Abstract
Some of the biochemical abnormalities underlying schizophrenia, involve differences in methylation and methylating enzymes, as well as other related target genes. We present results of a study of differences in mRNA expression in peripheral blood lymphocytes (PBLs) and post-mortem brains of chronic schizophrenics (CSZ) and non-psychotic controls (NPC), emphasizing the differential effects of sex and antipsychotic drug treatment on mRNA findings. We studied mRNA expression in lymphocytes of 61 CSZ and 49 NPC subjects using qPCR assays with TaqMan probes to assess levels of DNMT, TET, GABAergic, NR3C1, BDNF mRNAs, and several additional targets identified in a recent RNA sequence analysis. In parallel we studied DNMT1 and GAD67 in samples of brain tissues from 19 CSZ, 26 NPC. In PBLs DNMT1 and DNMT3A mRNA levels were significantly higher in male CSZ vs NPC. No significant differences were detected in females. The GAD1, NR3C1 and CNTNAP2 mRNA levels were significantly higher in CSZ than NPC. In CSZ patients treated with clozapine, GAD-1 related, CNTNAP2, and IMPA2 mRNAs were significantly higher than in CSZ subjects not treated with clozapine. Differences between CSZ vs NPC in these mRNAs was primarily attributable to the clozapine treatment. In the brain samples, DNMT1 was significantly higher and GAD67 was significantly lower in CSZ than in NPC, but there were no significant sex differences in diagnostic effects. These findings highlight the importance of considering sex and drug treatment effects in assessing the substantive significance of differences in mRNAs between CSZ and NPC.
Keywords: schizophrenia, mRNA biomarkers, lymphocyte vs brain, epigenetics, DNMTs, GABAergic
Introduction
Abnormalities in the DNA methylation/demethylation cycle and their effects on the transcriptional activity of genes heavily influenced by the extent of methylation have been reported in schizophrenia and related disorders. Our research group reported increased levels of DNMT1 and DNMT3A mRNAs (DNA writers) and of TET 1–3 (DNA erasers) in the brain and peripheral blood lymphocytes (PBLs) of patients with schizophrenia compared to non-psychotic controls (NPC) (Auta et al., 2013; Grayson & Guidotti, 2013; Zhubi et al., 2009). These changes are accompanied by decreased levels of the GAD67 mRNA in the brain, the enzyme which plays a central role in the production and tonic release of brain GABA (Guidotti et al., 2011a). Additional research also indicated the important role of reduced GABAergic function in schizophrenia pathophysiology (Bristow et al., 2015; Dienel & Lewis, 2019; Huang & Akbarian, 2007; Lewis & Hashimoto, 2007; Mitchell et al., 2015; Rocco et al., 2016). In PBLs from patients with schizophrenia we have previously reported decreases in glucocorticoid receptor (NR3C1) and BDNF mRNAs. These latter changes were seen in some of the same subjects exhibiting increased DNMT1 expression. Our findings suggested that some of the abnormalities in the brains of schizophrenic subjects are also present in PBLs. The extent to which dysfunction of the methylation-demethylation cycle genes and their putative corresponding targets detected in post-mortem brain samples from patients with schizophrenia also replicate in the PBLs of living schizophrenia subjects needs additional confirmation. In this report, we present results from a study of DNA methylation cycle related mRNAs and their targets in a larger cohort of patients with chronic schizophrenia (CSZ) and non-psychiatric control subjects (NPC). We also report qPCR analysis of mRNA’s in PBLs which showed significant changes from a RNA-Seq analysis, which was derived from a sub-sample of the same CSZ and NPC subjects. Finally, we present results of DNMT1 and GAD67 in a larger sample from brains of CSZ and NPC subjects. We examine effects of sex differences and treatment with antipsychotic drugs on the diagnostic differences in mRNA values in PBLs and brain. Ultimately, our goal is to determine the extent that abnormalities exist in the expression of the methylation/demethylation enzyme RNAs and their corresponding target genes in a larger sample of patients with CSZ and NPC and the extent that differences in PBLs and brain are similar.
2. Methods
2.1. Subjects
2.21. Subjects for Lymphocyte Samples Analysis
Subjects were 61 CSZ patients treated with antipsychotic medication, and 49 NPC. (Table 1). CSZ were recruited from the Nathan Kline Institute (NKI) or its associated state hospital, outpatient clinic, and residences. NPC subjects were recruited from NKI research clinic, or from the local community by advertisements. CSZ (35 outpatients, 26 inpatients) had a long history of illness with several hospitalizations and/or years of outpatient clinic treatment. Diagnoses of schizophrenia was made by review of hospital records, using checklists for DSM IV and later DSM V, and supplemented by SCID diagnostic interviews when these were available from other studies. NPC were subjects who never met criteria for schizophrenia, bipolar disorder, major depressive disorder, schizophreniform disorder, or brief or drug-induced psychosis and were not currently treated with antipsychotic or antidepressant medication (see supplement for additional details of selection criteria).
Table 1.
Characteristics of Subjects In Lymphocyte and Brain Samples
| Characteristic | Schizophrenia - CSZ | Control - NPC | Test |
|---|---|---|---|
| (A) LYMPHOCYTE SAMPLES | (n=60 −61) | (n= 49) | |
| Age (m) | 44.7± 9.7 | 35.6 ±10.8 | T=4.64, df=108, P=<0.001 |
| Sex (M/F) (n) | 52/9 | 33/16 | Χ2 4.96=, df=1 P=0.026 |
| Race (W/B/H/A) (n) | 17/39/3/2 | 13/32/2/2 | FET= 1.000 |
| Cigarette smoker (Y/N) (n) | 38/23 | 10/39 | Χ2 =19.38.df=1,P<0.001 |
| Cigarette smoked/wk (m) | 34.6 ±44.2 | 13.0± 28.7 | T=2.95, df=108,P=0.004 |
| Antipsychotic Treatment (lst Gen/2nd gen/combined (n) | 4/38/19 | NR | |
| On Clozapine (Y/N) (n) | 28/33 | NR | |
| On Antidepressant (Y/N/) (n) | 6/52 | 0/49 | |
| On Mood stabilizer (Y/N) (n) | 18/42 | 0/49 | |
| On Valproate (Y/N) (n) | 9/52 | 0/49 | |
| On Benzodiazepine (Y/N) (n) | 22/38 | 0/49 | |
| PANSS Total (m) | 72.0± 14.9 | NR | |
| Urine Tox THC (P/N) (n) | 1/25 | 5/28 | FET=0.22 |
| Urine Tox Cocaine (P/N) (n) | 0/28 | 1/34 | FET=1.00 |
| (B) BRAIN SAMPLES | CSZ (n=19) | NPC (n=26) | |
| Age (m) | 56.3± 18.4 | 57.6 ±18.1 | T=0.23,df=32,P=0.819 |
| Sex M/F (n) | 12/7 | 19/7 | χ2=0.504, df=1, P=0.478 |
| Age of Onset of Illness (m) | 22.6 ± 9.9 | NR | |
| Type of Antipsychotic (not on antipsychotic, 1st gen, 2nd gen, combined 1st and second gen) (n) |
6/7/5/1 | NR | |
| On Clozapine (Yes/No) (n) | 2/17 | NR | |
| On Haloperidol (Yes/No)(n) | 5/14 | NR | |
| On Valproate (Yes/No) (n) | 3/16 | NR | |
| On Mood Stabilizer (Yes/No) (n) | 4/15 | NR |
NR= not relevant; (n)=number of subjects, m= Mean ± S.D., (Y/N) = Yes/No. NR=Not relevant or not available (M/F) = male/female, Race/Ethnicity: W=Caucasian, B=black or African American H=Hispanic, A=Asian. Antipsychotic type: lst Generation antipsychotic, 2nd generation antipsychotic, combined lst and 2nd generation antipsychotic. Statistical tests: Χ2=chi-square, FET=Fishers’ Exact Test. T= t-test.
2.12. Subjects For Post-Mortem Brain Samples Analysis.
Post-mortem brain samples of prefrontal cortex, Brodmann area 9 were obtained from the Harvard-McLean brain resource center. There were 19 CSZ, and 26 NPC (Table 1). This represents a larger set of brain samples, and the full results of this enlarged sample have not been presented in our previous publications(Veldic et al., 2004; Veldic et al., 2005). The storage and preparation of brain samples and the diagnostic procedures were similar to those described in our previous publication(Veldic et al., 2004).
2.2. Clinical Assessments
Psychopathology in CSZ was assessed with the Positive and Negative Symptom Scale (PANSS) by interview by trained research psychiatrists, or research assistants who had achieved at least an ICC of .80 with total PANSS score rating agreement with psychiatrist’s ratings.
2.3. Lymphocyte Sample Collection Extraction and qPCR Analysis
Subject’s blood was collected in 4–8 10 ml EDTA tubes, put in an ice bucket, and rapidly processed. Lymphocyte pellets were extracted and frozen using Ficoll gradient procedures similar to that described previously (Auta et al 2013) (see supplement). RNA was extracted from lymphocyte pellets with a TRIzol procedure (see supplement). First strand cDNA with prepared using the Invitrogen SuperScript VILO cDNA Synthesis kit; up to 2.5 μg RNA was reacted with reagent mix, incubated at 42° C for 60 min, and terminated at 85° C for 5 min. The mixture was frozen (−80° C). For qPCR, TaqMan Universal PCR Master Mix was used for target amplification using the cDNA template and using primer/probes from the TaqMan Gene Expression Assay mix (see Table 2 for probes). Samples were assayed in triplicate, normalized against β-actin as the housekeeping gene, and ddCt=2^(-dt) values calculated. The quality of mRNA was measured for each sample determining RIN quality as previously reported (Gatta et al., 2019).
Table 2-.
Gene Symbols and TaqMan Primers for mRNAs Assayed
| Primer Probe Gene Symbol | Taqman Assay Gene Expression | Gene Name |
|---|---|---|
| ACTB | Hs01060665_g1 | Actin beta |
| DNMT1(custom, cus) | Hs00945875_m1 AIFAT65 | DNA (cytosine-5-)-methyltransferase 1 DNMT1 based on primer sequence F: 5′-CGTCTAGAAAACGGGAACCAAGCAAG-3′ R: 5′-TCTAATCCCAGTTACTTGGGAGGCTG-3′ |
| DNMT1(original, or) | Hs00154749_m1 | DNA (cytosine-5-)-methyltransferase 1 Transcript variant 1 (DNMT1b), 2 (DNMT1a), and variants 3,4 |
| DNMT3A | Hs01027166_m1 | DNA methyltransferase 3 alpha |
| GAD1 | Hs01065893_m1 | glutamate decarboxylase 1 (full length, multiple transcripts) |
| GAD1 (variant GAD67) | Hs01065886_m1 | glutamate decarboxylase 1 (truncated form GAD67) |
| GAD1 (variant GAD25) | Hs00247564_m1 | glutamate decarboxylase 1 (truncated form GAD25) |
| TET1 | Hs00286756_m1 | tet methylcytosine dioxygenase 1 |
| TET2 | Hs00325999_m1 | tet methylcytosine dioxygenase 1 |
| TET3 | Hs00379125_m1 | tet methylcytosine dioxygenase 1 |
| BDNF (broad spectrum-bs) | Hs02718934_s1 | brain derived neurotrophic factor (Taqman Best Coverage) BDNF transcript variants 1–11,12,13,14,17,16,18,x1 |
| BDNFIX (variant 1) | Hs04186202_s1 | brain derived neurotrophic factor BDNF, transcript variant 1 (BDNFIX) |
| Glucocorticoid (NR3C1-best coverage- bc) | Hs00353740_m1 | nuclear receptor subfamily 3 group C member 1 (Taqman Best Coverage) Transcript Variants 1,2,3,4,5,6,7,8,x1,x2,x3,x5,x6,x7 |
| Glucocorticoid (NR3C1)-(custom,cus) |
AIY90ZY custom | nuclear receptor subfamily 3 group C member 1 (NR3C1)-based on primer sequence: F: 5′-CAGCTCCTCAACAGCAACAACA-3′ R: 5′-GTGCTGTCCTTCCACTGCTCT-3 Transcript Variants 1,2,3,4,5,6, x5,x6,x7 |
| MBD4 | AID1VOX | methyl-CpG binding domain 4, DNA glycosylase |
| IMPA2 | Hs00274110_m1 | inositol monophosphatase 2 |
| APBB2 | Hs00921383_m1 | amyloid beta precursor protein binding family B member 2 |
| CPT1A | Hs00912671_m1 | carnitine palmitoyltransferase 1A |
| APOBEC3A | AICXUP | apolipoprotein B mRNA editing enzyme catalytic subunit 3A |
| CCR1 | Hs00928897_s1 | C-C motif chemokine receptor 1 |
| FPRL2 | Hs00266666_s1 | formyl peptide receptor 3 |
| CNTNAP2 | Hs04975510_cn | contactin associated protein-like 2 |
| CD4 | Hs01058407_m1 | CD4 molecule |
Some of the duplicate or custom made probes - DNMT1(cus), (NR3C1-cus)-were chosen so sequences would more closely resemble the primer sequences used in our previous published results (Auta et al, 2013) which used a different method than the TaqMan probes utilized in this report. The differing portions of the gene covered in the original and these duplicate TaqMan probes for the same main gene are further specified in the information under the Gene Name column in the table. The GAD1 full length TaqMan probe contains the transcripts for the variants GAD67, GAD25, and multiple other transcripts (see supplementary data for complete content of all variants).
2.5. Lymphocyte Samples for RNA Seq Analysis
18 NPC and 19 CSZ subjects from the larger sample were used for RNA-Seq analysis(Gatta et al., 2021). Total RNA, including small RNAs, were purified from frozen lymphocyte pellets using miRNeasy Mini kit (QIAGEN) followed by 15 min DNase digestion. The quality of isolated RNAs, assessed by RNA integrity number (RIN) measurements, were between 7 and 9. Details of the methods of library preparation and RNA-Seq analysis of these samples are presented in a separate paper which also presents full results of the RNA-Seq analysis(Gatta et al., 2021). The expression values were analyzed using a statistical model that accounts for sex and age. Measurements of ACTB mRNA levels were performed and used as a normalizing standard. The current report presents results of qPCR analysis in the larger original sample, from which the RNA-Seq sub sample was derived, of a few selected genes which showed significant changes in the RNA -Seq analysis.
2.6. DNMT1 and GAD67 Analysis in Brain Samples
In these experiments, DNMT1 and GAD1 mRNA expression were measured by in situ hybridization. To visualize the DNMT1 mRNA signal, 40-mm-thick free-floating sections were incubated with a mixture of two antisense oligonucleotide probes complementary to 1627–1650 and 4801–4824 bps of the human DNMT1 cDNA (accession no. NM001379)). To detect GAD67 mRNAs, we hybridized the slices with antisense probes 1063–1086 and 2674–2697 of the human GAD67 cDNA (GenBank accession no. NM000817). The specificity of these probes was previously described in detail in Veldic et al(Veldic et al., 2004). In situ hybridization was performed according to Rodriguez et al(Rodriguez et al., 2002) and Veldic et al.(Veldic et al., 2004). The criteria used for unbiased, three dimensional counts and for determining the reproducibility of our measurements, are described in detail in the supplementary text which is published on the PNAS web site corresponding to Veldic et al(Veldic et al., 2004). Cell counts were performed using confocal microscopy at a magnification of 40 μm to obtain serial optical sections from each. This method allows counts of diaminobenzidine-positive cells in a counting box (100 × 100 × 20 μm) by using the modified procedure by Veldic et al.(Veldic et al., 2004) of the three-dimensional cell-counting procedure described by Williams and Rakic (Williams & Rakic, 1988). The cells counted in each box were corrected for tissue shrinkage, which in our experimental conditions was between 30% and 35%. The thickness of each section was measured by confocal microscopy, focusing from the upper to the lower surface of the specimen on sections with a division spacing of 2 μM according to the specifications of the manufacturer (Leica, Wetzlar, Germany). To count strongly stained cells and exclude weakly stained cells or nonspecifically labeled cells from the analysis, the threshold intensity of staining was established at 3X the background (measured by Leica Confocal Software). Final values are expressed as DNMT1 or GAD67 mRNA positive neurons/mm3×102.
2.7. Statistical Analysis
Statistical analysis was performed with SPSS 25, using parametric (t-tests, general linear model [GLM] Anova) and non-parametric (Mann-Whitney U [MWU], Chi-square) analyses. Statistical significance was set at P<.05, 2 -tailed. We examined mRNA and other variables for normality, and where the distributions markedly deviated from normality we attempted transforms (log, Ln, square root) to normalize the distributions. Transforms were performed on original variables multiple by 103 or 104 where appropriate. Where the distributions remained markedly abnormal even after transformations, we used a non-parametric test (MWU, Kruskal-Wallis). To assess the relationship between mRNA variables or between other variables we used Pearson (r) and/or Spearman (rho) correlation analysis and scatter plots. To assess corrected significance levels in multiple comparisons for a specific variable or correlations, we used BH procedures with protected significance level (at α=.05) (Benjamini & Hochberg, 1995; Hsueh et al., 2003). Logistic regression analysis was performed with programs in Stata 15 and SPSS.
3. Results
3.1. Subjects Characteristics
Subject characteristics in the CSZ and NPC lymphocyte and post-mortem brain groups are presented in Table 1. Although we had tried to match our PBL CSZ and NPC subjects on relevant background characteristics, our CSZ subjects were significantly older and had more males than our control subjects, and had a higher rate of cigarette smoking; 62% of our CSZ were current cigarette smokers, compared to the 20% of our NPC subjects. No CSZ subjects in this sample had very high levels of cigarette smoking (i.e. >20 cigarettes/day), in contrast to our previously reported sample (Auta et al., 2013). PANSS scores varied from low and well stabilized to highly symptomatic (PANSS mean 73, range 32–102). 46% of the CSZ patients were treated with clozapine. Subject characteristics from whom brain samples were obtained were considerably older than the subjects in the PBL sample, and only 2 of the 19 (10.5%) CSZ were on clozapine.
3.2. mRNA Levels in PBLs of CSZ vs NPC
Table 3 shows the main results of the comparison between mRNA levels in PBLs of CSZ vs NPC subjects. DNMT3A was significantly higher in in CSZ than NPC, and DNMT1 was higher in CSZ but only in male subjects. The GABAergic mRNA levels were much lower in PBLs than most of the mRNA of other genes. However, GAD1, which more broadly covers multiple GAD transcripts, showed significantly higher levels in CSZ than NPC. The more specific GAD67 or GAD25 mRNAs transcripts and the ratio of GAD25/GAD67 transcripts showed no statistical difference between CSZ and NPC; when an outlier in the NPC group was removed the GAD67 mRNA in male CSZ was higher than NPC (Z=2.09, P=0.037) (see figure S1). We also studied whether there were changes in the expression of various TET protein members that contribute to DNA demethylation (Dong et al., 2012). There were no significant differences in TET enzyme levels in CSZ vs NPC, although TET3 showed a trend (P=0.056) for higher levels in CSZ than NPC. Results on PBLs from 12 CSZ and 12 NPC showed no statistical differences in MBD4 or APOBEC3A mRNA levels between the two groups (Table S1).
Table 3.
Comparisons of Lymphocyte mRNA Values in Schizophrenics and Controls
| mRNA | Schizophrenics (CSZ) | Controls (NPC) | Test |
|---|---|---|---|
| DNMT | |||
| DNMT1 (or) | |||
| All Subjects | 128.92 ± 53.17 (n=29) | 115.21 ± 53.38 (n=31) | TL= −1.202, df=58, P=0.234 |
| Males | 136.65 ± 56.61 (n=22) | 101.45 ± 25.49 (n=19) | TL= 2.544, df=39, P=0.015* |
| Females | 104.61 ± 32.70 (n=7) | 136.98 ± 76.57 (n=12) | TL= 1.001, df=17, P=0.331 |
| DNMT1 (cus) | |||
| All Subjects | 13.69 ± 8.16 (n=38) | 10.83 ± 5.02 (n=33) | T= 1.746, df= 69, P=0.085 |
| Males | 14.38 ± 8.66 (n=31) | 10.05 ± 3.98 (n=22) | TU=2.446, df= 44.86, P=0.018 |
| Females | 10.65 ± 4.75 (n=7) | 12.41 ± 6.56 (n=11) | T=0.611, df= 16, P=0.550 |
| DNMT3A | |||
| All Subjects | 201.27 ± 83.28 (n=36) | 162.27 ± 48.76 (n =31) | TU=2.376, df= 57.73, P=0.021* |
| Males | 213.09 ± 86.81 (n=28) | 157.17 ± 42.46 (n=19) | TU=2.931, df= 41.63, P=0.005*A |
| Females | 159.90 ± 55.69 (n=8) | 170.35 ± 58.45 (n=12) | T= 0.399, df=18, P= 0.695 |
| GABAERGIC RELATED | |||
| GAD1 | |||
| All subjects | 0.24 ± 0.28 (n=36) | 0.083 ± 0.092 (n=31) | TUL=2.965, df=60.56, P=0.004*A |
| Males | 0.28 ± 0.30 (n=28) | 0.094 ± 0.11 (n=19) | TUL=2.749, df=45.00, P=.009* |
| Females | 0.12 ± 0.15 (n=8) | 0.065 ± 0.050 (n=12) | TL=0.700, df=18, P=0.493 |
| GAD67 | |||
| All subjects | Median 0.024 (n=37) | Median 0.019 (n=33) | MWU Z= 1.571, P=0.116b |
| Males | Median 0.035 (n=30) | Median 0.020 (n=22) | MWU Z= 1.741, P=0.082b |
| Females | Median 0.018 (n=7) | Median 0.019 (n=11) | MWU Z= −0.556, P= 0.596c |
| GAD25 | |||
| All subjects | 0.164 ± 0.322 (n=37) | 0.068 ± 0.106 (n=33) | TL= 1.623, df= 68, P= 0.109 |
| Males | 0.195 ± 0.351 (n=30) | 0.066 ± 0.085 (n= 22) | TL= 1.813, df= 50, P= 0.076 |
| Females | 0.032 ± 0.025 (n=7) | 0.074 ± 0.144 (n=11) | TL=0.718, df= 16, P= 0.483 |
| GAD25/GAD67 Ratio | |||
| All subjects | Median 1.65 (n=37) | Median 1.92 (n=33) | MWU Z= −1.171, P= 0.242c |
| Males | Median 1.52 (n=30) | Median 2.18 (n=22) | MWU Z=- 0.963, P=0.335c |
| Females | Median 1.76 (n=7) | Median 1.79 (n=11) | MWU Z= −0.618, P= 0.659c |
| TET | |||
| TET1 | |||
| All subjects | 28.26 ± 13.02 (n=36) | 26.71 ± 12.84 (n=31) | T= 0.487, df=65, P=0 .628 |
| Males | 29.42 ± 13.75 (n=28) | 28.76 ± 13.89 (n=19) | T= 0.161 df=45, P= 0.873 |
| Females | 24.18 ± 9.69 (n=8) | 23.47 ± 10.72 (n=12) | T= 0.151, df= 18, P=0.882 |
| TET2 | |||
| All subjects | 327.90 ± 128.82 (n=36) | 287.93 ± 145.45 (n=30) | T= 1.184, df= 64, P= 0.241 |
| Males | 327.87 ± 123.53(n=28) | 286.88 ± 108.14 (n=19) | T= 1.17, df=45, P= 0.247 |
| Females | 328.01 ± 155.52 (n=8) | 289.74 ± 200.74 (n=11) | T=−0.44, df=17, P=0. 659 |
| TET3 | |||
| All subjects | 194.97 ± 67.74 (n=36) | 164.45 ± 59.35 (n=31) | T=1.947, df= 65, P=0.056 |
| Males | 198.50 ± 66.67 (n=28) | 176.80 ± 57.32 (n=19) | T=1.157, df= 45, P=0.253 |
| Females | 182.62 ± 74.63 (n=8) | 144.88 ± 59.57 (n=12) | T= 0.449, df= 17, P=0.659 |
| Other Genes Expression Influenced by Methylation of CpG Targets of Promotor Gene | |||
| BDNF | |||
| BDNF (bs) | |||
| All subjects | 20.62 ± 8.45 (n=36) | 18.03 ± 11.67 (n=31) | TL= 1.593, df=65, P=0.116 |
| Males | 21.72 ± 9.10 (n=28) | 19.82 ± 14.38 (n=19) | TL= −1.074, df=45, P=0.289 |
| Females | 16.77 ± 4.00 (n=8) | 15.20 ± 4.36 (n=12) | TL= 0.887, df=18, P= 0.387 |
| BDNFIX | |||
| All subjects | 3.52 ± 2.32 (n=38) | 3.01 ± 2.22 (n=33) | TL= 1.373, df=69, P= 0.174 |
| Males | 3.79 ± 2.47 (n=31) | 2.93 ± 2.12 (n=22) | TL= 1.637, df=51, P= 0.108 |
| Females | 2.32 ± 0.81 (n=7) | 3.16 ± 2.51 (n=11) | TL= 0.52, df=16, P= 0.609 |
| GLUCOCORTICOID RECEPTOR | |||
| NR3C1(bc) | |||
| All subjects | 281.30 ± 117.50(n= 35) | 175.45 ± 89.50 (n=31) | TSR= 4.393, df=64, P<0. 001*A |
| Males | 288.02 ± 116.54 (n=27) | 168.61 ± 97.15 (n=19) | TSR= 4.157, df=44, P<0.001*A |
| Females | 258.6 ± 125.92(n=8) | 186.28 ± 78.69 (n=12) | TSR= 1.461, df=18, P= 0.161 |
| NR3C1 (cus) | |||
| All subjects | 144.26 ± 54.36 (n=38) | 119.25 ± 51.13 (n=33) | T= 1.988, df=69, P= 0.051 |
| Males | 148.01 ± 58.24 (n=31) | 119.31 ± 43.51 (n=22) | T= 1.954, df=51, P=0.056 |
| Females | 127.64 ± 29.49 (n=7) | 119.11 ± 66.25 (n=11) | T= .318, df=16, P= 0.754 |
| mRNA’s Which Were Preliminary Hits from RNA Sequence Analysis | |||
| FPRF3 | |||
| All subjects | 20.45 ± 22.78 (n=36) | 28.53 ± 20.97 (n=30) | TL= 2.276, df=64, P= 0.026 |
| Males | 20.29 ± 20.27 (n=28) | 30.29 ± 23.41 (n=18) | TL= 1.964, df= 44, P= 0.056 |
| Females | 20.98 ± 31.78 (n=8) | 25.88 ± 17.32 (n=12) | TL= 1.290, df=18, P= 0.213 |
| CD4 | |||
| All subjects | 139.22 ± 118.11 (n=35) | 124.64 ± 113.89 (n=30) | TL= 0.824, df=63, P= 0.413 |
| Males | 137.08 ± 127.37 (n=27) | 121.14 ± 89.48 (n=18) | TL= 0.402, df=44, P=0.689 |
| Females | 146.44 ± 86.16 (n=8) | 129.87 ± 147.56 (n=12) | TL=0.628, df=18, P= 0.435 |
| CCR1 | |||
| All subjects | Median 4.27 (n=36) | Median 6.95 (n=29) | MWU Z= 0.132, P= 0.895b |
| Males | Median 3.93 (n=28) | Median 6.61 (n=18) | MWU Z=−0.450, P= 0.653c |
| Females | Median 11.84 (n=8) | Median 7.00 (n=11) | MWU Z= 1.239, P= 0.215b |
| APPB2 | |||
| All subjects | 8.13 ± 7.93 (n=38) | 6.25 ± 5.30 (n=33) | TL=1.298, df=69, P=0.199 |
| Males | 8.22 ± 7.30 (n=31) | 6.61 ± 5.76 (n=22) | TL=1.117, df=51, P= 0.269 |
| Females | 7.78 ± 11.01 (n=7) | 5.52 ± 4.40 (n=11) | TL= 0.377, df= 16, P=0.711 |
| CPT1 | |||
| All subjects | 226.53 ± 174.22 (n=36) | 281.15 ± 211.04 (n=33) | T=1.176, df=67, P=0.244 |
| Males | 229.64 ± 180.02 (n=33) | 269.19 ± 229.24 (n=22) | T= 0.703, df=51, P=0. 485 |
| Females | 207.20 ± 148.33 (n=5) | 305.06 ± 176.69 (n=11) | T= 1.073, df= 14, P= 0.301 |
| CNTNAP2 | |||
| All subjects | 0.37 ± 1.15 (n=36) | 0.04 ± 0.05 (n=33) | TLU= 3.047, df= 59.10, P=0.003*A |
| Males | 0.44 ± 1.27 (n=29) | 0.04 ± 0.05 (n=22) | TLU= 3.126, df= 47.19, P=0.003*A |
| Females | 0.06 ± 0.08 (n=7) | 0.04 ± 0.07 (n=11) | TL=0.283, df= 16, P=0.781 |
| IMPA2 | |||
| All subjects | 38.38 ± 32.89 (n=36) | 26.10 ± 18.76 (n=33) | TSR= 1.603, df=67, P=0.114 |
| Males | 38.63 ± 33.30 (n=29) | 28.28 ± 18.92 (n=22) | TSR= 1.063, df= 49, P= 0.293 |
| Females | 37.37 ± 33.69 (n-7) | 21.74 ± 18.52 (n=11) | TSR= 1.007, df= 16, P= 0.329 |
Each number presents Mean ± S.D. For non-normal distributions analyzed by MWU test Median values are presented. Values are ddCt=2^(-dt) * 104; B-Actin was used as housekeeping gene. Values reported in the table are the original values, although for some tests the analysis T-test was performed on the normalized transformed (log, sq root) values. T=independent sample T-test, TL=independent samples t-test based on log transformed values; TU= independent samples T-Test for unequal variances, TSR=independent samples T-test based on square root transformed values, MWU=non-parametric test based on Mann-Whitey U.
statistically significant at BH corrected significance levels (α=.05) using 3 test comparison =i.e. for all subjects, males, females.
= statistically significant BH corrected significant levels (α=.05), when tests for 19 mRNAs were included in protected BH comparison test.
In MWU test mean rank of CSZ higher than NPC.
In MWU test mean rank of CSZ lower than NPC. For full picture of GAD67 mRNA values also see Figure S1 in supplement.
Among the genes which previous research has suggested are substantially influenced by level of methylation of their promoter genes, BDNF and glucocorticoid receptor, mRNA levels of the two BDNF transcripts (BDNF-bs and BDNFIX) were not significantly different in CSZ vs NPC. However, glucocorticoid receptor levels were higher in CSZ than NPC with the NR3C1-bc transcript showing a highly significant difference (P<.001) and the custom transcript NR3C1-cus showing a trend in the same direction (P=.051).
We did additional qPCR analysis on mRNA levels in the entire PBL samples on a few genes which came out as more significant hits in CSZ vs NPC from preliminary results of RNA sequence analysis in PBLs (see Table S2 for details). Of these genes only CNTNAP2 and FPRF3 showed a statistically significant difference between CSZ and NPC. CSZ had lower FPRF3 mRNA levels than NPC, and CSZ had higher levels of CNTNAP2 than NPC. Because the characteristics of the larger PBL sample might differ from the restricted sample we used for RNA-Seq, we also did a similar qPRC analysis in PBLs from those subjects used for RNA Seq. Results of qPCR analysis in this sub-sample were similar to that reported for the full sample in Table 3, but CD4 mRNA was also higher in CSZ than NPC at borderline significance (CSZ- 152.2 ± 128.6, NPC- 81.8 ± 51.9, TL=2.00, df=24, P=0.057).
3.3. Sex Effects On the Differences in mRNA Expression in Lymphocytes of CSZ and NPC Subjects
Sex appeared to be an important mediator of the extent of mRNA differences between CSZ and NPC. Whereas there were significant differences between CSZ and NPC for DNMTs and NR3C1 for male subjects, and strong trends for male subjects in several other mRNAs, there were no significant differences between CSZ and NPC for any mRNA for female subjects. Furthermore, for some of the mRNA comparisons (DNMT1, DNMT3A, GAD25, BDNFIX, CCR1) female CSZ vs NPC showed an opposite trend from males. In GLM analyses there was a statistically significant interaction effect of diagnosis*sex for mRNAs DMNT1(or) (F=5.144, df=1,56, P=0.028) and a trend for DMNT3A (F=3.159, df=1,63, P=0.080). Furthermore, the male/female difference influencing the diagnostic difference between CSZ and NP was not limited to the current sample. Re-analysis of data from an earlier independent sample we published (Auta et al., 2013) revealed a significant difference between CSZ and NPC for DNMT1, TET1, and BDNF mRNA in the male subjects, but not in the female subjects (Table 4).
Table 4.
Re-analysis of Additional Male Female Difference In Lymphocyte mRNA in Earlier Published Sample (Auta et al, 2013)
| mRNA | Schizophrenics (CSZ) | Controls (NPC) | Test |
|---|---|---|---|
| DNMT1 | |||
| Total Sample | 65.80 ± 24.43 (n=27) | 49.65 ±16.88 (n=20) | TU=2.68, df=44.8, P=0.01 |
| Male | 71.93 ± 23.73 (n=21) | 54.60 ± 17.78 (n=10) | T=2.05 df=29, P=0.05 |
| Female | 44.33 ± 11.76 (n=6) | 44.70 ±15.20 (n=10) | T=.050, df=14, P=0.96 |
| TET1 | |||
| Total Sample | 2.73 ±1.09 (n=25) | 1.82± 0.79 (n=18) | T=3.00, df=41, P=0.005 |
| Male | 2.82 ±1.17 (n=20) | 1.76 ± 0.70 (n=7) | T=2.25, df=25, P=0.034 |
| Female | 2.35 ± 0.69 (n=5) | 1.86± 0.87 (n=11) | T=1.110, df=14, P=0.29 |
| BDNF | |||
| Total Sample | 0.26 ± 0.15 (n=18) | 0.38 ± 0.16 (n=11) | T=2.01, df=27, P=0.055 |
| Male | 0.22 ± 0.10 (n=11) | 0.44 ± 0.18 (n=5) | T=3.32, df=14, P=0.005 |
| Female | 0.34 ± 0.19 (n=7) | 0.33 ± 0.13 (n=6) | T=0.11, df=11, P=0.91 |
Each value represents Mean ± S.D. T= independent sample T-test. TU= independent sample T-test for unequal variances.
3.4. Potential Drug Effects on mRNAs-Clozapine Treatment
Studies suggest that the potential effects of antipsychotic or other psychotropic drugs on promoter methylation/demethylation or on histone acetylation or methylation processes may be relevant to explore whether drugs interacting with the epigenome contribute to any differences we detect between CSZ and NPC. Since all the CSZ were treated with antipsychotics, and some with other psychotropic medications, we were unable to compare results from drug free versus drug treated CSZ. However, we were able to compare the mRNA levels in CSZ treated with a few specific antipsychotics, clozapine, olanzapine, or haloperidol, to CSZ not treated with these antipsychotics when there were sufficient subjects to make comparisons. Since there is strong evidence from animal studies that valproate may affect methylation and methylation enzymes(Dong et al., 2010; Guidotti et al., 2011b), we also compared mRNA in CSZ concurrently treated with valproate to those not treated with valproate.
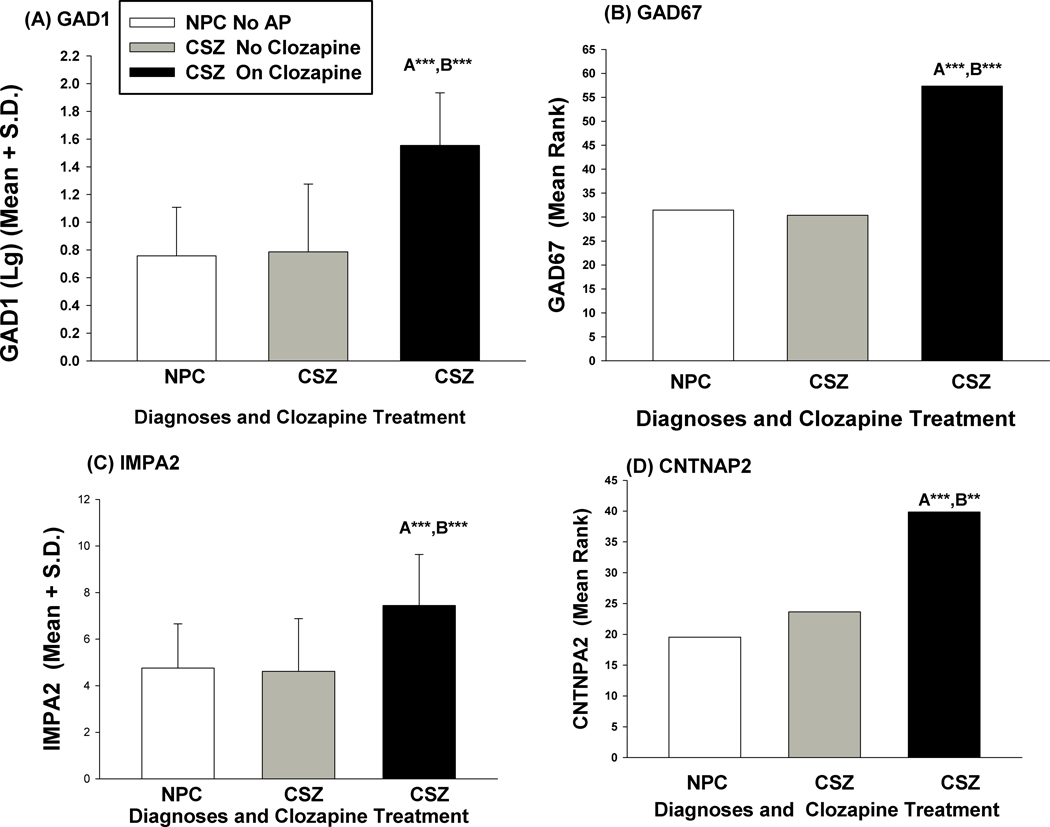
There were no significant effects of concomitant treatment with valproate vs non-valproate on any of the mRNAs we assessed in PBLs. However, there were significant effects of clozapine on levels of some mRNA levels in CSZ (Table 5). In CSZ on clozapine, GABAergic (GAD1, GAD67, GAD25), CNTNAP2, and IMPA2 mRNAs were significantly higher and TET 1 significantly lower than in patients not on clozapine. When we analyzed differences between CSZ and NPC subjects, incorporating the clozapine treatment variable, it was clear that for the GABAergic mRNAs (GAD1, GAD67, GAD25), and for NR3C1-cus, CNTNAP2, and IMPA2, clozapine treatment was primarily responsible for the difference of CSZ vs. NPC, whereas non-clozapine treated CSZ had mRNA values more similar to NPC controls (Figure 1 and Table S4). For DNMT3A there was still a significant difference between CSZ and NPC in males when clozapine status was added as an additional factor in a complex GLM analysis (diagnosis effect F=9.16, df=1,44, P=0.004), but those CSZ not on clozapine showed a larger difference from NPC. There was no effect of treatment vs non-treatment with olanzapine or haloperidol on mRNA values (see supplement).
Table 5.
Effects Of Clozapine On mRNAs In Subjects With Schizophrenia
| mRNA | On Clozapine | Not on Clozapine | Comparison Test |
|---|---|---|---|
| DNMT3A(m) | 188.87±84.45 (n=13) | 231.49±86.36 (n=15) | T=−1.36, df=1,26, P=.200 |
| GAD1 | 0.469±0.296 (n=14) | 0.102±0.156 (n=22) | TL=5.70, df=1,34, P<.001 |
| GAD67 | 0.195± 0.148 (n=12) | 0.035± 0.062 (n=25) | MWU Z=3.76, P<.001 |
| GAD25 | 0.388±0.497 (n=12) | 0.006±0.007 (n=25) | TL=4.01, df=1,35, P<.001 |
| TET1 | 22.16 ±11.44 (n=14) | 32.61±12.98 (n=22) | T=−2.46, df=1,34, P=.019 |
| NR3C1-cus | 170.50 ±73.00 (n=13) | 130.61±36.40 (n=25) | TU=1.85, df=1,15.2, P=.083 |
| CNTNAP2 | 0.80 ± 1.83 (n=13) | 0.07 ± 0.11 (n=23) | TL= 4.12, df=34, P<.001 |
| IMPA2 | 59.89 ± 36.73 (n=13) | 26.23 ± 23.61 (n=23) | TSR= 3.640, df=34, P=.001 |
Each umber represents Mean ± S.D. See legend to Table 4 for interpretation of symbols in comparison test results and interpretation of mean values. (m)=male subjects only.
Figure 1. Effects of Clozapine on Difference Between Schizophrenics (CSZ) and Controls (NPC).
‘A’ significance of difference CSZ On Clozapine vs. NPC, ‘B’ significance of difference CSZ On Clozapine vs CSZ No-Clozapine ***P<.001, ** P<.01.
(A) GAD1 (log), Univariate GLM- Overall Group Effect: F= 24.32, df=2,64, P<.001
(B) IMPA2 (square root), Univariate GLM Overall Group effect: F= 9.223, df=2,66, P<.001
(C) GAD67, non-parametric Kruskal-Wallis test, Test Statistic 16.71, df=2, P<.001. Pairwise Comparisons tested (with Bonferroni corrections) CSZ On Clozapine vs NPC standardized test statistic=3.772, P<.001, CSZ On Clozapine vs CSZ No Clozapine standardized test statistic =3.774, P<.001.
(D) CNTNAP2, non-parametric Kruskal-Wallis test, Test Statistic 18.06, df=2, P<.001. Pairwise Comparisons tested (with Bonferroni corrections) CSZ On Clozapine vs NPC standardized test statistic=4.218, P<.001, CSZ On Clozapine vs CSZ No Clozapine standardized test statistic =3.240, P=.004.
No AP = No antipsychotic medication.
3.6. Effects of Other Background Variables
Table 1 indicated that there were significant differences in age, and cigarette smoking between the CSZ and NPC subjects in the PBL sample, and we, therefore, investigated whether these differences significantly influenced the differences between mRNA levels between CSZ and NPC. Cigarette smoking modulated the effects of CSZ vs NPC diagnosis on differences in several mRNAs. There was a correlation between number of cigarettes smoked/wk and DNMT1-cus levels in patients with schizophrenia (r=0.389 p=0.016), and GLM analysis showed a significant interaction of effects of diagnosis*cigarette smoker status for the total sample (F=5.693, df=,1,67 p=0.020) and the male subjects (F=4.382, df=1,49, p=0.042) with CSZ smokers showing a greater increase in DNMT1 compared to NPC than non-smokers. There were some small (r’s=.24–30) but statistically significant correlations between smoking on some other mRNAs - GABAergic, NR3C1, FPRF3- but there were no statistically significant interactions of diagnosis x cigarette smoking status for these mRNAs (see supplement). There were modest (r’s=.20 −.30) statistically significant correlations between age and DNTM1(or) or NR3C1-bc, but age was not a significant effect when it was added as a covariate to the GLM analysis (see supplement).
3.7. Relationship Between DNMT, GABAergic, Glucocorticoid and BDNF mRNAs.
In post-mortem brain samples of CSZ, increased DNMT1mRNA has been accompanied by decrease in GABAergic mRNA, specifically GAD67. In addition, control of the expression of glucocorticoid receptor and BDNF genes are thought to be heavily influenced by degree of methylation of their promotor genes. We had previously reported a decrease in glucocorticoid and BDNF mRNAs, in the same PBL samples in which we found an increase in DNMT1 and a negative correlation between higher levels of DNMT1 and BDNF mRNAs (Auta et al., 2013). On the basis of these prior results we hypothesized that there would be a negative correlation between DNMT1 or DNMT3A with the GABAergic, NR3C1 and BDNF mRNAs in PBLs. However, we found significant positive correlations between DNMT1 or DNMT3A and the GABAergic, NR3C1, and BDNF mRNAs (Table 6, Figure S2).
Table 6.
Correlations Between DNMT mRNAs and GABAergic, BDNF and Glucocorticoid mRNAs.
| mRNA | DNMT1(or) (L) | DNMT1-cus | DNMT3A | ||||||
|---|---|---|---|---|---|---|---|---|---|
| All Subjects | CSZ | NPC | All Subjects | CSZ | NPC | All Subjects | CSZ | NPC | |
| GAD1(L) | +0.043 | −0.058 | +0.097 | +0.177 | +0.117 | −0.074 | +0.023 | −0.010 | −0.268 |
| GAD67(Ln) | +0.282* | +0.070 | +0.492** | +0.075 | +0.052 | +0.022 | +0.201 | +0.120 | +0.079 |
| BDNF (bc) (L) | +0.053 | +0.27O | −0.210 | +0.156 | +0.089 | +0.009 | +0.514***B | +0.600***B | +0.344 |
| BDNFIX (L) | +0.352**B | +0.373 | +0.296 | +0.266*B | +0.256 | +0.253 | +0.544***B | +0.589***B | +0.502**B |
| NR3C1 (bc) (SR) | +0.106 | +0.319 | +0.106 | +0.537***B | +.0.465**B | +0.504**B | +0.459***B | +0.394*B | +0.437*B |
| NR3C1-cus | +0.359**B | +0.451* | +0.220 | +0.560***B | +0.543***B | +0.561***B | +0.541***B | +0.518**B | 0.490**B |
Each number is Pearson (r) correlation between indicated mRNAs measured in lymphocytes. N’s All subjects 52–71, Schizophrenics 25–38, NPC controls 27–33. Transformations: (L)=log10, (Ln)= Log2, SR= square root. Significance:
P<.05,
P≤.01,
P≤.001.
= survived protected significance level with BH procedure (α=.05) using the 6 mRNA comparisons. Nonparametric correlations (rho) on non-transformed values were in the same range.
3.8. Relationship Between mRNA Levels and Psychopathology Scores in CSZ
The only correlation between PANSS symptom scores and mRNA levels in PBLs, which survived BH corrected significance levels, was a positive correlation between FPLR3 and PANSS Positive symptoms (r FPLR3(L)= +0.397, P=0.016, rho FPLR3 =+0.449, P=0.006) There were two other nominally significant correlations (PANSS positive symptoms and CCR1 mRNA rho=+0.392, p=.018, PANSS Total score and GAD1 rho=+0.351, P=0.036).
3.9. DNMT1 and GAD67 mRNA in Post-Mortem Brain Samples
DNMT1 mRNA was significantly higher and GAD67 mRNA was significantly lower, in layers 1 and 2 of Brodmann’s area 9 of frontal cortex, in CSZ subjects than NPC. However, there were no significant sex differences in the diagnostic difference effect between CSZ vs NPC (Table 7). DNMT1 was not significantly different in CSZ vs NPC in layer 5. Furthermore, logistic regression analysis showed that most of the difference between CSZ and NPC was explained by differences in the DNMT1 and GAD67 mRNA levels only in layer 2 of the frontal cortex. This analysis showed that GAD67 and DNMT1 levels in layer 2 resulted in correct classification of 17 or 19 schizophrenics and 24 of 26 of the controls (91.1% of subjects) with excellent discrimination (c-statistic =0.97). Higher GAD67 in layer 2 was associated with a lower odds of schizophrenia (OR 0.95; 95% CI 0.92, 0.99) and higher DNMT1 in layer 2 was associated with a higher odds of schizophrenia (OR 1.05; 95% CI 1.01, 1.08) (see supplement for details of analysis). Reelin mRNA was significantly lower in CSZ vs NPC but there was no sex effect on brain reelin values (Table S5). In contrast to PBL results, there was a significant negative correlation between DNMT1 and GAD67 mRNA values in the same subjects for the total sample (r=−0.33, P=.03). There were not sufficient numbers of CSZ on a single antipsychotic to validly perform valid statistical comparisons involving potential antipsychotic drug effects on the brain DNMT1 or GAD67 values and there were no subjects who were drug free. Some trends were in the same direction as seen in the drug effects in PBLs (see supplement).
Table 7.
DNMT1 and GAD67 mRNA in Frontal Cortex in Brains of Subjects with Schizophrenia and Controls
| mRNA | Schizophrenia (CSZ) | Controls (NPC) | Analysis Of Variance or T-Test |
|---|---|---|---|
| DNMT1 Layer 1 | FD= 27.453, df=1,41, P=<.001 FS= 0. 308, df=1,41, P=0.582 FD*S= 0.169, df=1,41, P=0.683 |
||
| All Subjects | 233.11± 43.74 (n=19) | 164.77± 34.70 (n=26) | |
| Males | 237.67±49.22 (n=12) | 165.26 ± 39.160(n=19) | T= 4.540, df=29, P <0.001 |
| Females | 225.29 ± 34.41(n=7) | 163.43 ± 20.354(n=7) | T=−4.094, df=12 P=0.001 |
| DNMT1 Layer 2 | FD= 24.455, df=1,41, P<.001 FS= .207, df=1,41, P=0.652 FD*S= 563, df=1,41, P=0.458 |
||
| All Subjects | 469.89± 67.59 (n=19) | 363.85 ± 68.609 (n=26) | |
| Males | 459.92±50.61 (n=12) | 365.63 ±74.19 (n=19) | T=3.860, df=29, P=0. .001 |
| Females | 487.00 ± 92.04* (n=7) | 359.00± 55.34 (n=7) | T=3.153, df=12, P=0.008 |
| DNMT1 Layer 5 | FD= 0.007, df=1,41, P 0.936 FS= 0.674, df=1,41, P=0.417 FD*S=0.707, df=1,41, P=0.405 |
||
| All Subjects | 189.37± 38.24 (n=19) | 185.08 ±46.23 (n=26) | |
| Males | 198.00 ± 32.91 (n=12) | 185.00± 50.55 (n=19) | T= 0.789, df=29, P=0.437 |
| Females | 174.57 ±44.68 (n=7) | 185.29± 35.16 (n=7) | T= 0.499 df=12, P=0. 627 |
| GAD67 Layer 1 | FD= 12.093, df=1,41, P=.001 FS= 2.577, df=1,41, P=0.116 FD *S= 0.193, df=1,41, P=0 663 |
||
| All Subjects | 160.79 ± 30.70 (n=19) | 201.65 ± 35.37 (n=26) | |
| Males | 165.42± 34.37 (n=12) | 207.58 ± 35.97 (n=19) | T= 3.233, df=29, P=.003 |
| Females | 152.86 ± 23.38 (n=7) | 185.57 ± 30.33 (n=7) | T=2.260, df=12, P=0. 043 |
| GAD67 Layer 2 | FD= 24.452, df=1,41, P<.001 FS= 0. 146, df=1,41, P=0.704 FD *S= 0.981, df=1,41, P=0.328 |
||
| All Subjects | 355.15± 62.61 (n=19) | 439.88 ± 52.28 (n=26) | |
| Males | 359.33± 78.26 (n=12) | 433.00± 48.64 (n=19) | T=3.245, df=29, P=.003 |
| Females | 348.00±± 20.88 (n=7) | 458.57 ± 61.11(n=7) | TU=4.530, df=7.382, P=0.002 |
Values are Mean± S.D. DNMT1 and GAD67 mRNA positive neurons/mm3×102
FD=F diagnosis effect; FS=F sex effect; FD*S=F interaction-diagnosis
sex effect. T= 2-sample t-test. TU= two sample
t-test with unequal variances.
4. Discussion
Our study shows that the DNMT1 and DNMT3A abnormalities previously observed in post-mortem brain samples of schizophrenia patients may also exist in lymphocytes of these patients (Auta et al., 2013; Grayson & Guidotti, 2013; Guidotti et al., 2011a; Veldic et al., 2004; Zhubi et al., 2009) and further confirm DNMT1 and GAD67 changes in the frontal cortex of CSZ post-mortem brains. In addition, our results demonstrate that sex and antipsychotic drug treatment may be important variables to consider when interpreting the significance of gene expression in CSZ vs. NPC, especially in PBLs and the concordance of results in brain and PBLs.
Previous research has reported sex differences in the age-related incidence of schizophrenia and response to treatment, the latter especially in relation to adjunctive treatment with estradiol. Females show a lower incidence of schizophrenia compared to males in the younger years, but show similar or increased incidence in older ages above 40 years (Abel et al., 2010). Several studies (Kulkarni et al., 2015; Weiser et al., 2019) have shown that the addition of estradiol to antipsychotic treatment of female schizophrenia improves clinical response, and the most recent study (Weiser et al., 2019) showed that this effect was much greater in female schizophrenics above the age of 38. Our study showed that significant differences between CSZ and NPC in levels of DNMT1, DNMT3A, GAD1, and NRC3C in PBLs were only found in males and not females. Although the smaller number of female subjects could have made finding a statistically significant difference in the female sub-sample more difficult, the pattern of the DNMT results in females showed an opposite direction to that found in males, making such an explanation less likely for these mRNAs. Furthermore, re-analysis of our earlier published data on DNMT1 (Table 4), which showed an effect in PBLs of male CSZ but not female CSZ, confirms this finding in an independent sample. The lower levels of DNMTs in lymphocytes of female schizophrenics may be related to an effect of female sex hormones on DNMT expression. This is supported by a study by Yamagata and associates (Yamagata et al., 2009) which suggested that estrogen and progesterone may down regulate DNMT1 and DNMT3a. The fact that we didn’t find any sex difference in the diagnostic effects of DNMT1 in the post-mortem brain samples may be due to the much older age of females in the brain samples. None of the female controls, and only 1 of the female schizophrenics, was below age 50, suggesting most of these female subjects were likely past menopause and would have had much lower levels of serum estrogen and progesterone than premenopausal women. If lower levels of DNMTs in lymphocytes of younger females with schizophrenics is also reflected in brain levels of these enzymes, this could be one mechanism contributing to the lower incidence of schizophrenia in younger females vs males. Since higher DNMT levels could result in hypermethylation of the GAD67 promoter and consequently lead to lower synthesis of brain GABA which has been implicated as one of the biological mechanism underlying schizophrenia (Grayson & Guidotti, 2013), lower DNMT levels may reduce the level of GABA deficits in younger female schizophrenics and be a plausible explanation for the lower incidence of schizophrenia in younger females. However, an alternative explanation of the lack of sex differences in our brain data could be that DNMT and GAD67 are differentially regulated in brain and peripheral tissue.
The absence of significantly higher DNMT1 and DNMT3A mRNA expression in PBLs female CSZ vs controls, with a trend to lower DNMT levels in female CSZ, compared to the significantly higher level in male CSZ, is also consistent with some other clinical differences in response to antipsychotics in females, especially younger or premenopausal females. Several reviews indicate that women with schizophrenia often have better response to antipsychotics or better overall outcome than men; females often require lower doses to achieve clinical response, although dose differences are limited to certain antipsychotics (Lange et al., 2017; Seeman, 2020). One study showed that the lower doses for female patients may be limited to younger female patients (age 20–39) and older females may require equal or higher doses than men (Seeman, 1983). Another study reported that premenopausal females had a better response to haloperidol or olanzapine than postmenopausal females (Goldstein et al., 2002). If lower levels of DNMT in PBLs of younger females with schizophrenia were also found in their brains, and were associated with less of a deficit in GAD67 and GABA in their brains, this may in part be a plausible explanation for achieving better clinical response using lower doses of antipsychotics. Clozapine in often utilized in patients with schizophrenia who have shown a poor response to several antipsychotics. In our current sample only 11% of female patients with schizophrenia were treated with clozapine whereas 52% of the male patients in the sample were treated with clozapine, which would be consistent with a better response first line antipsychotics in female patients.
Another major influence on the difference between CSZ and NPC in PBLs was the effect of clozapine treatment. This is also likely to involve epigenetic mechanisms. A previous study in mice demonstrated that chronic methionine-induce hypermethylation of reelin and GAD67 and other GABAergic promoters is reversed by clozapine-mediated DNA demethylation of these promoters (Dong et al., 2008). An in-vitro and clinical study by Swathy and associates(Swathy et al., 2018) present evidence that clozapine and several other antipsychotics may alter methylation and DNMT expression in cultured cells, as well as DNA extracted from whole peripheral blood. Another study (Kinoshita et al., 2017) showed both increases and decreases in DNA methylation of a large number of genes in leukocyte samples of patients on long-term clozapine treatment. Our study showed significant effects of clozapine treatment on several PBL mRNA levels in CSZ, and that the difference in GABAergic mRNAs between CSZ and NPC could be explained by clozapine treatments, since only the clozapine treated patients showed a significant increase in PBL mRNA expression. The differences in CNTNAP2 expression between CSZ and NPC was also due to clozapine treatment. In addition, the extent of the NR3C1-cus difference was influenced by clozapine treatment. Although the IMPA2 mRNA expression levels overall were not significantly difference between CSZ and NPC in the total sample, those CSZ on clozapine did show a difference from NPC. We are uncertain whether or how these differences due to clozapine effects are related to any specific epigenetic effects of clozapine cited in the previous literature. Although we did not collect hemological data on our subjects, a previous study (Lee et al., 2015) suggests that clozapine does not affect lymphocyte levels, so differences in lymphocyte number are not a likely explanation of the clozapine drug effects. If the increase GAD1 and GAD67 expression observed in PBLs of CSZ on clozapine were are indicative of similar changes occurring in brain, this could suggest improvement of GABAergic function in brains, and would be consistent with a GABAergic mediation of better clinical response in CSZ treated with clozapine vs. other antipsychotics. Although the data in our brain sample showed slightly higher GAD67 levels in the 2 CSZ on clozapine, this difference from other CSZ not on clozapine was not statistically significant (P>.10). Furthermore, our data suggest that GAD levels in brain and PBLs may not be that closely related (see below) and, therefore, the clinical significance of increase GAD levels in PBLs of clozapine treated CSZ is less clear. Higher GAD1 or GAD67 mRNA levels in PBLs were not negatively correlated with (lower) PANSS scores, as might be expected if higher GAD brain levels were associated with lower psychotic symptoms.
However, our study’s findings emphasize that it is important to consider sex and drug treatment effects in analyzing differences in mRNA and related epigenetic markers between patients with schizophrenia and controls, and that some of the differences in results from different studies may be explained by the differences in the number of patients on clozapine treatment. A survey of some previous studies (Table S6), which focused on GABAergic or related methylation effects in schizophrenia vs controls, found that many studies have not considered these factors systematically (see supplement).
Our results also suggest that the concordance of differences between CSZ and NPC in both brain and PBLs may be limited. Although the increase in DNMT expression in PBLs of male CSZ were similar to the increase in DNMT1 in our brain samples, the changes in GABAergic and glucocorticoid mRNAs in PBLs in this sample of CSZ are not in agreement with some results of differences in CSZ vs NPC in brain. Whereas in the frontal cortex of brains of CSZ we found significantly decreased GAD67 mRNA compared to NPC, in PBLs our results showed significantly increased GAD1 mRNA in CSZ and a trend for increase of the more specific GAD67 which became significant (P=0.037) when one outlier value in the NPC sample was removed. Increased DNMT expression could result in increased methylation of the GAD67 promoter and this epigenetic influence on GAD67 mRNA expression would be consistent with an expected negative correlation between DNMT and GAD67 mRNA. However, although there was a significant negative correlation between DNMT1 and GAD67 values in brain, there were positive correlations between DNMTs and GABAergic mRNAs in PBL samples. These results suggest that GABAergic markers may be differently regulated in PBLs than in brain. However, differences in antipsychotic drug treatment could influence some of the differences between brain and PBL, since 46% of our PBL CSZ were on clozapine compared to 10.5% in the brain sample, and clozapine treatment had a major effect of GABAergic levels in PBLs. Some researchers have also reported decreases in glucocorticoid receptor mRNAs in the frontal cortex of CSZ vs controls (Knable et al., 2002; Sinclair et al., 2012; Sinclair et al., 2011) whereas our results showed increased NR3C1 mRNAs in PBLs of CSZ vs NPC. There was also a positive correlation of DNMT mRNA and glucocorticoid receptor mRNA, which was contrary to our theoretical expectations of negative methylation effects since the glucocorticoid receptor mRNA is thought to be heavily influenced by the degree of methylation of its gene. These results also suggest that glucocorticoid receptor regulation in PBLs may be different than in brain. However, another recent study of CSZ in China using mixed PMCs did report decreased glucocorticoid receptor mRNA in CSZ not currently on antipsychotics vs controls (Liu et al., 2020). Differences in extent of clozapine drug treatment of the CSZ, sex differences in sample composition, as well as differences in assay techniques may contribute to these divergent results (see supplement for further discussion).
One reason we replicated only a few CSZ vs NPC differences in our qPCR analysis of mRNA levels from RNA sequence hits, may relate to important differences in the gene transcripts traditionally measured by qPCR vs RNA Seq analysis. The qPCR product is usually only 100–200 bp long, whereas RNA-Seq measures the entire length of the transcripts and combine multiple transcripts of a gene. So, it is possible that our qPRC assay with the TaqMan probes did not measure the most relevant portions of these genes as compared with the RNA-Seq results. .
Conclusions
Our results show a sex difference in higher DNMT1 and DMNT3A mRNA expression in PBLs in male CSZ vs NPC, both in the current sample and in a re-analysis of those from a previous cohort(Auta et al., 2013) which showed similar sex difference effects. The higher DNMT1 expression in males is similar to the higher DNMT1 in CSZ detected in our post-mortem brain samples. There were also significant effects of clozapine treatment on the expression levels of several mRNAs in PBLs of CSZ, and the clozapine treatment effect was a major influence on the difference between CSZ and NPC in these mRNAs. These factors need further investigation in clinical studies, post-mortem brain analysis, and animal models. Most importantly, the impact of these two variables could also influence the apparent degree of concordance between brain and PBL potential markers in schizophrenia.
Supplementary Material
Acknowledgments
Funding: this research was supported in part by NIH grant 1R01MH101043 and a philanthropic grant to Dr. Smith’s section at NKI.
Role of funding source:
The funding source had no influence over the design, analysis or conclusions of the research.
Footnotes
Conflict of Interest: The authors report no conflicts of interest potentially related to the research reported in this paper.
References:
- Abel KM, Drake R, & Goldstein JM (2010). Sex differences in schizophrenia. Int Rev Psychiatry, 22(5), 417–428. 10.3109/09540261.2010.515205 [DOI] [PubMed] [Google Scholar]
- Auta J, Smith RC, Dong E, Tueting P, Sershen H, Boules S, Lajtha A, Davis J, & Guidotti A. (2013). DNA-methylation gene network dysregulation in peripheral blood lymphocytes of schizophrenia patients. Schizophr Res, 150(1), 312–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y. (1995). Controlling for the false discovery Rate: A practical and power approach to multiple testing. J Royal Statistical Soc., 57(1), :289–300. [Google Scholar]
- Bristow GC, Bostrom JA, Haroutunian V, & Sodhi MS (2015, September). Sex differences in GABAergic gene expression occur in the anterior cingulate cortex in schizophrenia. Schizophr Res, 167(1–3), 57–63. 10.1016/j.schres.2015.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel SJ, & Lewis DA (2019, November). Alterations in cortical interneurons and cognitive function in schizophrenia. Neurobiol Dis, 131, 104208. 10.1016/j.nbd.2018.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong E, Chen Y, Gavin DP, Grayson DR, & Guidotti A. (2010, Nov-Dec). Valproate induces DNA demethylation in nuclear extracts from adult mouse brain. Epigenetics, 5(8), 730–735. 10.4161/epi.5.8.13053 [DOI] [PubMed] [Google Scholar]
- Dong E, Gavin DP, Chen Y, & Davis J. (2012). Upregulation of TET1 and downregulation of APOBEC3A and APOBEC3C in the parietal cortex of psychotic patients [Research Support, Non-U S Gov’t]. Transl Psychiatry, 4(2), 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong E, Nelson M, Grayson DR, Costa E, & Guidotti A. (2008, September 9). Clozapine and sulpiride but not haloperidol or olanzapine activate brain DNA demethylation. Proc Natl Acad Sci U S A, 105(36), 13614–13619. 10.1073/pnas.0805493105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatta E, Grayson DR, Auta J, Saudagar V, Dong E, Chen Y, Krishnan HR, Drnevich J, Pandey SC, & Guidotti A. (2019, June 25). Genome-wide methylation in alcohol use disorder subjects: implications for an epigenetic regulation of the cortico-limbic glucocorticoid receptors (NR3C1). Mol Psychiatry. 10.1038/s41380-019-0449-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatta E, Saudagar V, Drnevich J, Forrest MA,J, , , Clark L, Sershen H, Smith R, Grayson D, Davis J, & Guidotti A. (2021). Concordance of immune-related markers in lymphocytes and prefrontal cortex in schizophrenia. Schizophr Bull Openi, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Cohen LS, Horton NJ, Lee H, Andersen S, Tohen M, Crawford A, & Tollefson G. (2002, May 15). Sex differences in clinical response to olanzapine compared with haloperidol. Psychiatry Res, 110(1), 27–37. 10.1016/s0165-1781(02)00028-8 [DOI] [PubMed] [Google Scholar]
- Grayson D, & Guidotti A. (2013). The dynamics of DNA methylation in schizophrenia(SZ)and related psychiatric disorders. Neropsychopharmacology Reviews, 38(1), 138–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Chen Y, Davis JM, Dong E, Gavin DP, Grayson DR, Matrisciano F, Pinna G, Satta R, Sharma RP, Tremolizzo L, & Tueting P. (2011a). Epigenetic GABAergic targets in schizophrenia and bipolar disorder. Neuropharmacology, 60(7–8), 1007–1016. http://sfx.med.nyu.edu/sfxlcl3?sid=Entrez%3APubMed&id=pmid%3A21074545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Chen Y, Davis JM, Dong E, Gavin DP, Grayson DR, Matrisciano F, Pinna G, Satta R, Sharma RP, Tremolizzo L, & Tueting P. (2011b, June). Epigenetic GABAergic targets in schizophrenia and bipolar disorder. Neuropharmacology, 60(7–8), 1007–1016. 10.1016/j.neuropharm.2010.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh HM, Chen JJ, & Kodell RL (2003). Comparison of methods for estimating the number of true null hypotheses in multiplicity testing [Comparative Study]. J Biopharm Stat, 13(4), 675–689. [DOI] [PubMed] [Google Scholar]
- Huang HS, & Akbarian S. (2007, August 29). GAD1 mRNA expression and DNA methylation in prefrontal cortex of subjects with schizophrenia. PLoS One, 2(8), e809. 10.1371/journal.pone.0000809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M, Numata S, Tajima A, Yamamori H, Yasuda Y, Fujimoto M, Watanabe S, Umehara H, Shimodera S, Nakazawa T, Kikuchi M, Nakaya A, Hashimoto H, Imoto I, Hashimoto R, & Ohmori T. (2017, March 14). Effect of Clozapine on DNA Methylation in Peripheral Leukocytes from Patients with Treatment-Resistant Schizophrenia. Int J Mol Sci, 18(3). 10.3390/ijms18030632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knable MB, Barci BM, Bartko JJ, Webster MJ, & Torrey EF (2002). Molecular abnormalities in the major psychiatric illnesses: Classification and Regression Tree (CRT) analysis of post-mortem prefrontal markers. Mol Psychiatry, 7(4), 392–404. 10.1038/sj.mp.4001034 [DOI] [PubMed] [Google Scholar]
- Kulkarni J, Gavrilidis E, Wang W, Worsley R, Fitzgerald PB, Gurvich C, Van Rheenen T, Berk M, & Burger H. (2015, June). Estradiol for treatment-resistant schizophrenia: a large-scale randomized-controlled trial in women of child-bearing age. Mol Psychiatry, 20(6), 695–702. 10.1038/mp.2014.33 [DOI] [PubMed] [Google Scholar]
- Lange B, Mueller JK, Leweke FM, & Bumb JM (2017, March). How gender affects the pharmacotherapeutic approach to treating psychosis - a systematic review. Expert Opin Pharmacother, 18(4), 351–362. 10.1080/14656566.2017.1288722 [DOI] [PubMed] [Google Scholar]
- Lee J, Takeuchi H, Fervaha G, Powell V, Bhaloo A, Bies R, & Remington G. (2015, October). The Effect of Clozapine on Hematological Indices: A 1-Year Follow-Up Study. J Clin Psychopharmacol, 35(5), 510–516. 10.1097/jcp.0000000000000387 [DOI] [PubMed] [Google Scholar]
- Lewis D, & Hashimoto T. (2007). Deciphering the disease process of schizophrenia: the contribution of cortical gaba neurons. Int Rev Neurobiol, 78, 109–131. [DOI] [PubMed] [Google Scholar]
- Liu Y, Tang Y, Li C, Tao H, Yang X, Zhang X, & Wang X. (2020). Altered Expression of Glucocorticoid Receptor and Neuron-Specific Enolase mRNA in Peripheral Blood in First-Episode Schizophrenia and Chronic Schizophrenia. Front Psychiatry, 11, 760. 10.3389/fpsyt.2020.00760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AC, Jiang Y, Peter C, & Akbarian S. (2015, September). Transcriptional regulation of GAD1 GABA synthesis gene in the prefrontal cortex of subjects with schizophrenia. Schizophr Res, 167(1–3), 28–34. 10.1016/j.schres.2014.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocco BR, Lewis DA, & Fish KN (2016, June 15). Markedly Lower Glutamic Acid Decarboxylase 67 Protein Levels in a Subset of Boutons in Schizophrenia. Biol Psychiatry, 79(12), 1006–1015. 10.1016/j.biopsych.2015.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez MA, Caruncho HJ, Costa E, Pesold C, Liu WS, & Guidotti A. (2002, September 23). In Patas monkey, glutamic acid decarboxylase-67 and reelin mRNA coexpression varies in a manner dependent on layers and cortical areas. J Comp Neurol, 451(3), 279–288. 10.1002/cne.10341 [DOI] [PubMed] [Google Scholar]
- Seeman MV (1983, Mar-Apr). Interaction of sex, age, and neuroleptic dose. Compr Psychiatry, 24(2), 125–128. 10.1016/0010-440x(83)90100-1 [DOI] [PubMed] [Google Scholar]
- Seeman MV (2020, February). Men and women respond differently to antipsychotic drugs. Neuropharmacology, 163, 107631. 10.1016/j.neuropharm.2019.05.008 [DOI] [PubMed] [Google Scholar]
- Sinclair D, Fullerton J, Webster M, & Shannon Weickert C. (2012). Glucocorticoid receptor 1B and 1C mRNA transcript alterations in schizophrenia and bipolar disorder, and their possible regulation by GR gene variants. PLoS One, 7(3), e31720-e31720. http://sfx.med.nyu.edu/sfxlcl3?sid=Entrez%3APubMed&id=pmid%3A22427805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair D, Tsai SY, Woon HG, & Weickert CS (2011, December). Abnormal glucocorticoid receptor mRNA and protein isoform expression in the prefrontal cortex in psychiatric illness. Neuropsychopharmacology, 36(13), 2698–2709. 10.1038/npp.2011.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swathy B, Saradalekshmi KR, Nair IV, Nair C, & Banerjee M. (2018, March). Understanding the influence of antipsychotic drugs on global methylation events and its relevance in treatment response. Epigenomics, 10(3), 233–247. 10.2217/epi-2017-0086 [DOI] [PubMed] [Google Scholar]
- Veldic M, Caruncho H, Liu W, CDavis J, Satta R, Grayson D, Guidotti A, & Costa E. (2004). DNA methyltransferase(DNMT1) is selectively overexpressed in telencephalic cortical GABAergic interneurons in schizophrenic brains. PNAS, 101, 348–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldic M, Guidotti A, Maloku E, Davis J, & Costa E. (2005). In psychosis, cortical interneurons over express DNA-methyltransferase 1. PNAS, 102, 2152–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser M, Levi L, Zamora D, Biegon A, SanGiovanni JP, Davidson M, Burshtein S, Gonen I, Radu P, Slobozean Pavalache K, Nastas I, Hemi R, Ryan T, & Davis JM (2019, October 1). Effect of Adjunctive Estradiol on Schizophrenia Among Women of Childbearing Age: A Randomized Clinical Trial. JAMA Psychiatry, 76(10), 1009–1017. 10.1001/jamapsychiatry.2019.1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RW, & Rakic P. (1988, December 15). Three-dimensional counting: an accurate and direct method to estimate numbers of cells in sectioned material. J Comp Neurol, 278(3), 344–352. 10.1002/cne.902780305 [DOI] [PubMed] [Google Scholar]
- Yamagata Y, Asada H, Tamura I, Lee L, Maekawa R, Taniguchi K, Taketani T, Matsuoka A, Tamura H, & Sugino N. (2009, May). DNA methyltransferase expression in the human endometrium: down-regulation by progesterone and estrogen. Hum Reprod, 24(5), 1126–1132. 10.1093/humrep/dep015 [DOI] [PubMed] [Google Scholar]
- Zhubi A, Veldic M, Puri NV, Kadriu B, Caruncho H, Loza I, Sershen H, Lajtha A, Smith RC, Guidotti A, Davis JM, & Costa E. (2009). An upregulation of DNA-methyltransferase 1 and 3a expressed in telencephalic GABAergic neurons of schizophrenia patients is also detected in peripheral blood lymphocytes. Schizophrenia Research, 111(1–3), 115–122. http://sfx.med.nyu.edu/sfxlcl3?sid=Entrez%3APubMed;id=pmid%3A19386473 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.