Abstract
Background
Measurements of plasma free metanephrines are recommended for diagnosing pheochromocytomas and paragangliomas (PPGL). Metanephrines can be detected in saliva with LC-MS/MS with sufficient analytical sensitivity and precision. Because collecting saliva is noninvasive and less cumbersome than plasma or urine sampling, we assessed the diagnostic accuracy of salivary metanephrines in diagnosing PPGL.
Methods
This 2-center study included 118 healthy participants (44 men; mean age: 33 years (range: 19--74 years)), 44 patients with PPGL, and 54 patients suspected of PPGL. Metanephrines were quantified in plasma and saliva using LC-MS/MS. Diagnostic accuracy; correlation between plasma and salivary metanephrines; and potential factors influencing salivary metanephrines, including age, sex, and posture during sampling, were assessed.
Results
Salivary metanephrines were significantly higher in patients with PPGL compared with healthy participants (metanephrine (MN): 0.19 vs 0.09 nmol/L, P < 0.001; normetanephrine (NMN): 2.90 vs 0.49 nmol/L, P < 0.001). The diagnostic sensitivity and specificity of salivary metanephrines were 89% and 87%, respectively. Diagnostic accuracy of salivary metanephrines was 88%, with an area under the ROC curve of 0.880. We found a significant correlation between plasma and salivary metanephrines (Pearson correlation coefficient: MN, 0.86, P < 0.001; NMN, 0.83, P < 0.001). Salivary NMN concentrations were higher when collected in a seated position compared with supine (P < 0.001) and increased with age (P < 0.001).
Conclusions
Salivary metanephrines are a promising tool in the biochemical diagnosis of PPGL. Salivary metanephrines correlate with plasma free metanephrines and are increased in patients with PPGL. At this time, however, salivary metanephrines cannot replace measurement of plasma free metanephrines.
Introduction
Pheochromocytomas and sympathetic paragangliomas are rare neuroendocrine tumors arising from chromaffin cells that can synthesize and secrete catecholamines. Approximately 40% of these tumors are associated with an underlying inherited mutation (1, 2). Early detection of sympathetic pheochromocytomas and paragangliomas (PPGL) in clinically suspected patients is warranted to prevent potentially lethal cardiovascular complications. Therefore, screening for PPGL is recommended for patients with typical signs and symptoms and/or cardiovascular events, young lean individuals with type 2 diabetes, patients with an adrenal incidentaloma, germline mutation carriers, and patients with a previous history or family history of PPGL (3, 4).
Measurement of plasma free metanephrines is currently considered to be the most accurate method for diagnosing these tumors, with diagnostic sensitivity and specificity of 90%–100% and 79%–98%, respectively (3, 5). However, the concentration of plasma free metanephrines can be affected by several factors, including age, sex, caffeine, medication (e.g., tricyclic antidepressants), salt intake, season, adrenalectomy, smoking, method of sampling, and posture during blood sampling (3, 6–10). Blood sampling in the seated position, for example, can falsely increase plasma metanephrines, with approximately 30% due to activation of the sympathoadrenal system (7). Therefore, the US Endocrine Society recommends letting patients rest in the supine position for 20–30 min before blood sampling (3).Consequently, these recommendations require determination of reference intervals for plasma free metanephrines in the supine position and special facilities in the hospital for blood sampling, which can be cumbersome and lead to increased costs (11).
Saliva has a potential diagnostic function and offers advantages over plasma because it can be collected easily and noninvasively. Saliva contains various enzymes, hormones, and antibodies that enter the saliva from the blood by active and passive diffusion or extracellular ultrafiltration. Metanephrines also pass across the acinar cells via ultrafiltration into saliva through gap junctions between cells of secretory units (12). Previously, we showed that high-performance LC-MS/MS was a highly sensitive technique that enabled the measurement of very low concentrations of catecholamines and metanephrines in saliva (13). Because saliva can be collected at home, specimen collection could also be more convenient.
The primary aim of this study was to assess the diagnostic accuracy of salivary metanephrines in diagnosing PPGL. Secondary objectives were to assess the correlation between plasma and salivary metanephrines and to evaluate potential influencing factors like sex, age, and posture during sampling on salivary metanephrines.
Methods
Study Design
This 2-center study was performed at the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) at the National Institutes of Health (Bethesda, MD, USA) and the University Medical Center Groningen (UMCG; Groningen, the Netherlands).
Participants
Group 1 consisted of healthy, normotensive participants (blood pressure ≤130/80 mmHg without the use of antihypertensive medication) with a negative history of cardiovascular events.
Patients at the NICHD and UMCG with a confirmed sympathetic PPGL were included in group 2. Patients were eligible for inclusion at the NICHD if they were diagnosed with a sympathetic PPGL. This diagnosis was based on increased (according to the reference intervals of the NICHD) plasma free metanephrine (MN) and/or normetanephrine (NMN) collected via an indwelling catheter after 30 min of supine rest (upper reference limit of the NICHD: MN, 0.31 nmol/L; NMN, 0.61 nmol/L). In addition, imaging by computed tomography (CT) or magnetic resonance imaging (MRI) and nuclear imaging had to be compatible with the PPGL diagnosis. In the case of resection of the tumor, the histopathology had to be compatible with the PPGL diagnosis.
Patients were eligible for inclusion at the UMCG if they were diagnosed with a sympathetic PPGL. This diagnosis was based on increased (according to the reference intervals of the UMCG) plasma free MN and/or NMN collected via direct venipuncture after 30 min of supine rest (upper reference limit of the UMCG: MN, 0.28 nmol/L; NMN, 0.79 nmol/L) and imaging by CT or MRI and nuclear imaging and, in case of resection of the tumor, the histopathology compatible with the PPGL diagnosis.
Group 3 consisted of patients suspected of and tested for a sympathetic PPGL (i.e., germline mutation carriers) and patients with a previous history or family history of PPGL and in whom a PPGL was ruled out. Absence of PPGL was defined as negative CT or MRI studies and negative results of follow-up biochemical testing (i.e., plasma free MN and plasma NMN both within reference range).
Exclusion criteria for all 3 groups were as follows: age <18 years and use of medications that could influence plasma free metanephrines, including tricyclic antidepressants, phenoxybenzamine, monoamine-oxidase inhibitors, sympathomimetics, cocaine, and methyldopa. Patients in group 2 were excluded when their histology reports were not compatible with a diagnosis of a PPGL.
The study protocol was approved by the medical ethics committee of the UMCG and approved by the Institutional Review Board of the NICHD (protocol 00-CH-0093). All patients provided written informed consent. Recommendations of the Standards for the Reporting of Diagnostic Accuracy Studies were followed.
Specimen Collection
Participants were asked to not drink caffeine-containing beverages, to abstain from smoking, and to fast for at least 1 h before saliva collection. Blood pressure was measured in a seated position using an automated oscillometric device. Saliva was collected using a polyethylene swab (Salivette®; Starstedt). After that, participants at the UMCG were placed in the supine position for 30 min, and blood was drawn via venipuncture (using Becton Dickinson Vacutainer® tubes containing K2-EDTA as an anticoagulant) together with saliva collection in a supine position. At the NICHD, saliva was collected only in the supine position.
Analytical Method
Salivettes were centrifuged at 1000g for 2 min, and the filtrate was collected and stored at −80 °C until analysis. Plasma free and salivary metanephrines were analyzed by LC-MS/MS with automatic solid-phase extraction sample preparation (14). All assays were performed in batches at the Department of Laboratory Medicine of the UMCG.
The analytical method, extensively validated for plasma free metanephrines, was validated for saliva as described previously, using saliva pools with low, medium, and high concentrations of MN and NMN (13).
Intra- and interassay CVs were 1.4%–7.0% and 3.2%–8.3%, respectively, for salivary MN 1.7%–1.8% and 1.7%–3.2%, respectively, for salivary NMN. Lower limits of quantification in saliva were 0.03 and 0.035 nmol/L for MN and NMN, respectively. Recovery was 106% (SD: 10%) for MN and 101% (SD: 5%) for NMN. Linearity was excellent over the different calibration ranges (MN: 0.03–10 nmol/L; NMN: 0.035–26 nmol/L) with R2 > 0.99 (13).
Assessment of Short-Term Stability of MN/NMN in Saliva
For assessment of short-term stability of MN and NMN in saliva, saliva samples from 6 volunteers were used. The saliva samples were divided into aliquots, which were stored for periods of 1, 3, 6 and 8 days at −20 °C, 6 °C, and room temperature until storage at −80°C for batch sample processing and LC-MS/MS analysis. The control group included aliquots that were stored at −80 °C right after obtaining the saliva samples.
Repeated-measures ANOVA was used to elucidate changes and differences in analyte concentrations (Bonferroni correction).
Statistical Analysis
Data are presented as mean (±SD) or median with interquartile range (IQR), as appropriate. Analyses were performed using SPSS statistics (version 23; IBM/SPSS).
Reference intervals for salivary metanephrines were established in the healthy participants. Gaussian distributions of salivary metanephrines were obtained after logarithmic transformation of the data. The antilogarithm of the mean (plus or minus 2 SD) of the transformed data was used to obtain the lower and upper reference limits.
The diagnostic sensitivity of salivary and plasma metanephrines was calculated from the percentage of true-positive over total true-positive plus false-negative test results in patients with PPGL (group 2). A true positive test result was defined as MN and/or NMN concentrations above the upper reference limit. Diagnostic specificity was calculated from the percentage of true-negative over the total of true-negative plus false-positive test results in patients with suspected PPGL and in whom a PPGL was excluded (group 3). Diagnostic accuracy of test performance was defined as the sum of true positive and true negative test results over the sum of the number of test results. ROC curves were constructed based on a binary logistic regression model including MN and NMN as covariates. The area under the curve was calculated to determine the discrimination power. The difference in test performance was calculated using the method of Hanley and McNeil.
After log transformation of the data, a parametric correlation analysis (Pearson correlation coefficient) was used to calculate the correlation between salivary and plasma free metanephrines.
To assess several influencing factors on salivary metanephrines, intra- and intergroup differences were analyzed using the Wilcoxon signed-rank test and the Mann-Whitney U test. A 1-sided significance level of 0.05 was used only to reject the null hypothesis that position during sampling does not influence the concentration of metanephrines.
Results
Participants
In group 1, we included 118 healthy participants (44 men and 74 women) with a mean age of 33 years (range: 19 - 74 years).
Forty-eight patients with PPGL were eligible for inclusion at the NICHD, and 20 patients with PPGL were eligible at the UMCG. Twenty-four patients were excluded due to the use of interfering medication, missing data, or a pathology diagnosis incompatible with PPGL (Fig. 1). Forty-four patients were included in group 2 (24 men and 20 women) with a mean age of 51 years (±SD: 14 years). Twenty-four patients had sympathetic paraganglioma and 20 patients had pheochromocytoma. Twenty-four patients had metastatic PPGL.
Fig. 1.
Flowchart of the inclusion of healthy participants, patients with PPGL, and patients with suspected PPGL.
Sixty patients tested for PPGL were eligible for inclusion in group 3. Six patients were excluded due to missing data. In total, 54 patients (23 men and 31 women) were included, with a mean age of 46 years (±SD: 15.7 years). Fifty patients had a germline mutation in one of the susceptibility genes for PPGL. Four patients had a previous history of PPGL. In all patients, a PPGL was ruled out after a median follow-up period of 4.5 years.
The characteristics of the patients in groups 2 and 3 are shown in Table 1.
Table 1.
Patient characteristics for groups 2 and 3.
| Patients with PPGL (n = 44) | Patients with suspected PPGL (n = 54) | |
|---|---|---|
| Age in years, mean (SD) | 51 (13.9) | 46 (15.7) |
| Sex (M/F) | 24/20 | 23/31 |
| PCC, n (%) | 20 (45) | 0 |
| sPGL, n (%) | 24 (55) | 0 |
| Metastatic PPGL, n (%) | 24 (55) | 0 |
| Germline mutation confirmed: | ||
| SDHx | 8 | 28 |
| RET | 6 | 13 |
| HIF2α | 3 | 0 |
| VHL | 1 | 11 |
| NF1 | 1 | 1 |
| MDH2 | 1 | 0 |
HIF2α, hypoxia-inducible factor-2α; MDH2, malate dehydrogenase 2; NF1, neurofibromatosis; PCC, pheochromocytoma; RET, rearranged during transfection; SDHx, succinate dehydrogenase; sPGL, sympathetic paraganglioma; VHL, von Hippel Lindau.
SALIVARY METANEPHRINES
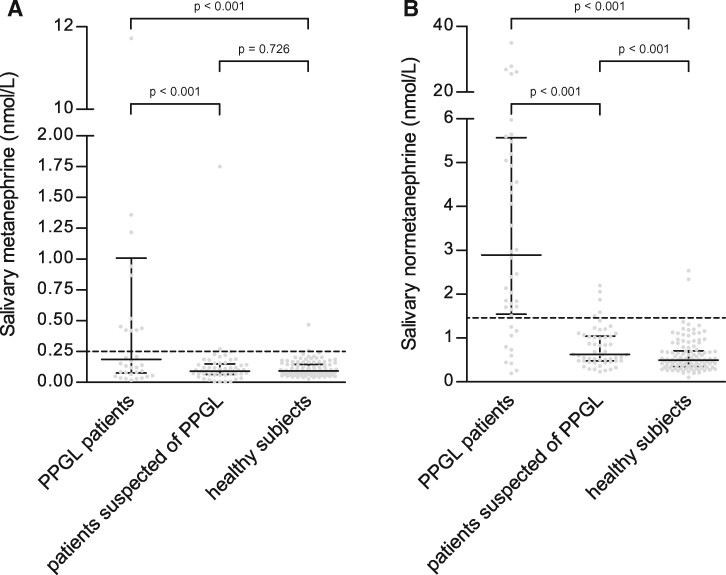
Reference intervals for salivary metanephrines in a supine position were 0.04–0.25 nmol/L for MN and 0.20–1.46 nmol/L for NMN (Table 2). Supine salivary MN concentrations in patients with PPGL, patients with suspected PPGL, and healthy participants were 0.19 nmol/L (IQR: 0.08–1.41 nmol/L), 0.09 nmol/L (IQR: 0.06–0.15 nmol/L), and 0.09 nmol/L (IQR: 0.07–0.14 nmol/L; P < 0.001), respectively. There was a statistically significant difference for salivary MN concentrations for patients with PPGL vs healthy participants (P < 0.001) and between patients with PPGL vs patients with suspected PPGL (P < 0.001). There was no statistically significant difference for salivary MN concentrations between healthy participants vs patients with suspected PPGL (P = 0.726).
Table 2.
Concentrations of salivary metanephrines in healthy participants (n = 118).
| MN (nmol/L) | P value | NMN (nmol/L) | P value | |
|---|---|---|---|---|
| Posture | ||||
| Supine | 0.09 (0.07–0.14) | 0.312 | 0.49 (0.34–0.71) | <0.001 |
| Seated | 0.09 (0.07–0.13) | 0.62 (0.46–0.86) | ||
| Age | ||||
| <40 y | 0.09 (0.06–0.15) | 0.092 | 0.42 (0.32–0.59) | <0.001 |
| >40 y | 0.10 (0.08–0.15) | 0.63 (0.48–0.95) | ||
| Sex | ||||
| Male | 0.10 (0.07–0.17) | 0.060 | 0.55 (0.40–0.82) | 0.071 |
| Female | 0.09 (0.07–0.12) | 0.47 (0.33–0.67) |
Data presented as median and interquartile ranges.
Supine salivary NMN concentrations in patients with PPGL, patients with suspected PPGL, and healthy participants were 2.90 nmol/L (IQR: 1.54–5.57 nmol/L), 0.63 nmol/L (IQR: 0.48–1.08 nmol/L), and 0.49 nmol/L (IQR: 0.34–0.71 nmol/L), respectively. There was a statistically significant difference for salivary NMN concentrations of patients with PPGL vs healthy participants (P < 0.001) and between patients with PPGL vs patients with suspected PPGL (P < 0.001) and between patients with suspected PPGL and healthy participants (P < 0.001) (Fig. 2).
Fig. 2.
Concentrations (median and IQR) of salivary MN (A) and NMN (B) in patients with PPGL, patients with suspected PPGL, and healthy participants. The dotted line shows the upper reference limit of salivary MN (0.25 nmol/L) and NMN (1.46 nmol/L).
CORRELATION OF PLASMA AND SALIVARY METANEPHRINES
We found a statistically significant correlation between salivary and plasma concentrations of MN and NMN in the supine position (Pearson correlation coefficient: 0.86 and 0.83, respectively; P < 0.001 for both) (Fig. 3).
Fig. 3.
Correlation between salivary and plasma MN (A) and NMN (B) concentrations.
INFLUENCING FACTORS ON SALIVARY METANEPHRINES IN HEALTHY PARTICIPANTS
Salivary MN collected in the seated position (median: 0.09 nmol/L (IQR:0.07–0.13 nmol/L)) was not statistically different compared with salivary MN collected in the supine position (median: 0.09 nmol/L (IQR: 0.07--0.13 nmol/L)) (P = 0.312) (Table 2). Salivary NMN was statistically significant higher in the seated position (median: 0.62 nmol/L (IQR: 0.46–0.86 nmol/L)) compared with collection in the supine position (median: 0.49 nmol/L (IQR: 0.34--0.71 nmol/L)) (P < 0.001) (Table 2).
Salivary MN in healthy participants <40 years (median: 0.09 nmol/L (IQR: 0.06-0.15 nmol/L)) was not statistically different compared with salivary MN concentrations of healthy participants >40 years (median: 0.10 nmol/L (IQR: 0.08–0.15 nmol/L)) (P = 0.092). Salivary NMN in healthy participants >40 years (median: 0.63 nmol/L (IQR: 0.48--0.95 nmol/L)) was statistically higher than in participants <40 years (median: 0.42 nmol/L (IQR: 0.32--0.59 nmol/L)) (P < 0.001).
There was a trend, albeit not statistically significant, that salivary MN and NMN concentrations were higher in men (median: MN, 0.10 nmol/L (IQR: 0.07--0.17 nmol/L); NMN, 0.55 nmol/L (IQR: 0.40--0.82 nmol/L)) compared with women (median: MN, 0.09 nmol/L (IQR: 0.07--0.12 nmol/L)); NMN, 0.47 nmol/L (IQR: 0.33--0.67 nmol/L)), respectively (P = 0.060 and P = 0.071).
Short-Term Stability MN/NMN in Saliva
MN and NMN in saliva did not show a significant time-dependent increase or decrease up to 8 days, even at room temperature (online Supplemental Fig. 1). However, we did see in one individual a slightly increasing trend for NMN after 3 days at 6 °C and room temperature. Consequently, we suggest that the saliva should be sent to the laboratory with cooling packs and within 3 days after collection.
DIAGNOSTIC ACCURACY OF SALIVARY AND PLASMA METANEPHRINES
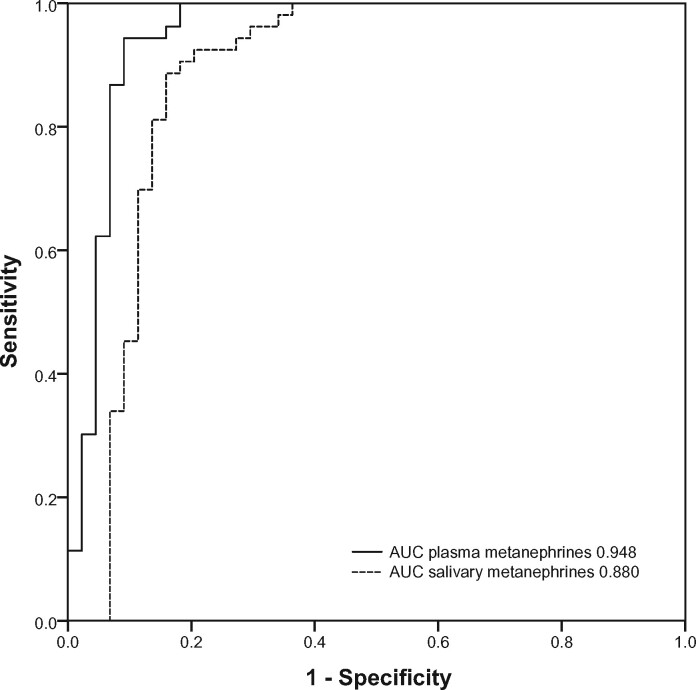
The diagnostic sensitivity of salivary metanephrines in the supine position was 89% (39/44 patients with PPGL), and diagnostic specificity was 87% (47/54). The diagnostic accuracy of salivary metanephrines in the supine position was 88% (86/98). The area under the ROC curve for combined salivary MN and NMN in the supine position was 0.880 (Fig. 4). Five of 44 patients with PPGL did not have increased supine salivary metanephrines concentrations (online Supplemental Table 1).
Fig. 4.
ROC curve for salivary and plasma metanephrines in the supine position, constructed according to logistic regression model with a combination of NMN and MN, including patients with PPGL and patients with suspected PPGL in whom PPGL was excluded. AUC, area under the curve.
Because only patients with PPGL who had increased plasma metanephrines were included, the diagnostic sensitivity of plasma MN was 100%. The diagnostic specificity of plasma metanephrines was 87% (47/54 patients with PPGL). The area under the ROC curve for combined plasma MN and NMN in the supine position was 0.948 (Fig. 4), which was not statistically significantly different compared with salivary metanephrines (P = 0.086).
Discussion
In this study, we assessed the accuracy of salivary metanephrines measured by LC-MS/MS as an alternative, noninvasive tool in the biochemical diagnosis of PPGL. We showed that salivary metanephrines has diagnostic accuracy of 88%, with diagnostic sensitivity of 89% and diagnostic specificity of 87%. Furthermore, we found a strong correlation between plasma and salivary metanephrines, and the concentration of salivary metanephrines was significantly higher in the group of patients with PPGL than in healthy participants. In addition, salivary NMN concentrations were higher when saliva was collected in the seated position compared with supine and increased with age in healthy participants.
In a previously published study (15), salivary metanephrines was determined in 30 patients with pheochromocytoma and 70 healthy controls, using ELISA. In agreement with our data, this study found a significantly higher concentration of salivary metanephrines in patients with pheochromocytoma compared with healthy controls and a strong correlation between plasma and salivary metanephrines. The calculated diagnostic sensitivity and specificity of salivary metanephrines for diagnosing pheochromocytoma were both 100%. However, the previous study included only patients with a pheochromocytoma with plasma concentrations of MN and NMN that were, on average, 9 and 4 times higher, respectively, than the values in our study participants. The patients in our study were included in 2 tertiary referral centers, and >50% of patients had a sympathetic PPGL or metastatic disease.
The diagnostic accuracy of plasma metanephrines was higher than salivary metanephrines, although the difference was not statistically significant. The lack of statistical significance is probably due to the low number of participants. In the present study, 5 patients had false-negative results for salivary metanephrines. It should be noted that 3 patients had only mildly increased plasma metanephrines concentrations. Three of the patients with false-negative test results demonstrated very low concentrations of salivary metanephrines. This might be explained by potential preanalytical factors on salivary metanephrines, such as dilution of saliva due to a high salivary flow rate. Until now, a marker to correct for this dilution phenomenon is lacking. Next to dilution and influencing factors that are already well known for plasma metanephrines, several preanalytical factors should be considered when measuring analytes in saliva. Our previous study assessed the influence of the collection device, posture, eating, and awakening response on salivary metanephrines (13). In line with 2 other studies, patients need proper instructions not to chew on the Salivette, as this will lead to decreased concentrations of salivary metanephrines (16, 17).
Comparable to plasma free metanephrines, we found higher salivary NMN, but not MN concentrations, collected in the seated position compared with the supine position, and this result confirms our previous observation (13). Patients should be instructed to collect their saliva in the supine position.
The difference between salivary NMN in the seated and supine positions but the lack of difference between salivary MN in the seated and supine positions might be explained by the fact that norepinephrine (but not epinephrine) in saliva originates not only from the bloodstream but also from salivary sympathetic nerve endings (17). This locally released norepinephrine adds to the measured salivary NMN concentration. This phenomenon also explains our finding that the NMN concentration is higher in saliva than plasma. Unfortunately, this phenomenon might compromise the diagnostic accuracy of salivary metanephrines.
For clinical practice, saliva collection offers many advantages over plasma or urinary collection, as salivary collection is noninvasive, inexpensive, and minimally stressful. This makes it potentially suitable for elderly patients, patients with needle phobia, and children. In addition, saliva can be collected at home. Salivary NMN and MN were stable up to 3 days at 6 °C and room temperature.
Our study has some limitations. As mentioned, the diagnostic sensitivity of salivary metanephrines was calculated using a design with participation of consecutive patients with PPGL who fulfilled our selection criteria, including increased plasma metanephrines as the reference test. A fair comparison between these 2 diagnostics would require a study with larger sample size, including consecutive patients with suspected PPGL. Furthermore, comparable to plasma free NMN concentrations, we found that higher age was associated with increased salivary NMN concentrations (18). This suggests that age-adjusted reference intervals for salivary NMN seem justified. However, the relatively low number of patients in the present study did not allow assessment of such age-adjusted reference intervals. Reference intervals are also required for children because it has recently been demonstrated that plasma free NMN concentrations show dynamic changes during early childhood (19). We did not include patients with hypertension in group 1 to calculate specific reference intervals because another study has shown that, after multivariate analysis, the differences between participants who are hypertensive and normotensive are not considered to be clinically relevant (6).
In conclusion, determining salivary metanephrines is a promising, novel tool in the biochemical diagnosis of PPGL. Salivary metanephrines correlates with plasma free metanephrines and is elevated in patients with PPGL. At this moment, however, salivary metanephrines cannot replace the measurement of plasma free metanephrines.
Supplemental Material
Supplemental material is available at Clinical Chemistry online.
Supplementary Material
Nonstandard Abbreviations:
- PPGL
pheochromocytoma and sympathetic paraganglioma
- MN
metanephrines
- NICHD
National Institute of Child Health and Human Development
- UMCG
University Medical Center Groningen
- NMN
normetanephrines
- IQR
interquartile range
Author Contributions
All authors confirmed they have contributed to the intellectual content of this paper and have met the following 4 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; (c) final approval of the published article; and (d) agreement to be accountable for all aspects of the article thus ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved.
Study concept and design: Eijkelenkamp, Osinga, van Faassen, Kema, Kerstens, Sluiter, Links, van der Horst-Schrivers. Acquisition, analysis, or interpretation of data: Eijkelenkamp, Osinga, van Faassen, Kema, Sluiter, Links, van der Horst-Schrivers. Drafting of the manuscript: Eijkelenkamp, van der Horst-Schrivers. Critical revision of the manuscript for important intellectual content: all authors. Administrative, technical, or material support: van Faassen, Kema, Pacak. Final approval of the version to be published: all authors.
Authors’ Disclosures or Potential Conflicts of Interest
Upon manuscript submission, all authors completed the author disclosure form.
Wim J. Sluiter's author disclosure form was completed on his behalf by Michiel N. Kerstens. Disclosures and/or potential conflicts of interest:
Employment or Leadership
None declared.
Consultant or Advisory Role
None declared.
Stock Ownership
None declared.
Honoraria
None declared.
Research Funding
This work was funded by the Von Hippel-Lindau Alliance and supported by the Intramural Research Program of the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Expert Testimony
None declared.
Patents
None declared.
Role of Sponsor
The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, preparation of manuscript, or final approval of manuscript.
Acknowledgments
We gratefully acknowledge the contribution of J. Seventer for her critical appraisal of the manuscript.
References
- 1. Fishbein L, Nathanson KL.. Pheochromocytoma and paraganglioma susceptibility genes: estimating the associated risk of disease. JAMA Oncol 2017;3:1212–3. [DOI] [PubMed] [Google Scholar]
- 2. Dahia PL. Pheochromocytoma and paraganglioma pathogenesis: learning from genetic heterogeneity. Nat Rev Cancer 2014;14:108–19. [DOI] [PubMed] [Google Scholar]
- 3. Lenders JWM, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SKG, Murad MH, et al. ; Endocrine Society. Pheochromocytoma and paraganglioma: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2014;99:1915–42. [DOI] [PubMed] [Google Scholar]
- 4. Lenders JWM, Kerstens MN, Amar L, Prejbisz A, Robledo M, Taieb D, et al. Genetics, diagnosis, management and future directions of research of phaeochromocytoma and paraganglioma: a position statement and consensus of the Working Group on Endocrine Hypertension of the European Society of Hypertension. J Hypertens 2020;38:1443–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eisenhofer G, Prejbisz A, Peitzsch M, Pamporaki C, Masjkur J, Rogowski-Lehmann N, et al. Biochemical diagnosis of chromaffin cell tumors in patients at high and low risk of disease: plasma versus urinary free or deconjugated O-methylated catecholamine metabolites. Clin Chem 2018;64:1646–56. [DOI] [PubMed] [Google Scholar]
- 6. Eisenhofer G, Peitzsch M, Kaden D, Langton K, Mangelis A, Pamporaki C, et al. Reference intervals for LC-MS/MS measurements of plasma free, urinary free and urinary acid-hydrolyzed deconjugated normetanephrine, metanephrine and methoxytyramine. Clin Chim Acta 2019;490:46–54. [DOI] [PubMed] [Google Scholar]
- 7. Lenders JWM, Willemsen JJ, Eisenhofer G, Ross HA, Pacak K, Timmers HJLM, et al. Is supine rest necessary before blood sampling for plasma metanephrines? Clin Chem 2007;53:352–4. [DOI] [PubMed] [Google Scholar]
- 8. de Jong WHA, Eisenhofer G, Post WJ, Muskiet FAJ, Vries EGE, Kema IP.. Dietary influences on plasma and urinary metanephrines: implications for diagnosis of catecholamine-producing tumors. J Clin Endocrinol Metab 2009;94:2841–9. [DOI] [PubMed] [Google Scholar]
- 9. Deutschbein T, Unger N, Jaeger A, Broecker-Preuss M, Mann K, Petersenn S.. Influence of various confounding variables and storage conditions on metanephrine and normetanephrine levels in plasma. Clin Endocrinol 2010;73:153–60. [DOI] [PubMed] [Google Scholar]
- 10. Eijkelenkamp K, van Geel EH, Kerstens MN, Faassen M, Kema IP, Links TP, et al. Blood sampling for metanephrines comparing venipuncture vs. indwelling intravenous cannula in healthy subjects. Clin Chem Lab Med 2020;58:1681–6. [DOI] [PubMed] [Google Scholar]
- 11. Chortis V, Bancos I, Crowley RK, Arlt W.. Supine or sitting? Economic considerations regarding patient position during plasma metanephrine analysis for the exclusion of chromaffin tumours. Clin Endocrinol 2015;82:462–3. [DOI] [PubMed] [Google Scholar]
- 12. Chiappin S, Antonelli G, Gatti R, Palo EF.. Saliva specimen: a new laboratory tool for diagnostic and basic investigation. Clin Chim Acta 2007;383:30–40. [DOI] [PubMed] [Google Scholar]
- 13. Osinga TE, van der Horst-Schrivers ANA, van Faassen M, Kerstens MN, Dullaart RPF, Pacak K, et al. Mass spectrometric quantification of salivary metanephrines—a study in healthy subjects. Clin Biochem 2016;49:983–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Faassen M, Bischoff R, Eijkelenkamp K, Jong WHA, Ley CP, Kema IP.. In matrix derivatization combined with LC-MS/MS results in ultrasensitive quantification of plasma free metanephrines and catecholamines. Anal Chem 2020;92:9072–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stefanescu AM, Schipor S, Paun DL, Dumitrache C, Badiu C.. Salivary free catecholamines metabolites as possible biochemical markers in pheochromocytoma diagnosis. Acta Endocrinol 2011;7:431–42. [Google Scholar]
- 16. Higashi T, Hijikuro M, Yamagata K, Ogawa S.. Influence of saliva flow rate stimulated by gum-chewing on salivary concentrations of catecholamine metabolites. Clin Chim Acta 2012;414:248–52. [DOI] [PubMed] [Google Scholar]
- 17. Kennedy B, Dillon E, Mills PJ, Ziegler MG.. Catecholamines in human saliva. Life Sci 2001;69:87–99. [DOI] [PubMed] [Google Scholar]
- 18. Eisenhofer G, Lattke P, Herberg M, Siegert G, Qin N, Darr R, et al. Reference intervals for plasma free metanephrines with an age adjustment for normetanephrine for optimized laboratory testing of phaeochromocytoma. Ann Clin Biochem 2013;50:62–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peitzsch M, Mangelis A, Eisenhofer G, Huebner A.. Age-specific pediatric reference intervals for plasma free normetanephrine, metanephrine, 3-methoxytyramine and 3-O-methyldopa: particular importance for early infancy. Clin Chim Acta 2019;494:100–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.