Abstract
A chemically programmed bispecific antibody (cp-bsAb) that targeted cysteine protease legumain and αvβ3 integrin has been prepared using the aldolase antibody chemical programming (AACP) strategy. In vitro evaluation of the anti-legumain, anti-integrin cp-bsAb and its comparison with cpAbs targeting either integrin or legumain have shown that the former possesses superior functions, including receptor binding and inhibitory effects on cell proliferation as well as capillary tube formation, among all three cpAbs. The anti-legumain, anti-integrin cp-bsAb also inhibited growth of primary tumor more effectively than either anti-legumain or anti-integrin cpAb as observed in the MDA-MB-231 human breast cancer mouse model. The AACP-based cp-bsAb, which contains a generic aldolase antibody, can also serve as a suitable platform for combination therapy, where two equally potent compounds are used to target extracellular receptors.
Keywords: aldolase, antibody, 38C2, alpha(v)beta(3), integrin, bispecific, chemical, legumain, protease
INTRODUCTION
Cysteine protease legumain and heterodimeric glycoprotein αvβ3 integrin regulate many biological functions in both normal and pathological cells.1,2 Legumain protease, also known as asparaginyl endopeptidase (AEP), is produced by the pH-dependent autoactivation of pro-legumain,3 and is localized in endolysosomes and in the nucleus. It catalyzes activation of several essential proteases, including cathepsin B, H, and L,4 and progelatinase A,5 which may be involved in maintaining protein homeostasis through the regulated proteolysis of intracellular and extracellular proteins.6 Legumain protease also catalyzes proteolysis of the extracellular matrix (ECM) protein fibronectin,7 which binds to integrins besides other ECM components. Integrin αvβ3 is one of 24 heterodimeric integrins that mediate cell–cell and cell–extracellular matrix interactions and play important roles in cell signaling.2,8 However, both legumain and αvβ3 integrin overexpress in many human cancers on tumor cells and/or in angiogenic tumor vasculature, and have emerged as potential targets for the treatment of primary and metastatic human cancers.9,10 Expressions of these proteins strongly correlate with the invasive and metastatic phenotype of a number of solid tumors.2,5,11 The relevance of legumain and αvβ3 integrin as potential cancer targets is further supported, showing that their inhibitors could reduce tumor growth and metastases in animal models of human cancers.9,12
Legumain protease is known to secrete into tumor microenvironments under hypoxia, and to colocalize with αvβ3 integrin on tumor cell surfaces.13 Interaction of legumain and αvβ3 integrin is mediated through the characteristic RGD motif present in the protease. This process leads to an increase in catalytic activity of the fully activated legumain14 and upward shift in the pH profile optimum from pH 5.5 to 6.0 through conformational stabilization of the protease.15 Evidently, legumain protease’s and αvβ3 integrin’s functions are intertwined. We argued that targeting of legumain protease and αvβ3 integrin together using their inhibitors could have superior effects on tumor growth and/or metastasis than when only one of the two proteins is inhibited. To examine this hypothesis, we used a chemically programmed bispecific antibody (cp-bsAb)16,17 that targets legumain protease and αvβ3 integrin, and is prepared by using the aldolase Ab-chemical programming (AACP) strategy.18–21 In this communication, we have described synthesis and evaluation of an anti-legumain, anti-integrin cp-bsAb as well as the analogous anti-legumain and anti-integrin monospecific cpAbs, and show that the former indeed mediated superior therapeutic effects compared to either of the latter two cpAbs.
RESULTS AND DISCUSSION
Chemical programming of an Ab or Ab fragment is an emerging concept that allows one to generate multiple cpAbs using one good Ab and a variety of synthetic inhibitors (organics) possessing a designed linker as the Ab-programming agents (Ab-PAs).22 In some cases, the linker is replaced with an antigen of circulating Abs such that the latter can be recruited to a target of the organics used.23 Upon chemical programming, plasma half-life of a small molecule increases, and the cpAb can discern Ab-dependent cell-mediated cytotoxicity, which we have shown earlier with the anti-integrin cpAbs.10,12,18 For the AACP approach, an Ab-PA is equipped with a lactam24 that reacts selectively with the lysine residues into aldolase Ab, such as 38C2 (or humanized 38C2, i.e., 38C2h), binding sites giving a cpAb, I (Scheme 1). A diketone (DK) or vinyl ketone (VK, as a pro-vinyl ketone) linker19 can also be used; a lactam linker has been used here in most cases for ease of synthesis and stability under reactive conditions. Similarly, a heterodimeric Ab-PA, prepared by combining two equally potent drugs or compounds (comp1 and comp2) through an appropriately functionalized bidentate lactam linker, conjugates with Ab 38C2 giving a cp-bsAb, II. Alternatively, Ab 38C2 can be programmed using a monofunctional lactam linker (LNK-FG), bifunctional lactam linker (LNK-*FG2), or one already armed with a compound (comp1), i.e., (LNK-comp1/FG) to give a cpAb intermediate III (Ab-(NH-LNK-FG)2), IV (Ab-(NH-LNK-*FG2)2), and V (Ab-(NH-LNK-comp1/FG)2), respectively. Subsequently, intermediate III can react with an appropriately functionalized inhibitor or compound (comp1) affording cpAb I, or with a heterodimeric compound (comp1/comp2) giving cp-bsAb II, whereas intermediate IV can react with two equally potent drugs or compounds (comp1 and comp2) and intermediate V with comp2 affording the cp-bsAb II.
Scheme 1. Production of a cpAb or cp-bsAb by Aldolase Ab Programming (AACP) Strategy Using a Fully Functionalized Antibody-Programming Agent (Ab-PA) or Precursorsa.
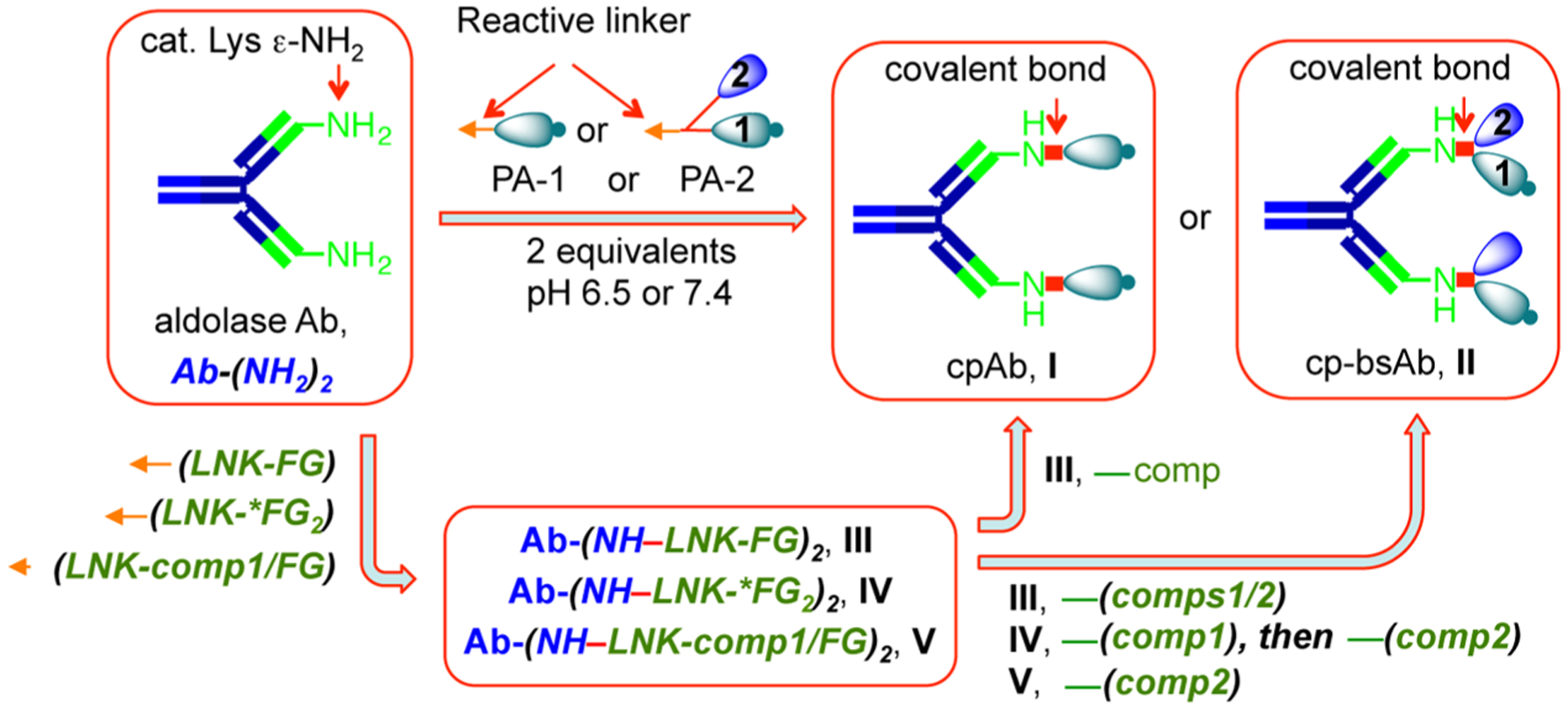
aAb PA or a functionalized linker (* stands for two different linker-functional groups) that can react covalently through a β-lactam (L) function with reactive lysine residues in the aldolase Ab binding sites yielding cpAb, cp-bsAb, and cpAb intermediates. CpAb intermediates further react with compounds (comp1/2) affording a cpAb or cp-bsAb. Linkers and Ab PAs can also react with Ab through the β-diketone (DK) or vinylketone (VK) function. (Ab, antibody. PA, programming agent: a compound fully functionalized with lactam, vinyl ketone, or diketone linker. LNK, linker. FG, functional group.).
First, we prepared a series of mono- and bifunctional lactam linkers (LNK), including LNK1, LNK2, and LNK3 (see Supporting Information, Figure S-1), and examined their conjugation to Ab 38C2. We found that they all conjugated to Ab 38C2 efficiently before or after arming with a small molecule, which could be confirmed using the Methodol assay (see latter, and Supporting Information Figure S-2, left).19 Therefore, we used the fully functionalized Ab-PAs 3, 4, and 5, to prepare anti-legumain and anti-integrin cpAbs, and an anti-legumain, anti-integrin cp-bsAb, respectively (Figure 1). Ab-PA 3 is a legumain inhibitor containing compound 125 as the targeting head and a lactam linker for conjugation with the Ab, and 412 is an αvβ3 integrin inhibitor with compound 226 as the targeting head and a pro-vinylketone linker for conjugation to the Ab; whereas, Ab-PA 5 is a heterodimeric legumain-integrin inhibitor composed of compounds 1 and 2, attached together through a “Y” linker, and a lactam function for conjugation with the Ab. Compounds 3 and 5 were synthesized as described (see Supporting Information Schemes S-1,2). All Ab-PAs, 3–5, were next reacted with aldolase Ab 38C227 affording cpAbs 38C2-3, 38C2-4, and 38C2-5. Here, pro-vinylketone (pro-VK) linker in compound 4 underwent aldolase Ab 38C2-catalyzed retro-aldol reaction yielding VK [4a],28 which subsequently reacted with Ab 38C2 like the LNKs giving 38C2-4.12 After chemical programming of Ab 38C2 using the PAs was complete as assessed by loss of catalytic activity of the reaction mixture using the Methodol assay19,29 (Supporting Information Figure S-2, right), the programmed Abs 38C2-3, 38C2-4, and 38C2-5 were dialyzed using PBS buffer, and used for performing the in vitro and in vivo experiments.
Figure 1.
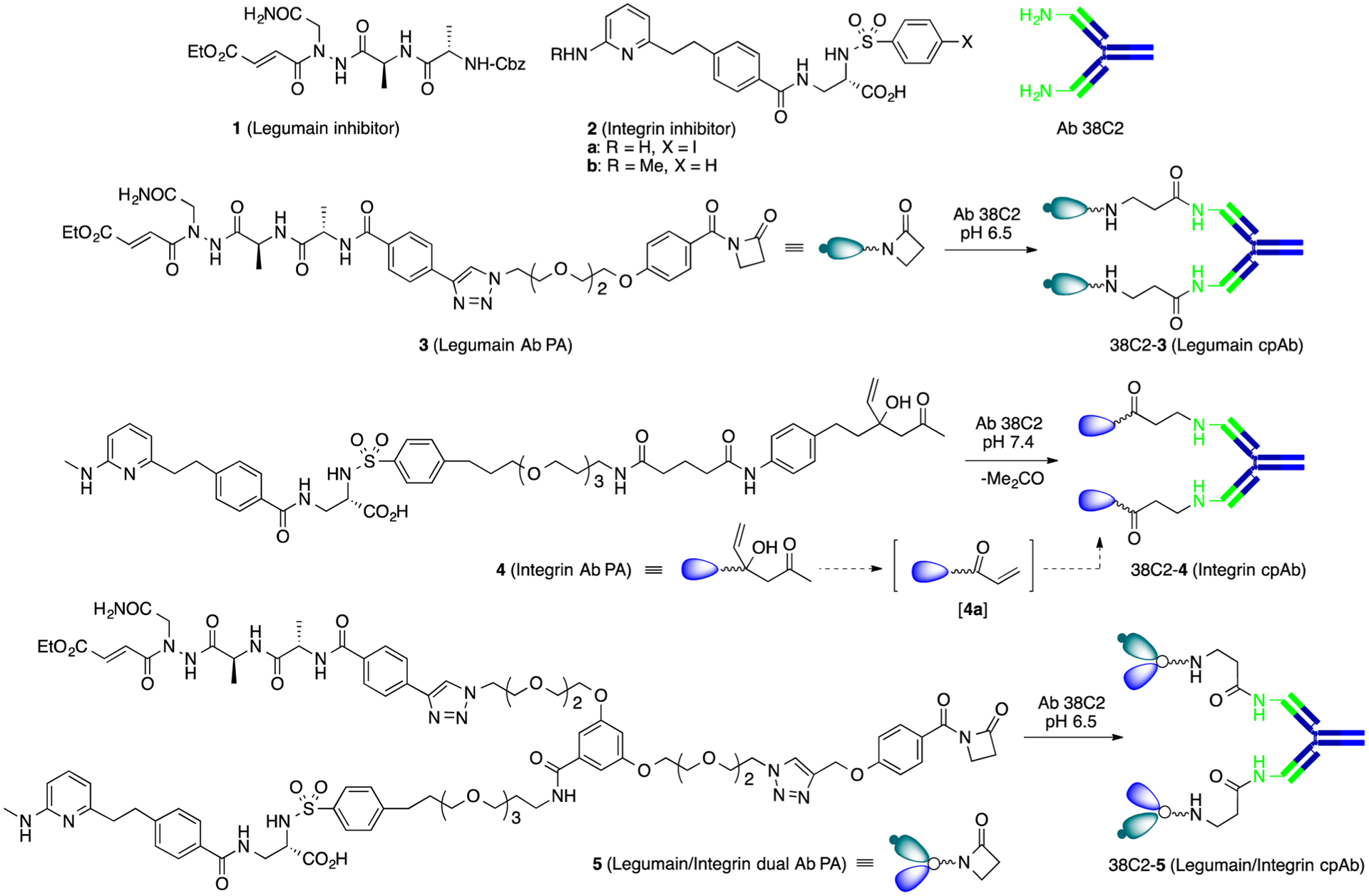
Structure of legumain and αvβ3 integrin inhibitors, the antibody programming agents, and chemically programmed antibodies. Compounds 3–5 reacted with Ab 38C2 at 37 °C for 2–24 h affording anti-legumain, anti-integrin cpAbs (38C2-3, 38C2-4) and cp-bsAb (38C2-5). Compound [4a] is an intermediate produced by an in situ retro aldol reaction of compound 4 before the latter reacts with the Ab. Key: legumain and integrin inhibitors are portrayed in green and blue, respectively.
With the cpAbs and cp-bsAb (38C2-3, 38C2-4, and 38C2-5) in hand, their binding to αvβ3 integrin and legumain protease was first examined using human U87 glioblastoma,30 MDA-MB-231 human breast cancer,31 and LNCaP prostate adenocarcinoma cells.32 Expression and colocalization of both αvβ3 integrin and legumain protease on these cells was confirmed using specific Abs. Using flow cytometry assay, both cpAbs 38C2-3 and 38C2-4 and cp-bsAb 38C2-5 were found binding to these cell lines, and no binding was recorded with the negative control Ab 38C2 (Figure 2A–C). Importantly, the cell stain level was stronger with 38C2-5 as compared to 38C2-3 and 38C2-4, which suggested a superior binding of 38C2-5 to either or both integrin and protease on cell surface. Using ELISA assay, it was also confirmed that 38C2-5 bound to both the purified αvβ3 integrin and legumain protease, whereas 38C2-3 bound to only legumain and 38C2-4 to αvβ3 integrin, as expected (Figure 2D). A superior binding of 38C2-5 compared to 38C2-3 and 38C2-4 in both the cell-based and the purified protein-based in vitro assays is presumably because the former possesses a large linker size than the latter two cpAbs.
Figure 2.
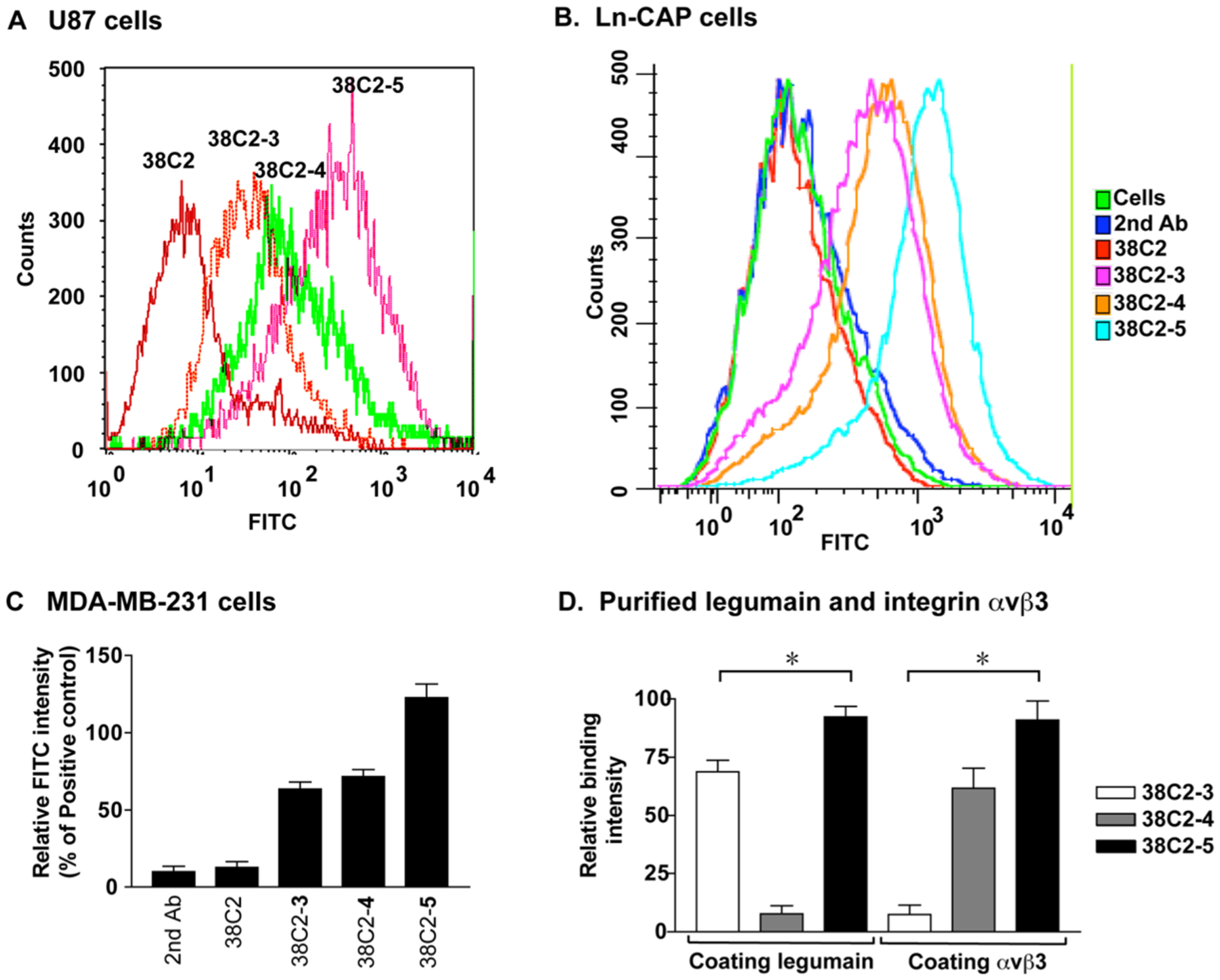
In vitro cellular assays. Flow cytometry histograms showing binding of 38C2-3, 38C2-4, and 38C2-5 to (A) U87 glioblastoma, (B) Ln-CAP prostate cancer, and (C) MDA-MB-231 breast cancer cells. 38C2-5 has a stronger cell binding affinity than 38C2-3 and 38C2-4. The 38C2 Abs and FITC-labeled goat anti-mouse secondary Abs were used as negative controls. (D) ELISA histogram showing specific binding of 38C2-3, 38C2-4, and 38C2-5 to legumain and/or human integrin αvβ3 (*p < 0.001).
Inhibitors of integrin αvβ3 and legumain protease are known to suppress proliferation of tumor cells expressing these proteins, but we have found that chemical programming increases activity of compounds in resulting cpAbs, which we have shown in this study using compound 3 and cpAb 38C2-3 (Supporting Information Figure S-3). An examination of the cpAbs 38C2-3 and 38C2-4 and cp-bsAb 38C2-5 using U87 cells also confirmed that all three chem-Abs inhibited cell proliferation at 1 μM concentration, but effects of 38C2-5 were significantly higher than those of 38C2-3 or 38C2-4. While 38C2-5 completely inhibited proliferation of U87 cells, there was approximately 20% and 60% inhibition by 38C2-3 and 38C2-4, respectively, at the end of day 4 (Figure 3A, left). Cells treated with 38C2-4 or 38C2-5 detached and rounded up to form clusters, i.e., spheroids (Figure 3A, right). In contrast, fewer U87 cells formed spheroids in control (Ab 38C2) and 38C2-3 treated groups. The results were similar to those observed with the integrin α5β1 inhibitors and anti-α5β1 cPAbs.33
Figure 3.
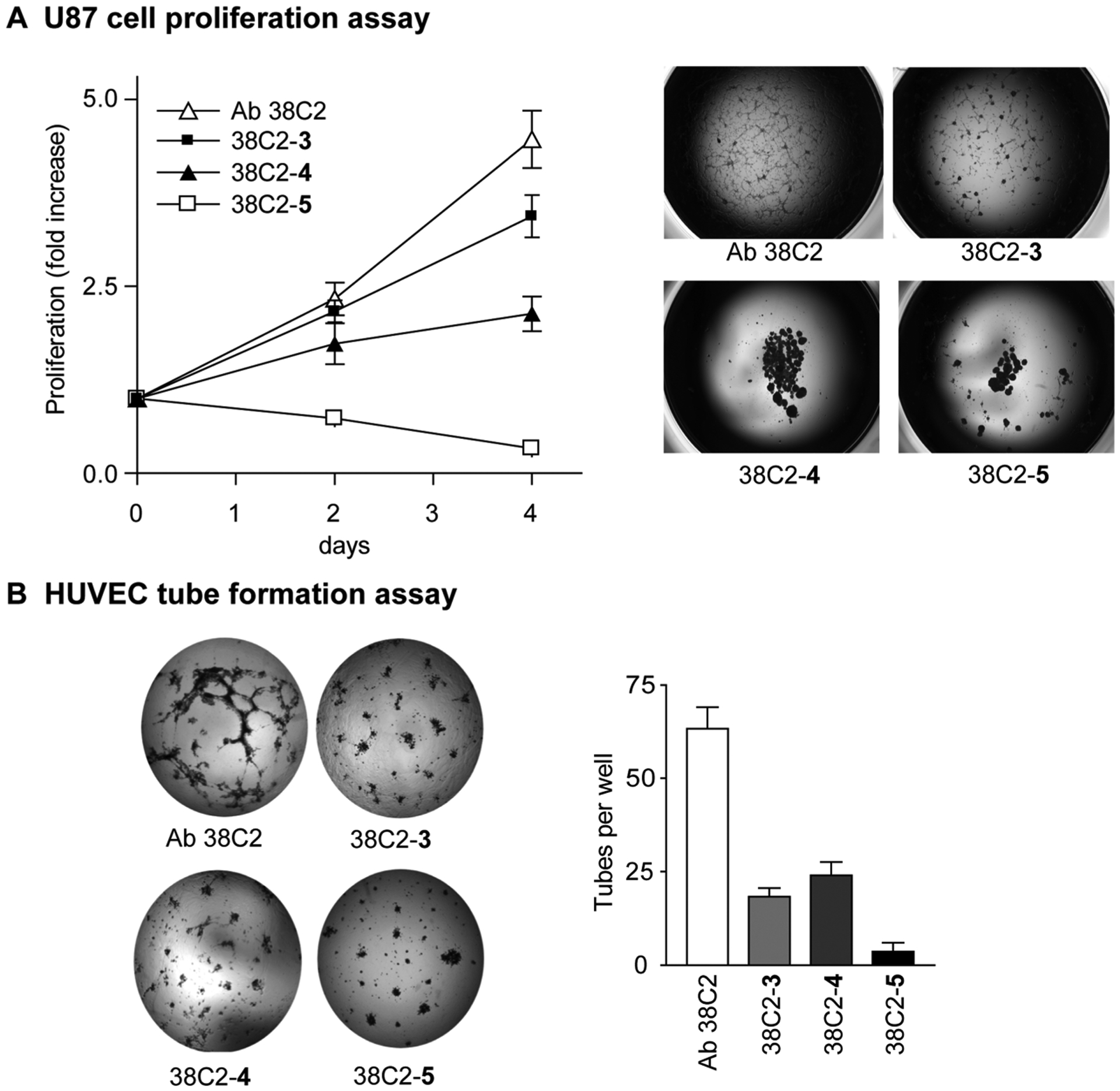
In vitro cell proliferation and tube formation assays. (A) A line graph showing the proliferation of human U87 cells treated with Ab 38C2, cpAbs 38C2-3 and 38C2-4, and cp-bsAb 38C2-5 (left) (38C2-5 vs 38C2-3, p < 0.001; 38C2-5 vs 38C2-4, p < 0.001). Microscope image showing morphology of the human U87 cells on day 6 after cells were treated with Ab 38C2 or the chem-Abs (right). (B) 38C2-3, 38C2-4, and 38C2-5 inhibit HUVEC tube formation in vitro (left). Tube formation was quantified by comparing pixel tube number in each image by using the NIH Image program (right). The experiments were repeated three times. (38C2-5 vs 38C2-3, p < 0.001; 38C2-5 vs 38C2-4, p < 0.001.) Data are represented as mean ± SEM.
Development of functional vasculatures requires precise spatial-temporal regulation of endothelial cell proliferation and invasion. To determine and analyze the effects that can be engendered by using the anti-legumain, anti-integrin cp-bsAb 38C2-5 on tumor vascularization, human endothelial cell functions were assessed in vitro in Matrigel using the tube formation assay, as described in the literature.34 Vascular tube formation was observed as early as 5 h after assay initiation, and formed within 24 h. As anticipated, both cpAbs 38C2-3 and 38C2-4 and cp-bsAb 38C2-5 inhibited the tube formation at 1 μM concentration as compared to the control experiments using Ab 38C2 (Figure 3B).
We further examined the therapeutic effects of 38C2-5 on tumor growth using human MDA-MB-231 breast cancer cells, in vivo, as compared to 38C2-3, 38C2-4, and 38C2 alone. As described previously,13 MDA-MB-231 tumor model was established on the right flank of eight-week-old Hsd:Athymic nude female mice by injecting 100 μL of single-cell suspension (5 × 105 viable cells/mL) subcutaneously. Starting on day 15 after the tumor challenge, mice were divided into four groups and treated with PBS, 38C2-3, 38C2-4, or 38C2-5 (125 μg in 200 μL of PBS) through ip injection every 5 days for a total of 8 injections per mouse. The results are shown in Figure 4A. Evidently, all three, 38C2-3, 38C2-4, and 38C2-5, inhibited tumor growth when compared to the Ab 38C2-treated control group, but 38C2-5 had a stronger inhibitory effect than 38C2-3 or 38C2-4 had. In fact, tumors in the 38C2-5-treated mice no longer grew, and instead started to shrink after 50 days of treatment (p < 0.001, n = 6). On day 55, tumors were removed and dissected to make frozen sections (5 μm) for immunofluorescent staining, which showed that legumain and integrin αvβ3 are highly expressed in the primary tumor sites (Figure 4B). CD31 staining of tumor sections revealed that the 38C2-5 treated tumors had lower vascular density than those in control mice, as well as the 38C2-3 or 38C2-4 treated groups (Figure 4C). The results were consistent with the tube formation assay results in vitro.
Figure 4.
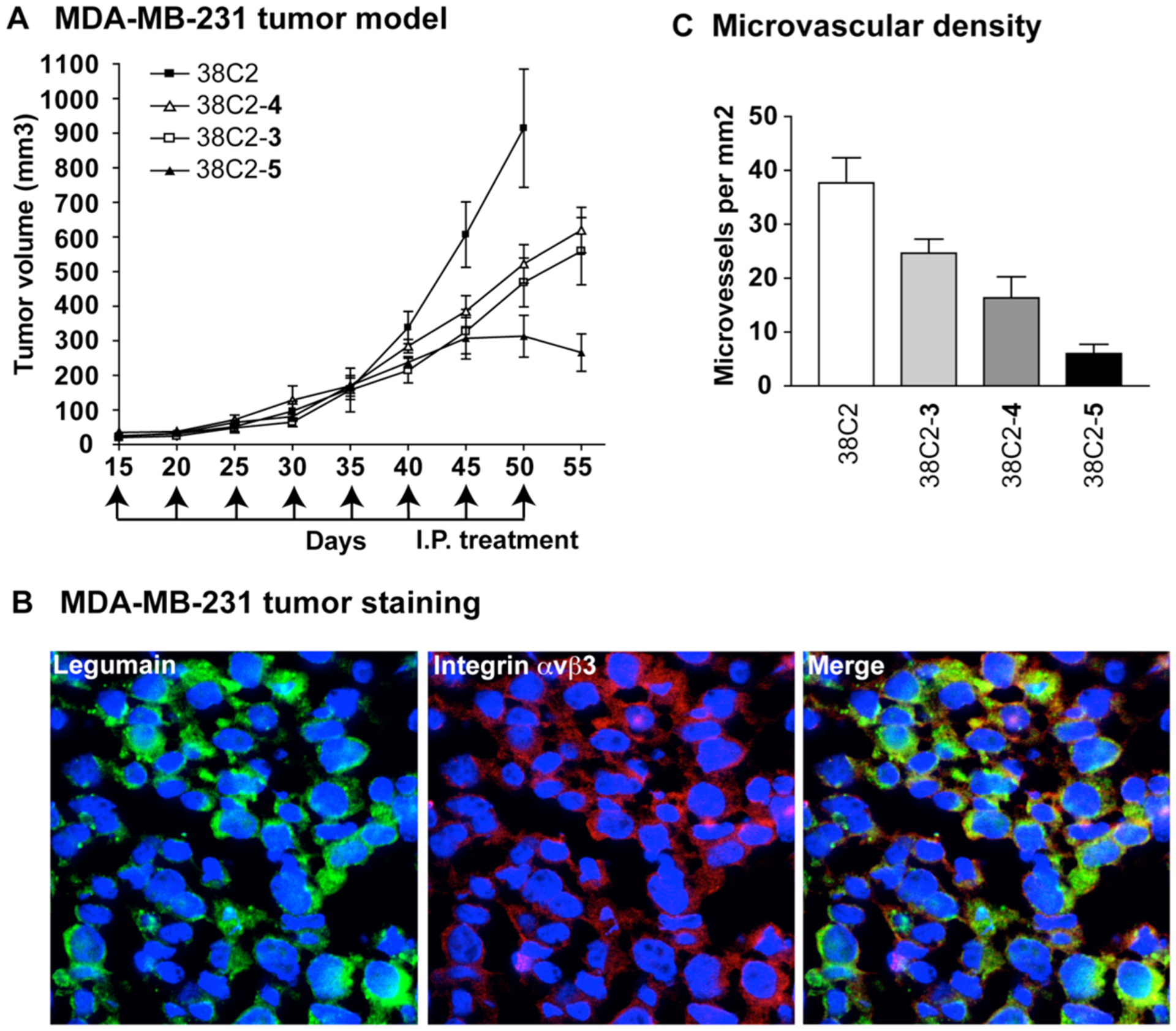
In vivo tumor growth study. (A) Inhibition of primary MDA-MB-231 tumor growth by 38C2-5. Nude mice were treated with 125 μg of 38C2, 38C2-3, 38C2-4, or 38C2-5 on day 15, 20, 25, …, 50 after 15 days of MDA-MB-231 carcinoma challenge. Primary tumor volumes were measured by micro caliper measurements (volume = (1/2)(width)2 × length) (n = 6 mice per group (38C2-5 vs 38C2-3, p < 0.01; 38C2-5 vs 38C2-4, p < 0.01)). (B) Immunofluorescence staining of MDA-MB-231 tumor tissue. Legumain and integrin αvβ3 are localized on MDA-MB-231 tumor cell surface. (C) Microvascular density quantified on tumor sections. Five sections from each mice and 4 mice from each treated group were examined. Number of microvessels per mm2 was counted under a light microscope.
Classical bispecific antibody (bsAb) is a seminatural monoclonal Ab (mAb) that can mediate superior effects compared to a monospecific mAb through binding two or more targets of interest.35 A variety of bsAbs have been designed and produced, yet development and large-scale production of a high affinity bsAb for defined targets has been difficult. In contrast, the aldolase Ab-based cp-bsAbs can be prepared on a large scale, rapidly for a variety of targets using the AACP strategy, and they are likely to bind targets with high affinity due to the bidentate character of the ligands and because the latter can reach deep inside the receptor binding sites. The cp-bsAbs can also serve as a platform for combination therapy, which will be more effective than using single agents.36 Further studies are directed to create a new Ab space using the AACP approach in the form of cp-bsAbs, which is less likely to be occupied by the classical Abs and bsAbs.
CONCLUSION
In summary, AACP has emerged as a unique strategy that allows rapid development of a desired cpAb, and has been successfully used to prepare a cp-bsAb 38C2-5 that targets legumain protease and αvβ3 integrin. In vitro and in vivo studies with 38C2-5, and the related anti-legumain and anti-αvβ3 integrin cpAbs 38C2-3 and 38C2-4, revealed that targeting legumain protease and αvβ3 integrin provides an effective treatment of human cancers. The results concur with a hypothesis that targeting two or more related pathways may be necessary to achieve effective therapy for a complex disease like cancer that progresses by numerous pathways.36 Presumably, other cell surface receptors, colocalizing on cell surface or present on two interacting cells, could be targeted similarly using the aldolase Ab-derived cpAbs for the therapeutic intervention and treatment of human cancers. The AACP approach may complement classical methods where bsAbs are prepared using antibody engineering by somatic hybridization of two different hybridomas37 or by chemical conjugation of the Ab fragments.38
Supplementary Material
ACKNOWLEDGMENTS
We thank the US Department of Defense (W81XWH-09-1-0690 to S.C.S.) for the financial support. We also thank Professor Richard A. Lerner and the late Professor Carlos F. Barbas III of the Scripps Research Institute for the helpful suggestions, and Dr. William Netzer of the Rockefeller University for reading the manuscript.
ABBREVIATIONS USED
- AACP
aldolase antibody chemical programming
- Ab
antibody
- Ab-PAs
Ab-programming agents
- AEP
asparaginyl endopeptidase
- cpAb
chemically programmed antibody
- cp-bsAb
chemically programmed bispecific antibody
- DK
diketone
- ECM
extracellular matrix
- FG
functional group
- LNK
linker
- RGD
arginine-glycine-aspartic acid
- VK
vinyl ketone
Footnotes
Supporting Information
Detailed description of the experimental procedures and additional figures. The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.molpharmaceut.5b00257.
The authors declare no competing financial interest.
REFERENCES
- (1).Chen JM; Dando PM; Rawlings ND; Brown MA; Young NE; Stevens RA; Hewitt E; Watts C; Barrett AJ Cloning, isolation, and characterization of mammalian legumain, an asparaginyl endopeptidase. J. Biol. Chem 1997, 272, 8090–98. [DOI] [PubMed] [Google Scholar]
- (2).Desgrosellier JS; Cheresh DA Integrins in cancer: biological implications and therapeutic opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Haugen MH; Johansen HT; Pettersen SJ; Solberg R; Brix K; Flatmark K; Maelandsmo GM Nuclear legumain activity in colorectal cancer. PLoS One 2013, 8, e52980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Shirahama-Noda K; Yamamoto A; Sugihara K; Hashimoto N; Asano M; Nishimura M; Hara-Nishimura I Biosynthetic processing of cathepsins and lysosomal degradation are abolished in asparaginyl endopeptidase-deficient mice. J. Biol. Chem 2003, 278, 33194–9. [DOI] [PubMed] [Google Scholar]
- (5).Chen JM; Fortunato M; Stevens RA; Barrett AJ Activation of progelatinase A by mammalian legumain, a recently discovered cysteine proteinase. Biol. Chem 2001, 382, 777–83. [DOI] [PubMed] [Google Scholar]
- (6).Brix K; McInnes J; Al-Hashimi A; Rehders M; Tamhane T; Haugen MH Proteolysis mediated by cysteine cathepsins and legumain-recent advances and cell biological challenges. Protoplasma 2015, 252 (3), 755–74. [DOI] [PubMed] [Google Scholar]
- (7).Morita Y; Araki H; Sugimoto T; Takeuchi K; Yamane T; Maeda T; Yamamoto Y; Nishi K; Asano M; Shirahama-Noda K; Nishimura M; Uzu T; Hara-Nishimura I; Koya D; Kashiwagi A; Ohkubo I Legumain/asparaginyl endopeptidase controls extracellular matrix remodeling through the degradation of fibronectin in mouse renal proximal tubular cells. FEBS Lett. 2007, 581, 1417–24. [DOI] [PubMed] [Google Scholar]
- (8).Hynes RO Integrins: bidirectional, allosteric signaling machines. Cell 2002, 110, 673–87. [DOI] [PubMed] [Google Scholar]
- (9).Luo Y; Zhou H; Krueger J; Kaplan C; Lee SH; Dolman C; Markowitz D; Wu W; Liu C; Reisfeld RA; Xiang R Targeting tumor-associated macrophages as a novel strategy against breast cancer. J. Clin. Invest 2006, 116, 2132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Popkov M; Rader C; Gonzalez B; Sinha SC; Barbas CF III. Small molecule drug activity in melanoma models may be dramatically enhanced with an antibody effector. Int. J. Cancer 2006, 119, 1194–207. [DOI] [PubMed] [Google Scholar]
- (11).Liu C; Sun C; Huang H; Janda K; Edgington T Overexpression of legumain in tumors is significant for invasion/metastasis and a candidate enzymatic target for prodrug therapy. Cancer Res. 2003, 63, 2957–64. [PubMed] [Google Scholar]
- (12).Guo F; Das S; Mueller BM; Barbas CF III; Lerner RA; Sinha SC Breaking the one antibody-one target axiom. Proc. Natl. Acad. Sci. U.S.A 2006, 103, 11009–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Liu Y; Bajjuri KM; Liu C; Sinha SC Targeting cell surface alpha(v)beta(3) integrin increases therapeutic efficacies of a legumain protease-activated auristatin prodrug. Mol. Pharmaceutics 2012, 9, 168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Dall E; Brandstetter H Mechanistic and structural studies on legumain explain its zymogenicity, distinct activation pathways, and regulation. Proc. Natl. Acad. Sci. U.S.A 2013, 110, 10940–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Dall E; Brandstetter H Activation of legumain involves proteolytic and conformational events, resulting in a context- and substrate-dependent activity profile. Acta Crystallogr., Sect. F: Struct. Biol. Cryst. Commun 2012, 68, 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Gavrilyuk JI; Wuellner U; Salahuddin S; Goswami RK; Sinha SC; Barbas CF III. An efficient chemical approach to bispecific antibodies and antibodies of high valency. Bioorg. Med. Chem. Lett 2009, 19, 3716–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Doppalapudi VR; Huang J; Liu D; Jin P; Liu B; Li L; Desharnais J; Hagen C; Levin NJ; Shields MJ; Parish M; Murphy RE; Del Rosario J; Oates BD; Lai J-Y; Matin MJ; Ainekulu Z; Bhat A; Bradshaw CW; Woodnutt G; Lerner RA; Lappe RW Chemical generation of bispecific antibodies. Proc. Natl. Acad. Sci. U.S.A 2010, 107, 22611–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Rader C; Sinha SC; Popkov M; Lerner RA; Barbas CF III. A chemically programmed monoclonal antibody for cancer therapy. Proc. Natl. Acad. Sci. U.S.A 2003, 100, 5396–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Sinha SC; Das S; Li L-S; Lerner RA; Barbas CF III. Preparation of integrin alpha(v)beta(3)-targeting Ab 38C2 constructs. Nat. Protoc 2007, 2, 449–56. [DOI] [PubMed] [Google Scholar]
- (20).Li L-S; Rader C; Matsushita M; Das S; Barbas CF III; Lerner RA; Sinha SC Chemical-Adaptor Immunotherapy: Design, synthesis and evaluation of novel integrin-targeting devices. J. Med. Chem 2004, 47, 5630–40. [DOI] [PubMed] [Google Scholar]
- (21).Goswami RK; Bajjuri KM; Forsyth JS; Das S; Hassenpflug W; Huang ZZ; Lerner RA; Felding-Habermann B; Sinha SC Chemically Programmed Antibodies Targeting Multiple Alpha(V) Integrins And Their Effects On Tumor-Related Functions In Vitro. Bioconjugate Chem. 2011, 22, 1535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Rader C Chemically programmed antibodies. Trends Biotechnol. 2014, 32, 186–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).McEnaney PJ; Parker CG; Zhang AX; Spiegel DA Antibody-recruiting molecules: an emerging paradigm for engaging immune function in treating human disease. ACS Chem. Biol 2012, 7, 1139–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Gavrilyuk JI; Wuellner U; Barbas CF III. β-Lactam-based approach for the chemical programming of aldolase antibody 38C2. Bioorg. Med. Chem. Lett 2009, 19, 1421–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Götz MG; James KE; Hansell E; Dvorák J; Seshaadri A; Sojka D; Kopacek P; McKerrow JH; Caffrey CR; Powers JC Aza-peptidyl Michael acceptors. A new class of potent and selective inhibitors of asparaginyl endopeptidases (legumains) from evolutionarily diverse pathogens. J. Med. Chem 2008, 51, 2816–32. [DOI] [PubMed] [Google Scholar]
- (26).Duggan ME; Duong LT; Fisher JE; Hamill TG; Hoffman WF; Huff JR; Ihle NC; Leu CT; Nagy RM; Perkins JJ; Rodan SB; Wesolowski G; Whitman DB; Zartman AE; Rodan GA; Hartman GD Nonpeptide alpha(v)beta(3) antagonists. 1. tumors is significant for invasion/alpha-(IIb)beta(3) antagonist into a potent alpha(v)beta(3) antagonist. J. Med. Chem 2000, 43, 3736–45. [DOI] [PubMed] [Google Scholar]
- (27).Wagner J; Lerner RA; Barbas CF III. Efficient aldolase catalytic antibodies that use the enamine mechanism of natural enzymes. Science 1995, 270, 1797–800. [DOI] [PubMed] [Google Scholar]
- (28).Goswami RK; Huang Z-Z; Forsyth JS; Felding-Habermann B; Sinha SC Multiple Catalytic Aldolase Antibodies Suitable For Chemical Programming. Bioorg. Med. Chem. Lett 2009, 19, 3821–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).List B; Barbas CF III; Lerner RA Aldol sensors for the rapid generation of tunable fluorescence by antibody catalysis. Proc. Natl. Acad. Sci. U.S.A 1998, 95, 15351–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Friedlander DR; Zagzag D; Shiff B; Cohen H; Allen JC; Kelly PJ; Grume M Migration of brain tumor cells on extracellular matrix proteins in vitro correlates with tumor type and grade and involves av and β1 integrins. Cancer Res. 1996, 56, 1939–47. [PubMed] [Google Scholar]
- (31).Walker-Nasir E; Codington JF; Jahnke MR; Fuller TC; Jeanloz RW Isolation and partial characterization of surface components of cell line MDA-MB-231 derived from a human metastatic breast carcinoma. J. Natl. Cancer Inst 1982, 69, 371–80. [PubMed] [Google Scholar]
- (32).Horoszewicz JS; Leong SS; Kawinski E; Karr JP; Rosenthal H; Chu TM; Mirand EA; Murphy GP LNCaP model of human prostatic carcinoma. Cancer Res. 1983, 43, 1809–18. [PubMed] [Google Scholar]
- (33).Goswami RK; Liu Y; Liu C; Lerner RA; Sinha SC Synthesis and evaluation of the aldolase antibody-derived chemical-antibodies targeting α5β1 integrin. Mol. Pharmaceutics 2013, 10, 538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Matsumura T; Wolff K; Petzelbauer P Endothelial cell tube formation depends on cadherin 5 and CD31 interactions with filamentous actin. J. Immunol 1997, 158, 3408–16. [PubMed] [Google Scholar]
- (35).May C; Sapra P; Gerber HP Advances in bispecific biotherapeutics for the treatment of cancer. Biochem. Pharmacol 2012, 84, 1105–12. [DOI] [PubMed] [Google Scholar]
- (36).LoRusso PM; Canetta R; Wagner JA; Balogh EP; Nass SJ; Boerner SA; Hohneker J Accelerating cancer therapy development: The importance of combination strategies and collaboration. Summary of an Institute of Medicine Workshop. Clin. Cancer Res 2012, 18, 6101–9. [DOI] [PubMed] [Google Scholar]
- (37).Milstein C; Cuello AC Hybrid hybridomas and their use in immunohistochemistry. Nature 1983, 305, 537–40. [DOI] [PubMed] [Google Scholar]
- (38).Ellerman D; Scheer JM Generation of bispecific antibodies by chemical conjugation. In Bispecific Antibodies; Kontermann RE, Ed.; Springer; (www.springer.com): 2011; pp 47–63. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


