |
| C23—C19—C20 | 121.2 (4) | S1—C6—H6AB | 109.0 |
| C20—C19—C18 | 116.4 (4) | S1A—C6—H6BC | 109.3 |
| C19—C23—H23 | 117.9 | S1A—C6—H6BD | 109.3 |
| C19—C23—C24 | 124.3 (4) | C9—C13—H13A | 109.5 |
| C24—C23—H23 | 117.9 | C9—C13—H13B | 109.5 |
| C17—C10—H10 | 106.5 | C9—C13—H13C | 109.5 |
| C17—C10—C9 | 113.1 (3) | H13A—C13—H13B | 109.5 |
| C17—C10—C11 | 118.6 (4) | H13A—C13—H13C | 109.5 |
| C9—C10—H10 | 106.5 | H13B—C13—H13C | 109.5 |
| C11—C10—H10 | 106.5 | C26—C25—H25A | 109.6 |
| C11—C10—C9 | 104.9 (4) | C26—C25—H25B | 109.6 |
| H26A—C26—H26B | 107.7 | C24—C25—C26 | 110.4 (4) |
| C18—C26—H26A | 108.8 | C24—C25—H25A | 109.6 |
| C18—C26—H26B | 108.8 | C24—C25—H25B | 109.6 |
| C25—C26—H26A | 108.8 | H25A—C25—H25B | 108.1 |
| C25—C26—H26B | 108.8 | C15—C14—H14A | 108.8 |
| C25—C26—C18 | 113.8 (4) | C15—C14—H14B | 108.8 |
| H21A—C21—H21B | 107.8 | C9—C14—C15 | 113.8 (3) |
| C17—C21—H21A | 109.0 | C9—C14—H14A | 108.8 |
| C17—C21—H21B | 109.0 | C9—C14—H14B | 108.8 |
| C17—C21—C20 | 112.8 (3) | H14A—C14—H14B | 107.7 |
| C20—C21—H21A | 109.0 | O2—C7—C8 | 121.7 (5) |
| C20—C21—H21B | 109.0 | O2—C7—C6 | 120.7 (5) |
| C19—C18—C26 | 109.7 (3) | C6—C7—C8 | 117.5 (4) |
| C19—C18—C22 | 106.9 (3) | C18—C16—H16 | 104.1 |
| C19—C18—C16 | 107.4 (3) | C15—C16—C18 | 114.2 (3) |
| C26—C18—C22 | 109.7 (3) | C15—C16—H16 | 104.1 |
| C26—C18—C16 | 109.1 (3) | C17—C16—C18 | 113.6 (3) |
| C22—C18—C16 | 113.9 (3) | C17—C16—C15 | 114.9 (3) |
| O5—C24—C23 | 122.3 (4) | C17—C16—H16 | 104.1 |
| O5—C24—C25 | 121.1 (5) | C5—S1—C6 | 98.4 (2) |
| C23—C24—C25 | 116.6 (4) | C4—C1—C2 | 110.8 (8) |
| O4—C15—H15 | 107.3 | C4—C1—C3 | 109.7 (6) |
| O4—C15—C14 | 111.2 (3) | C4—C1—C5 | 109.0 (7) |
| O4—C15—C16 | 110.5 (3) | C3—C1—C2 | 109.0 (9) |
| C14—C15—H15 | 107.3 | C5—C1—C2 | 105.2 (7) |
| C14—C15—C16 | 113.0 (3) | C5—C1—C3 | 113.1 (5) |
| C16—C15—H15 | 107.3 | C1—C2—H2A | 109.5 |
| O3—C8—C9 | 107.5 (3) | C1—C2—H2B | 109.5 |
| O3—C8—C12 | 112.0 (4) | C1—C2—H2C | 109.5 |
| O3—C8—C7 | 109.5 (4) | H2A—C2—H2B | 109.5 |
| C12—C8—C9 | 103.3 (4) | H2A—C2—H2C | 109.5 |
| C7—C8—C9 | 112.7 (4) | H2B—C2—H2C | 109.5 |
| C7—C8—C12 | 111.7 (4) | C1—C4—H4A | 109.5 |
| C10—C17—C21 | 109.9 (3) | C1—C4—H4B | 109.5 |
| C10—C17—H17 | 109.5 | C1—C4—H4C | 109.5 |
| C10—C17—C16 | 108.8 (3) | H4A—C4—H4B | 109.5 |
| C21—C17—H17 | 109.5 | H4A—C4—H4C | 109.5 |
| C21—C17—C16 | 109.5 (3) | H4B—C4—H4C | 109.5 |
| C16—C17—H17 | 109.5 | C1—C3—H3A | 109.5 |
| C10—C9—C8 | 100.3 (3) | C1—C3—H3B | 109.5 |
| C13—C9—C10 | 112.3 (4) | C1—C3—H3C | 109.5 |
| C13—C9—C8 | 108.7 (3) | H3A—C3—H3B | 109.5 |
| C14—C9—C10 | 106.9 (3) | H3A—C3—H3C | 109.5 |
| C14—C9—C8 | 116.2 (4) | H3B—C3—H3C | 109.5 |
| C14—C9—C13 | 112.0 (3) | O1—C5—S1 | 121.9 (4) |
| C18—C22—H22A | 109.5 | O1—C5—C1 | 121.4 (5) |
| C18—C22—H22B | 109.5 | C1—C5—S1 | 116.6 (4) |
| C18—C22—H22C | 109.5 | C5A—S1A—C6 | 105.7 (6) |
| H22A—C22—H22B | 109.5 | C3A—C1A—C2A | 108 (3) |
| H22A—C22—H22C | 109.5 | C3A—C1A—C4A | 113.4 (19) |
| H22B—C22—H22C | 109.5 | C3A—C1A—C5A | 113.4 (17) |
| C10—C11—H11A | 110.8 | C4A—C1A—C2A | 105 (3) |
| C10—C11—H11B | 110.8 | C5A—C1A—C2A | 105 (2) |
| C10—C11—C12 | 104.6 (4) | C5A—C1A—C4A | 110.7 (17) |
| H11A—C11—H11B | 108.9 | C1A—C2A—H2AA | 109.5 |
| C12—C11—H11A | 110.8 | C1A—C2A—H2AB | 109.5 |
| C12—C11—H11B | 110.8 | C1A—C2A—H2AC | 109.5 |
| C19—C20—C21 | 113.3 (4) | H2AA—C2A—H2AB | 109.5 |
| C19—C20—H20A | 108.9 | H2AA—C2A—H2AC | 109.5 |
| C19—C20—H20B | 108.9 | H2AB—C2A—H2AC | 109.5 |
| C21—C20—H20A | 108.9 | C1A—C3A—H3AA | 109.5 |
| C21—C20—H20B | 108.9 | C1A—C3A—H3AB | 109.5 |
| H20A—C20—H20B | 107.7 | C1A—C3A—H3AC | 109.5 |
| C8—C12—H12A | 110.3 | H3AA—C3A—H3AB | 109.5 |
| C8—C12—H12B | 110.3 | H3AA—C3A—H3AC | 109.5 |
| C11—C12—C8 | 107.0 (4) | H3AB—C3A—H3AC | 109.5 |
| C11—C12—H12A | 110.3 | C1A—C4A—H4AA | 109.5 |
| C11—C12—H12B | 110.3 | C1A—C4A—H4AB | 109.5 |
| H12A—C12—H12B | 108.6 | C1A—C4A—H4AC | 109.5 |
| H6AA—C6—H6AB | 107.8 | H4AA—C4A—H4AB | 109.5 |
| H6BC—C6—H6BD | 108.0 | H4AA—C4A—H4AC | 109.5 |
| C7—C6—H6AA | 109.0 | H4AB—C4A—H4AC | 109.5 |
| C7—C6—H6AB | 109.0 | O1A—C5A—S1A | 119.5 (13) |
| C7—C6—H6BC | 109.3 | O1A—C5A—C1A | 121.7 (15) |
| C7—C6—H6BD | 109.3 | C1A—C5A—S1A | 118.6 (12) |
| O3—C8—C9—C10 | 78.4 (4) | C11—C10—C9—C13 | −71.3 (4) |
| O3—C8—C9—C13 | −163.7 (4) | C11—C10—C9—C14 | 165.5 (3) |
| O3—C8—C9—C14 | −36.2 (5) | C20—C19—C23—C24 | 169.9 (4) |
| O3—C8—C12—C11 | −92.8 (4) | C20—C19—C18—C26 | 169.2 (4) |
| O3—C8—C7—O2 | −150.6 (4) | C20—C19—C18—C22 | −72.0 (4) |
| O3—C8—C7—C6 | 33.2 (5) | C20—C19—C18—C16 | 50.7 (5) |
| O4—C15—C14—C9 | 77.5 (4) | C20—C21—C17—C10 | −172.5 (3) |
| O4—C15—C16—C18 | 51.7 (4) | C20—C21—C17—C16 | −53.0 (5) |
| O4—C15—C16—C17 | −82.2 (4) | C12—C8—C9—C10 | −40.1 (4) |
| O5—C24—C25—C26 | −147.6 (4) | C12—C8—C9—C13 | 77.8 (4) |
| C19—C23—C24—O5 | 177.9 (4) | C12—C8—C9—C14 | −154.8 (4) |
| C19—C23—C24—C25 | −4.1 (7) | C12—C8—C7—O2 | −25.9 (6) |
| C19—C18—C16—C15 | 170.8 (3) | C12—C8—C7—C6 | 157.8 (4) |
| C19—C18—C16—C17 | −54.7 (4) | C6—S1—C5—O1 | −8.4 (6) |
| C23—C19—C18—C26 | −14.2 (5) | C6—S1—C5—C1 | 169.9 (5) |
| C23—C19—C18—C22 | 104.7 (5) | C6—S1A—C5A—O1A | 4 (2) |
| C23—C19—C18—C16 | −132.7 (4) | C6—S1A—C5A—C1A | 179.1 (15) |
| C23—C19—C20—C21 | 133.4 (4) | C13—C9—C14—C15 | −67.5 (5) |
| C23—C24—C25—C26 | 34.4 (6) | C25—C26—C18—C19 | 45.3 (5) |
| C10—C17—C16—C18 | 177.5 (3) | C25—C26—C18—C22 | −71.9 (4) |
| C10—C17—C16—C15 | −48.4 (4) | C25—C26—C18—C16 | 162.7 (3) |
| C10—C9—C14—C15 | 55.9 (4) | C14—C15—C16—C18 | 177.0 (3) |
| C10—C11—C12—C8 | 4.4 (5) | C14—C15—C16—C17 | 43.2 (5) |
| C26—C18—C16—C15 | 52.0 (4) | C7—C8—C9—C10 | −160.8 (4) |
| C26—C18—C16—C17 | −173.5 (3) | C7—C8—C9—C13 | −42.9 (5) |
| C21—C17—C16—C18 | 57.3 (4) | C7—C8—C9—C14 | 84.5 (5) |
| C21—C17—C16—C15 | −168.5 (3) | C7—C8—C12—C11 | 143.9 (4) |
| C18—C19—C23—C24 | −6.6 (7) | C7—C6—S1—C5 | −90.2 (4) |
| C18—C19—C20—C21 | −49.9 (5) | C7—C6—S1A—C5A | 81.0 (8) |
| C18—C26—C25—C24 | −56.1 (5) | C16—C15—C14—C9 | −47.5 (5) |
| C8—C9—C14—C15 | 166.8 (3) | S1—C6—C7—O2 | 30.1 (6) |
| C17—C10—C9—C8 | 174.8 (3) | S1—C6—C7—C8 | −153.6 (3) |
| C17—C10—C9—C13 | 59.5 (5) | C2—C1—C5—S1 | −106.9 (8) |
| C17—C10—C9—C14 | −63.7 (4) | C2—C1—C5—O1 | 71.4 (10) |
| C17—C10—C11—C12 | −158.0 (4) | C4—C1—C5—S1 | 134.2 (6) |
| C17—C21—C20—C19 | 49.7 (5) | C4—C1—C5—O1 | −47.5 (9) |
| C9—C10—C17—C21 | 179.7 (3) | C3—C1—C5—S1 | 11.9 (7) |
| C9—C10—C17—C16 | 59.8 (4) | C3—C1—C5—O1 | −169.8 (6) |
| C9—C10—C11—C12 | −30.4 (4) | S1A—C6—C7—O2 | −27.2 (6) |
| C9—C8—C12—C11 | 22.6 (5) | S1A—C6—C7—C8 | 149.1 (4) |
| C9—C8—C7—O2 | 89.9 (5) | C2A—C1A—C5A—S1A | 92 (3) |
| C9—C8—C7—C6 | −86.4 (5) | C2A—C1A—C5A—O1A | −93 (3) |
| C22—C18—C16—C15 | −70.9 (4) | C3A—C1A—C5A—S1A | −149.7 (18) |
| C22—C18—C16—C17 | 63.5 (4) | C3A—C1A—C5A—O1A | 25 (3) |
| C11—C10—C17—C21 | −56.7 (5) | C4A—C1A—C5A—S1A | −21 (2) |
| C11—C10—C17—C16 | −176.6 (4) | C4A—C1A—C5A—O1A | 154 (2) |
| C11—C10—C9—C8 | 44.0 (4) |
(S)-{2-[(8S,9S,10R,11S,13S,14S,17R)-11,17-Dihydroxy-10,13-dimethyl-3-oxo-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-17-yl]-2-oxoethyl} 2,2-dimethylpropanethioate (SY20C174) . Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O3—H3···O5i | 0.84 | 1.96 | 2.802 (4) | 175 |
Symmetry code: (i) −x+1/2, −y+1, z+1/2.
References
- Bircher, A. J., Thürlimann, W., Hunziker, T., Pasche-Koo, F., Hunziker, N., Perrenoud, D., Elsner, P. & Schultheiss, R. (1995). Dermatology, 191, 109–114. [DOI] [PubMed]
- Bouley, E. (2013). Patent EP 2853528.
- Boultif, A. & Louër, D. (2004). J. Appl. Cryst. 37, 724–731.
- Bruker (2016). APEX3 and SAINT. Bruker AXS, Inc., Madison, Wisconsin, USA.
- Burden, A. D. & Beck, M. H. (1992). Br. J. Dermatol. 127, 497–500. [DOI] [PubMed]
- Cremer, D. & Pople, J. A. (1975). J. Am. Chem. Soc. 97, 1354–1358.
- Davies, J. E., Kellet, D. N., Staniforth, M. V., Torossian, R. & Grouhel, A. (1981). Arzneimittelforschung, 31, 453–459. [PubMed]
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
- Jezequel, J., Becuwe, B., Daniel, C. & Geraudel, O. (1979). J. Fr. Otorhinolaryngol. Audiophonol. Chir. Maxillofac. 28, 65–6, 68. [PubMed]
- Krause, L., Herbst-Irmer, R., Sheldrick, G. M. & Stalke, D. (2015). J. Appl. Cryst. 48, 3–10. [DOI] [PMC free article] [PubMed]
- Lauerma, A. I. (1991). Contact Dermatitis, 24, 123–130. [DOI] [PubMed]
- Le Bail, A., Duroy, H. & Fourquet, J. L. (1988). Mater. Res. Bull. 23, 447–452.
- Liddle, G. W. (1960). J. Clin. Endocrinol. Metab. 20, 1539–1560. [DOI] [PubMed]
- Macrae, C. F., Sovago, I., Cottrell, S. J., Galek, P. T. A., McCabe, P., Pidcock, E., Platings, M., Shields, G. P., Stevens, J. S., Towler, M. & Wood, P. A. (2020). J. Appl. Cryst. 53, 226–235. [DOI] [PMC free article] [PubMed]
- Mazauric, F. X. & Alligier, B. (1978). J. Fr. Otorhinolaryngol. Audiophonol. Chir. Maxillofac. 27, 721–723. [PubMed]
- Nugent, C. A., Macdiarmid, W. D., Nelson, A. R. & Tyler, F. H. (1963). J. Clin. Endocrinol. Metab. 23, 684–693. [DOI] [PubMed]
- Palmer, D. C. (2015). Z. Kristallogr. Cryst. Mater. 230, 9–10.
- PANalytical (2011). X’Pert Data Collector and X’Pert HighScore Plus. PANalytical BV, Almelo, The Netherlands.
- Parsons, S., Flack, H. D. & Wagner, T. (2013). Acta Cryst. B69, 249–259. [DOI] [PMC free article] [PubMed]
- Petříček, V., Dušek, M. & Palatinus, L. (2014). Z. Kristallogr. 229, 345–352.
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Uphill, P. F. (1981). Arzneimittelforschung, 31, 459–462.
- Visser, J. W. (1969). J. Appl. Cryst. 2, 89–95.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) PL358, SY20C174, New_Global_Publ_Block. DOI: 10.1107/S2056989021007167/dj2019sup1.cif
Structure factors: contains datablock(s) PL358. DOI: 10.1107/S2056989021007167/dj2019PL358sup2.hkl
Supporting information file. DOI: 10.1107/S2056989021007167/dj2019PL358sup4.cdx
Structure factors: contains datablock(s) SY20C174. DOI: 10.1107/S2056989021007167/dj2019SY20C174sup3.hkl
Supporting information file. DOI: 10.1107/S2056989021007167/dj2019SY20C174sup5.cdx
Additional supporting information: crystallographic information; 3D view; checkCIF report