Abstract
Though peer influence is a strong predictor of adolescents’ risk-taking behaviors, not all adolescents are susceptible to their peer group. 136 adolescents (Mage=12.79 years) completed an fMRI scan, measures of perceived peer group norms and engagement in risky behavior. Ventral striatum sensitivity when anticipating social rewards and avoiding social punishments significantly moderated the association between negative perceived peer norms and adolescents’ own risk behaviors. Negative perceived peer norms were associated with increased risky behavior only for those with high ventral striatum sensitivity; adolescents with low ventral striatum sensitivity were resilient to negative peer norms, showing low risk taking regardless of peer context. Findings provide a novel contribution to the study of peer influence susceptibility.
Keywords: adolescence, influence, peers, risk taking, fMRI
Adolescence is a time of heightened vulnerability for risk-taking behavior that gives rise to later substance abuse, substance dependence, related health risk behaviors, and has critical implications for morbidity and mortality throughout the lifespan (see Telzer et al., 2017). Perceptions of peers’ risk behavior strongly predicts adolescents’ initiation and escalation of risk-taking behaviors (Brechwald & Prinstein, 2011; Prentice, 2008). Yet, substantial work suggests notable variability in adolescents’ peer influence susceptibility; for some, perceptions of peers’ engagement in substance use is a strong predictor of adolescents’ own substance use trajectories, while other adolescents are remarkably resilient to peer socialization pressures (Brechwald & Prinstein, 2011). However, it remains unclear which underlying processes make some adolescents more susceptible to peer influence. Unfortunately, individuals, and perhaps especially adolescents, are remarkably unaware of, and unable to report the implicit processes that contribute to conformity (Nisbett & Wilson, 1977). Self-reported measures of susceptibility may thus be limited. Therefore, for effective prospective identification of at-risk youth we need a process-based understanding of peer influence that does not rely solely on self-report. The psychological processes underlying individual differences in susceptibility may be best understood through the examination of neural processes.
Peer Influence Susceptibility in Adolescence
Peer influences, whether prosocial or deviant, may be especially salient during adolescence, when a reorientation from parental to peer contexts occurs (Blakemore & Mills, 2014; Nelson, Jarcho, & Guyer., 2016). Increased time spent with peers, as well as a greater emphasis on gaining peer acceptance, may place adolescents at risk for conforming to the norms and behaviors of their peer group in an effort to enhance their social belonging (i.e., “fitting in”; Do, Prinstein, & Telzer, in press). Perceptions of negative peer norms (e.g., peers who encourage deviant, risky, behaviors) can influence adolescents’ attitudes regarding the acceptability of such behaviors, thereby encouraging engagement in health risk behaviors. As such, adolescence is generally a time of heightened susceptibility to peer influence. For instance, experimental studies have shown that compared to children and adults, adolescents engage in more risk taking in the presence of peers versus alone (e.g., Gardner & Steinberg, 2005) and tend to conform to the attitudes of their peers about risky behaviors (Cohen & Prinstein, 2005; Knoll, Magis-Weinberg, Speekenbrink, & Blakemore, 2015). Moreover, many prospective longitudinal studies have demonstrated that in addition to adolescents’ tendency to befriend peers who engage in similar levels of risk taking (i.e., selection effects), adolescents’ perception that peers are engaging in health risk behaviors significantly predicts adolescents’ later engagement in health risk behaviors via socialization effects (Brechwald & Prinstein, 2011). Taken together, a large body of research shows that adolescents are generally susceptible to negative peer influence.
However, while some adolescents are indeed likely to emulate peers’ risk-taking behaviors, others are resilient to conformity pressures (Brechwald & Prinstein, 2011; Steinberg & Monahan, 2007). For instance, research using both self-reported and performance-based approaches to study peer influence susceptibility have shown that susceptibility is a normally distributed construct (Prinstein, Brechwald, & Cohen, 2011; Widman et al., 2016), suggesting approximately equal proportions of youth who are extremely high or low in susceptibility. This variability in adolescents’ susceptibility prospectively predicts peer influence effects. For adolescents who are high in susceptibility (i.e., greater changes in behavior following exposure to experimentally manipulated peer norms), perceptions of friends’ risky behavior are associated with adolescents’ risky behavior. In contrast, adolescents who are low in susceptibility do not show peer-related changes in risky behavior over time (Prinstein et al., 2011; Teunissen et al., 2016). Moreover, late adolescents who self-report high susceptibility show a stronger link between perceived peer norms and their own drinking behavior compared to their low susceptibility peers (DiGuiseppi et al., 2018). Taken together, the perceived norms of peers can play a powerful role in predicting adolescents’ risky behavior, but only for those who are highly susceptible.
Sensitivity to Social Rewards and Punishments
The Social Reward/Social Punishment Framework (Falk, Way, & Jasinka, 2012) suggests two pathways by which susceptibility to normative social influence occurs. One pathway occurs via the drive to pursue social rewards conferred by conforming, and the second pathway occurs via the avoidance of social punishment, including social exclusion. Individual differences in adolescents’ propensity to seek social rewards (e.g., approval by others) and avoid social punishment (e.g., disapproval by others) may be one factor that differentiates adolescents who are susceptible to peer influence and adolescents who are not. A stronger drive to gain social rewards and avoid social punishments may increase conformity to peer group norms, such that youth engage in the behaviors that they think are encouraged by the peer group as a means to attain peer approval and avoid peer rejection (Do, Prinstein, & Telzer, in press). Importantly, social rewards and punishments need not be directly experienced but only anticipated to elicit social conformity (Falk, Way, & Jasinka, 2012). Indeed, the mere threat of peer rejection is enough to limit group deviance, and increase adherence to social norms (Juvonen & Gross, 2005). Thus, approaching rewards and avoiding punishments are key motivational drivers and are reinforced via approval/acceptance and disapproval/rejection from peers. Peer influence susceptibility is therefore likely driven both by the motivation to affiliate, be accepted, and maintain a positive self-concept as well to avoid exclusion and negative self-concept in the peer group (Falk, Way, & Jasinka, 2012).
Given the limited efficacy of self-reports for understanding implicit processes of conformity (Nisbett & Wilson, 1977), examining neurobiological sensitivity may be a promising avenue for probing individual differences in susceptibility to peer influence. The ventral striatum (VS) is a key brain region central to incentive-based, motivated behaviors (Smith et al., 2011) and is implicated in encoding both appetitive (i.e., rewarding, approach-related) and aversive (i.e., punishing, avoidance-related) social cues in the environment (Kohls et al., 2013). Across rodents, non-human primates, and humans, increases in dopamine signaling peak during adolescence (Wahlstrom et al., 2010), influencing motivated behaviors that are altered in adolescence (Padmanabhan & Luna, 2014). For instance, adolescents exhibit greater ventral striatum activation than children and adults when receiving primary (e.g., sweet liquid; Galván & McGlennen, 2013) and secondary rewards (e.g., money; Galván et al., 2006; Van Leijenhorst et al., 2010; Schreuders et al., 2018), as well as when anticipating social rewards and avoiding social punishments (e.g., social acceptance or rejection from peers; Chein, Albert, O’Brien, Uckert, & Steinberg, 2011; Guyer, McClure-Tone, Shiffrin, Pine, & Nelson, 2009; Somerville, Hare, & Casey, 2011; 32 33). Thus, conformity to peers may be driven, in part, by a desire to attain social rewards and avoid social punishments, which may be substantiated via alterations in ventral striatum sensitivity.
Preliminary evidence suggests that individual differences in ventral striatum activation interact with the social context to predict adolescent behavior. While traditionally viewed as a vulnerability biomarker, heightened ventral striatum activation can also promote resilience depending on the social context (see Telzer, 2016). Indeed, the ventral striatum processes the motivational salience of both positive and negative contexts (Levita et al., 2009; Lindquist et al., 2016), including anticipated positive outcomes like rewards as well as avoidance of negative and aversive outcomes such as punishments (Kohls et al., 2013). For instance, heightened ventral striatum activation in a risky context, particularly in the presence of peers or social rewards, is associated with compromised cognitive control and increases in risk taking (Chein et al., 2011; Galván, Hare, Voss, Glover, & Casey, 2007; Qu et al., 2015; Perino, Miernicki, & Telzer, 2016; Telzer, Fuligni, Lieberman, & Galván, 2013). However, heightened ventral striatum activation in positive, prosocial contexts is associated with decreases in risk taking (Telzer et al., 2013), underscoring that individual differences in ventral striatum sensitivity interact with the social context to promote or impede adolescent adjustment.
Current Study
We examined how variability in VS activation when anticipating the receipt of social rewards and the avoidance of social punishments moderates the link between deviant peer group norms and adolescent risk taking. This approach diverges from prior research that has either examined the neural correlates of social influence itself (e.g., Cascio, O’Donnell, Bayer, Tinney, & Falk, 2015; Nook & Zaki, 2015; Welborn et al., 2016) or tested how VS activation predicts concurrent or future risk taking and peer conformity effects (e.g., Cascio, Carp, et al., 2015; Qu et al., 215), which assumes that neurobiological sensitivity applies equally to all adolescents and fails to consider that the environment may determine if, and how, neurobiological sensitivity influences developmental outcomes. Thus, considering neurobiological sensitivity as a moderator of social context is a promising avenue for identification of the most susceptible and at-risk youth (Schriber & Guyer, 2016).
In the current study, adolescents completed the Social Incentive Delay (SID) task during an fMRI scan to measure VS sensitivity when anticipating the receipt of social rewards and avoidance of social punishments, as well as measures of perceived peer group norms and self-reported risk-taking behaviors. Adolescents rated the extent to which the peer norms they encountered in daily life encouraged prosocial activities (e.g., try hard in school, volunteer for a good cause) and deviant activities (e.g., drink alcohol, lie to adults). We focused on adolescents’ perceptions of peers’ behaviors since perceptions of friends’ risk behaviors are a stronger predictor of adolescents’ own risk behavior compared to actual peer behavior (Iannotti & Bush, 1992; Slagt, Dubas, Deković, Haselager, & van Aken, 2015; DiGuiseppi et al., 2018). Although prior research has measured both adolescents’ perceptions of their friends’ behavior, as well as friends’ own reported behavior (Prinstein & Giletta, 2016), the extent literature suggests that peer norms need only be perceived to influence adolescents’ behavior.
We hypothesized that adolescents who perceive more deviant relative to prosocial peer norms would show heightened risk taking, but this would be moderated by neurobiological sensitivity to social rewards and punishments. In particular, we predicted that adolescents who perceived that their peers were engaging in relatively more risky behaviors (e.g., deviant peer norms) than positive behaviors (e.g., prosocial peer norms) would be particularly vulnerable to risk taking, but only if those adolescents had heightened VS activation when anticipating receiving social rewards and avoiding social punishments. For these same neurobiologically sensitive youth, we hypothesized that when they perceived that their peers engaged in relatively more positive than deviant behaviors, they would be buffered from risk taking. We additionally predicted that adolescents with low VS activation would be resilient to peer influence effects, insofar as those adolescents would not be motivated to adhere to peer norms, whatever peer context they found themselves in. By measuring VS activation when anticipating social rewards and punishments, we were able to test whether peer influence susceptibility is characterized by a drive to obtain social rewards, a heightened motivation to avoid social punishments, or both.
In addition to examining neurobiological sensitivity as a moderator of peer norms on adolescent risk taking, we examined whether self-reported susceptibility (assessed with the Resistance to Peer Influence (RPI) scale; Steinberg & Monahan, 2007) moderates this link. Self-report instruments, such as the RPI, allow adolescents an opportunity to reflect on their own tendencies to resist pressures from peers, with reported changes in youths’ responses across development (Steinberg & Monahan, 2007). However, self-reported susceptibility may be less predictive of adolescents’ behaviors, as youth are often unaware of, unable to, or unwilling to report on their conformity tendencies (Nisbett & Wilson, 1977; Prinstein et al., 2011; Prinstein & Giletta, 2016). Indeed, prior neuroimaging work has begun to reveal how neural activation predicts risk-taking behavior even more powerfully than adolescents’ own self-reports (Falk et al., 2014; Telzer et al., 2013). Thus, we hypothesized that neurobiological susceptibility would moderate the link between peer group norms and adolescent risk taking, above and beyond self-reported susceptibility.
Methods
Participants
Participants were recruited from three rural public middle schools in the southeast United States. Between 66.7–72.1% of students in these schools were classified as economically disadvantaged (NCDPI, 2017), and 69.5% of students in the district were eligible for free or reduced-price lunch based on district reports. Of the 148 participants who completed the fMRI session, three were excluded from analyses due to not completing the scan, two for excessive motion (>2mm in any direction), one for technical errors, one for an MRI artifact, and five for missing data on the peer group norms questions, leading to a total sample of 136 adolescents (70 female) ages 11–14 (Mage = 12.79, SD = .59). We focus on early adolescents (ages 11–14) given that this developmental period is marked by increased behavioral (i.e., conformity) and neural (i.e., ventral striatum activation) sensitivity to peers. For instance, early adolescents show greater peer influence effects in both prosocial (van Hoorn et al., 2016; Foulkes et al., 2018) and deviant (Knoll et al., 2017) peer contexts, and report lower resistance to peer influence (Steinberg & Monahan, 2007) relative to older adolescents.
Adolescents were from diverse racial/ethnic backgrounds (47 Hispanic/Latinx, 40 White, 31 Black/African-American, 12 multi-racial, 6 other). Overall, the sample was from low to middle socioeconomic status in terms of parental reported household income (31% less than $30,000, 34% $30-$60,000, 35% over $60,000), parental education (25% less than high school, 16% high school diploma, 30% some college, 29% associate’s degree or higher), and census-based area deprivation index (percentiles compared to national average with higher scores meaning greater deprivation: Range = 22–97; M = 68.0, SD = 17.8; University of Wisconsin, 2018). Adolescents and parents gave written assent/consent in accordance with the university’s Institutional Review Board.
Procedures
Participants were recruited from a larger study of 873 students in 6th and 7th grade. Participants from the larger study provided interest in being contacted for a future fMRI study. Interested participants were then called and screened on the phone for eligibility (i.e., MRI contraindications) and recruited for the fMRI study within the same academic year as the larger study. We screened 284 families, of whom 91 were ineligible due to learning disabilities, braces, head trauma or other MRI contraindications, and 45 were eligible but did not participate due to scheduling difficulties or no longer interested in participating, resulting in a final sample of 148 adolescents. Thus, of those contacted and eligible, 77.5% participated.
For the current study, adolescents and their primary caregiver attended the fMRI session, during which consent and assent were obtained. Participants completed an fMRI scan that lasted approximately 1.5 hours, during which they completed the SID task (described below), as well as four other tasks that are not the focus of the current manuscript. Following the scan, participants completed several self-report measures using computer-assisted software in a private room, including perceived peer group norms and risk-taking behaviors, as well as other measures which are not the focus of this manuscript. Adolescents were compensated with a monetary remuneration of $90, small prizes for completing the full scan and staying still (e.g., headphones, candy; worth $20), snacks during the visit, and a meal. Parents were compensated with a monetary remuneration of $50, as well as a meal, compensation for gas, and parking.
Questionnaire Measures
Perceived peer group norms.
Participants completed a revised version of the Perception of Peer Group Norms Questionnaire (Marshall-Denton, Véronneau, & Dishion, 2016). Participants indicated how many of their close friends participate in 16 behaviors on a 1- (none) to 6- (almost all) point scale, including eight negative (e.g., “may have tried or use tobacco”; “lie to adults”; “fight or bully others”) and eight positive (e.g., “try to set goals for school success”; “volunteer for a good cause”; “resist peer activities involving tobacco, drugs, and alcohol use”) behaviors. All positive statements were reverse coded and a total mean score was calculated so that higher scores indicate perceptions of relatively more negative peer group norms, and lower numbers indicate perceptions of relatively more positive peer group norms. The scale demonstrated good reliability (α = .827).
Risk-taking behaviors.
Participants completed a modified version of the Adolescent Risk-taking Scale (Alexander et al., 1990). Adolescents reported on their frequency of engaging in 14 risky behaviors on a 4-point scale (0=never, 1=once or twice, 2=several times, 3=many times). The scale included questions about rule breaking (e.g., “I have snuck out of my house without my parents knowing”), sexual activity (e.g., “I have had sex with someone I just met”), substance use (e.g., “I have gotten drunk or high at a party”), and dangerous behavior (e.g., “I did something risky or dangerous on a dare”). A total mean score for all items was calculated (α = .769).
Resistance to peer influence.
To examine self-reported peer susceptibility, we utilized the Resistance to Peer Influence Scale (Steinberg & Monahan, 2007) that included 10 items (e.g., “I go along with my friends just to keep my friends happy.”). The original scale uses a two-question tree-structure for each item to derive final scores on a 4-point Likert scale. In order to simplify readability for participants, items used in this study were condensed into one question each on a 1- (really true) to 4- (not at all true) point scale, with higher scores representing higher resistance to peer influence and lower scores representing greater peer susceptibility. Additionally, all items were modified to be ‘I’ statements rather than “Some people” statements. The scale demonstrated good internal consistency (α = .858).
Social Incentive Delay Task
Participants completed the Social Incentive Delay Task while undergoing fMRI to measure neural responses when anticipating receiving social rewards and avoiding social punishments. The SID is modified from the widely used Monetary Incentive Delay Task (Knutson, Westdorp, Kaiser, & Hommer, 2000), and reliably engages the VS (Cremers, Veer, Spinhoven, Rombouts, & Roelofs, 2015; Kohls et al., 2013). For instance, anticipation of both social and monetary rewards recruits the VS (Spreckelmeyer et al., 2009), and the anticipation of avoidable social punishments recruits the VS similarly to VS activation during the anticipation of social reward gain (Kohls et al., 2013).
Each trial of the SID began with a cue that signaled whether the anticipated feedback would be a reward, punishment, or neutral (500 ms; see Figure 1). The cue was a different shape for each condition. The cue was followed by a jittered crosshair (between .48 and 3.9 seconds, M = 2.0 seconds), which was followed by the target (a white square; 300 ms), at which point participants were instructed to press a button as quickly as possible. The display of social feedback (1450 ms) was dependent on the trial type and participants’ reaction time. In the reward condition, a hit (i.e., fast enough response) earned the feedback of a happy face (i.e., social reward feedback), and a miss (i.e., too slow response) earned a blurred face (i.e., neutral feedback). During the punishment condition, a hit earned a blurred face (i.e., neutral feedback) and a miss earned an angry face (i.e., social punishment feedback). Both hits and misses were followed by a blurred face in the neutral condition. After the feedback, another jittered crosshair (between .51 and 4.2 seconds, M = 2.3 seconds) was presented before the next trial began. Trials were presented in an event-related design, with reward, punishment, and neutral conditions randomly ordered. Participants completed two rounds of the task, totaling 116 trials (48 reward, 48 punishment, 20 neutral).
Figure 1.

Social Incentive Delay Task. Each trial consists of a cue (circle, diamond, or triangle), a jittered crosshair delay, a target (white square) signaling participants to press a button, and feedback (e.g., happy face). Each cue and corresponding feedback depicted in lower panel of figure.
To prevent a ceiling or floor performance effect and ensure participants performed roughly at 50% accuracy so that they received relatively equal amount of positive and negative feedback, the time required for a successful hit was adaptive, starting at .30 seconds for the first trial and adding or subtracting .02 seconds after a miss or hit, respectively, with an upper bound of .50 seconds and a lower bound of .16 seconds. In order to make the task motivationally salient, age-matched adolescent faces posing emotional facial expressions were utilized as rewards and punishments. The faces were photographs of ethnically diverse male and female adolescents (24 faces, 12 female) taken from the National Institute of Mental Health Child Emotional Faces Picture Set (NIMH-ChEFS). Participants were trained on the meaning of each cue and completed 12 practice trials prior to entering the scanner. Three participants only had one round of usable fMRI data from the task (due to early exit from scanner or technical issues), but were included in analyses because they met a priori requirements for the number of trials needed per condition (8 hits, or above a 15% hit rate).
fMRI Data Acquisition and Preprocessing
Imaging data were collected using a 3 Tesla Siemens Prisma MRI scanner. The SID was presented on a computer screen and projected through a mirror. A high-resolution structural T2*-weighted echo-planar imaging (EPI) volume (TR = 2000ms; TE = 25ms; matrix = 92 x 92; FOV = 230mm; 37 slices; slice thickness = 3mm; voxel size 2.5 x 2.5 x 3 mm3) was acquired coplanar with a T2*-weighted structural matched-bandwidth (MBW), high-resolution, anatomical scan (TR = 5700ms; TE = 65ms; matrix = 192 x 192; FOV = 230mm; 38 slices; slice thickness = 3mm). In addition, a T1* magnetization-prepared rapid-acquisition gradient echo (MPRAGE; TR = 2400ms; TE = 2.22ms; matrix = 256 x 256; FOV = 256mm; sagittal plane; slice thickness = 0.8mm; 208 slices) was acquired. The orientation for the EPI and MBW scans was oblique axial to maximize brain coverage and to reduce noise. Preprocessing was conducted using FSL (FMRIB’s Software Library, version 6.0; www.fmrib.ox.ac.uk/fsl) and included the following steps: Skull stripping using BET (Smith, 2002); motion correction with MCFLIRT (Jenkinson, Bannister, Brady, & Smith, 2002); spatial smoothing with Gaussian kernel of full width at half maximum (FWHM) 6 mm; high-pass temporal filtering with a filter width of 128 s (Gaussian-weighted least-squares straight line fitting, with sigma=64.0s); grand-mean intensity normalization of the entire 4D dataset by a single multiplicative factor; and individual level ICA denoising for motion and physiological noise using MELODIC (version 3.15; Beckmann & Smith, 2004), combined with an automated signal classifier (Tohka et al., 2008; Neyman-Pearson threshold = .3). For the spatial normalization, the EPI data were registered to the T1 image with a linear transformation, followed by a white-matter boundary based transformation (BBR; Greve & Fischl, 2009) using FLIRT, linear and non-linear transformations to standard Montreal Neurological Institute (MNI) 2-mm brain were performed using Advanced Neuroimaging Tools (ANTs; Avants et al, 2011), and then spatial normalization of the EPI image to the MNI.
fMRI Data Analysis
Individual level, fixed-effects analyses were estimated using the general linear model convolved with a canonical hemodynamic response function in SPM8. The task was modeled as event-related with eight conditions, including three anticipation conditions (reward, punishment, neutral), two outcome conditions for both reward (hit, miss) and punishment (hit, miss), and one outcome condition for neutral. Anticipation conditions were modeled as the onset of the cue and the duration of the cue and jitter prior to the target, and outcome conditions were modeled at the onset of and for the full duration of the feedback. Six motion parameters were modeled as regressors of no interest. Using the parameter estimates from the GLM, linear contrast images comparing each of the conditions of interest were calculated for each individual. The primary contrasts of interest for this study was reward anticipation vs. neutral anticipation and punishment anticipation vs. neutral anticipation.
Individual subject contrasts were then submitted to random effects, group-level analyses using GLMFlex (McLaren, Schultz, Locascio, Sperling, & Atri, 2011), which corrects for variance–covariance inequality, removes outliers and sudden activation changes in the brain, partitions error terms, and analyzes all voxels containing data (http://mrtools.mgh.harvard.edu/index.php/GLM_Flex). Exploratory, whole-brain analyses for each contrast are presented in Supporting Information Table 1 and are available on Neurovault (Gorgolewski et al., 2015: https://neurovault.org/collections/LXPKHSIX/). Notably, the ventral striatum was activated more to anticipating social rewards and social punishments than to neutral. Our primary, confirmatory analyses employed a region-of-interest (ROI) approach with the bilateral ventral striatum using a mask based on Neurosynth by searching “ventral striatum” (http://neurosynth.org/analyses/terms/ventral%20striatum/). The resulting automated meta-analytic image was based on 415 studies, and was thresholded at Z = 14 (see Supporting Information, Figure 1). Using this mask, we extracted parameter estimates of signal intensity from the primary contrasts of interest (reward anticipation vs. neutral anticipation and punishment anticipation vs. neutral anticipation). Parameter estimates from each of these contrasts therefore represent neural activation in the ventral striatum when anticipating social rewards and social punishments, each controlling for anticipation to neutral. Parameter estimates from the ventral striatum ROI were used as a moderator in subsequent analyses to test our primary hypotheses.
Analysis Plan
Moderation analyses were conducted in SPSS (version 25, IBM) using the PROCESS macro (Hayes, 2013). This is a path-analysis approach to moderation that simultaneously models multiple conditional effects using ordinary least squares regression for continuous outcomes. Bootstrap bias-corrected confidence intervals (95%) are estimated, where nonzero overlapping confidence intervals indicate a significant effect. Moderation was conducted by standardizing the predictor and moderator variables prior to analysis. Adolescent risk taking served as the dependent variable, and age was entered as a covariate. We first tested whether self-reported susceptibility (i.e., RPI) moderates the link between peer group norms and adolescent risk taking. We then tested whether ventral striatum activation to anticipating social rewards and punishments moderates the link between peer group norms and adolescent risk taking. We further included RPI as a covariate to ensure ventral striatum activation serves as a moderator above and beyond self-reported peer influence susceptibility. For probing the significant moderation effects, we used the Johnson-Neyman technique and marginal-effects plots in conjunction with visual depictions of simple slope using small multiples (created with the R-based interActive data visualization tool; McCabe, Kim & King, 2018).
Results
Descriptive Statistics
Table 1 presents correlations among all study variables. Adolescents who perceived relatively more negative than positive peer group norms reported greater risk taking. Ventral striatum activation when anticipating social rewards or punishments was not associated with peer group norms or risk taking. Age was positively correlated with risk taking, and marginally related to more negative peer group norms. Males and females did not differ on any of the study variables (ps > .16). We therefore controlled for age but not sex in our primary analyses.
Table 1.
Descriptives and Correlations Among Study Variables
| Variable | 1. | 2. | 3. | 4. | 5. | 6. |
|---|---|---|---|---|---|---|
| 1. Peer Group Norms | 1 | −.03 | −.09 | −.16 | .44*** | .14+ |
| 2. VS Reward-Neutral | 1 | .72*** | .07 | .11 | −.04 | |
| 3. VS Punish-Neutral | 1 | .10 | .02 | .08 | ||
| 4. Resistance to Peer Influence | 1 | −.10 | .06 | |||
| 5. Risk Taking | 1 | .24** | ||||
| 6. Age | 1 | |||||
| M (SE) range |
2.16 (.06) 1.0–4.13 |
.11 (.02) −.61–.76 |
.06 (.02) −.89–.88 |
3.33(.05) 1.0–4.0 |
.24 (.02) 0–1.43 |
|
Note.
p < .001;
p < .005;
p < .10
Moderation of Link between Peer Norms and Adolescent Risk Taking
Self-reported resistance to peer influence did not moderate the association between perceived peer group norms and adolescent risk taking (B=.024, SE=.019, p=.22, 95% CI [−.014, .061]). In contrast, ventral striatum activation when anticipating receiving social rewards and avoiding social punishments each moderated the association between peer group norms and risk taking, above and beyond self-reported RPI (Table 2).
Table 2.
Ventral Striatum Sensitivity to Social Rewards and Social Punishments Moderates Link Between Perceived Peer Group Norms and Adolescent Risk Taking
| ΔR2 | B (SE) | p | 95% CI | |
|---|---|---|---|---|
| Reward - Neutral | ||||
| Step 1, Covariates | .072 | .007 | ||
| RPI | −.108 (.080) | .177 | [−.266, .050] | |
| Age | .243 (.081) | .003 | [.082, .403] | |
| Step 2, Main Effects | .176 | .000 | ||
| Peer Group Norms (PGN) | .394 (.075) | .000 | [.246, .542] | |
| VS Reward-Neutral | .130 (.072) | .074 | [−.013, .273] | |
| Step 3, Interaction | .027 | .030 | ||
| PGN * VS | .161 (.073) | .030 | [.016, .306] | |
| Total R2 | .275 | |||
| Punishment - Neutral | ||||
| Step 1, Covariates | .072 | .007 | ||
| RPI | −.108 (.080) | .177 | [−.266, .050] | |
| Age | .243 (.081) | .003 | [.082, .403] | |
| Step 2, Main Effects | .160 | .000 | ||
| Peer Group Norms (PGN) | .396 (.076) | .000 | [.246, .546] | |
| VS Punish-Neutral | .048 (.078) | .537 | [−.105, .201] | |
| Step 3, Interaction | .050 | .003 | ||
| PGN * VS | .229 (.076) | .003 | [.078, .380] | |
| Total R2 | .282 | |||
Note. PGN=peer group norms; VS= ventral striatum activation; RPI=resistance to peer influence
We probed this interaction by using the Johnson–Neyman technique (Bauer & Curran, 2005; Hayes & Matthes, 2009), which mathematically derives the “regions of significance”, where the conditional effect of the predictor variable transitions between not statistically significant to statistically significant. The simple slope of perceived peer group norms on adolescent risk taking is no longer significant at .95 standard deviations below the mean on ventral striatum activation to social rewards (78.65% of observations are within the region of significance) and .90 standard deviations below the mean on ventral striatum activation to social punishments (66.85% of observations are within the region of significance).
For visualization purposes, we used small multiples to plot a broad range of simple slope effects, which displays the observed data that is most representative of each simple slope (McCabe et al., 2018). As shown in Figure 2, for adolescents with relatively higher ventral striatum activation (.5SD below the mean and higher), peer group norms were significantly associated with adolescent risk taking, such that those perceiving relatively more deviant peer norms showed heightened risk taking, whereas those perceiving relatively more prosocial peer norms were buffered from heightened risk taking. In contrast adolescents with relatively lower ventral striatum activation were resilient to peer norms, such that prosocial and deviant peer norms were not associated with risk taking. As shown in Figure 2, the crossover point (small diamond on each line) captures where the groups (i.e., high and low neurobiologically sensitive youth) are no longer significantly different from one another. The crossover point occurs when youth report relatively more prosocial peer group norms, suggesting that high and low neurobiologically sensitive youth only differ in their risk-taking behaviors when they are in more deviant peer contexts.
Figure 2.
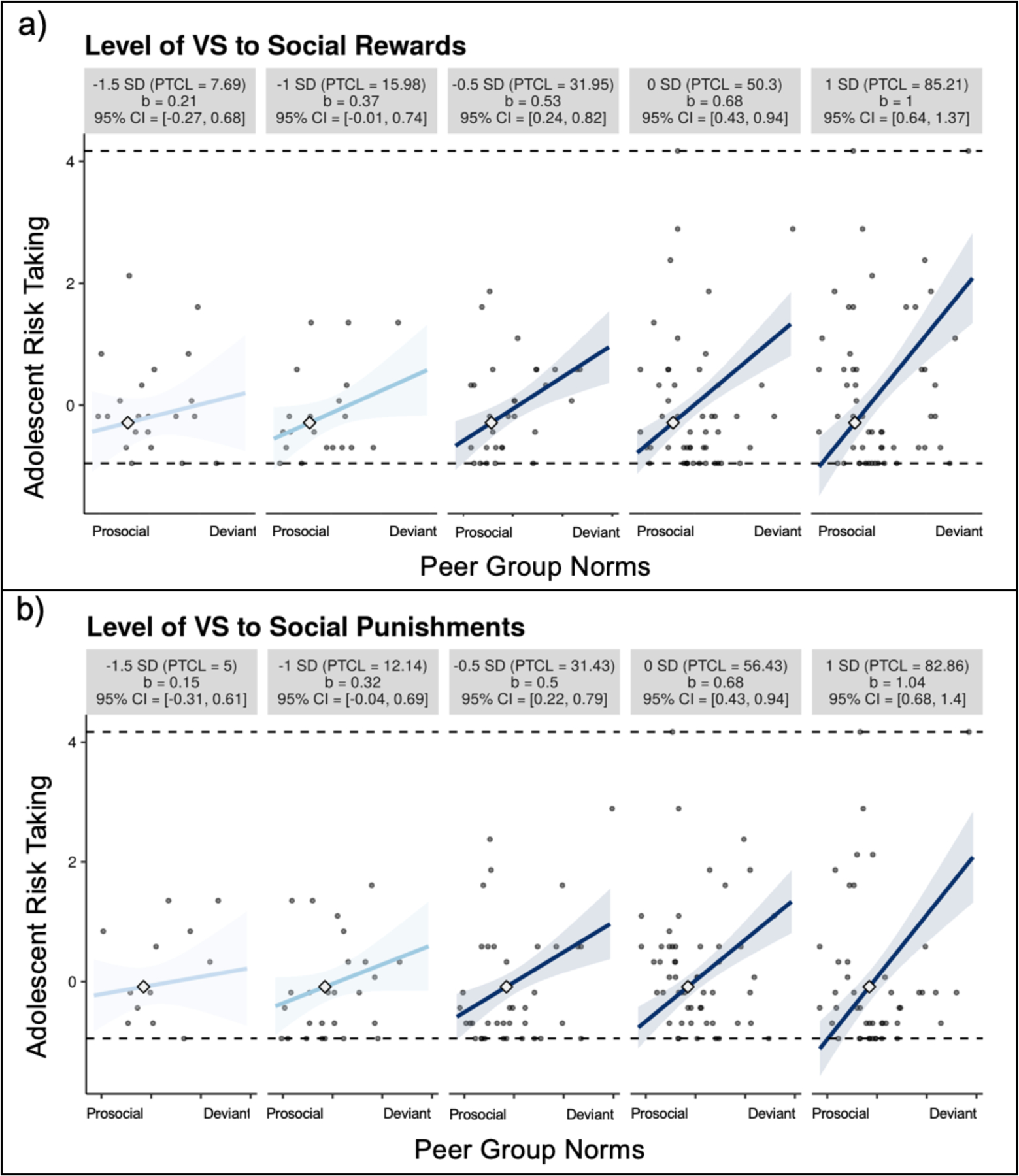
Small-multiples depictions of the interaction effect of perceived peer group norms and ventral striatum activation on adolescent risk taking. The small multiples illustrate the interaction across the range from 1.5 SD below to 1 SD above the mean in VS activation to (a) social rewards and (b) social punishments. Each graphic shows the computed 95% confidence region (shaded area), the observed data (gray circles), the maximum and minimum values of the outcome (dashed horizontal lines), and the crossover point (diamond). CI = confidence interval; PTCL = percentile. We z-transformed risk taking, such that negative scores represent below average risk taking, whereas positive scores represent above average risk taking.
Discussion
Adolescence is marked by increases in susceptibility to peers, heightened risk taking, and rapid changes in ventral striatum activation. The current study examined how variability in ventral striatum sensitivity to social rewards and punishments moderates individual differences in susceptibility to peers. We found that heightened ventral striatum activation when anticipating social rewards and avoiding social punishments moderated the association between perceived peer norms and risk taking, suggesting that the ventral striatum is signaling the motivational relevance of anticipating social feedback, regardless of valence, placing youth at risk when they are in negative peer contexts but buffering them in positive peer contexts.
While prior research has examined the neural correlates of peer conformity (e.g., Cascio, O’Donnell, Bayer, Tinney, & Falk, 2015; Nook & Zaki, 2015; Welborn et al., 2016), or how heightened striatal activation predicts concurrent or future peer influence and conformity effects (e.g., Cascio, Carp, et al., 2015), the current study provides a novel contribution by examining how variability in ventral striatum sensitivity to social rewards and punishments moderates the link between social context and risk taking. In particular, we found that perceptions of more deviant peer norms were associated with increased risk-taking behavior but only for adolescents with high ventral striatum activation when anticipating both social rewards and punishments. Adolescents with high ventral striatum sensitivity who perceived more prosocial peer norms engaged in less risk taking. In contrast, adolescents with low ventral striatum activation were resilient in the face of negative peer norms, showing low risk taking regardless of peer context. Together, these findings suggest that ventral striatum sensitivity to social rewards and punishments does not serve as a monolithic negative susceptibility marker, but instead may tune adolescents to the social norms of their peer context, amplifying peer influence effects in both positive and negative ways. These effects persisted above and beyond self-reported peer influence susceptibility, suggesting that neurobiological sensitivity to social rewards and punishments may be a more sensitive index of heightened susceptibility to peer influence, and may capture more implicit aspects of susceptibility that are not accessible to or reported by individuals.
This method of using the brain as a moderator of social context diverges from prior research emphasizing that neurobiological sensitivity applies equally to all adolescents. This perspective offers two important advantages which may be especially relevant for prevention efforts. First, this perspective underscores that not all adolescents are vulnerable to peer influence effects, and that adolescents with low neurobiological sensitivity (i.e., low ventral striatum activation) will be resilient to conformity pressures, as they may not be motivated to adhere to peer norms, no matter the peer context they find themselves in. Second, this perspective underscores that high ventral striatum sensitivity does not equally place all youth at risk; only for adolescents in negative peer contexts (e.g., deviant peer norms) is heightened ventral striatum activation linked to adolescents’ own risk behaviors. Adolescents with high ventral striatum activation who are in positive peer environments (e.g., prosocial peer norms) are buffered from engaging in risk taking. Importantly, our results may not only identify adolescents most at risk, but also perhaps those who may be the most open to positive socializing influences from prosocial peers. Future research should examine the extent to which sensitivity to social rewards and punishments moderates the links between prosocial norms and prosocial behaviors.
Heightened VS activation reinforces the rewarding nature of engaging in motivated appetitive behaviors and avoiding aversive behaviors (Kohls et al., 2013). Although speculative, individuals with heightened VS activation to both anticipated social reward gain and social punishment avoidance may experience greater motivation to adhere to peers’ behavior, perhaps out of a purported desire to gain peer acceptance, increase social connection, and avoid peer disapproval and rejection (Falk et al., 2012). As such, those with heightened VS activation in negative peer contexts (e.g., perceived deviant peers) may adhere to those norms and engage in more risk taking, whereas those with heightened VS activation in positive peer contexts (e.g., perceived prosocial peers) may adhere to those norms and avoid risk taking, engaging in levels similar to their peers with low VS sensitivity. Collectively, these findings implicate high VS activation to both anticipated social reward gain and social punishment avoidance as a potential biomarker that modulates the perceived value associated with peer influence.
These findings have implications for interventions seeking to decrease adolescent risk taking. While some youth will be impervious to interventions, those who are neurobiologically sensitive (i.e., have heightened VS activation to social rewards and punishments) will be more likely to benefit from interventions that focus on changing adolescents’ social context and the perceived norms of their peers. Indeed, prior work suggests that peer influence may be driven by misperceptions of norms and overestimations of risk attitudes among peers (i.e., pluralistic ignorance) (Prentice & Miller, 1993; Prinstein & Wang, 2005). Thus, among youth who are neurobiologically sensitive (i.e., heightened VS activation), interventions that focus on helping adolescents seek alternate socially rewarding stimuli (e.g., engagement with prosocial peers) may have the largest impact. These findings suggest social contextual processes that might be most relevant to target in psychosocial preventive approaches, and greater awareness of the biologically reinforcing properties of peer conformity.
Limitations and Future Directions
Although the adolescent transition represents a key period of heightened susceptibility to peers (Brechwald & Prinstein, 2011; Prentice, 2008) in both deviant and prosocial contexts (Foulkes et al., 2018; Knoll et al., 2017; van Hoorn et al., 2016), remarkably little prospective longitudinal work has been conducted, especially examining the developmental psychobiological precursors of peer influence susceptibility. Because our study was cross-sectional, we are unable to examine how peer influence and neurobiological susceptibility unfold over time. Specifically, we cannot test the direction of effects, and it is possible that adolescents engaging in risk taking seek out peers who endorse the same behaviors (i.e., selection effects). Given substantial reorganization of the adolescent brain (Nelson, Jarcho, & Guyer., 2016), including significant changes in ventral striatum activation across adolescence (Telzer, 2016), it is possible that there are sensitive periods during which heightened ventral striatum activation is a particularly salient susceptibility marker. Longitudinal research will be key to unpack whether early striatal sensitivity, sensitivity at particular developmental periods, and/or longitudinal increases in ventral striatum sensitivity similarly serve as particular risk factors. A developmental longitudinal investigation of peer influence susceptibility will allow us to examine how changes in psychobiological processes might be associated with changes in peer influence susceptibility and correspondingly, prospective risk-taking behaviors.
In addition, peer influence susceptibility likely is the product of complex and interacting networks throughout the brain. While this study examined regional brain activity within the ventral striatum, this research reflects the assumption that brain regions operate in isolation. Compared to work on adults (e.g., O’Donnell et al., 2017; Wasylyshyn et al., 2018), more research is needed among youth using a network neuroscience approach. For instance, greater connectivity between regions involved in executive control (e.g., dorsolateral prefrontal cortex) and motivational relevance (e.g., ventral striatum) may be associated with less peer influence susceptibility, as heightened connectivity may signal effective top-down cognitive control. In contrast, greater connectivity between regions involved in affective salience (e.g., amygdala) and motivational relevance (e.g., ventral striatum) may be associated with greater peer influence susceptibility, as heightened connectivity may signal greater attention to socio-affective stimuli and a motivation to seek social rewards. Focus on connectivity within and between these networks will allow us to begin to identify the psychobiological processes associated with peer influence susceptibility across development.
Finally, future research would benefit from the use of peers’ self-reported norms and behaviors, which would reduce concerns about methods variance. Nonetheless, the use of adolescents’ perceived peer norms offers an advantage in examining a more proximal predictor of risk behavior, since prior work shows that adolescents’ perceptions of their peers’ behavior mediates the association between peers’ actual reported behavior and adolescents’ own behavior (Fromme & Ruela, 1994; Prinsteim & Wang, 2005).
Conclusions
Adolescents vary considerably in peer influence susceptibility (Brechwald & Prinstein, 2011), however remarkably little work has adequately operationalized, measured, or examined the predictive validity of peer susceptibility markers. This work revealed that ventral striatum sensitivity to both anticipated social reward gain and social punishment avoidance significantly moderates the association between exposure to perceived peer norms and adolescents’ own risk behaviors. These findings provide a novel and innovative contribution to the study of peer influence susceptibility, and to work revealing how individual differences in neural responses may be associated with developmental adaptation.
Supplementary Material
Acknowledgments
This research was supported by the National Institutes of Health (R01DA039923 to E.H.T.) and the National Science Foundation (BCS 1539651 to E.H.T.).
References
- Alexander CS, Kim YJ, Ensminger M, Johnson KE, Smith BJ, & Dolan LJ (1990). A measure of risk taking for young adolescents: Reliability and validity assessments. Journal of Youth and Adolescence, 19, 559–569. 10.1007/BF01537176 [DOI] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, & Gee JC (2011). A reproducible evaluation of ANT’s similarity metric performance in brain image registration. Neuroimage, 54, 2033–44. 10.1016/j.neuroimage.2010.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer DJ, & Curran PJ (2005). Probing interactions in fixed and multilevel regression: Inferential and graphical techniques. Multivariate Behavioral Research, 40, 373–400. doi: 10.1207/s15327906mbr4003_5 [DOI] [PubMed] [Google Scholar]
- Beach SRH, Brody GH, Lei M-K, & Philibert RA (2010). Differential susceptibility to parenting among African American youths: Testing the DRD4 hypothesis. Journal of Family Psychology, 24(5), 513–521. 10.1037/a0020835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, & Smith SM (2004). Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Transactions on Medical Imaging, 23, 137–152. doi: 10.1109/TMI.2003.822821 [DOI] [PubMed] [Google Scholar]
- Blakemore S-J, & Mills KL (2014). Is adolescence a sensitive period for sociocultural processing? Annual Review of Psychology, 65, 187–207. 10.1146/annurev-psych-010213-115202 [DOI] [PubMed] [Google Scholar]
- Brechwald WA, & Prinstein MJ (2011). Beyond homophily: A decade of advances in understanding peer influence processes. Journal of Research on Adolescence, 21, 166–179. 10.1111/j.1532-7795.2010.00721.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio CN, Carp J, O’Donnell MB, Tinney FJ Jr, Bingham CR, Shope JT,… Falk EB (2015). Buffering social influence: Neural correlates of response inhibition predict driving safety in the presence of a peer. Journal of Cognitive Neuroscience, 27(1), 83–95. 10.1162/jocn_a_00693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio CN, O’Donnell MB, Bayer J, Tinney FJ, & Falk EB (2015). Neural correlates of susceptibility to group opinions in online word-of-mouth recommendations. Journal of Marketing Research, 52, 559–575. 10.1509/jmr.13.0611 [DOI] [Google Scholar]
- Chein J, Albert D, O’Brien L, Uckert K, & Steinberg L (2011). Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Developmental Science, 14(2), F1–F10. 10.1111/j.1467-7687.2010.01035.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen GL, & Prinstein MJ (2006). Peer contagion of aggression and health risk behavior among adolescent males: An experimental investigation of effects on public conduct and private attitudes. Child Development, 77(4), 967–983. 10.1111/j.1467-8624.2006.00913.x [DOI] [PubMed] [Google Scholar]
- Cremers HR, Veer IM, Spinhoven P, Rombouts SARB, & Roelofs K (2015). Neural sensitivity to social reward and punishment anticipation in social anxiety disorder. Frontiers in Behavioral Neuroscience, 8, 1–9. 10.3389/fnbeh.2014.00439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGuiseppi GT, Meisel MK, Balestrieri SG, Ott MQ, Cox MJ, Clark MA, & Barnett NP (2018). Resistance to peer influence moderates the relationship between perceived (but not actual) peer norms and binge drinking in a college student social network. Addictive Behaviors, 80, 47–52. 10.1016/j.addbeh.2017.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do KT, Prinstein MJ, & Telzer EH (in press). Neurobiological susceptibility to peer influence in adolescence. In Kadosh KC (Ed). The Handbook of Developmental Cognitive Neuroscience. Oxford University Press, New York, NY. [Google Scholar]
- Falk EB, Cascio CN, O ’Donnell MB, Carp J, Tinney FJ, Bingham CR, … Simons-Morton BG (2014). Neural responses to exclusion predict susceptibility to social influence. Journal of Adolescent Health, 54, S22–S31. 10.1016/j.jadohealth.2013.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk E, Way B, & Jasinska A (2012). An imaging genetics approach to understanding social influence. Frontiers in Human Neuroscience, 6, 1–13. 10.3389/fnhum.2012.00168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkes L, Leung JT, Fuhrmann D, Knoll LJ, & Blakemore S-J (2018). Age differences in the prosocial influence effect. Developmental Science, 21(6), e12666. 10.1111/desc.12666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromme K, & Ruela A (1994). Mediators and moderators of young adults’ drinking. Addiction, 89, 63–71. 10.1111/j.1360-0443.1994.tb00850.x [DOI] [PubMed] [Google Scholar]
- Galván A, & McGlennen KM (2013). Enhanced striatal sensitivity to aversive reinforcement in adolescents versus adults. Journal of Cognitive Neuroscience, 25, 284–296. 10.1162/jocn_a_00326 [DOI] [PubMed] [Google Scholar]
- Galván A, Hare TA, Parra CE, Penn J, Voss H, Glover G, & Casey BJ (2006). Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. Journal of Neuroscience, 26(25), 6885–6892. 10.1523/jneurosci.1062-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galván A, Hare T, Voss H, Glover G, & Casey BJ (2007). Risk-taking and the adolescent brain: Who is at risk? Developmental Science, 10(2), F8–F14. 10.1111/j.1467-7687.2006.00579.x [DOI] [PubMed] [Google Scholar]
- Gardner M, & Steinberg L (2005). Peer influence on risk taking, risk preference, and risky decision making in adolescence and adulthood: An experimental study. Developmental Psychology, 41(4), 625–635. 10.1037/0012-1649.41.4.625 [DOI] [PubMed] [Google Scholar]
- Gorgolewski KJ, Varoquaux G, Rivera G, Schwarz Y, Ghosh SS, Maumet C, … Margulies DS (2015). NeuroVault.org: A web-based repository for collecting and sharing unthresholded statistical maps of the human brain. Frontiers in Neuroinformatics, 9, 8. 10.3389/fninf.2015.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve DN, & Fischl B (2009). Accurate and robust brain image alignment using boundary-based registration. NeuroImage, 48, 63–72. doi: 10.1016/j.neuroimage.2009.06.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, McClure-Tone EB, Shiffrin ND, Pine DS, & Nelson EE (2009). Probing the neural correlates of anticipated peer evaluation in adolescence. Child Development, 80(4), 1000–1015. 10.1111/j.1467-8624.2009.01313.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF (2013). Introduction to mediation, moderation, and conditional process analysis. A regression-based approach. New York, NY: Guilford Press. [Google Scholar]
- Iannotti RJ, & Bush PJ (1992). Perceived vs. actual friends’ use of alcohol, cigarettes, marijuana, and cocaine: Which has the most influence? Journal of Youth and Adolescence, 21(3), 375–389. 10.1007/BF01537024 [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, & Smith S (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage, 17, 825–841. 10.1006/nimg.2002.1132 [DOI] [PubMed] [Google Scholar]
- Juvonen J, and Gross EF (2005). The rejected and the bullied: lessons about social misfits from developmental psychology. In Williams KD, Forgas JP, and Von Hippel W (Eds). The Social Outcast: Ostracism, Social Exclusion, Rejection, and Bullying. (pgs 155–170). Psychology Press: New York, NY. [Google Scholar]
- Knoll LJ, Leung JT, Foulkes L, & Blakemore S-J (2017). Age-related differences in social influence on risk perception depend on the direction of influence. Journal of Adolescence, 60, 53–63. 10.1016/j.adolescence.2017.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll LJ, Magis-Weinberg L, Speekenbrink M, & Blakemore SJ (2015). Social influence on risk perception during adolescence. Psychological Science, 26(5), 583–592. 10.1177/0956797615569578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohls G, Perino MT, Taylor JM, Madva EN, Cayless SJ, Troiani V, … Schultz RT (2013). The nucleus accumbens is involved in both the pursuit of social reward and the avoidance of social punishment. Neuropsychologia, 51, 2062–2069. 10.1016/j.neuropsychologia.2013.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, & Hommer D (2000). fMRI visualization of brain activity during a monetary incentive delay task. NeuroImage, 12(1), 20–27. 10.1006/nimg.2000.0593 [DOI] [PubMed] [Google Scholar]
- Levita L, Hare TA, Voss HU, Glover G, Ballon DJ, & Casey BJ (2009). The bivalent side of the nucleus accumbens. NeuroImage, 44, 1178–1187. doi: 10.1016/j.neuroimage.2008.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist KA, Satpute AB, Wager TD, Weber J, & Barrett LF (2016). The brain basis of positive and negative affect: Evidence from a meta-analysis of the human neuroimaging literature. Cerebral Cortex, 26, 1910–1922. 10.1093/cercor/bhv001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall-Denton R, Véronneau MH, & Dishion TJ (2016). Brief report: A confirmatory approach to the validation of the peer group norm questionnaire. Journal of Adolescence, 50, 16–21. 10.1016/j.adolescence.2016.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe CJ, Kim DS, & King KM (2018). Improving present practices in the visual display of interactions. Advances in Methods and Practices in Psychological Science, 1(2), 147–165. 10.1177/2515245917746792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren DG, Schultz AP, Locascio JJ, Sperling RA, & Atri A (2011). Repeated-measures designs overestimate between-subject effects in fMRI packages using one error term. Paper presented at the 17th Annual Meeting of Organization for Human Brain Mapping, Quebec City, Canada, 6, 26–30, 2011. Retrieved from http://mrtoolsmghharvar-dedu/indexphp/GLM_Flex [Google Scholar]
- Nelson EE, Jarcho JM, & Guyer AE (2016). Social re-orientation and brain development: An expanded and updated view. Developmental Cognitive Neuroscience, 17, 118–127. 10.1016/j.dcn.2015.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisbett RE, & Wilson TD (1977). The halo effect: Evidence for unconscious alteration of judgments. Journal of Personality and Social Psychology, 35(4), 250–256. 10.1037/0022-3514.35.4.250 [DOI] [Google Scholar]
- Nook EC, & Zaki J (2015). Social norms shift behavioral and neural responses to food. Journal of Cognitive Neuroscience, 27, 1412–1426. 10.1162/jocn_a_00795 [DOI] [PubMed] [Google Scholar]
- O’Donnell MB, Bayer JB, Cascio CN, & Falk EB (2017). Neural bases of recommendations differ according to social network structure. Social Cognitive and Affective Neuroscience, 12, 61–69. 10.1093/scan/nsw158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan A, & Luna B (2014). Developmental imaging genetics: Linking dopamine function to adolescent behavior. Brain & Cognition, 89, 27–38. 10.1016/j.bandc.2013.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perino MT, Miernicki ME, & Telzer EH (2016). Letting the good times roll: adolescence as a period of reduced inhibition to appetitive social cues. Social Cognitive and Affective Neuroscience, 11(11), 1762–1771. 10.1093/scan/nsw096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice DA, & Miller DT (1993). Pluralistic ignorance and alcohol use on campus: Some consequences of misperceiving the social norm. Journal of Personality and Social Psychology, 64(2), 243–256. 10.1037//0022-3514.64.2.243 [DOI] [PubMed] [Google Scholar]
- Prentice DA (2008). Mobilizing and weakening peer influence as mechanisms for changing behavior: Implications for alcohol intervention programs. In Prinstein M & Dodge K (Eds.), Understanding peer influence in children and adolescents (pp. 161–180). Guilford Press: New York, NY, US. [Google Scholar]
- Prinstein MJ, & Giletta M (2016). Peer relations and developmental psychopathology. In Cicchetti D (Ed.), Developmental psychopathology (pp. 527–579). Wiley: Hoboken, NJ. 10.1002/9781119125556.devpsy112 [DOI] [Google Scholar]
- Prinstein MJ, & Wang SS (2005). False consensus and adolescent peer contagion: Examining discrepancies between perceptions and actual reported levels of friends’ deviant and health risk behaviors. Journal of Abnormal Child Psychology, 33(3), 293–306. 10.1007/s10802-005-3566-4 [DOI] [PubMed] [Google Scholar]
- Prinstein MJ, Brechwald WA, & Cohen GL (2011). Susceptibility to peer influence: Using a performance-based measure to identify adolescent males at heightened risk for deviant peer socialization. Developmental Psychology, 47(4), 1167–1172. 10.1037/a0023274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, Galván A, Fuligni AJ, Lieberman MD, & Telzer EH (2015). Longitudinal changes in prefrontal cortex activation underlie declines in adolescent risk taking. Journal of Neuroscience, 35(32), 11308 –11314. 10.1523/JNEUROSCI.1553-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreuders E, Braams BR, Blankenstein NE, Peper JS, Güroğlu B, & Crone EA (2018). Contributions of reward sensitivity to ventral striatum activity across adolescence and early adulthood. Child Development, 89(3), 797–810. doi: 10.1111/cdev.13056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schriber RA, & Guyer AE (2016). Adolescent neurobiological susceptibility to social context. Developmental Cognitive Neuroscience, 19, 1–18. 10.1016/j.dcn.2015.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slagt M, Dubas JS, Deković M, Haselager GJT, & van Aken MAG (2015). Longitudinal associations between delinquent behaviour of friends and delinquent behaviour of adolescents: Moderation by adolescent personality traits. European Journal of Personality, 29(4), 468–477. 10.1002/per.2001 [DOI] [Google Scholar]
- Smith KS, Berridge KC, & Aldridge JW (2011). Disentangling pleasure from incentive salience and learning signals in brain reward circuitry. Proceedings of the National Academy of Sciences, 108(27), E255–E264. 10.1073/pnas.1101920108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM (2002). Fast robust automated brain extraction. Human Brain Mapping, 17, 143–155. doi: 10.1002/hbm.10062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreckelmeyer KN, Krach S, Kohls G, Rademacher L, Irmak A, Konrad K,… Gründer G (2009). Anticipation of monetary and social reward differently activates mesolimbic brain structures in men and women. Social Cognitive and Affective Neuroscience, 4(2), 158–165. 10.1093/scan/nsn051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Hare T, & Casey BJ (2011). Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. Journal of Cognitive Neuroscience, 23(9), 2123–2134. 10.1162/jocn.2010.21572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, & Monahan KC (2007). Age differences in resistance to peer influence. Developmental Psychology, 43(6), 1531–43. 10.1037/0012-1649.43.6.1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH (2016). Dopaminergic reward sensitivity can promote adolescent health: A new perspective on the mechanism of ventral striatum activation. Developmental Cognitive Neuroscience, 17, 57–67. 10.1016/j.dcn.2015.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Fuligni AJ, Lieberman MD, & Galván A (2013). Ventral striatum activation to prosocial rewards predicts longitudinal declines in adolescent risk taking. Developmental Cognitive Neuroscience, 3, 45–52. 10.1016/j.dcn.2012.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Rogers CR, & van Hoorn J (2017). Neural correlates of social influence on risk taking and substance use in adolescents. Current Addiction Reports, 4, 333–341. doi: 10.1007/s40429-017-0164-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teunissen HA, Kuntsche E, Scholte RHJ, Spijkerman R, Prinstein MJ, & Engels RCME (2016). Friends’ drinking norms and male adolescents’ alcohol consumption: The moderating role of performance-based peer influenc susceptibility. Journal of Adolescence, 53, 45–64. 10.1016/j.adolescence.2016.08.017 [DOI] [PubMed] [Google Scholar]
- Tohka J, Foerde K, Aron AR, Tom SM, Toga AW, and Poldrack RA (2008). Automatic independent component labeling for artifact removal in fMRI. NeuroImage, 39, 1227–1245. doi: 10.1016/j.neuroimage.2007.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- University of Wisconsin School of Medicine and Public Health (2018) Area Deprivation Index v2.0. Downloaded from https://www.neighborhoodatlas.medicine.wisc.edu/ 5/1/2018.
- Van Leijenhorst L, Zanolie K, Van Meel CS, Westenberg PM, Rombouts SARB, & Crone EA (2010). What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cerebral Cortex, 20, 61–69. 10.1093/cercor/bhp078 [DOI] [PubMed] [Google Scholar]
- Wahlstrom D, White T, & Luciana M (2010). Neurobehavioral evidence for changes in dopamine system activity during adolescence. Neuroscience and Biobehavioral Reviews, 34(5), 631–648. 10.1016/j.neubiorev.2009.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylyshyn N, Falk BH, Garcia JO, Cascio CN, O’Donnell MB, Bingham CR, … Falk EB (2018). Global brain dynamics during social exclusion predict subsequent behavioral conformity. Social Cognitive and Affective Neuroscience, 13, 182–191. 10.1093/scan/nsy007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welborn BL, Lieberman MD, Goldenberg D, Fuligni AJ, Galván A, & Telzer EH (2016). Neural mechanisms of social influence in adolescence. Social Cognitive and Affective Neuroscience, 11(1), 100–109. 10.1093/scan/nsv095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widman L, Choukas-Bradley S, Helms SW, & Prinstein MJ (2016). Adolescent susceptibility to peer influence in sexual situations. Journal of Adolescent Health, 58, 323–329. 10.1016/j.jadohealth.2015.10.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


